
I.Epidemiology. Acute myocardial infarction (MI) is the leading cause of death in North America and Europe. Each year, an estimated 650,000 Americans will sustain a new MI, and another 300,000 will have a recurrent MI. Coronary artery disease is the leading cause of death in the United States and has been for the past 90 years. However, the incidence of and mortality associated with acute MI have declined dramatically over the last 30 years with the advent of the coronary care unit, fibrinolytic therapy, catheter-based reperfusion, and lipid-modifying therapy. The aging of the population in advanced economies and the global increased incidence of diabetes and obesity will, however, increase the burden of atherosclerotic coronary artery disease in the future.
II.Pathophysiology. In most patients, coronary plaque rupture is the initiating event of acute MI. Rupture of the thin fibrous cap of a coronary atheroma exposes the underlying subendothelial matrix to formed elements of circulating blood, leading to activation of platelets, thrombin generation, and thrombus formation. Erosion of a coronary plaque without rupture can also lead to thrombus formation and is estimated to cause up to 25% of MIs. Acute coronary syndrome (ACS) is a dynamic process that involves cyclical transitioning among complete vessel occlusion, partial vessel occlusion, and reperfusion. Occlusive thrombus in the absence of significant collateral vessels most often results in acute ST-segment elevation myocardial infarction (STEMI). The pathophysiology of STEMI and non-STEMI (NSTEMI) is similar, and this explains the substantial overlap in ACSs with regard to ultimate outcome, extent of necrosis, and mortality rates. The recognition of ST-segment elevation is particularly important because it generally mandates the need for emergent reperfusion therapy.
III.Definition. A 2012 expert consensus document defined acute MI as evidence of myocardial necrosis in a clinical setting consistent with acute myocardial ischemia. This concept was further described as the detection of a rise and/or fall in cardiac troponin (cTn) with at least one value above the 99th percentile of the upper reference limit (URL) together with evidence of ischemia. Ischemia was defined as any symptom supportive of ischemia, electrocardiographic changes suggestive of new ischemia, development of pathologic Q-waves on electrocardiogram (ECG), and imaging evidence of infarction or identification of an intracoronary thrombus on angiography or autopsy. Included in the definition were sudden cardiac death (SCD) with evidence of myocardial ischemia (new ST-elevation, left bundle branch block [LBBB], or coronary thrombus), documented stent thrombosis by angiography or autopsy, and biomarker elevation >5× URL for post–percutaneous coronary intervention (PCI) patients with normal baseline values or a rise of >20% if baseline values are stable or falling and biomarker elevation >10× URL for post–coronary artery bypass grafting (post-CABG) patients (Table 1.1). Established MI was defined as any one criterion that satisfies the following: development of new pathologic Q-waves on ECG with or without symptoms in the absence of nonischemic causes, imaging evidence of MI, or pathologic findings of healed or healing MI.
TABLE 1.1 Clinical Classification of Different Types of MI |
|
Type 1 |
Spontaneous MI related to ischemia from a coronary plaque rupture, erosion, or dissection |
Type 2 |
MI because of ischemia resulting from increased oxygen demand or decreased supply (“demand ischemia”) |
Type 3 |
SCD with symptoms of ischemia, new ST-elevation, or LBBB, but biomarkers are unavailable |
Type 4a |
MI associated with PCI |
Type 4b |
MI associated with stent thrombosis |
Type 5 |
MI associated with CABG |
CABG, coronary artery bypass grafting; LBBB, left bundle branch block; MI, myocardial infarction; PCI, percutaneous coronary intervention; SCD, sudden cardiac death.
Adapted from Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035; American Heart Association, Inc.
IV.Clinical Diagnosis. In any patient with a clinical history of chest pain suspected to be of cardiac origin, an ECG should be promptly obtained. Ideally, this should be within 10 minutes of presentation to an emergency room or outpatient center or first medical contact and interpreted promptly to determine eligibility for reperfusion therapy. If the ECG demonstrates acute ST-segment elevation or new LBBB, emergent reperfusion treatment with primary PCI or fibrinolysis is indicated. During this evaluation period, a targeted medical history and physical examination should be performed. If the patient’s history is compatible with cardiac ischemia and the ECG does not meet the criteria for emergent reperfusion therapy, the patient may have unstable angina or NSTEMI. These syndromes are discussed in Chapter 2.
A.Signs and symptoms
1.The classic symptoms of an acute MI is characterized by severe, crushing substernal chest pain described as a squeezing or constricting sensation with frequent radiation to the left arm, often associated with an impending sense of doom. The discomfort is similar to that of angina pectoris, but it is typically more severe, of longer duration (usually >20 minutes), and is not relieved with rest or nitroglycerin. Peak intensity is usually gradual and not instantaneous, as it would be with other cardiac emergencies like a pulmonary embolism (PE) or acute aortic syndrome.
a.The chest discomfort may radiate to the neck, jaw, back, shoulder, right arm, and epigastrium. Pain in any of these locations without chest pain is possible. Myocardial ischemic pain localized to the epigastrium is often misdiagnosed as indigestion. Symptoms may be atypical in the elderly, in women, and in patients with diabetes mellitus.
b.If the pain is sudden, radiates to the back, and is described as tearing or knifelike, aortic dissection should be considered.
2.Associated symptoms may include diaphoresis, dyspnea, fatigue, lightheadedness, palpitations, acute confusion, indigestion, nausea, or vomiting. Gastrointestinal symptoms are especially common with inferior infarction. Ischemic chest pain rarely radiates below the umbilicus.
B.Physical examination. Although the physical examination does not add much to the diagnosis of acute MI, the presence of heart failure on examination identifies patients at heightened clinical risk. The examination is also extremely important in excluding other diagnoses that may mimic acute MI, in risk stratification, and in serving as a baseline examination to monitor for mechanical complications of acute MI that may develop. The mechanical complications of papillary muscle rupture with acute mitral regurgitation and ventricular septal defect are often heralded by a new systolic murmur (see Chapter 3). Early diagnosis of these complications relies on well-documented examination findings at baseline and during the hospital course.
V.Differential diagnosis. The differential diagnosis of ST-elevation on a surface ECG includes conditions with comorbid ischemia such as acute aortic dissection involving the root, conditions with ST-elevation but no ischemia such as left ventricular (LV) hypertrophy or early repolarization abnormality, and conditions with chest pain but no ischemia such as myopericarditis (Table 1.2). The most common differential diagnostic considerations are discussed in the following text.
TABLE 1.2 Differential Diagnostic Considerations for STEMI |
||
Comorbid Ischemia |
ST-Elevation but No Ischemia |
Chest Pain but No Ischemia |
Aortic dissection |
Early repolarization |
Aortic dissection |
Stress cardiomyopathy |
LV hypertrophy |
Stress cardiomyopathy |
Systemic arterial embolism |
LBBB |
Myopericarditis |
Hypertensive crisis |
Hyperkalemia |
Pleuritis |
Aortic stenosis |
Brugada syndrome |
PE |
Cocaine use |
Costochondritis |
|
Arteritis |
Gastrointestinal disorders |
|
LBBB, left bundle branch block; LV, left ventricular; PE, pulmonary embolism; STEMI, ST-segment elevation myocardial infarction.
Adapted with permission from Christofferson RD. Acute ST-elevation myocardial infarction. In: Shishehbor MH, Wang TH, Askari AT, et al, eds. Management of the Patient in the Coronary Care Unit. New York, NY: Lippincott Williams & Wilkins; 2008.
A.Pericarditis. Chest pain that is worse when the person is supine and improves when the person is sitting upright or slightly forward is typical of pericarditis. Care must be taken in excluding acute MI, however, because pericarditis can complicate acute MI. The electrocardiographic abnormalities of acute pericarditis may also be confused with acute MI. Diffuse ST-segment elevation is the hallmark of acute pericarditis, but this finding may be seen in acute MI that involves the left main coronary artery or a large “wraparound” left anterior descending artery. PR-segment depression, peaked T-waves, or electrocardiographic abnormalities out of proportion to the clinical scenario may favor the diagnosis of pericarditis. The ST-segment elevations in pericarditis are often concave, whereas the ST-segment elevations in acute MI are usually convex. Reciprocal ST-depression does not occur in pericarditis, except in leads aVR and V1. Early T-wave inversion is not a feature of acute pericarditis. Echocardiography may be useful, not in evaluating for pericardial effusion, which may occur in either condition, but in documenting the lack of regional wall motion abnormalities in a coronary distribution in the setting of ongoing pain and ST-elevation.
B.Myocarditis. As with pericarditis, the symptoms and electrocardiographic findings of myocarditis may be similar to those of acute MI. Echocardiography is less useful in differentiating this syndrome from acute MI, because segmental LV dysfunction may be encountered in either condition. With myocarditis, a complete history may reveal a more insidious onset and an associated viral syndrome. The diagnosis of myocarditis can be confirmed with cardiac magnetic resonance imaging (MRI) with gadolinium. A patchy distribution of delayed hyperenhancement in the epicardium and mid-myocardium with sparing of the endocardium is the characteristic MRI finding with myocarditis.
C.Stress cardiomyopathy (Takotsubo). Following an acute emotional or physical stress, patients may present with typical chest pain, ischemic ECG changes, a mild troponin elevation and new onset, transient regional LV wall motion abnormalities. In the absence of obstructive epicardial coronary artery disease, this clinical presentation may be explained by a stress-induced cardiomyopathy. The presenting signs and symptoms of this syndrome can be identical to an acute MI, making stress cardiomyopathy a diagnosis of exclusion. Stress cardiomyopathy is also called apical ballooning syndrome as the regional wall motion abnormalities tend to preferentially affect the apex while sparing the basal and mid-ventricular segments, although many other variants have been described. The term “takotsubo” comes from the Japanese word for “octopus pot” as the apical ballooning as seen on imaging represents the shape of this octopus trap. Stress cardiomyopathy occurs most commonly in women and in older adults. In addition to an ECG and an echo, the evaluation of stress-induced cardiomyopathy generally requires coronary angiography to document the absence of obstructive coronary artery disease. The LV wall motion usually but not invariably returns to normal over time and recurrence may occur following another stressful event.
