Bradyarrhythmias, Atrioventricular Block, Asystole, and Pulseless Electrical Activity
I.Introduction. Bradyarrhythmias and conduction blocks are common electrocardiographic findings. Many of these arrhythmias are asymptomatic and do not require specific therapy, whereas others can be life threatening, requiring rapid intervention.
II.Anatomy
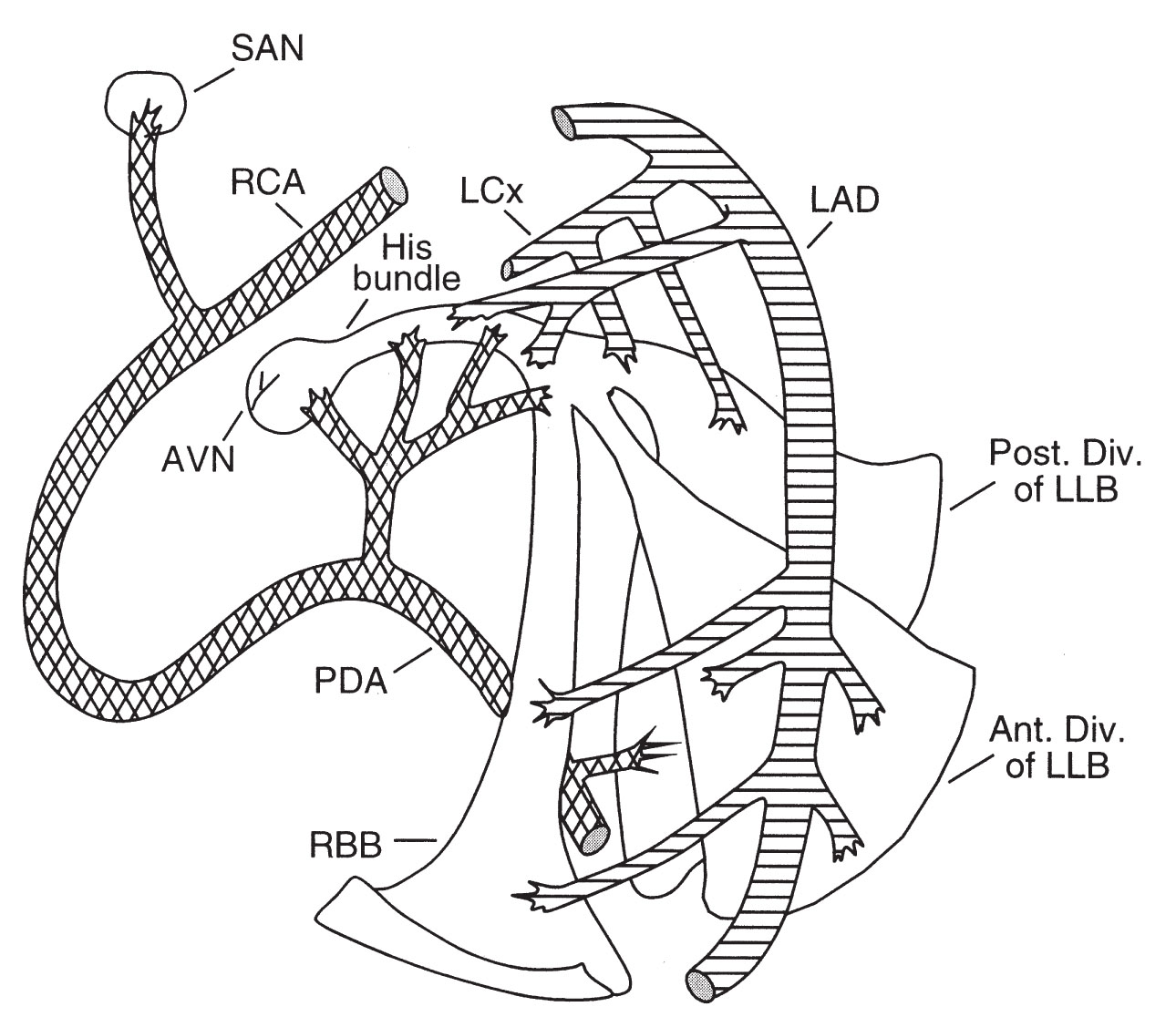
A. Sinoatrial node. The normal sinus beat originates in the sinoatrial (SA) node, a focus of automatic cells near the junction of the superior vena cava and right atrium.
1.The blood supply to the SA node is from the sinus node artery, which arises from the proximal right coronary artery (RCA) in 55% of the population (Fig. 22.1) and the left circumflex artery (LCx) in 35%. The SA node receives a dual supply of blood from both the RCA and the LCx in 10% of the population.

FIGURE 22.1 Diagrammatic representation of the conduction system and its blood supply. AVN, atrioventricular node; LAD, left anterior descending; LCx, left circumflex artery; LLB, left lateral branch; PDA, patent ductus arteriosus; RBB, right bundle branch; RCA, right coronary artery; SAN, sinoatrial node.
2.The automaticity of the SA node is affected by both the parasympathetic and sympathetic nervous systems. If the SA node fails to generate an impulse, other foci in the atrium, atrioventricular (AV) node, or ventricle can act as “backup” pacemaker sites.
B. AV node. The AV node is located in the anteromedial portion of the right atrium just anterior to the coronary sinus.
1.The impulse generated by the SA node progresses through the atrium to the AV node. The AV node is also innervated by both the parasympathetic and sympathetic nervous systems.
2.The AV node receives its blood supply from the AV node artery, which arises from the posterior descending artery (PDA) in 80% of the population (Fig. 22.1), from the LCx in 10%, and from both arteries in 10%.
3.Collateral blood supply from the left anterior descending artery (LAD) makes the AV node somewhat less prone to ischemic damage than the SA node.
C. His bundle and bundle branches
1.After a normal delay of <200 ms in the AV node, the electrical impulse is propagated down the His bundle to the right and left bundle branches. The left bundle branch splits further into anterior and posterior fascicles. The autonomic nervous system does not have a major effect on conduction below the AV node.
2.The His bundle and right bundle branch receive their blood supply from the AV nodal artery and from septal perforating branches of the LAD. The anterior fascicle of the left bundle branch receives blood from the septal perforating branches of the LAD alone. The posterior fascicle of the left bundle branch has a dual blood supply from the septal perforating branches of the LAD and branches of the PDA.
III.Sinus node dysfunction. Sinus node dysfunction encompasses any dysfunction of the sinus node and includes inappropriate sinus bradycardia, SA exit block, SA arrest, and tachycardia–bradycardia syndrome.
A.Clinical presentation. There is a wide range of presentations, and some patients’ disease may be asymptomatic.
1.Syncope and presyncope are the most dramatic presenting symptoms. Fatigue, angina, and shortness of breath are more subtle consequences of sinus node dysfunction.
2.In the tachycardia–bradycardia syndrome, the primary complaint may be palpitations. Documentation of the symptomatic tachyarrhythmia may be difficult because of the sporadic and fleeting nature of the problem.
B.Etiology. The intrinsic and extrinsic causes of sinus node dysfunction are listed in Table 22.1. Idiopathic degenerative disease is the most common cause of intrinsic sinus node dysfunction, and the incidence increases with age. Acute coronary syndromes are another common cause of bradyarrhythmias, occurring in 25% to 30% of patients with myocardial infarction (MI) (Table 22.2).