D.Acute aortic dissection. Sharp, tearing chest pain that radiates through the chest to the back is typical of aortic dissection. Chest pain with new neurologic deficits or symptoms may also be a presenting sign of an aortic dissection with both coronary and carotid involvement. This type of chest pain pattern should be investigated thoroughly before administration of antithrombotic, antiplatelet, or fibrinolytic therapy. Proximal extension of the dissection into either coronary ostium can account for acute MI. A chest radiograph may reveal a widened mediastinum. Transthoracic echocardiography (TTE) may reveal a dissection flap in the proximal ascending aorta. If aortic dissection is suspected, a computerized tomography (CT) scan of the chest with contrast should promptly be obtained. In the presence of acute renal failure or if CT is unavailable, transesophageal echocardiography is an alternative imaging modality to evaluate for aortic dissection.
E.PE. Shortness of breath associated with pleuritic chest pain but without evidence of pulmonary edema is suggestive of PE. Echocardiography helps to rule out wall motion abnormalities and may identify right ventricular (RV) dilatation and dysfunction in the setting of PE. A clot in transit may rarely be seen on TTE. In the absence of renal dysfunction, a CT PE protocol helps confirm the diagnosis.
F.Esophageal disorders. Gastroesophageal reflux disease, esophageal motility disorders, and esophageal hyperalgesia can cause chest pain, the character of which can mimic cardiac ischemic pain. These disorders can often coexist in patients with coronary disease, thereby complicating the diagnosis. A workup for coronary disease should precede evaluation of esophageal disorders. Symptoms that may be suggestive but not diagnostic of chest pain of an esophageal origin include postprandial symptoms, relief with antacids, and a lack of radiation of the pain.
G.Acute cholecystitis can occasionally mimic the symptoms and ECG findings of inferior acute MI, but rarely can the two coexist. Tenderness in the right upper quadrant, fever, and an elevated leukocyte count favor cholecystitis. Confirmatory imaging should be obtained if acute cholecystitis is suspected.
H.Brugada syndrome. In this setting, a genetic defect in myocardial sodium channels predisposes patients to ventricular fibrillation and SCD. Patients with Brugada do not typically present with acute chest pain, but may present with SCD. Twelve lead ECG may show a pseudo-right bundle branch block (RBBB) pattern and ST-elevation in V1–V2, typical findings for type 1 Brugada pattern. The mechanism of SCD in Brugada is not intracoronary thrombus, but believed to be secondary to the altered sodium channels and variability in refractory periods in adjacent myocardium.
VI.Laboratory examination (Fig. 1.1)
A.Cardiac troponin. Both fourth-generation and high-sensitivity troponin T and troponin I assays are particularly useful in the diagnosis and management of non–ST-elevation ACS because of their high sensitivity to detect myocardial injury. In the setting of ST-elevation on ECG, waiting for confirmatory testing with cTn is not indicated. A single troponin fourth-generation T concentration measured 72 hours after acute MI may be predictive of MI size, independent of reperfusion. Troponin elevation in the absence of ischemic heart disease can be found in a number of clinical settings including congestive heart failure (CHF), aortic dissection, hypertrophic cardiomyopathy, PE, acute neurologic disease, cardiac contusion, or drug toxicity.
B.High-sensitivity cardiac troponin (hs-cTn). Recently approved in the United States, the development of hs-cTn has increased our ability to detect myocardial injury early and accurately. By definition, hs-cTn must be able to detect concentrations below the 99th percentile above the assay’s lower limit of detection for more than 50% of healthy population and should have a coefficient of variance of <10% at the 99th percentile value. Hs-cTn assays are, however, best utilized to rapidly and reliably rule out myocardial ischemia, especially in patients presenting acutely with chest pain because of the test’s high negative predictive value. If the initial hs-cTn value is negative, a second value can be measured as early as 1 to 3 hours without compromising sensitivity or negative predictive value. The shortened time interval to a second biomarker measurement can translate into a more rapid rule out for acute MI and shorten emergency room stays for patients presenting with suspected acute chest pain.
C.Creatine kinase and creatine kinase myocardial band (CK and CK-MB). An elevated level of CK and CK-MB is rarely helpful in making the diagnosis of acute MI for a patient with ST-segment elevation, and both assays are now of historical significance. All major societal guidelines recommend the use of cTn over both CK and CK-MB as the preferred marker of myocardial injury. Because it usually takes 4 to 6 hours to see an appreciable rise in CK levels, an initial normal value does not exclude recent complete occlusion. CK levels remain helpful in gauging the size and timing of acute MI. CK levels peak at 24 hours, but early peaking is noted among patients who undergo successful reperfusion of the infarct-related artery. The presence of a positive CK, CK-MB assay in the setting of negative troponin almost certainly indicates a noncardiac source, including skeletal muscle disease or trauma (e.g., rhabdomyolysis).

FIGURE 1.1 Timing of biomarker release after acute myocardial infarction. AMI, acute myocardial infarction; CK, creatine kinase; CK-MB, creatine kinase myocardial band; CV, coefficient of variation; MI, myocardial infarction. (Reprinted from Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction—executive summary. J Am Coll Cardiol. 2007;50(7):652–726. Copyright © 2007 American College of Cardiology Foundation and the American Heart Association, Inc. With permission.)
VII.Diagnostic testing
A.Electrocardiography
1.Definitive electrocardiographic diagnosis of acute MI requires ST-elevation of 1 mm or more in two or more contiguous leads, often with reciprocal ST-depression in the contralateral leads. In leads V2–V3, ST-elevation of at least 2 mm in men ≥40 years, 2.5 mm in men <40 years, and 1.5 mm in women are required for accurate diagnosis.
2.ECG subsets. ST-segment elevations can be divided into subgroups that may be correlated with the infarction-related artery and risk of death. These five subgroups are listed in Table 1.3 and illustrated in Figure 1.2.
TABLE 1.3 Acute MI: ECG Subsets and Correlated Infarct-Related Artery and Mortality |
||||
Category |
Anatomy of Occlusion |
ECG Findings |
30-D Mortality Rate (%)a |
1-Y Mortality Rate (%) |
1. Proximal LAD |
Proximal to first septal perforator |
ST ↑ V1–V6, I, aVL, and fascicular or bundle branch block |
19.6 |
25.6 |
2. Mid-LAD |
Proximal to large diagonal but distal to first septal perforator |
ST ↑ V1–V6, I, aVL |
9.2 |
12.4 |
3. Distal LAD or diagonal |
Distal to large diagonal or diagonal itself |
ST ↑ V1–V4, or I, aVL, V5, V6 |
6.8 |
10.2 |
4. Moderate to large inferior (posterior, lateral, RV) |
Proximal RCA or left circumflex |
ST ↑ II, III, aVF, and any of the following: (a) V1, V3R, V4R; (b) V5, V6; (c) R > S in V1, V2 |
6.4 |
8.4 |
5. Small inferior |
Distal RCA or left circumflex branch |
ST ↑ II, III, aVF only |
4.5 |
6.7 |
ECG, electrocardiogram; LAD, left anterior descending (coronary artery); MI, myocardial infarction; RCA, right coronary artery; RV, right ventricular; ↑, increased.
aMortality rate based on GUSTO I cohort population in each of the 5-year categories, all receiving reperfusion therapy.
Reprinted with permission from Topol EJ, Van de Werf FJ. Acute myocardial infarction: early diagnosis and management. In: Topol EJ, ed. Textbook of Cardiovascular Medicine. 1st ed. New York, NY: Lippincott-Raven; 1998.

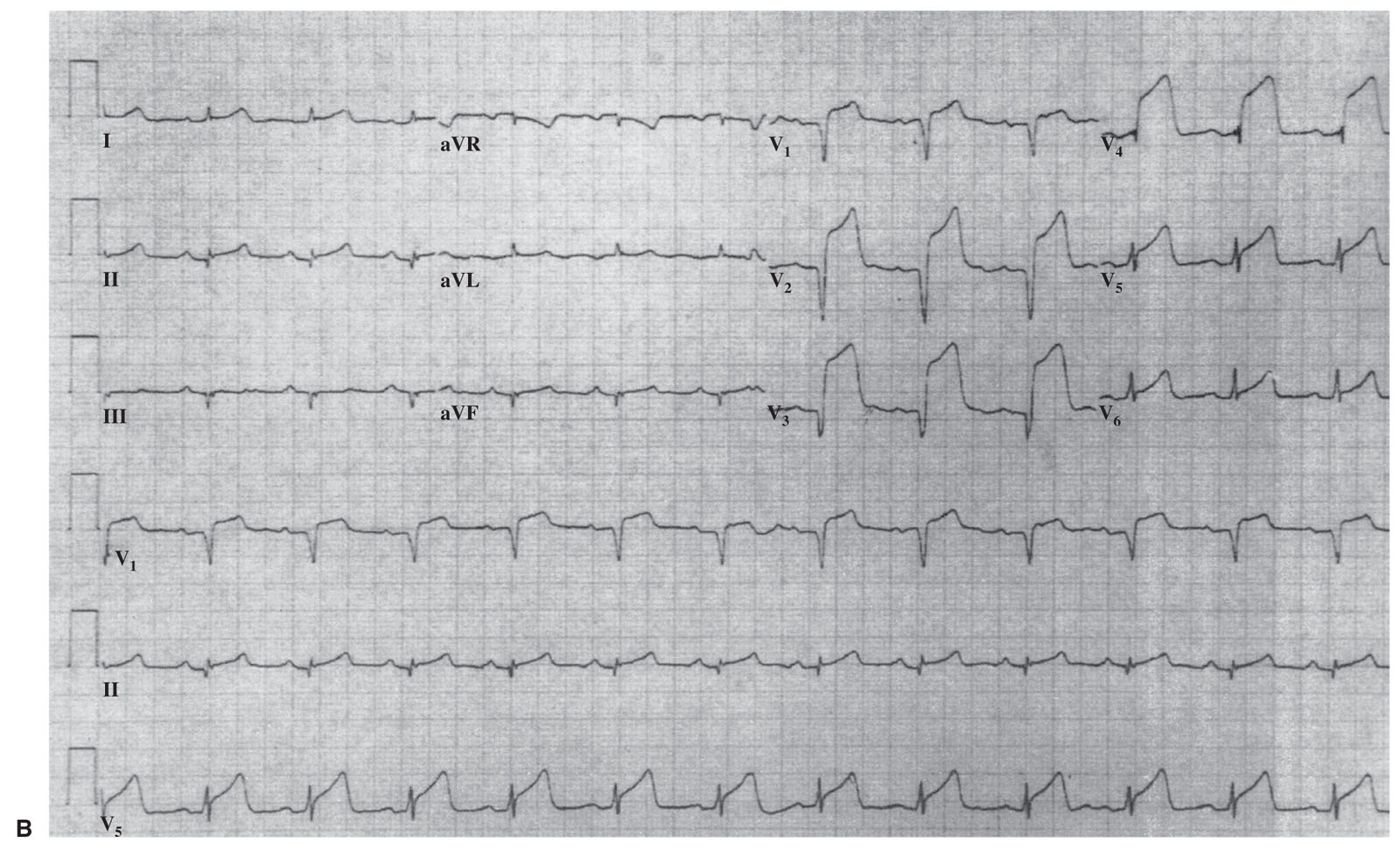
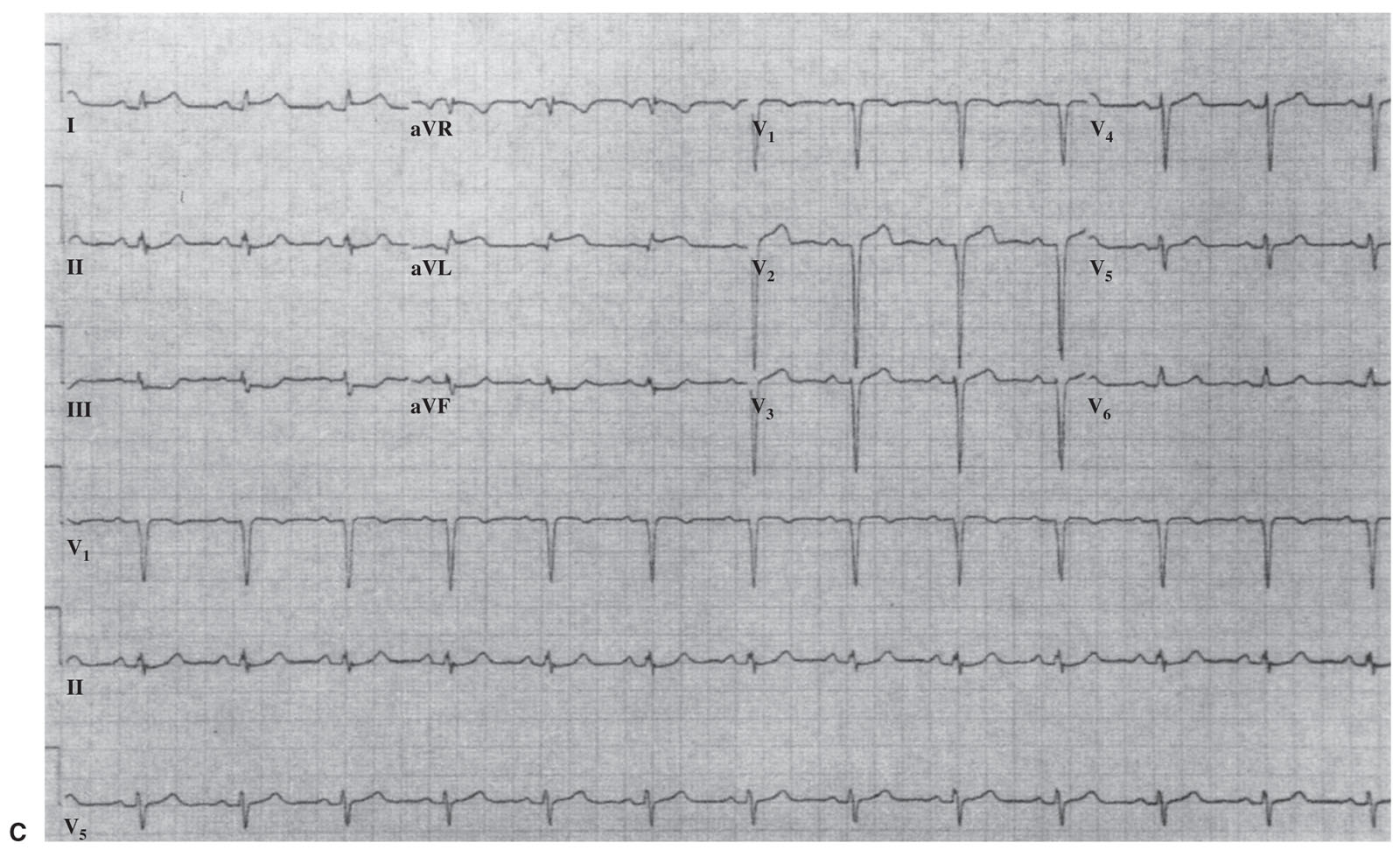
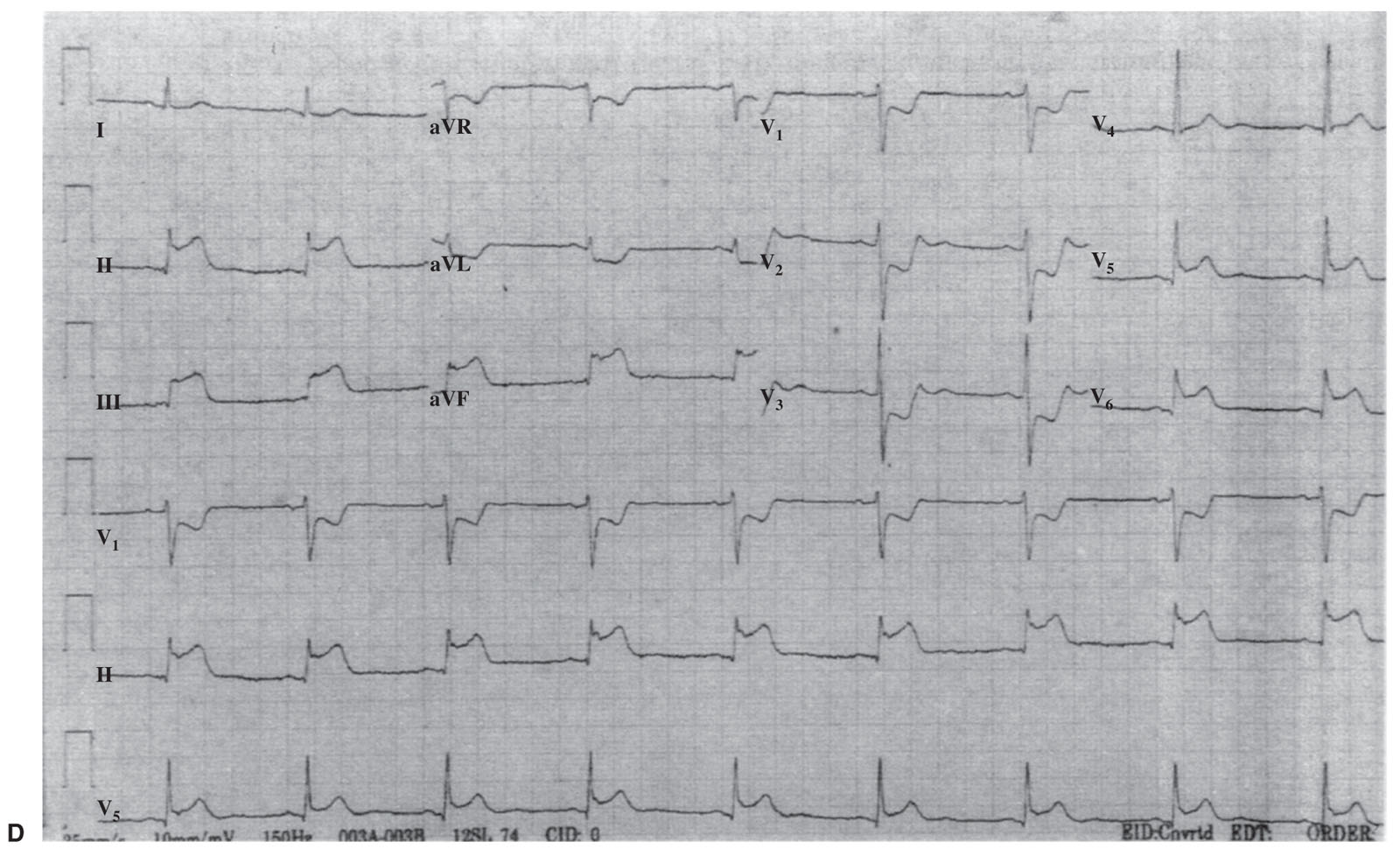

FIGURE 1.2 Electrocardiographic subsets of acute myocardial infarction (MI). A: Large anterior MI with conduction disturbance (proximal left anterior descending [LAD] coronary artery). B: Anterior MI without conduction disturbance (mid-LAD). C: Lateral MI (distal LAD, diagonal branch, or left circumflex branch). D: Large inferior MI with reciprocal changes (proximal right coronary artery [RCA]). E: Small inferior MI (distal RCA). (Reprinted with permission from Topol EJ, Van de Werf FJ. Acute myocardial infarction: early diagnosis and management. In: Topol EJ, ed. Textbook of Cardiovascular Medicine. 2nd ed. New York, NY: Lippincott-Raven; 2002.)
a.New LBBB in the setting of symptoms consistent with acute MI may indicate a large, anterior wall, acute MI involving the proximal left anterior descending coronary artery and should be managed as acute STEMI.
b.In the absence of an old ECG or in the presence of LBBB at baseline, the diagnosis of acute STEMI can be made with >90% specificity on the basis of the criteria listed in Table 1.4 and illustrated in Figure 1.3.
c.RBBB may challenge interpretation of ST-elevation in leads V1 through V3. RBBB does not, however, obscure ST-segment elevation, and criteria for ST-elevation apply.
B.Echocardiography may be helpful in the evaluation of LBBB of undetermined duration in that the lack of regional wall motion abnormality in the presence of continuing symptoms makes the diagnosis of acute MI unlikely. It is worth noting that abnormal septal motion is often observed in the setting of LBBB even in the absence of ischemia. Echo may also be useful in detecting extensive wall motion abnormalities in the posterior circulation when the presenting electrocardiogram (EKG) is normal or reveal ST-depression consistent with the presence of a true posterior infarction.