TABLE 22.1 Etiologies of Sinus Node Dysfunction |
Intrinsic Causes |
Idiopathic degenerative disease |
Coronary artery disease |
Cardiomyopathy |
Hypertension |
Infiltrative disorders (amyloidosis, hemochromatosis, and tumors) |
Collagen vascular disease (scleroderma and systemic lupus erythematosus) |
Inflammatory processes (myocarditis and pericarditis) |
Surgical trauma (valve surgery and transplantation) |
Musculoskeletal disorders (myotonic dystrophy and Friedreich ataxia) |
Congenital heart disease (postoperative or in the absence of surgical correction) |
Extrinsic Causes |
Drug effects |
β-Blocking agents |
Calcium channel blocking agents |
Digoxin |
Sympatholytic antihypertensives (clonidine, methyldopa, and reserpine) |
Antiarrhythmic drugs |
Type Ia (quinidine, procainamide, and disopyramide) |
Type Ic (flecainide and propafenone) |
Type III (sotalol and amiodarone) |
Others (lithium, cimetidine, amitriptyline, and phenytoin) |
Autonomic influences |
Excessive vagal tone |
Carotid sinus syndrome |
Vasovagal syncope |
Well-trained athletes (normal variant and not dysfunction) |
Electrolyte abnormalities |
Hyperkalemia |
Hypercarbia |
Endocrine disorders—hypothyroidism |
Increased intracranial pressure |
Hypothermia |
Sepsis |
Reprinted with permission from Topol EJ, ed. Textbook of Cardiovascular Medicine. Philadelphia, PA: Lippincott–Raven.
TABLE 22.2 Incidence of Bradyarrhythmia in the Setting of Acute Myocardial Infarction |
|
Rhythm |
Incidence (%) |
Sinus bradycardia |
25 |
Junctional escape rhythm |
20 |
Idioventricular escape rhythm |
15 |
First-degree AV block |
15 |
Second-degree, Mobitz type I AV block |
12 |
Second-degree, Mobitz type II AV block |
4 |
Third-degree AV block |
15 |
Right bundle branch block |
7 |
Left bundle branch block |
5 |
Left anterior fascicular block |
8 |
Left posterior fascicular block |
0.5 |
AV, atrioventricular.
C.Electrocardiographic findings
1.Inappropriate sinus bradycardia, also known as “chronotropic incompetence,” is defined as a sinus rate of <60 beats/min that does not increase appropriately with exercise. Inappropriate sinus bradycardia must be differentiated from a low resting heart rate, which may be normal in athletes and sleeping individuals.
2.Sinus arrest, or a sinus pause, occurs when the sinus node fails to depolarize on time. Pauses of <3 seconds may be seen on Holter monitoring in up to 11% of normal adults (especially athletes) and are not a cause for concern. However, pauses lasting longer than 3 seconds are generally considered abnormal and are suggestive of underlying pathology, especially if the patient is awake when they occur.
3.SA exit block, although similar to sinus arrest on the electrocardiographic tracing, may be distinguished by the fact that the duration of the pause is a multiple of the sinus PP interval. High-grade SA exit block cannot be differentiated from prolonged sinus arrest and is treated in the same manner.
4.Tachycardia–bradycardia syndrome, also referred to as “sick sinus syndrome,” is characterized by episodes of sinus or junctional bradycardia interspersed with an atrial tachyarrhythmia, usually paroxysmal atrial fibrillation.
D.Diagnostic testing. Invasive testing is used when noninvasive methods have failed to yield a diagnosis and sinus node dysfunction is still strongly suspected.
1.Noninvasive testing
a.Electrocardiogram (ECG). In evaluating sinus node dysfunction, the initial workup should include a 12-lead ECG, followed by ambulatory ECG monitoring. In most cases, 24 to 48 hours of monitoring (Holter) is sufficient, but frequently, extended monitoring for 2 to 4 weeks (event recorder) is required for diagnosis. Rarely, in more paroxysmal cases, prolonged monitoring for months to years via implantable loop recorders is indicated. Use of a diary during the recording period can help correlate symptoms with the cardiac rhythm. Stress testing can help document the severity of chronotropic incompetence.
b.Autonomic testing includes physical maneuvers, such as carotid sinus massage and tilt table testing, as well as pharmacologic interventions to test the autonomic reflexes.
(1) Carotid sinus massage distinguishes intrinsic sinus arrest from a pause because of carotid sinus hypersensitivity, which is a 3-second or longer pause and/or a ≥50 mm Hg or greater drop in blood pressure that occurs with massage of the carotid sinus (firm pressure applied to one carotid sinus at a time for 5 seconds). Carotid sinus massage should not normally precipitate sinus arrest, although it will decrease the rate of depolarization of the SA node and slow conduction in the AV node. Carotid sinus hypersensitivity is relatively common in older patients with atherosclerotic disease and can be provoked by neck motion, shaving, or a tight shirt collar. When carotid sinus hypersensitivity is accompanied by syncope or presyncope, it constitutes carotid sinus syndrome and may warrant permanent pacing for nonvasodepressive subtypes (Table 22.3).
(2) Tilt table testing may help differentiate between syncope caused by sinus node dysfunction and that because of autonomic dysfunction (neurocardiogenic syncope). Bradycardic episodes precipitated by tilt table testing are usually caused by autonomic dysfunction and not by sinus node dysfunction.
(3) Pharmacologic testing may be used to differentiate between sinus node dysfunction and autonomic dysfunction. Total autonomic blockade is achieved after administration of atropine 0.04 mg/kg and propranolol 0.2 mg/kg. The resulting intrinsic heart rate represents the sinus node rate, devoid of autonomic influences. Assuming that the normal intrinsic heart rate (in beats/min) is defined by the formula:
Intrinsic heart rate = 118.1 − (0.57 × age)
then (a) an intrinsic heart rate lower than predicted using this formula is consistent with sinus node dysfunction and (b) an intrinsic heart rate close to the predicted rate with a clinical presentation similar to sinus node dysfunction is suggestive of autonomic dysfunction as the cause of bradyarrhythmia.
2.Invasive testing. Electrophysiologic study (EPS) is rarely used in modern practice for the diagnosis of sinus node dysfunction. However, when performed, the two most common tests use indirect measurements of SA node function, as direct measurement of SA node function is laborious.
a.Sinus node recovery time (SNRT) is the time it takes the SA node to recover following paced overdrive suppression of the node.
(1) A delay of longer than 1,400 ms is considered abnormal. This measurement may be corrected by subtracting the intrinsic sinus cycle length (in milliseconds) from the recovery time. A corrected SNRT >550 ms is suggestive of sinus node dysfunction.
(2) The limitations of this test are as follows:
(a) It is an indirect measurement of SA node function and reflects both sinoatrial node conduction time (SACT) and automaticity.
(b) It may be falsely shortened by an SA node entrance block during atrial pacing (because of failure of the paced impulse to reset the sinus node) or falsely prolonged by an SA node exit block (the sinus node is normal but the impulse cannot leave the node), which affects its specificity.
(c) The SNRT is not prolonged in all patients with sinus node dysfunction, which affects its sensitivity.
b.Sinoatrial node conduction time
(1) The steady-state atrial rate is determined (A1–A1 interval or the time between P-waves). Then, premature atrial extra stimuli (A2) are introduced by pacing high in the right atrium, starting in late diastole at progressively shorter intervals until atrial refractoriness is found (i.e., A2 does not result in a P-wave). The duration before the next spontaneous atrial impulse (A3) is measured and the baseline rate is subtracted.