VIII.Risk stratification. Early risk stratification in patients with acute MI is crucial, especially in those being considered for fibrinolysis, because the risks of therapy must be weighed against the potential benefits. Five simple baseline parameters have been reported to account for >90% of the prognostic information for 30-day mortality. These characteristics are given in descending order of importance: age, systolic blood pressure, Killip classification (Table 1.5), heart rate, and location of MI (Table 1.3; Fig. 1.2). Timely, optimally performed primary PCI is the preferred reperfusion strategy for all patients with STEMI, but patients with evidence of acute heart failure or cardiogenic shock are especially well suited for treatment with primary PCI. In addition, various risk models have been created to improve risk prediction.
A.The Global Registry of Acute Coronary Events score is used to predict in-hospital mortality and postdischarge to 6-month mortality in all patients with ACS. Risk is calculated based on Killip class, heart rate, systolic blood pressure, serum creatinine, age, presence of cardiac arrest at admission, presence of cardiac biomarkers, and ST-segment deviation. Patients with a score of ≤60 have a ≤0.2% probability of in-hospital mortality, whereas patients with a score of ≥250 have a ≥52% probability of in-hospital mortality.
B.The thrombolysis in myocardial infarction (TIMI) risk score was developed in patients with ST-elevation who received fibrinolysis and incorporates eight variables obtained from the history, physical examination, and ECG (Table 1.6). In patients treated with fibrinolysis, a TIMI score of 9 or greater predicts a 30-day mortality of approximately 35%. In patients with a TIMI score of 0 or 1, the 30-day mortality rate is <2%. The strongest predictor of poor prognosis is advanced age (where age ≥75 years receives 3 points and age 65 to 74 years receives 2 points). Other variables that predict a poor prognosis include hypotension, Killip class II–IV at presentation, tachycardia, history of diabetes or hypertension, anterior ST-elevation (also complete LBBB), low body weight, and a time to treatment of >4 hours.
TABLE 1.4 Electrocardiographic Criteria for the Diagnosis of Acute MI in the Presence of LBBB |
|
Criterion |
Scorea |
ST-segment elevation ≥ 1 mm concordant with QRS |
5 |
ST-segment depression ≥ 1 mm in leads V1, V2, or V3 |
3 |
ST-segment elevation ≥ 5 mm discordant with QRS |
2 |
LBBB, left bundle branch block; MI, myocardial infarction.
aPoint scores for each criterion met are added. Total point score of 3 yields ≥90% specificity and an 88% positive predictive value.
From Sgarbossa EB, Pinski SL, Barbagelata A, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle branch block. N Engl J Med. 1996;334:481–487. Copyright © 1996 Massachusetts Medical Society. Adapted with permission from Massachusetts Medical Society.
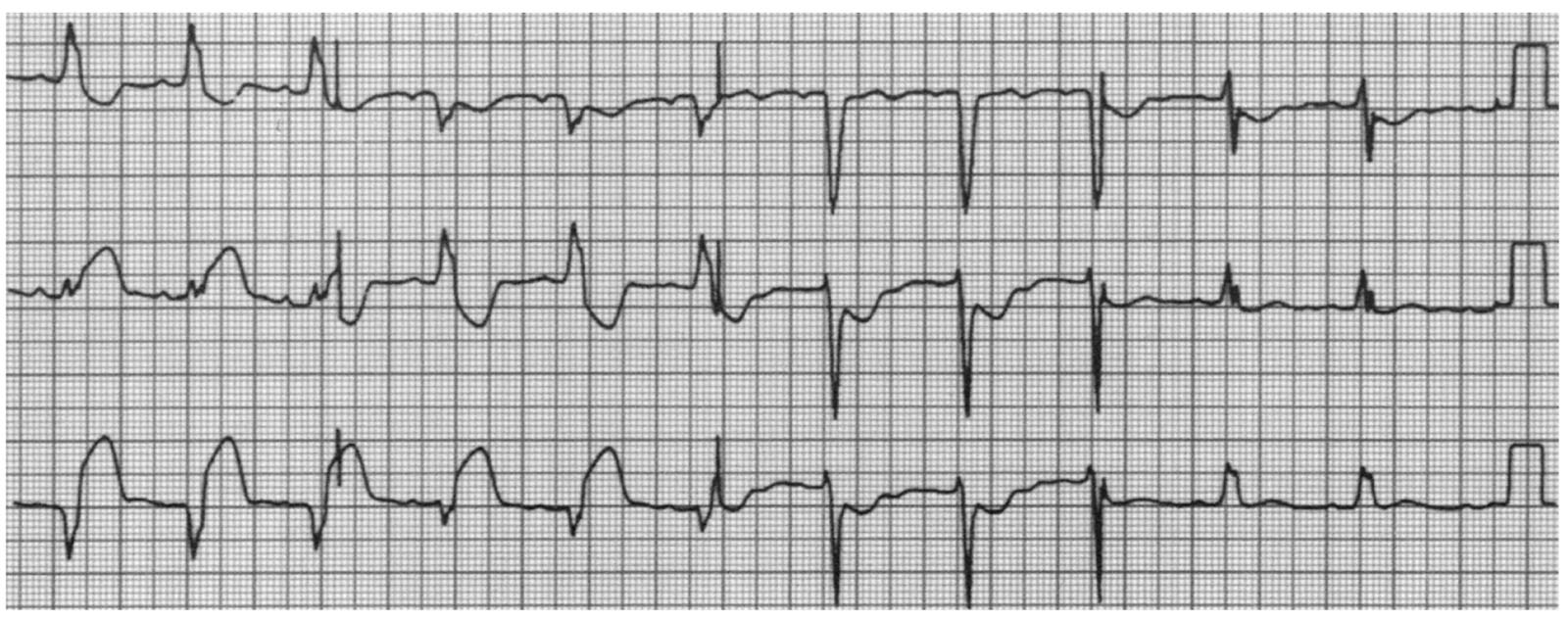
FIGURE 1.3 Electrocardiogram displays all of the criteria for the diagnosis of acute myocardial infarction (MI) in the setting of left bundle branch block: ST-segment elevation > 1 mm, concordant with QRS in lead II (5 points); ST-segment depression > 1 mm in leads V2 and V3 (3 points); and ST-segment elevation > 5 mm, discordant with QRS in leads III and VF (2 points). A score of 10 points indicates an extremely high likelihood of inferior MI. (From Sgarbossa EB, Pinski SL, Barbagelata A, et al. Electrocardiographic Diagnosis of Evolving Acute Myocardial Infarction in the Presence of Left Bundle-Branch Block. N Engl J Med. 1996;334:481–487. Copyright © 1996 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.)
TABLE 1.5 30-Day Mortality Based on Hemodynamic (Killip) Class |
|||
Killip Class |
Characteristics |
Patients (%) |
Mortality Rate (%) |
I |
No evidence of CHF |
85 |
5.1 |
II |
Rales, ↑JVD, or S3 |
13 |
13.6 |
III |
Pulmonary edema |
1 |
32.2 |
IV |
Cardiogenic shock |
1 |
57.8 |
CHF, congestive heart failure; ↑, increased; JVD, jugular venous distention; S3, third heart sound.
Adapted with permission from Lee KL, Woodlief LH, Topol EJ, et al. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. GUSTO-I Investigators. Circulation. 1995;91(6):1659–1668.
IX.Therapy
A.Prior to reperfusion
1.Aspirin. Immediate administration of aspirin is indicated for all patients with suspected acute MI, unless there is a clear history of true aspirin allergy (not intolerance). Aspirin therapy conveys as much mortality benefit as streptokinase (SK) and the combination provides additive benefit. The dose should be four 81 mg chewable tablets (for more rapid absorption) or one 325 mg nonchewable tablet. If oral administration is not possible, a rectal suppository can be given. If true aspirin allergy is present, clopidogrel monotherapy is the best alternative.
2.Oxygen. The routine administration of supplemental oxygen via nasal cannula to all patients with suspected MI is no longer warranted as there is some emerging data regarding the deleterious effects of supplemental oxygen. Instead, arterial oxygen saturations should be checked in all patients and if <94%, oxygen therapy should be initiated. Supplemental oxygen should also be supplied to patients who are visibly cyanotic or are in respiratory distress. Administration through a face mask or endotracheal tube may be necessary for patients with severe pulmonary edema or cardiogenic shock.
TABLE 1.6 TIMI Risk Model for Prediction of Short-Term Mortality in STEMI Patients |
|
History |
|
Age 65–74 y |
2 points |
Age ≥ 75 y |
3 points |
Angina or DM/HTN |
1 point |
Physical Examination |
|
HR > 100 beats/min |
2 points |
SBP < 100 mm Hg |
3 points |
Killip class II–IV |
2 points |
Weight < 67 kg |
1 point |
Presentation |
|
Anterior ST-elevation or LBBB |
1 point |
Time to treatment > 4 h |
1 point |
|
TIMI risk score = Total points (0–14) |
|
DM, diabetes mellitus; HR, heart rate; HTN, hypertension; LBBB, left bundle branch block; SBP, systolic blood pressure; STEMI, ST-segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
Adapted with permission from Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102(17):2031–2037.
3.Nitroglycerin. Nitroglycerin can be useful in the management of acute MI complicated by heart failure, persistent chest pain, or hypertension. Nitroglycerin should not be administered to patients who are hypotensive, to patients in whom RV infarction is suspected, or to those with recent use of phosphodiesterase inhibitors (24 to 48 hours). Administration of sublingual nitroglycerin may also identify whether the ST-segment elevation represents coronary artery spasm while arrangements for reperfusion therapy are being initiated. A meta-analysis performed before the age of routine reperfusion suggested a mortality benefit with intravenous nitroglycerin, although routine use of oral nitrates after MI had no benefit in two large randomized trials in the modern era. A 30% reduction in systolic blood pressure can be expected with appropriately aggressive dosing (10 to 20 µg/min with 5 to 10 µg/min increases every 5 to 10 minutes). Intravenous therapy may be continued for up to 24 to 48 hours.
4.Oral platelet P2Y12 receptor antagonists should be used routinely in all patients with STEMI, regardless of whether or not primary PCI is performed. Currently, the three agents available for treatment of STEMI are clopidogrel, prasugrel, and ticagrelor. Clopidogrel and prasugrel are thienopyridines that irreversibly inhibit the platelet adenosine diphosphate P2Y12 receptor, and ticagrelor is a reversible direct inhibitor of this same receptor. In patients in whom PCI is planned, a loading dose should be given as early as possible or at the time of PCI. The Prehospital Ticagrelor in ST-Segment Elevation Myocardial Infarction, or ATLANTIC, trial demonstrated that the administration of ticagrelor in the prehospital setting (ambulance) is safe, may prevent postprocedural acute stent thrombosis, but does not improve preprocedural coronary reperfusion. In contrast, the Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention or as Pretreatment at the Time of Diagnosis in Patients with Non-ST Elevation Myocardial Infarction, or ACCOAST trial, studied the administration of prasugrel before or after diagnostic angiography in patients with NSTEMI. Pretreatment with prasugrel in these patients did not reduce major ischemic events within the first 30 days, but did increase the risk of bleeding. The recommended loading dose of clopidogrel is 600 mg. The recommended loading dose of prasugrel is 60 mg and ticagrelor is given as a 180 mg loading dose. The absorption of prasugrel and ticagrelor may be significantly impaired in patients with STEMI. This may be overcome by crushing these tablets prior to administration for more rapid absorption. Current U.S. guidelines do not endorse one agent over another except in patients receiving fibrinolysis. In fibrinolysis patients, clopidogrel is the thienopyridine of choice, at a loading dose of 300 mg if the patient is ≤75 years and a loading dose of 75 mg if age >75 years. The duration of clopidogrel following fibrinolysis should be at least 14 days and ideally up to 12 months. The maintenance dose of clopidogrel and prasugrel is 75 mg daily and 10 mg daily, respectively. The maintenance dose for ticagrelor is 90 mg twice a day. It is currently recommended that clopidogrel and ticagrelor be held for 5 days and prasugrel be held for 7 days prior to CABG, unless the need for urgent revascularization outweighs the risk of potential excessive bleeding. Following CABG, dual antiplatelet therapy (DAPT) should be reintroduced and continued for at least a year in this setting (Class I). In patients with STEMI receiving DAPT following stent implantation, it is reasonable to use ticagrelor or prasugrel over clopidogrel for maintenance, especially in patients with diabetes. Prasugrel should, however, not be utilized in patients with a prior history of stroke or transient ischemic attack. The planned duration for DAPT following STEMI should be for 12 months (class I) at which point the risk and benefit for further continuation may be reassessed. For patients at recognized high risk for bleeding, discontinuation of DAPT at 6 months may be considered (class IIb).
5.Intravenous platelet P2Y12 receptor antagonists. Cangrelor is the only available intravenous P2Y12 inhibitor and binds reversibly to the receptor. Cangrelor is given as a 30 µg/kg IV bolus followed by an infusion of 4 µg/kg/min and provides potent platelet inhibition almost immediately (<2 minutes). The short plasma half-life of <5 minutes allows for near complete restoration of platelet function within 1 to 2 hours after stopping the infusion. The Effect of Platelet Inhibition with Cangrelor during PCI on Ischemic Events, or CHAMPION PHOENIX Trial, showed that preprocedural cangrelor reduced ischemic events including stent thrombosis at 48 hours compared with a loading dose of clopidogrel in patients undergoing urgent or elective PCI. Cangrelor currently has a Food and Drug Administration indication for patients undergoing PCI who have not yet been treated with an oral P2Y12 inhibitor and are not receiving glycoprotein (GP) IIb/IIIa inhibitors. This may be most effective in patients with extremely short door-to-balloon times because administration in this setting will ensure reliable platelet inhibition.
6.Parenteral anticoagulants. Unless there is a contraindication, all STEMI patients should receive intravenous antithrombotic therapy. Traditionally in patients who are undergoing primary PCI, this has been accomplished with unfractionated heparin (UFH). The dose of UFH is 70 to 100 U/kg as a bolus (maximum 4,000 U), followed by 12 U/kg/h infusion (maximum 1,000 U/h) to achieve a therapeutic activated clotting time (ACT). Routine administration of GP IIb/IIIa inhibitors is no longer warranted, but can be used as a bailout strategy in patients with a large thrombus burden, inadequate DAPT, loading or poor reflow. If GP IIb/IIIa inhibitors are used, the dose of UFH is reduced to 50 to 70 U/kg (maximum 4,000 units). The current guidelines allow for the use of bivalirudin, a direct thrombin inhibitor, as an alternative with or without prior administration of UFH. Bivalirudin is given as a 0.75 mg/kg intravenous bolus, followed by a 1.75 mg/kg/h infusion. Additional boluses of 0.3 mg/kg can be given to maintain therapeutic ACT. However, the recent How Effective Are Antithrombotic Therapies in Primary Percutaneous Coronary Intervention trial randomized patients scheduled for emergent angiography to receive upstream UFH or bivalirudin. The results of the trial demonstrated that the rate of major adverse cardiovascular events (all-cause mortality, stroke, reinfarction, or unplanned target-lesion revascularization) was significantly lower in the UFH group (5.7%) versus the bivalirudin group (8.7%) with no difference in major bleeding at 28 days. Overall data suggest that the use of bivalirudin is associated with lower bleeding risk (this advantage is reduced by adopting radial access) in PCI STEMI while being associated with increased risk of ischemic complications, especially acute stent thrombosis. Enoxaparin and fondaparinux are alternative options in patients undergoing fibrinolysis, but fondaparinux is contraindicated in patients undergoing primary PCI because it has been associated with catheter thrombosis.
B.Reperfusion therapy. The primary goal in the management of acute MI is to institute reperfusion therapy as quickly as possible. All patients with ST-segment elevation or new LBBB MI who seek treatment within 12 to 24 hours from onset of continuous symptoms should be considered for immediate reperfusion therapy. Persistent ischemic symptoms after 12 hours may indicate a stuttering course of occlusion, spontaneous reperfusion, and reocclusion and may indicate potential continued benefit for early therapy.
1.Benefit. The benefit of reperfusion therapy has been well documented in the management of acute MI, regardless of age, gender, and most baseline characteristics. However, the patients who derive the most benefit are those treated earliest and those at highest risk, such as those with anterior MI.
2.Time to treatment is paramount. Although the current guidelines focus on door-to-balloon times, total ischemic time is the most important parameter. Patients treated in the first hour have the highest mortality benefit. There is an inverse relationship between time to treatment and survival benefit. This relationship appears more consistent with fibrinolytic therapy than with direct PCI. After 12 hours of continuous symptoms, there is little net benefit to pharmacologic reperfusion with fibrinolytics. The therapeutic window for PCI extends beyond that of fibrinolysis, but is not infinite. The Occluded Artery Trial (OAT) suggests that stenting of an occluded infarct-related artery >72 hours after the initial event is not associated with benefit and may be harmful. Currently, the American Heart Association (AHA) recommends against PCI of an occluded infarct-related artery >24 hours after STEMI if the patient is hemodynamically stable and does not have signs of severe ischemia.
3.Fibrinolysis versus direct PCI. After it has been determined that a patient is a candidate for reperfusion therapy, the decision to use fibrinolytic or direct PCI therapy must be made quickly. Optimally performed primary PCI is the preferred strategy in all patients who present with STEMI. Patients presenting with cardiogenic shock or those presenting late (>2 hours after symptom onset) are especially well suited for primary PCI, given the relative lack of efficacy of fibrinolysis in these settings. Pooled data from several large trials show a significant (22%) reduction in short-term mortality for patients treated with primary angioplasty. This benefit was durable because there were significant reductions in the incidence of death, nonfatal MI, and recurrent ischemia at long-term follow-up. PCI is also associated with a reduction in the incidence of intracerebral hemorrhage compared with fibrinolytic therapy.
a.If a patient presents to a PCI-capable facility, a door-to-device time of 90 minutes or less is recommended. If a patient presents to a non–PCI-capable hospital, immediate transfer to a PCI-capable one is recommended if the door-to-device time can be achieved within 120 minutes. If primary PCI or transfer for PCI cannot be performed within 120 minutes of presentation, fibrinolysis should be administered within 30 minutes assuming no contraindications to fibrinolysis (Table 1.7).
b.Several trials, including the Danish Multicenter Randomized Study on Thrombolytic Therapy Versus Acute Coronary Angioplasty in Acute Myocardial Infarction (DANAMI)-2, the Air-Primary Angioplasty in Myocardial Infarction, and the Primary Angioplasty in Patients Transferred from a General Community Hospital to Specialized PTCA Units, have investigated the benefit of on-site fibrinolysis compared with transfer to tertiary centers for direct PCI. These studies have found improved outcomes in patients randomized to a transfer strategy and direct PCI even after taking into account the increased time for patient transfer. For example, patients in DANAMI-2 randomized to transfer for PCI had a significantly lower 30-day incidence of death, MI, or stroke (8.5% vs. 14.3%; p = 0.002) despite a median time from randomization to balloon inflation of 112 minutes.
TABLE 1.7 Contraindications and Cautions for Use of Thrombolytic Agents to Manage MI |
Absolute Contraindications |
Previous hemorrhagic stroke at any time or ischemic stroke within 3 mo |
Known intracranial neoplasm, structural cerebral vascular lesion, or closed head injury within 3 mo |
Active bleeding or bleeding diathesis (excluding menses) |
Suspected aortic dissection |
Intracranial or intraspinal surgery within 2 mo |
Severe uncontrolled hypertension, unresponsive to medical therapy |
For SK, prior exposure within 6 mo |
Relative Contraindications |
Severe, uncontrolled hypertension at presentation (blood pressure > 180/110 mm Hg), or history of chronic severe hypertension |
History of ischemic stroke > 3 mo, dementia, or known intracerebral pathologic condition not covered in contraindications |
Oral anticoagulant therapy |
Traumatic or prolonged (>10 min) CPR or major surgery (<3 wk) |
Noncompressible vascular punctures |
Recent internal bleeding (2–4 wk) |
Pregnancy |
Active peptic ulcer |
CPR, cardiopulmonary resuscitation; MI, myocardial infarction; SK, streptokinase.
Adapted from Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). J Am Coll Cardiol. 2004;44(3):E1–E211. Copyright © 2004 American College of Cardiology Foundation. With permission.