SACT = (A2 − A3 interval) − (A1 − A1 interval)
(2) The test assumes that SA node automaticity is not affected by pacing, that conduction time into the node is equal to conduction time out of the node, and that there is no shift in the principal pacemaker site.
E.Therapy. Treatment for symptomatic sinus node dysfunction may be pharmacologic, pacing, or a combination of both.
1.Indications for pacing in sinus node dysfunction are largely determined by symptoms (e.g., correlation with a documented arrhythmia; Table 22.3).
TABLE 22.3 Indications for Permanent Pacing |
|||
Indication |
Class I |
Class II |
Class III |
SND |
1.SND documented in association with symptomatic bradycardia and because of factors that are irreversible or because of essential drug therapy 2.Symptomatic chronotropic incompetence |
IIa. No clear association between SND with heart rate <40 beats/min and symptoms can be documented IIb. In minimally symptomatic patients, chronic heart rate <40 beats/min while awake |
1.SND with marked sinus bradycardia or pauses but no associated symptoms, including because of long-term drug therapy 2.SND in patients with symptoms suggestive of bradycardia that are clearly documented as not associated with a slow heart rate 3.SND with symptomatic bradycardia because of nonessential drug therapy |
Acquired AV block |
1.Third-degree or advanced second-degree (Mobitz II) AV block at any anatomic level, associated with any one of the following conditions: a.Bradycardia with symptoms presumed to be due to AV block b.Arrhythmias and other medical conditions that require drugs that result in symptomatic bradycardia c.Documented periods of asystole ≥ 3.0 s, or an escape rhythm below the AV node, or any escape rate <40 beats/min in awake, symptom-free individuals in sinus rhythm d.Documented pauses >5 s in awake, symptom-free patients who are in atrial fibrillation e.After catheter ablation of the AV junction f.Postoperative AV block that is not expected to resolve g.Neuromuscular diseases with AV block such as myotonic muscular dystrophy, Kearns–Sayre syndrome, Erb dystrophy (limb girdle), and peroneal muscular dystrophy, with or without symptoms h.Asymptomatic persistent AV block at any anatomic site with average awake ventricular rates of 40 beats/min or faster if cardiomegaly or LV dysfunction is present or if the site of block is below the AV node i.Present during exercise in the absence of myocardial ischemia |
IIa 1.Asymptomatic third-degree AV block with average ventricular rates ≥40 beats/min without cardiomegaly 2.Asymptomatic type II second-degree AV block with narrow QRS. Becomes class I recommendation when wide non-RBBB QRS. 3.Asymptomatic second-degree AV block at intra-His or infra-His levels found at EP study 4.First- or second-degree AV block with symptoms similar to those of pacemaker syndrome or hemodynamic compromise IIb 1.Neuromuscular diseases with any degree of AV block (including first-degree) with or without symptoms 2.AV block in the setting of drug use or toxicity when the block is expected to recur even after drug withdrawal |
1.Asymptomatic first-degree AV block 2.Asymptomatic type I second-degree AV block (Wenckebach) at the supra-His (AV node) level or not known to be intra-Hisian or infra-Hisian 3.AV block expected to resolve and unlikely to recur (e.g., drug toxicity, Lyme disease) |
1.Persistent second-degree AV block in the His-Purkinje system with alternating bundle branch block or third-degree AV block within or below the His-Purkinje system after acute ST-elevation acute MI 2.Transient advanced (second- or third-degree) infranodal AV block and associated bundle branch block. If the site of block is uncertain, an EP study may be necessary 3.Persistent and symptomatic second- or third-degree AV block |
IIa. None IIb 1.Persistent second- or third-degree AV block at the AV node level, even in the absence of symptoms |
1.Transient AV block without intraventricular conduction defect 2.Transient AV block in the presence of isolated left anterior fascicular block 3.New bundle branch or fascicular block in the absence of AV block. Persistent asymptomatic first-degree AV block in the presence of bundle branch block |
|
Chronic bifascicular blocks |
1.Intermittent advanced second- or third-degree AV block Type II second-degree AV block Alternating bundle branch block |
IIa 1.Syncope not proved to be due to AV block when other likely causes have been excluded, specifically ventricular tachycardia 2.HV interval >100 ms on EP study in asymptomatic patients 3.Pacing-induced infra-His block on EP study that is nonphysiologic IIb 1.Neuromuscular disorders with any fascicular or bifascicular block with or without symptoms |
1.Fascicular block without AV block or symptoms 2.Fascicular block with first-degree AV block without symptoms |
1.Recurrent syncope caused by spontaneous carotid sinus stimulation inducing ventricular asystole >3 s |
IIa 1.Syncope without clear, provocative events and with a hypersensitive cardioinhibitory response of >3 s IIb 1.Significantly symptomatic neurocardiogenic syncope associated with bradycardia documented spontaneously or at time of tilt table testing |
1.Hyperactive cardioinhibitory response to carotid sinus stimulation in the absence of clear symptoms 2.Situational vasovagal syncope in which avoidance behavior is effective and preferred |
|
Class I: Conditions for which there is evidence and/or general agreement that pacing is beneficial, useful, and effective.
Class II: Conditions for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of pacing.
Class IIa: Weight of evidence/opinion is in favor of usefulness/efficacy.
Class IIb: Usefulness/efficacy is less well established by evidence/opinion.
Class III: Conditions for which there is evidence and/or general agreement that pacing is not useful/effective and in some cases may be harmful.
AV, atrioventricular; EP, electrophysiologic; HV, half-value; LV, left ventricular; MI, myocardial infarction; RBBB, right bundle branch block; SND, sinus node dysfunction.
Adapted from Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices). J Am Coll Cardiol. 2008;51:2085–2105; Epstein AE, Darbar D, DiMarco JP, et al. ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2012;126:1784–1800.
2.Pacing may also be indicated when essential drug therapy that causes sinus node dysfunction cannot be stopped or changed.
3.For patients with tachycardia–bradycardia syndrome, a pacemaker is often placed for management of the bradyarrhythmia, and antiarrhythmic or rate-controlling drugs are added for treatment of the tachycardia episodes.
4.Acute treatment for patients with symptomatic sinus node dysfunction includes the following:
a.Atropine (0.04 mg/kg intravenous [IV] bolus)
b.Isoproterenol (starting at 1 µg/min intravenously), which may be used as a bridge to pacemaker placement. Isoproterenol is not indicated in most patients with cardiac arrest
c.Temporary pacing for patients whose conditions fail to respond to drug therapy
5.For patients requiring permanent pacing for sinus node dysfunction, a dual-chamber (right atrium and ventricle) pacemaker should be implanted and programmed to DDD or AAI modes, which have been shown to be superior to VVI mode with respect to subsequent incidence of atrial fibrillation, heart failure, pacemaker syndrome, and pacing-induced systolic cardiomyopathy. Subsequent AV block occurs in up to 35% of patients with sinus node dysfunction over extended follow-up, and thus single-chamber AAI pacing is typically used in younger patient populations.
IV.AV conduction disturbances. These disturbances are classified as first-, second-, or third-degree block, depending on the severity of the conduction abnormality.
A.Classification
1.First-degree AV block is characterized by prolongation of the PR interval beyond 200 milliseconds. This finding may occur as a normal variant in 0.5% of asymptomatic young adults without overt heart disease. In older individuals, it is most often caused by idiopathic degenerative disease of the conducting system.