4.Primary PCI. Once the decision has been made to perform reperfusion with primary PCI, the patient should be moved to the cardiac catheterization laboratory and undergo angiography as rapidly as possible. After the culprit lesion has been identified, reperfusion should be achieved with standard PCI techniques.
a.Radial approach: The benefits of a radial over a femoral approach in STEMI have been noted in the Radial Versus Femoral Access for Coronary Intervention, Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome, and Minimizing Adverse Haemorrhagic Events by Transradial Access Site and Systemic Implementation of angioX trials. Consequently, radial access should be the default approach when performing primary PCI as it is associated with decreased rates of access site bleeding, vascular complications, and need for blood transfusion and has been associated with an overall decrease in mortality.
b.Culprit versus multivessel PCI: Performing PCI on a flow-limiting lesion in a noninfarct artery in the setting of a hemodynamically stable STEMI was considered a contraindication in the recent past (class IIIb). Advances in pharmacology, stent technology, and safety of interventional procedures have led to a series of small randomized controlled trials (RCTs) that have shown both safety and superior clinical outcomes with a multivessel over culprit-alone strategy. Consequently, PCI of a noninfarct artery may now be considered in select patients either immediately following revascularization of the culprit lesion or in a staged manner later in the index hospitalization, or on an elective basis following discharge (class IIb). A number of clinical trials are currently underway that will clarify this matter further.
c.Platelet GP IIb/IIIa inhibitors. There is no current role for routine GP IIb/IIIa inhibitors in this setting. The historical efficacy of GP IIb/IIIa inhibitors was largely demonstrated in the era prior to potent DAPT. Three major trials have evaluated the efficacy of GP IIb/IIIa therapy in STEMI patients receiving oral DAPT: bavarian reperfusion alternatives evaluation-3 (BRAVE-3), ongoing tirofiban in myocardial infarction evaluation (On-TIME-2), and harmonizing outcomes with revascularization and stents in acute myocardial infarction (HORIZONS-AMI). These studies did not demonstrate benefit of the routine administration of GP IIb/IIIa inhibitors in addition to DAPT in patients with STEMI undergoing primary PCI. It remains reasonable to consider the utilization of GP IIb/IIIa therapy as a bailout strategy in the presence of large thrombus burden, slow or no reflow, or inadequate oral P2Y12 loading. The risk of bleeding is significantly increased with use of these agents but this can be significantly reduced by adopting radial access for the procedure. Abciximab, tirofiban, and eptifibatide are GP IIb/IIIa inhibitors currently available in the United States and are considered equivalent options.
d.Thrombus aspiration. Although thought to be useful mechanistically in small trials, two large randomized trials (Thrombus Aspiration during ST-Segment Elevation Myocardial Infarction [TASTE] and the trial of routine aspiration thrombectomy with PCI versus PCI alone in patients with STEMI [TOTAL]) have failed to show any benefit with routine use of thrombus aspiration in patients with STEMI. Consequently, societal guidelines provide a class III recommendation against the routine use of aspiration thrombectomy, but do allow for its use as a bailout strategy if reperfusion cannot be successfully established with initial attempts.
e.Distal embolic protection devices (EPDs) have failed to show any benefit in multiple trials. These devices are not routinely recommended for acute PCI of native coronary arteries. If the culprit vessel is a saphenous vein bypass graft, an EPD should be used because it has been shown to reduce a 30-day composite outcome of death, MI, emergency CABG, and target-lesion revascularization.
f.Coronary stenting. Primary PCI is the preferred modality for all patients presenting with STEMI. Historically, primary PCI was initially achieved with balloon angioplasty, but with the development of intracoronary stents, the use of balloon angioplasty is extremely limited in STEMI. Bare-metal stents (BMSs) were the first stents developed and reduced the high rates of acute closure and long-term restenosis associated with balloon angioplasty. The STENT-Primary Angioplasty in Myocardial Infarction study found that coronary stenting significantly reduced the need for target vessel revascularization at 6 months (7.7% vs. 17.0%; p < 0.001). These findings were confirmed in the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications trial, which found that coronary stenting significantly reduced the incidence of restenosis at 6 months (40.8% vs. 22.2%; p < 0.0001), independent of abciximab use. BMS are also prone to in-stent restenosis (ISR), which led to the development of drug-eluting stents (DESs). DESs have antiproliferative agents attached to the struts that reduce neointimal hyperplasia and thus ISR. DESs have reduced the rates of long-term restenosis but they do require longer duration of DAPT because of concern for in-stent thrombosis. The placement of DES should be considered first-line therapy for all STEMI patients who are candidates for primary PCI unless they have a strong contraindication to or inability to comply with prolonged DAPT. New-generation DESs have proven superior to use of BMS in STEMI patients in the Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction as well as Everolimus-Eluting Stents versus Bare Metal Stents in ST Segment Myocardial Infarction trials mainly because of reduced reintervention rates.
5.Fibrinolysis
a.Fibrinolytic therapy. Fibrinolysis is considered a second-line option for patients presenting with STEMI when timely, optimally performed primary PCI is not available. In this context, it is worthwhile to review the data and clinical considerations associated with fibrinolysis. The lifesaving capability of early fibrinolytic therapy has been well established, beginning with the Gruppo Italiano per lo Studio della Sopravivenza nell’Infarto Miocardico (GISSI) 1 trial in 1986. Pooled data show a relative reduction in mortality of 18% and an absolute reduction of nearly 2%.
b.Contraindications. As discussed previously (Table 1.7), the only absolute contraindications to fibrinolytic therapies are recent cerebrovascular accident (CVA), hemorrhagic CVA, intracranial neoplasm, active internal bleeding, and suspected aortic dissection. The presence of one of these or one or more of the relative contraindications would favor PCI, even if it meant delaying reperfusion.
c.Choice of agent
(1)Alteplase (tPA). The Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) trial I showed that use of accelerated tPA significantly reduced 30-day mortality rate by 15% relative to SK plus subcutaneous or intravenous heparin. This mortality reduction correlated with significantly higher rates of TIMI 3 flow at 90 minutes compared with SK (54% vs. 31%; p < 0.001). The benefit was seen across all subgroups, although the patients at highest risk derived the most benefit. The accelerated protocol consisted of an intravenous bolus dose of 15 mg followed by 0.75 mg/kg (up to 50 mg) over 30 minutes and then 0.5 mg/kg over 60 minutes. tPA is considered a fibrin-specific agent because of its relative selectivity for clot-bound fibrin. The use of continuous infusion fibrinolytics has been replaced by bolus-dosed fibrinolytics, such as reteplase and tenecteplase, because of the ease of administration of bolus-dosed agents and a lower risk of medication error.
(2)Reteplase. The first of the third-generation fibrinolytic agents approved for use in the United States, reteplase is a less fibrin-specific mutation of tPA. Reteplase has a longer half-life than tPA and can be administered in a double bolus (10 mg each, 30 minutes apart). The GUSTO III trial showed no mortality benefit of reteplase over tPA, but its ease of use may help to reduce time to administration.
(3)Tenecteplase (TNK), another third-generation fibrinolytic, is characterized by its improved fibrin specificity, enhanced resistance to plasminogen activator inhibitor 1, and decreased plasma clearance. These properties allow it to be administered as a single bolus and it is the currently preferred agent. The Assessment of the Safety and Efficacy of a New Treatment Strategy for Acute Myocardial Infarction 2 (ASSENT 2) trial found no mortality difference between TNK and tPA at 30 days. However, TNK was associated with significantly less noncerebral bleeding and improved mortality in patients treated for >4 hours after symptom onset. The weight-adjusted dose of TNK is 30 to 40 mg (ASSENT 1). The recent Strategic Reperfusion Early after Myocardial Infarction (STREAM) trial compared primary PCI to prehospital fibrinolysis with TNK in early presenting (within 3 hours) STEMI patients who were unable to undergo primary PCI within 1 hour of first medical contact. Prehospital fibrinolysis coupled with timely angiography was found to be an effective reperfusion strategy in this select group of patients with similar rates of the primary end point (composite of death, shock, CHF, and reinfarction up to 30 days) in both groups, but there was a slightly higher risk of intracranial bleeding associated with fibrinolysis.
(4)SK is no longer commercially available in the United States and is of historical significance. Because of the possible development of antibodies, SK should not be administered to a patient who has received it in the past. Because the overall rate of intracerebral hemorrhage is lower with SK (0.5%) than with tPA (0.7%), some cardiologists advocate its use, if commercially available, in the care of high-risk patients, such as elderly patients with a history of a cerebrovascular event or severe hypertension. SK is a nonfibrin-specific agent capable of lysing circulating and clot-bound plasminogen to plasmin. This process results in substantial systemic fibrinogenolysis, fibrinogenemia, and elevation in fibrin degradation products.
d.Bleeding complications after fibrinolysis. The most serious complication of fibrinolytic therapy is intracerebral hemorrhage, which occurs in approximately 0.5% to 0.7% of patients receiving such therapy. The major risk factors for intracranial hemorrhage include age >75 years, hypertension, low body weight, female gender, and coagulopathy (e.g., prior Coumadin use). The diagnosis must be considered if a patient has severe headache, visual disturbances, new neurologic deficit, acute confusional state, or seizure. If the clinical suspicion is high, fibrinolytic, antithrombin, and antiplatelet therapy should be interrupted while emergency CT or MRI is performed and neurosurgical consultation is obtained. Surgical evacuation may be lifesaving. Even with prompt recognition and treatment, the mortality rate is higher than 60% and elderly patients (>75 years) have a mortality rate greater than 90%. There is controversy regarding the risk of fibrinolytic therapy in elderly patients. An observational study from the Medicare database found that patients older than 75 years had an increased risk of death at 30 days with fibrinolytic therapy (RR = 1.38; 95% confidence interval [CI] 1.12 to 1.71; p = 0.003). However, an updated meta-analysis of nine randomized trials found that the risk reduction with fibrinolysis in patients older than 75 years was 16% (odds ratio = 0.84; 95% CI 0.72 to 0.98; p < 0.05). There appears to be a decreasing relative benefit with fibrinolysis in the elderly, but an absolute gain in lives saved. The only randomized trial to specifically study management of STEMI in the elderly found that patients treated with PCI had significantly lower 30-day and 1-year mortality rates than patients treated with fibrinolysis. However, the Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment (ExTRACT)-TIMI 25 study suggested that fibrinolytic therapy may be safe in the elderly if a reduced dose of enoxaparin is used. The previously discussed STREAM trial noted an increased rate of intracranial hemorrhage (ICH) (8.1%) in patients older than 75 years early in the study enrollment period. This led to a study amendment and a dose reduction of TNK by 50%, which lowered the rate of ICH in patients >75 years to 0%. Gastrointestinal, retroperitoneal, and access site bleeding may also complicate fibrinolytic therapy but are usually not life-threatening if promptly recognized and managed. In any case, the best treatment of acute STEMI in elderly patients appears to be primary PCI.
6.Rescue percutaneous revascularization is defined as the use of PCI when fibrinolytic therapy has proved unsuccessful. Despite the proven mortality benefit, >30% of patients who received lytic therapy have TIMI 0 to 1 flow at 90 minutes, whereas patency at 90 minutes has been shown to correlate with long-term survival. If reperfusion is not clearly evident 90 minutes after initiation of lytic therapy, particularly among patients with a large acute MI, the decision to perform emergency angiography and mechanical reperfusion should be made promptly. Patients in cardiogenic shock, with severe heart failure, or with compromising arrhythmias after lytic therapy should undergo immediate coronary angiography and should not await clinical assessment of reperfusion.
a.Clinical determination of successful reperfusion. It can be difficult to determine clinically whether a patient has successful reperfusion with fibrinolytic therapy. Resolution of chest pain is an inaccurate measure of reperfusion, because the pain may be blunted by narcotic analgesia or the partial denervation that is known to occur among some patients with MI. Serial assessment of 12-lead ECGs is a more reliable indicator of reperfusion, although it is also suboptimal. An accelerated idioventricular rhythm (AIVR) is fairly specific for reperfusion, but arrhythmias other than AIVR are not reliable indicators because a variety of ventricular and supraventricular arrhythmias may be observed in patients with nonreperfused infarction-related artery. The complete resolution of chest pain and electrocardiographic changes (defined as >70% resolution of ST-segment elevation), accompanied by a run of AIVR, is highly specific for successful reperfusion, but it occurs in <10% of patients receiving lytic therapy. Resolution of ST-segment elevation by >70% is correlated with effective tissue-level reperfusion, and this finding has been correlated with better clinical outcomes and angiographic reperfusion.
b.Benefit. It has been shown in the Randomized Evaluation of Salvage Angioplasty with Combined Utilization of Endpoints trial that patients with anterior MI who have unsuccessful thrombolysis (TIMI 0 or 1 flow) have a significant benefit from rescue angioplasty. In addition, the Rapid Early Action for Coronary Treatment trial demonstrated that among patients with failed reperfusion with lytics, treatment with rescue angioplasty with or without PCI is associated with a ~50% reduction in death, reinfarction, stroke, and severe heart failure. The Grupo de Análisis de la Cardiopatía Isquémica Aguda I trial evaluated an early invasive strategy (within 24 hours) versus an ischemia-guided approach among patients with STEMI treated with fibrinolytic therapy. This trial primarily demonstrated a reduction in revascularization events with the early invasive approach, although a trend was seen toward fewer deaths and reinfarctions. Based on the above data, an early angiography strategy (within 24 hours) is a reasonable approach in all patients who receive lytic therapy.
7.Pharmacoinvasive strategy. Although it is clear that routine fibrinolysis prior to transfer for PCI in all patients who present with STEMI (facilitated PCI) results in worse outcomes, fibrinolysis is still necessary to achieve early reperfusion in some patients who present to non–PCI-capable facilities. More recent data suggest that high-risk patients who receive fibrinolytic therapy benefit from immediate transfer for PCI. The Combined Abciximab Reteplase Stent Study in Acute Myocardial Infarction trial randomized patients presenting to a non–PCI-capable facility, who received half-dose fibrinolytics and abciximab, to either immediate transfer for PCI or rescue PCI. Patients who were transferred immediately for PCI had a significant reduction in the primary end point of death, reinfarction, or refractory ischemia at 30 days. In addition, the Trial of Routine Angioplasty and Stenting after Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction study showed that high-risk patients benefit from this pharmacoinvasive strategy. This trial looked at 1,059 high-risk patients with STEMI who presented to a non–PCI-capable facility within 12 hours of symptom onset. All patients received fibrinolysis with TNK and were then randomized to immediate transfer for PCI or rescue PCI dictated by continued chest pain, <50% resolution of ST-elevation, or hemodynamic instability. The primary end point was 30-day composite of the first occurrence of death, MI, recurrent ischemia, new or worsening heart failure, and cardiogenic shock. The primary end point was significantly less common in the pharmacoinvasive group compared with the group who received rescue PCI (11% vs. 17.2%; RR = 0.64; 95% CI 0.47 to 0.87; p = 0.004). Immediate transfer to a PCI-capable hospital should occur for all patients with cardiogenic shock or acute severe heart failure. Urgent transfer to a PCI-capable hospital should occur for patients with evidence of failed reperfusion or acute reocclusion. Hemodynamically stable patients with evidence of successful reperfusion should be transferred to a PCI-capable hospital for coronary angiography to be performed within 24 hours.
8.The late open artery hypothesis postulates that benefit in terms of improved ventricular function, increased electrical stability, and provision of collaterals can be gained by late patency of occluded infarct arteries. However, OAT failed to show benefit of angioplasty for late total occlusion within 3 to 28 days after MI. It should be noted that the OAT excluded high-risk patients with New York Heart Association (NYHA) class III or IV heart failure, rest angina, clinical instability, multivessel disease (left main or three-vessel disease), or severe inducible ischemia on stress testing. Patients in OAT were hemodynamically stable, were asymptomatic, and had TIMI 0 flow in the infarct-related artery. This study has led to a class III recommendation against PCI of a totally occluded artery >24 hours after STEMI in OAT-eligible patients (asymptomatic without the previously noted high-risk criteria).
9.Emergency coronary bypass surgery may be the treatment of choice for patients in whom the intent is to perform direct or rescue percutaneous mechanical reperfusion but who are found to have a critical left main or severe three-vessel disease unapproachable with percutaneous revascularization. Studies of this strategy are fairly encouraging, especially when patients can be taken to the operating room early in the course of infarction, before severe myocardial necrosis has occurred. RV infarction is a relative contraindication to bypass surgery because it complicates the discontinuation of cardiopulmonary support.
10.PCI in hospitals without surgical backup. Several trials and two meta-analyses have shown no difference in in-hospital or 30 day mortality in patients undergoing primary PCI at sites with or without surgical backup. Consensus guidelines recommend that primary PCI without on-site surgical backup is reasonable with the intent of providing timely, high-quality STEMI care (class IIa, Level of Evidence B).
C.Adjuvant therapy
1.β-Blockers. Extensive data from the era before reperfusion established the usefulness of β-blockers in reducing recurrent ischemia, arrhythmias, and mortality. Several small randomized trials performed in the fibrinolytic reperfusion era confirmed the anti-ischemic and antiarrhythmic benefits, although short-term mortality was not affected. As a result, prior recommendations have stated that β-blockers should be administered to all patients within the first 24 hours of acute MI, unless contraindicated by severe reactive airway disease, hypotension, bradycardia, or cardiogenic shock. However, more recent data from the PCI era have shown no difference in mortality and no difference in the composite end point of death, reinfarction, or ventricular fibrillation arrest. The Clopidogrel and Metoprolol in Myocardial Infarction Trial/Second Chinese Cardiac Study trial, a large (n = 22,929) RCT, found that the metoprolol group had more ventricular fibrillation arrest (2.5% vs. 3.0%; p = 0.001) and shock (5.0% vs. 3.9%; p < 0.001). The incidence of shock was most notable in patients with Killip class II and III heart failure. This has led to a change in guidelines recommending more judicious use of early (<24 hours) β-blockers, avoiding use in patients with significant signs of heart failure, low cardiac output, risk of cardiogenic shock, or other relative contraindications to their use.
a.For ongoing ischemia with tachycardia or hypertension, after rapid evaluation of ventricular function, intravenous metoprolol may be given (5 mg every 5 minutes until the desired blood pressure and pulse are achieved). Patients who tolerate the intravenous loading can begin moderate oral doses (12.5 to 50 mg of metoprolol, two to four times daily). The dose should be subsequently titrated upward to the maximally tolerated dose (200 mg of sustained-release metoprolol, once daily). Use of β-blockers should be avoided in patients with tachycardia of unclear origin, as this can represent decompensated heart failure with a compensatory tachycardia.
2.Angiotensin-converting enzyme (ACE) inhibitors can be started orally in the first 24 hours for all patients without hypotension, acute renal failure, or other contraindications. These medications were shown to reduce mortality in the GISSI 3 and International Study of Infarct Survival-4 trials. ACE inhibitors should be continued indefinitely in patients with LV dysfunction or clinical CHF, because these patients have been shown to derive a mortality benefit. In addition, the Heart Outcomes Prevention Evaluation study found that high-risk patients, including those with prior MI but normal LV function, still had long-term benefit from ramipril. Intravenous formulations of these agents should not be used because they have not demonstrated benefit and may increase mortality. Rather, a graded oral regimen is advised. Angiotensin-receptor blockers remain a viable option for ACE inhibitor–intolerant patients.
3.Calcium channel blockers. Evidence for a potential increase in mortality has limited the use of calcium channel blockers in the care of patients with acute MI. They are indicated for the management of supraventricular tachyarrhythmia, cocaine-induced MI, or relief of postinfarction angina unresponsive to β-blockade. Otherwise, these agents should be avoided. Short-acting agents, such as nifedipine, are contraindicated because of their reflex sympathetic activation. Verapamil and diltiazem should be avoided in patients with LV dysfunction or heart failure. Amlodipine is an effective antianginal agent and appears safe to use for this indication in patients with CHF.
4.Aldosterone antagonists. The use of aldosterone-blocking agents has been shown to be beneficial in post-MI patients. The Randomized Aldactone Evaluation Study found a reduction in all-cause mortality with the use of aldactone in patients with ischemic cardiomyopathy and NYHA class III or IV heart failure. However, the only randomized trial to address the use of such agents among patients with ventricular dysfunction after STEMI is the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study, where eplerenone was found to reduce death, cardiovascular death, and hospitalization for heart failure.