2.Second-degree AV block
a.Second-degree AV block is characterized by a failure of one or more, but not all, atrial impulses to conduct to the ventricles. The block may be at any level of the AV conduction system.
b.When more than one atrial impulse is present for each ventricular complex, the rhythm may be described as a ratio of the number of atrial impulses to the number of ventricular complexes (for two P-waves preceding each QRS complex, 2:1 second-degree AV block is present).
(1) Lesser degrees of AV block (i.e., 4:3 or 3:2) with a prolonging PR interval prior to a nonconducted atrial impulse are described as Mobitz type I AV block (also known as Wenckebach block).
(a) The conducted impulse of a Mobitz type I block will generally be narrow, and the site of block is often in the AV node above the His bundle.
(b) A Mobitz type I block with a bundle branch block is still likely to be above the His bundle, but a His bundle electrogram is needed to confirm the level of block.
(2) High-grade AV block (3:1, 4:1, or greater) is typically described as Mobitz type II AV block. The conducted impulses will generally be preceded by constant PR intervals and have a wide QRS morphology (right bundle branch block [RBBB] or left bundle branch block [LBBB] pattern). The site of block is often below the AV node. A Mobitz type II block is usually intra-Hisian or infra-Hisian and has a greater propensity for progressing to third-degree AV block.
(3) Pure 2:1 conduction patterns cannot be reliably classified as Mobitz type I or type II by ECG alone, and if diagnostic maneuvers (such as exercise) are not able to elucidate one type of second-degree block versus the other, an EPS may be warranted.
3. Third-degree AV block, or complete heart block, may be acquired or congenital and is characterized by AV electrical dissociation.
a.A total of 60% to 90% of cases of congenital complete heart block result from neonatal lupus. The vast remainder occur concurrently with congenital structural heart defects.
b.Acquired AV block occurs most commonly (~50%) because of progressive degeneration of the conduction system, but the differential includes a myriad of reversible and irreversible, iatrogenic and noniatrogenic causes (Table 22.4).
TABLE 22.4 Causes of Atrioventricular Block |
Drug effects |
Digoxin |
β-Blockers |
Certain calcium channel blockers (nondihydropyridines) |
Membrane-active antiarrhythmic drugs |
Ischemic heart disease |
Acute myocardial infarction |
Chronic coronary artery disease |
Idiopathic fibrosis of the conduction system |
Lenegre disease |
Lev disease |
Congenital heart disease |
Congenital complete heart block |
Ostium primum atrial septal defect |
Transposition of the great vessels |
Maternal systemic lupus erythematosus |
Calcific valvular disease |
Cardiomyopathy |
Infiltrative disease |
Amyloidosis |
Sarcoidosis |
Hemochromatosis |
Infectious/inflammatory diseases |
Endocarditis |
Myocarditis (Chagas disease, Lyme disease, rheumatic fever, tuberculosis, measles, and mumps) |
Collagen vascular diseases (scleroderma, rheumatoid arthritis, Reiter syndrome, systemic lupus erythematosus, ankylosing spondylitis, and polymyositis) |
Metabolic |
Hyperkalemia |
Hypermagnesemia |
Endocrine—Addison disease |
Trauma |
Cardiac surgery |
Radiation |
Catheter trauma |
Catheter ablation |
Tumors |
Mesothelioma |
Hodgkin disease |
Malignant melanoma |
Rhabdomyosarcoma |
Neurally mediated |
Carotid sinus syndrome |
Vasovagal syncope |
Neuromyopathic disorders |
Myotonic muscular dystrophy |
Slowly progressive X-linked muscular dystrophy |
Reprinted with permission from Topol EJ, ed. Textbook of Cardiovascular Medicine. Philadelphia, PA: Lippincott–Raven; 1998.
B.Clinical presentation
1.Signs and symptoms
a.First-degree AV block is generally not a cause of symptoms.
b.Second-degree AV block may result in symptoms; however, high-grade second-degree AV block may progress to third-degree AV block, which can frequently cause symptoms.
c.Depending on the escape rate, patients with third-degree AV block may experience fatigue or syncope.
2.Physical findings. The amplitude of the arterial pulse and venous waveform varies, depending on the timing of atrial filling of the ventricles.
a.Second-degree AV block is associated with a periodic change in amplitude. In patients with third-degree AV block, amplitude is constantly changing, with periodic appearance of cannon a-waves (large-amplitude waves in the venous pulsations seen in the neck when the atria contracts against a closed tricuspid valve).
b.Heart sounds are similarly affected by the change in filling duration of the ventricles.
(1) The first heart sound (S1) becomes softer as the PR interval is prolonged, resulting in a soft S1 in first-degree AV block, a progressive softening of S1 in type I second-degree AV block, and a constantly changing S1 in third-degree AV block.
(2) Third-degree AV block may also result in a functional systolic ejection murmur.
C.Etiology. The causes of AV block are listed in Table 22.4; the most common cause is idiopathic degenerative fibrosis. Acute MI results in AV block in 14% of patients with inferior infarction and 2% of those with anterior infarction, usually within the first 24 hours.
D.Diagnostic testing
1.First-degree AV block. Measuring a PR interval longer than 200 ms in adults and 180 ms in children makes the diagnosis. A P-wave precedes each QRS, and both the P and the QRS are morphologically normal.
2.Second-degree AV block
a.The diagnosis of Mobitz type I is made when the following criteria are met on the ECG:
(1) Sequential and gradual prolongation of the PR interval terminated by a nonconducted P-wave
(2) Prolongation of the PR interval occurring in progressively shorter increments in “typical” Wenckebach, which results in progressive shortening of the RR intervals prior to the nonconducted atrial impulse
(3) Duration of the pause following the nonconducted P-wave is less than the sum of any two consecutively conducted beats.
(4) Decreased PR interval following the pause when compared with the prepause PR interval
(5) “Grouped beating,” a pattern of repeated groups of QRS complexes characteristic of Wenckebach block
b.Mobitz type II second-degree AV block is less common than type I.
(1) The PR and PP intervals are constant with a sudden nonconducted P-wave (Fig. 22.2), in contrast to nonconducted (blocked) premature atrial contractions that have varying PR and PP intervals.
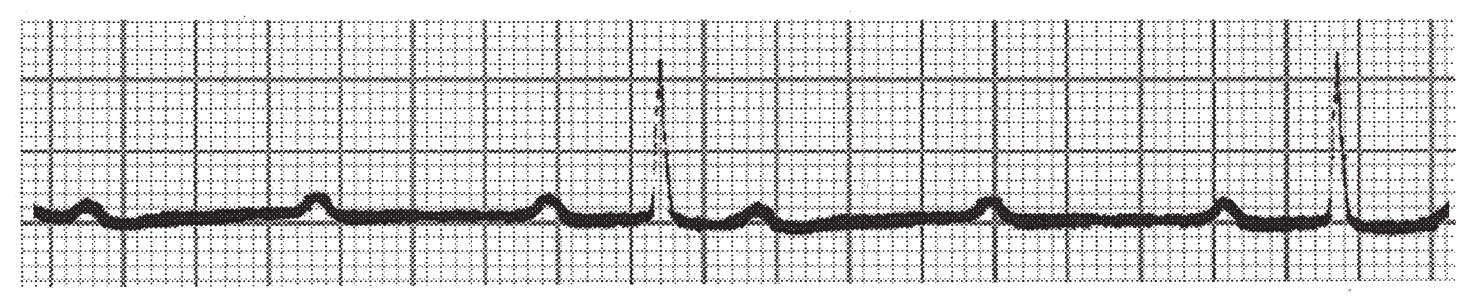
FIGURE 22.2 Mobitz type II second-degree atrioventricular block with 3:1 conduction.