5.Diabetes control. The Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction study found a significantly lower mortality rate at 1 year compared with standard therapy (8.6% vs. 18.0%; p = 0.020) in diabetic patients treated with aggressive blood glucose reduction with an insulin infusion during hospitalization, followed by multidose subcutaneous insulin injections. However, a small trial Organization to Assess Strategies in Ischemic Syndromes-6 Glucose-insulin-potassium (OASIS-6 GIK) and a large (>20,000 patients) randomized trial (CREATE-ELCA) failed to show any benefit to glucose–insulin–potassium (GIK) infusions. As a result, it appears prudent to institute sound glucose control, but it is not necessary to aggressively pursue glucose control with GIK infusions. Current guidelines suggest insulin therapy to achieve and maintain blood glucose levels <180 mg/dL while avoiding hypoglycemia.
6.Antiarrhythmics. The use of lidocaine or other antiarrhythmic agents is not warranted for the prophylactic suppression of ventricular tachycardia (VT) and fibrillation. Although lidocaine may decrease tachyarrhythmias, there is no survival benefit. There is also evidence to suggest an increase in mortality related to an increased incidence of bradycardia and asystole. The Resuscitation Outcomes Consortium recently published the “Amiodarone, Lidocaine or Placebo in Out-of-Hospital Cardiac Arrest,” a randomized trial comparing intravenous amiodarone versus lidocaine versus placebo in adults with nontraumatic out-of-hospital cardiac arrest with VT or ventricular fibrillation. Neither lidocaine nor amiodarone significantly improved survival or favorable neurologic outcomes compared to placebo.
7.Intra-aortic balloon pump (IABP). The utility of IABP to provide circulatory support in patients with cardiogenic shock complicating acute MI is now uncertain. A mortality benefit of IABP for MI complicated by cardiogenic shock was not demonstrated in the IABP-II trial which randomized 600 patients with MI and cardiogenic shock to IABP or no IABP. Subsequently, the guideline recommendation for IABP in patients with cardiogenic shock after MI was downgraded to a class IIa recommendation (Level of Evidence B). The counterpulsation to reduce infarct size pre-PCI acute myocardial infarction CRISP-AMI study evaluated 337 patients with anterior STEMI without shock and randomized them to a prophylactic IABP prior to primary PCI versus primary PCI alone. The primary end point was infarct size as measured by cardiac MRI and there was no demonstrable benefit for a prophylactic IABP in these patients.
8.Implantable cardioverter–defibrillators (ICDs). Posited to reduce the risk of sudden death following acute MI, the utility of ICDs implanted at an average of 18 days following the index MI was tested in patients with reduced ventricular function and autonomic dysfunction Defibrillator in Acute Myocardial Infarction (DINAMIT trial). Although there was a decrease in cardiovascular death, this study failed to demonstrate any reduction in all-cause mortality. Similarly, no difference in overall mortality was noted in the Immediate Risk Stratification Improves Survival trial that randomized 898 patients with recent MI (5 to 31 days) and either left ventricular ejection fraction (LVEF) <40% with initial heart rate >90 beats/min, or non sustained ventricular tachycardia (NSVT) >150 beats/min over a mean follow-up of 37 months. The Multicenter Automatic Defibrillator Implantation Trial II evaluated the benefit of delayed ICD insertion in patients with prior MI. The trial enrolled 1,232 patients with a history of MI at least 1 month prior to enrollment (90 days if bypass surgery was performed) and an LVEF ≤30%. Patients were randomized to prophylactic ICD insertion or standard medical therapy. At an average follow-up of 20 months, prophylactic insertion of an ICD significantly reduced all-cause mortality (14.2% vs. 19.8% for standard therapy; HR = 0.69; 95% CI 0.51 to 0.93; p = 0.016). According to the current American College of Cardiology/AHA guidelines, an ICD should be inserted in patients with ventricular fibrillation or hemodynamically significant sustained VT that occurs 48 hours after acute MI, assuming there is no recurrent ischemia or MI. Patients with an EF ≤35% and NYHA class II or III heart failure secondary to an MI that occurred at least 40 days prior should also receive an ICD. In addition, patients who are 40 days post-MI with NYHA class I heart failure and an EF ≤30% are candidates for ICD insertion. Lastly, any patient with a prior MI, nonsustained VT, EF ≤ 40%, and inducible ventricular fibrillation or sustained VT during an electrophysiologic study are candidates for an ICD. Patients who have received CABG should have their LVEF and NYHA functional class reassessed 90 days after the procedure to determine ICD candidacy.
9.Wearable cardioverter–defibrillators. Patients who are considered at risk for SCD but do not meet the above criteria, such as patients waiting for reassessment of LVEF after percutaneous or surgical revascularization, can be given the option of a wearable cardiac defibrillator as a bridge to ICD implantation or recovery of LVEF. The results of the randomized VEST Prevention of Early Sudden Death trial, however, showed no benefit utilizing this approach in post-MI survivors with LVEF <35% over 90 days of follow-up.
10.Anticoagulation for large anterior wall MIs. Historical teaching (not based on randomized data) has advocated anticoagulating patients for 6 weeks after a large anterior wall MI, with the goal of preventing LV thrombus development. However, in the era of primary PCI, the rates of LV thrombus have been reduced. Therapeutic anticoagulation is recommended in the presence of objective imaging evidence of LV thrombus. Prophylactic anticoagulation, however, is not currently recommended, because the routine addition of oral anticoagulation in all patients with STEMI would lead to “triple therapy”—warfarin and DAPT—leaving patients at an increased risk of bleeding without any demonstrable benefit.
X.Acute MI associated with cocaine abuse. The pathophysiologic process and management of acute MI associated with cocaine use differ from those of classic MI.
A.Pathophysiology
1.The underlying pathophysiologic factor in acute MI associated with cocaine abuse is believed to be coronary spasm or thrombus formation caused by α-adrenergic stimulation. This can occur in a normal segment of artery or be superimposed on mild to moderate atherosclerosis. Atherosclerosis is accelerated by chronic cocaine use.
2.Increased oxygen demand caused by β-adrenergic stimulation of heart rate and contractility also contributes to the onset of ischemia.
B.Clinical presentation. Chest pain caused by infarction after cocaine ingestion typically occurs within 3 hours, although it can vary from minutes to days, and depends on the route of administration (median of 30 minutes with intravenous cocaine, 90 minutes with crack smoking, and 135 minutes with nasal inhalation). More than 80% of persons with infarction are also cigarette smokers. Studies with animals have demonstrated a synergistic effect between cigarette smoking and cocaine use.
C.Therapy
1.The initial management of ST-segment elevation associated with cocaine use includes the routine administration of antiplatelets and heparin. Aggressive use of sublingual and intravenous nitroglycerin or intravenous calcium channel blockers is advised in an effort to relieve coronary spasm. Intravenous benzodiazepines can also be given because they not only relieve cocaine-induced chest pain but can also improve cardiac hemodynamics.
2.a-Blockers are contraindicated in patients with cocaine-induced acute MI. Although they block undesirable β-adrenergic effects, these agents allow unopposed α-adrenergic stimulation and have been associated with increased mortality in nonrandomized analyses.
3.Reperfusion therapy must be considered if vasodilator therapy is unsuccessful in relieving symptoms and ST-segment changes.
4.Immediate angiography and mechanical revascularization as appropriate may be even more beneficial in cocaine-induced MI patients. Many patients who use cocaine have contraindications to thrombolysis, such as severe hypertension or persistent vasospasm without thrombosis, which is not amenable to thrombolytic therapy.
XI.Postoperative acute MI
A.Etiology and pathophysiology. Acute MI following noncardiac operations most commonly occurs on the third or fourth postoperative day. Conventional theory was that MI was caused by a combination of increased oxygen demand and arterial shear stress associated with the increased adrenergic drive that accompanies pain and ambulation in the postoperative period. Intravascular volume shifts caused by redistribution of fluids, intravenous administration of fluids, and decreased enteral intake all contribute to the risk of postoperative MI. It is apparent that there is a postoperative inflammatory state associated with hypercoagulability, marked by an increase in fibrinogen and other acute-phase reactants. Significant myocardial injury has been observed in postoperative patients in the absence of traditional signs and symptoms of ACS, often labeled as a “troponin leak” or demand ischemia. These events are termed myocardial injury after noncardiac surgery (MINS) and are associated with an increase in long-term mortality when compared with patients without evidence of myocardial injury. There are currently no identifiable therapeutic targets for patients with MINS.
B.Therapy. Management of true postoperative MI is complicated by limitations on the use of fibrinolytic agents and anticoagulant therapies. Therapy relies more heavily on the intravenous use of β-blockers and urgent angiography and mechanical reperfusion. The optimal antiplatelet or anticoagulation regimen for recent (<1 year) DES patients undergoing noncardiac surgery is not known.
XII.Simplified reperfusion strategy. The wealth of data regarding reperfusion strategies and adjunctive therapies in acute MI detailed previously may lead to confusion regarding the optimal approach. Based on guideline recommendations, a simplification of the STEMI management strategy can be achieved.
A.For patients presenting with STEMI where primary PCI is available with 90 minutes, a reasonable strategy would involve administration of aspirin, parenteral anticoagulation, and an oral platelet P2Y12 receptor inhibitor, as well as nitrates and β-blocker therapy if not contraindicated, and immediate transfer to the catheterization laboratory for primary PCI with DES.
B.For patients presenting to a hospital where primary PCI is not available, but immediate transfer (medical contact to balloon time <120 minutes) to a PCI facility is available, a similar strategy is employed, with initiation of aspirin, platelet P2Y12 receptor inhibitors, and anticoagulation prior to transfer to the PCI facility.
C.If anticipated transfer times will exceed the medical contact-to-PCI time of 120 minutes, then fibrinolytic therapy should be instituted in eligible patients within 30 minutes of first medical contact in addition to therapy with aspirin, clopidogrel, and parenteral anticoagulation. The choice of UFH or enoxaparin remains operator dependent, with either option reasonable. Among patients receiving fibrinolytics, immediate transfer to a PCI facility is preferable, and early angiography (<24 hours) is recommended. Following the administration of fibrinolytics, any patient with cardiogenic shock, ventricular arrhythmias causing hemodynamic compromise, or evidence of failed reperfusion should be immediately transferred for urgent coronary angiography.
ACKNOWLEDGMENTS: The author thanks Drs. Deepak P. Vivekananthan, Michael A. Lauer, Ryan D. Christofferson, and Christopher Huff for their contributions to earlier editions of this chapter.
ESSENTIAL READING
Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–177.
Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2016;67(10):1235–1250.
O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–e425.
Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035.