(2) Each QRS complex may have multiple P-waves, which are designated by the number of P-waves before each conducted QRS (3:1, 4:1, etc.). The QRS complex is typically not narrow (a narrow QRS complex is suggestive of a Mobitz type I block). It is often difficult to distinguish between 2:1 Mobitz I versus Mobitz II patterns by ECG alone, particularly if the QRS complex is narrow. A commonly used discrimination tool in this scenario is exercise ECG testing. Exercise improves (decreases) AV nodal conduction time but not infranodal conduction time, and thus may improve block from 2:1 to 3:2 in Mobitz I, whereas block should be unchanged to worsened (2:1 to 3:1) in Mobitz II.
3. Third-degree AV block (Fig. 22.3)
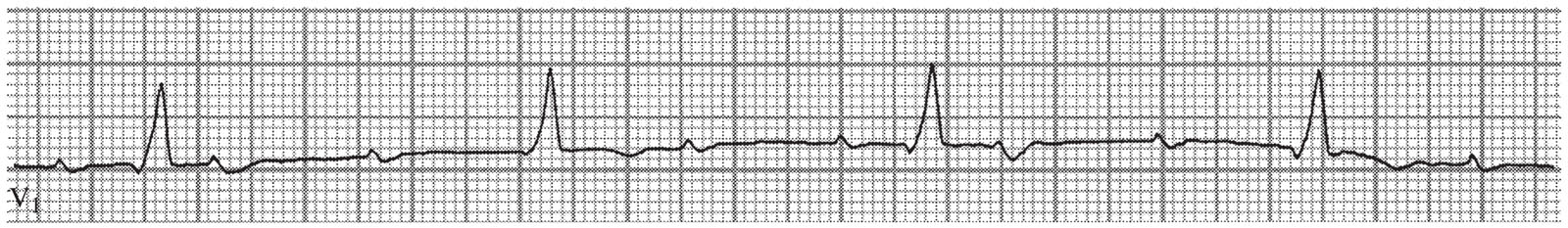
FIGURE 22.3 Third-degree atrioventricular block with sinus tachycardia and right bundle branch block.
a.Third-degree AV block is characterized by the identification of complete dissociation of the atrial and ventricular electrical activities (no temporal relationship exists between the P-waves and the QRS complexes), with atrial activity more rapid than ventricular activity. Using calipers, it is possible to march out the progression of the P-waves to determine the atrial rate.
b.Third-degree AV block is only one cause of AV dissociation; not all AV dissociation is third-degree AV block. For example, conditions where the ventricles are depolarizing faster than the atria—such as accelerated junctional rhythm or ventricular tachycardia—also result in AV dissociation if there is a lack of retrograde conduction over the AV node.
E.Therapy. Patients with first-degree AV block and Mobitz type I AV block usually do not require therapy. Permanent pacing is indicated for Mobitz type II AV block and third-degree AV block without reversible cause. (See Table 22.3 for complete indications for pacing.)
1.Medical therapy may be used as a bridge to pacing, but it has no role in long-term treatment.
a.The principal drug used as a bridge to pacing is atropine:
(1) It reduces heart block because of hypervagotonia but not because of AV nodal ischemia.
(2) It is more useful for AV block in inferior MI than anterior MI.
(3) It does not increase infranodal conduction (will not improve second-or third-degree AV block that is below the AV node).
(4) It is ineffective in the denervated hearts of transplant patients.
(5) It is used with caution (if at all) in Mobitz type II AV block because of a possible paradoxical decrease in heart rate (as atrial rate increases, AV conduction decreases, and a 2:1 block with an atrial rate of 80 beats/min and a ventricular rate of 40 beats/min may be converted to a 3:1 block with an atrial rate of 90 beats/min and a ventricular rate of 30 beats/min).
b.Targeted medical therapy ± temporary pacing is indicated for potential reversible causes prior to permanent pacemaker implantation. For example, cardiac Lyme should receive appropriate antibiotic therapy. Overmedication with AV nodal blocking agents should receive adequate time for drug metabolism/clearance ± administration of reversal agents (e.g., glucagon, IV calcium). Digoxin-specific Fab fragments may be used to treat patients with symptomatic AV blocks related to the use of digitalis.
2.Pacing
a.Third-degree AV block as a complication of inferior MI is usually temporary and thus usually only requires temporary pacing. However, complete heart block as a result of anterior MI often requires permanent pacing (Table 22.3).
b.Acquired third-degree AV block usually requires pacing, but patients with congenital third-degree AV block often have a sufficiently rapid escape rhythm to prevent symptoms and avoid permanent pacemaker implantation.
c.For irreversible third-degree AV block without permanent atrial tachyarrhythmia, a permanent dual-chamber pacemaker should be implanted and programmed to DDD mode. However, if the preimplant ejection fraction is ≤50%, implantation of a biventricular pacemaker (cardiac resynchronization therapy [CRT]) should be considered, due to lower composite rates of mortality, heart failure, and adverse left ventricular remodeling with CRT in this patient population (BLOCK-HF).
d.The location of the right ventricular permanent pacing lead, whether apical or nonapical, did not affect clinical outcomes or left ventricular remodeling in PROTECT-PACE, a recent prospective randomized control trial (RCT).
V.Junctional rhythms. Junctional rhythms arise from the area surrounding the AV node, including the supranodal fibers, the node itself, and the bundle of His. This area has an intrinsic rate of 30 to 60 beats/min and serves as a faster escape mechanism that supersedes the automaticity of ventricular myocardium (0 to 40 beats/min) in the case of complete AV block. Junctional rhythm that is faster than the sinus rhythm is referred to as accelerated junctional rhythm.
A.Clinical presentation. Patients usually do not develop symptoms that are directly attributable to accelerated junctional rhythm. The physical findings of AV dissociation may be noted and are the same as those seen in third-degree AV block.
B.Etiology
1.Accelerated junctional rhythm is seen in approximately 10% of patients with acute MI. More than one-half of these patients have inferior and about one-third have anterior MI.
2.Digitalis toxicity by itself does not seem to cause accelerated junctional rhythm, as evidenced in persons with normal hearts who take accidental overdoses of digoxin. Concomitant heart disease is required to develop accelerated junctional rhythm.
3.Other causes of accelerated junctional rhythm are valve surgery, acute rheumatic fever, direct current cardioversion, cardiac catheterization, serious infection, chronic obstructive pulmonary disease, systemic amyloidosis, and uremia with hyperkalemia.
C.ECG findings
1.Accelerated junctional rhythm
a.Unless the junctional rhythm causes retrograde activation of the atria, the P-wave is normal in morphologic characteristics. The QRS complex has a normal duration, unless there is concomitant bundle branch block. The distinguishing characteristic of accelerated junctional rhythm is the AV dissociation and changing PR interval (Fig. 22.4).
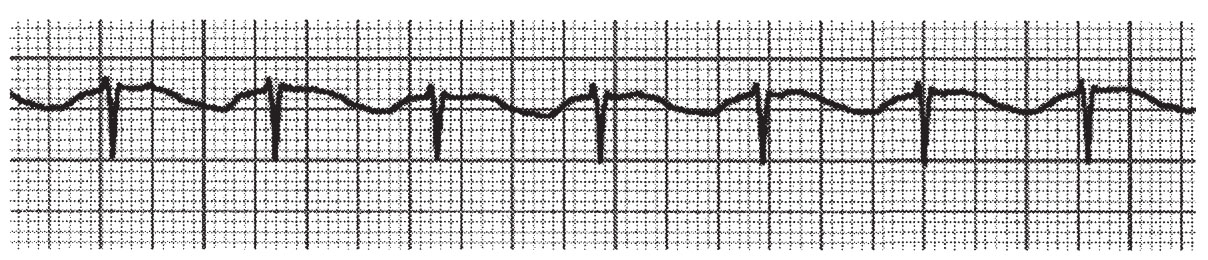
FIGURE 22.4 Accelerated junctional rhythm.
b.The difference between accelerated junctional rhythm and third-degree AV block is the fact that the ventricular rate is faster than the atrial rate in accelerated junctional rhythm and slower than the atrial rate in third-degree AV block.
2.Junctional rhythm. In the absence of a sinus beat, the AV node can act as a backup pacemaker. The ECG findings are classically an absence of P-waves (or retrograde P-waves with negative axis in inferior leads immediately before or after the QRS complex with short PR/RP intervals, respectively), a narrow QRS complex, and a rate of 30 to 60 beats/min.
D.Therapy
1.Therapy for junctional rhythm secondary to SA node failure or AV block is as previously outlined for AV conduction disturbances.
2.Patients with accelerated junctional rhythm do not usually require therapy for the arrhythmia, although management of the underlying cause is indicated.
3.Suppression of accelerated junctional rhythm may be achieved by increasing the atrial rate with drugs (e.g., atropine and adrenergics) or pacing.
4.Digoxin-induced accelerated junctional rhythm is an indication to stop digoxin, but it does not usually require administration of digoxin-specific Fab fragments.
VI.Intraventricular conduction disturbances. Conduction disturbances because of blockage below the AV node are classified on the basis of the intraventricular conduction system. An intraventricular conduction disturbance (IVCD) does not itself cause bradyarrhythmia, but it may be associated with any of the other rhythms that cause bradycardia. When associated with an acute MI, an IVCD predicts a worse outcome.
A.Etiology
1.The causes of IVCDs are similar to those that cause AV block (Table 22.4); idiopathic degenerative conduction disease and acute ischemic syndromes are the most common causes.
2.IVCDs increase with age and affect up to 2% of individuals older than 60 years.
3.The incidence of IVCDs is increased in persons with structural heart disease, especially those with coronary artery disease.
B.ECG findings
1.The ECG findings of IVCDs are summarized in Table 22.5 and examples are presented in Figures 22.5 to 22.8. As shown, IVCDs may be further classified by the number of fascicles they affect.
TABLE 22.5 Electrocardiographic Features for the Fascicular and Bifascicular Blocks |
||||||
ECG Finding |
LBBB |
LAFB |
LPFB |
RBBB |
RBBB and LAFB |
RBBB and LPFB |
QRS axis |
≥ −45° |
+90° to +120° |
−60° to −120° |
≥ +120° |
||
QRS duration leads I/aVL |
≥120 ms broad monophasic R |
Normal qR |
Normal rS |
≥120 ms qRS with wide terminal S |
≥120 ms qR |
≥120 ms rS |
Leads II, III, and aVF |
rS |
qR |
rS |
qR |
||
Leads V1 and V2 |
rS or QS |
rsR′ or rSR′ |
rsR′ or rSR′ |
rsR′ or rSR′ |
||
Leads V5 and V6 |
S |
no Q |
qRS |
|||
ECG, electrocardiogram; LAFB, left anterior fascicular block; LBBB, left bundle branch block; LPFB, left posterior fascicular block; RBBB, right bundle branch block.
2.Fascicular blocks
a.Unifascicular block affects only one of the three fascicles. Examples are RBBB, left anterior fascicular block (LAFB), and left posterior fascicular block (LPFB).
b.Bifascicular block is present when conduction disturbances affect two of the fascicles, most commonly the right bundle branch and the left anterior fascicle. Approximately, 6% of these patients progress to complete heart block. RBBB with LPFB is less common, but the progression to complete heart block is more common.
c.“Trifascicular block” is said to be present when there is a combination of bifascicular block and first-degree AV block (Fig. 22.8).
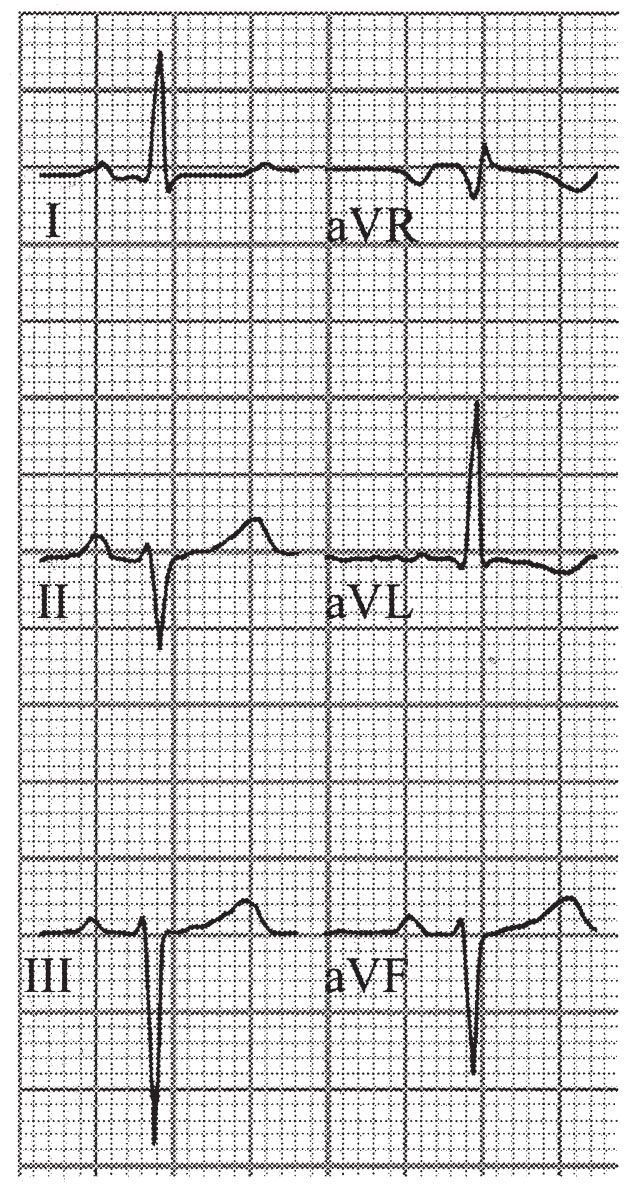
FIGURE 22.5 Left anterior hemiblock.
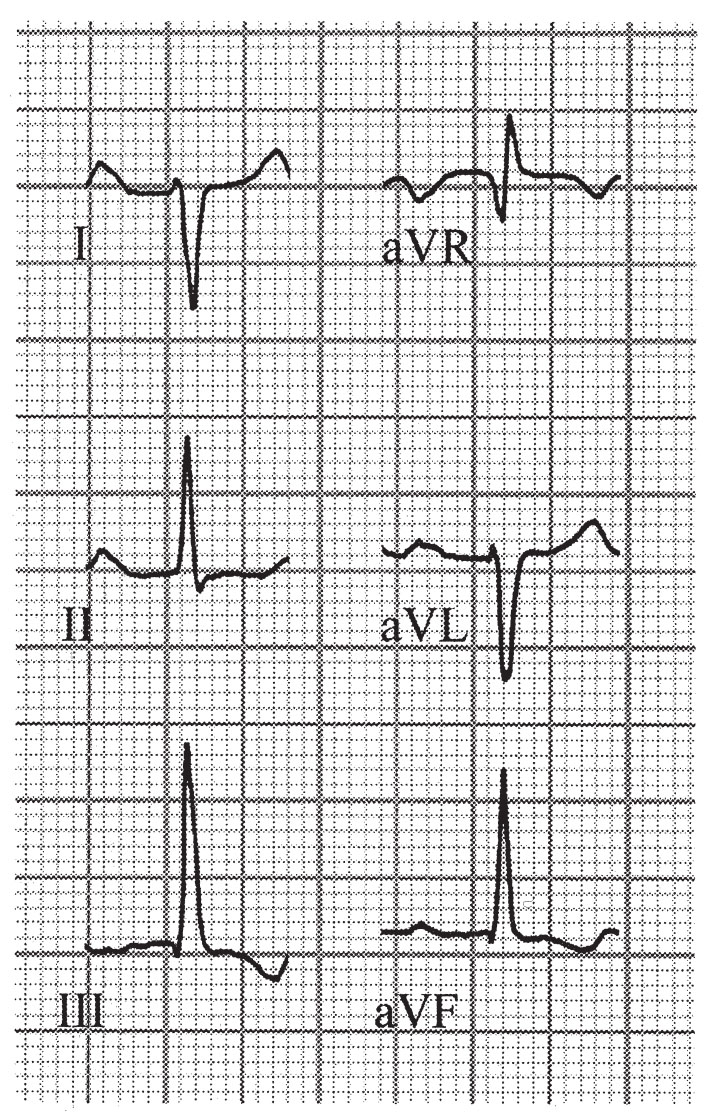
FIGURE 22.6 Left posterior hemiblock.
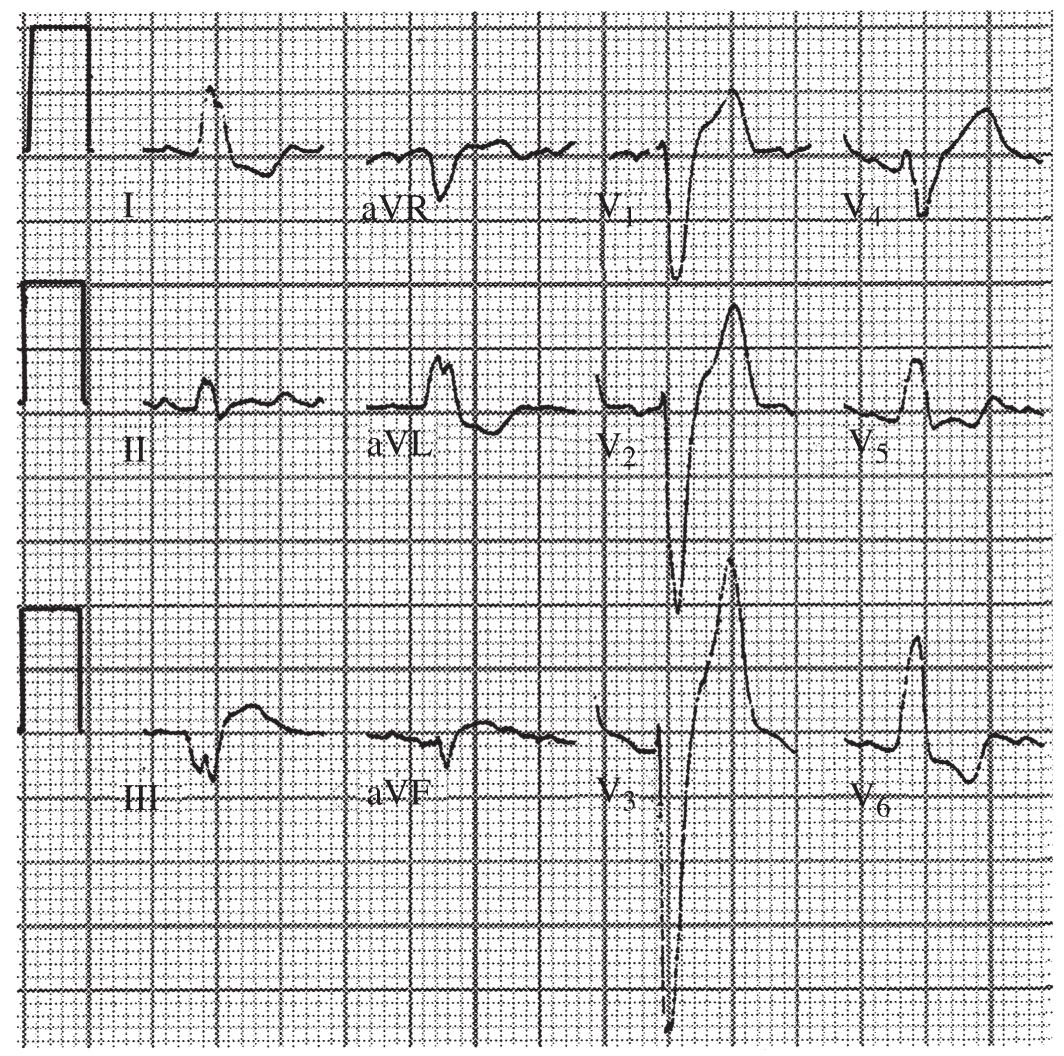
FIGURE 22.7 Left bundle branch block.

FIGURE 22.8 First-degree atrioventricular block with right bundle branch block and left anterior hemiblock.
C.Therapy. Pacing is indicated in patients with bifascicular block who have advanced AV block (Mobitz II or complete heart block) or alternating bundle branch block. Whereas an EPS is not routinely performed in this setting, pacing may also be indicated in asymptomatic patients if a prolonged HV interval >100 ms or nonphysiologic infra-Hisian block is found (Table 22.3).
VII.Postprocedural Bradyarrhythmias
A.Etiology. Bradyarrhythmias following cardiac surgery and endovascular procedures are not uncommon.
1.Cardiac surgery, in particular valvular surgery and myectomy, can cause mechanical damage to the conduction system, leading to new AV block and IVCD that are variably reversible. The estimated incidence of persistent high-grade AV block requiring permanent pacemaker implantation following cardiac surgery is 2% to 7%. The risk following transcatheter aortic valve replacement appears to be much greater, ranging from 10% to 40% and highly manufacturer specific. The risk of permanent high-grade AV block following nonurgent, coronary-exclusive interventions (coronary artery bypass grafting or percutaneous coronary intervention) is comparably low.
2.Prolonged ischemic time during cardiac surgery, most commonly cardiac transplantation, can similarly lead to sinus node dysfunction (SND) and subsequent permanent pacing requirement (up to 10% of posttransplant patients).
B.Therapy. Because postprocedural bradyarrhythmias are frequently temporary and resolve with time postprocedure, temporary pacing should be utilized initially, with the decision to proceed to permanent pacing made only after extended surveillance (institution and procedure-dependent, ranging from 5 to 14 days). The same criteria listed in Table 22.3 are used to determine the indications for permanent pacing postprocedure.
VIII.Pulseless Electrical Activity and Asystole
A.Pulseless electrical activity (PEA) is defined as the absence of a pulse or blood pressure, yet with the continued presence of electrical activity of the heart. Asystole is defined as the absence of both cardiac electrical (e.g., flat line) and mechanical activity. PEA and asystole exist on the same spectrum, classified by advanced cardiac life support (ACLS) as “nonshockable” rhythms and carry exceedingly poor prognoses, with survival 2% after out-of-hospital and 20% to 30% after in-hospital PEA/asystolic arrest.
B.Etiology
1.PEA results from electromechanical dissociation, a condition where cardiac electrical activation is ongoing (although not necessarily functioning normally) but produces inadequate (or absent) mechanical contraction/cardiac output. PEA can occur in a variety of rhythm disturbances, ranging from idioventricular escape rhythm to sinus tachycardia, although classically excluding ventricular tachycardia and ventricular fibrillation (VF) according to ACLS algorithms. PEA may degenerate to asystole, or asystole may occur directly.
2.A variety of potentially reversible clinical situations are classically associated with PEA and asystole, abbreviated as the H’s and T’s (Table 22.6).
TABLE 22.6 Conditions That Cause Pulseless Electrical Activity |
||
Condition |
Clues |
Management |
Hypovolemia |
History of poor oral intake and/or increased blood volume/GI/insensible losses, flat neck veins and poor skin turgor on physical examination, preceding sinus tachycardia, collapsible IVC on ultrasound |
Volume infusion (+ transfusion if concern for hemorrhage) |
Hypothermia |
History of exposure to cold, central body temperature, and ECG findings (bradycardia, Osborn waves) |
Gradual warming |
Hypoxia |
Baseline pulmonary pathology, cyanosis, increasing A–a gradient, airway concerns |
Increase oxygen delivery (noninvasive, positive pressure, intubation) |
Hypoglycemia |
History of diabetes (particularly insulin-requiring), fingerstick blood glucose, recent NPO status, acute kidney injury/renal failure |
Dextrose administration |
Hyperkalemia |
History of renal failure, ischemic presentation, uncontrolled diabetes, ECG findings (peaked T-waves, QRS prolongation, sine wave) |
(Immediate for electrical stabilization): Calcium chloride/gluconate, combination of IV insulin + glucose, sodium bicarbonate, albuterol (if controlled airway); (long term for potassium clearance): sodium polystyrene or dialysis |
Hydrogen ions (acidosis) |
History of renal failure, medical comorbidity predisposing to hypercapnia (COPD), acidosis or hypercapnia on arterial blood gas |
Sodium bicarbonate, hyperventilation |
Tension pneumothorax |
History (asthma, ventilator, COPD, recent procedure or trauma), no pulse with CPR, neck vein distention, tracheal deviation, absent breath sounds |
Needle decompression |
Thrombosis (MI) |
CAD history, ischemic symptoms, ST-changes on ECG, elevated cardiac enzymes, ventricular arrhythmia preceding or subsequent to PEA arrest |
Urgent revascularization |
Thrombosis (pulmonary embolism) |
Predisposing history (high Wells’ score), preceding hypoxia or elevated A–a gradient without clear alternative pulmonary pathology, ECG findings (sinus tachycardia, S1Q3T3, new RBBB, RV strain) |
(Periarrest): consider thrombolytics if high clinical suspicion and not contraindicated; (postarrest): confirm diagnosis via CT or V/Q scan imaging |
Tamponade (cardiac) |
History (recent cardiac procedure or trauma, renal failure, malignancy), vein distention; impending tamponade—tachycardia, hypotension, low pulse pressure with pulsus paradoxus, electrical alternans on ECG |
Urgent pericardiocentesis |
Toxins (drug overdose) |
History of ingestion, empty pill bottles at the scene, pupils, skin, and neurologic examination. ECG findings (brady- or tachycardia, PR + QRS + QTc intervals, aVR elevation) |
Toxicology consultation with targeted antidose administration, drug screening, airway protection (intubation) |
History of recent trauma, physical examination findings (absent breath sounds, overt fractures, ecchymoses, pupil asymmetry), FAST ultrasound findings, profound anemia |
Aggressive volume resuscitation ± transfusion, targeted trauma surgery consultation |
|
A–a, alveolar–arterial; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CPR, cardiopulmonary resuscitation; CT, computed tomography; ECG, electrocardiogram; FAST, focused assessment with sonography in trauma; GI, gastrointestinal; IV, intravenous; IVC, inferior vena cava; MI, myocardial infarction; NPO, nil per os; PEA, pulseless electrical activity; RBBB, right bundle branch block; RV, right ventricle/ventricular.
C.Therapy
1.Rapid management targeted at the suspected underlying cause is most likely to result in favorable outcome. The differential of PEA/asystolic arrest should always include the “H’s and T’s”: Hypovolemia, Hypothermia, Hypoxia, Hypoglycemia, Hyperkalemia, acidosis (“Hydrogen ions”), Tension pneumothorax, Thrombosis (coronary or pulmonary), Tamponade, Toxins, and Trauma (Table 22.6).
2.Emergency resuscitation should be initiated at once as per ACLS protocols:
a.Effective, uninterrupted cardiopulmonary resuscitation (CPR) prioritized over advanced airway management
b.Epinephrine, 1 mg IV push every 3 to 5 minutes
c.One-time IV push of 40 IU of vasopressin may be considered as an alternative to epinephrine during the first or second round of ACLS.
d.Atropine is no longer recommended for routine ACLS administration during PEA/asystole, nor is routine defibrillation for potential coarse VF masquerading as asystole, nor routine cardiac pacing for bradycardic rhythms or asystole.
e.Although not yet integrated into ACLS guidelines, 20 IU vasopressin + 1 mg ephinephrine for up to first 5 CPR cycles and 40 mg methylprednisolone during the first CPR cycle followed by stress-dose hydrocortisone with taper after return of spontaneous circulation demonstrated improved survival and neurologic outcomes after in-hospital PEA/asystolic arrest in a prospective RCT.
ACKNOWLEDGMENTS: The author thanks Drs. Santosh Oommen, Christopher Cole, Gregory Bashian, and Oussama Wazni for their significant contributions to earlier editions of this chapter.
Landmark Articles
Curtis AB, Worley SJ, Adamson PB, et al; for the BLOCK HF trial investigators. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585–1593.
Epstein AE, Darbar D, DiMarco JP, et al. ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2012;126:1784–1800.
Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices). J Am Coll Cardiol. 2008;51:2085–2105.
Field JM, Fazinski MF, Sayre MR, et al. 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S640.
Gillis AM, Russo AM, Ellenbogen KA, et al. HRS/ACCF expert consensus on pacemaker device and selection mode. J Am Coll Cardiol. 2012;60(7):682–703.
Kaye GC, Linker NJ, Marwick TH, et al; for the PROTECT-PACE investigators. Effect of right ventricular pacing lead site on left ventricular function in patients with high-grade atrioventricular block: results of the PROTECT-PACE study. Eur Heart J. 2015;36(14):856–862.
Kusumoto FM, Goldschlager N. Cardiac pacing. N Engl J Med. 1996;334:89–97.
Maloney JD, Jaeger FJ, Rizo-Patron C, et al. The role of pacing for the management of neurally mediated syncope: carotid sinus syndrome and vasovagal syncope. Am Heart J. 1994;127:1030–1037.
Mentzelopoulos SD, Malachias S, Chamos C, et al. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest. JAMA. 2013;310(3):270–279.
Neumar RW, Shuster M, Callaway CW, et al. 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S315.
Rotman M, Wagner GS, Wallace AG. Bradyarrhythmias in acute myocardial infarction. Circulation. 1972;45:703–722.