Venous Thromboembolism and Hypercoagulable States
I.Venous thromboembolism and hypercoagulable states
A.Venous thromboembolism (VTE). Deep-vein thrombosis (DVT) and pulmonary embolism (PE) represent different manifestations of the same clinical entity referred to as VTE. It is a common, lethal disease that affects hospitalized and nonhospitalized patients, recurs frequently, is often overlooked, and can result in long-term complications, including chronic thromboembolic pulmonary hypertension and the postthrombotic syndrome (PTS).
1.Deep-vein thrombosis. The lower extremities are the most common sites for DVT, but other affected sites include the upper extremities and the mesenteric and pelvic veins. The main goal in the management of DVT is the prevention of PE and PTS. Proximal lower extremity DVTs (popliteal vein and above) have an estimated risk of PE of 50% if not treated. Approximately 25% of calf vein thrombi propagate (in the absence of treatment) to involve the popliteal vein or above.
2.Pathogenesis and risk factors. Virchow’s triad still forms the best framework for understanding the pathogenesis of VTE. The triad includes stasis, hypercoagulability, and injury to the vessel wall. There are both inherited and acquired risk factors for hypercoagulability. The most common inherited risk factors include factor V Leiden (FVL) and prothrombin gene mutation (G20210A); deficiency of the natural anticoagulant protein C (PC), protein S (PS), and antithrombin (AT); hyperhomocysteinemia; and elevated factor VIII levels. Acquired risk factors include immobilization, surgery, trauma, pregnancy, use of oral contraceptives (OCPs) or hormone replacement therapy (HRT), malignancy, antiphospholipid syndrome (lupus anticoagulant and/or anticardiolipin antibodies), heparin-induced thrombocytopenia (HIT), myeloproliferative disorders, smoking, obesity (body mass index [BMI] > 30), inflammatory bowel disease, central venous catheters or pacemakers, and the nephrotic syndrome.
3.Clinical manifestations. Typical symptoms of DVT in the upper and lower extremities include pain and swelling. Signs of DVT on physical examination may include increased warmth, tenderness, edema, the presence of dilated veins (collaterals), erythema, and, in extreme situations, cyanosis or gangrene. A limb-threatening manifestation of DVT, phlegmasia cerulea dolens, occurs most often in the setting of malignancy, HIT, or other hypercoagulable conditions in which the thrombus completely occludes venous outflow, causing massive limb swelling, hypertension in the capillary bed, and eventually ischemia and necrosis. Phlegmasia cerulea dolens is a vascular emergency requiring leg elevation, anticoagulation, and, in select cases, thrombolysis or surgical or catheter-based thrombectomy. Fasciotomy may also be required to relieve associated compartmental syndromes.
4.Diagnosis
a.Clinical examination is unreliable in the diagnosis of DVT, as symptoms and signs are often insensitive and nonspecific. Pretest probability scores improve the utility of further testing. For example, using the Wells score (Table 25.1), patients in the low pretest probability category have a 96% negative predictive value for DVT (99% if the d-dimer is negative as well), but the positive predictive value in patients with a high pretest probability is <75%, supporting the need for further diagnostic testing to identify patients with thrombosis.
TABLE 25.1 Pretest Probability of Deep-Vein Thrombosis (Wells Score) |
|
Clinical Featurea |
Score |
Active cancer (treatment ongoing or within previous 6 mo of palliative treatment) |
1 |
Paralysis, paresis, or recent plaster immobilization of the lower extremities |
1 |
Recently bedridden for more than 3 d or major surgery, within 4 wk |
1 |
Localized tenderness along the distribution of the deep venous system |
1 |
Entire leg swollen |
1 |
Calf swelling by more than 3 cm when compared with the asymptomatic leg (measured 10 cm below tibial tuberosity) |
1 |
Pitting edema (greater in the symptomatic leg) |
1 |
Collateral superficial veins (nonvaricose) |
1 |
Alternative diagnosis as likely or greater than that of DVT |
−2 |
|
Score |
|
High |
3 or greater |
Moderate |
1 or 2 |
Low |
0 or less |
|
Modified Score (Adds One Point If There Is a Previously Documented DVT) |
|
Likely |
2 or more |
Unlikely |
1 or less |
DVT, deep-vein thrombosis.
aIn patients with symptoms in both legs, the more symptomatic leg is used.
Reprinted from Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350(9094):1795 (Copyright © 1997 Elsevier. All rights reserved.); Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349(13):1227. (Copyright © 2003 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.)
b.Venography has been the gold standard test for the diagnosis of DVT in the past.
However, because venography is invasive and requires the use of potentially harmful contrast agents, it has largely been replaced by noninvasive tests such as duplex ultrasonography.
c.Duplex ultrasonography has a sensitivity and specificity of about 95% and 98%, respectively, for the detection of proximal DVT in symptomatic patients; however, it is operator dependent and is less sensitive in asymptomatic individuals and for thrombi located in the calf veins. An inability to compress the vein with the ultrasound transducer is diagnostic for DVT. Diagnosis of recurrent DVT is more challenging, given the high incidence of persistently noncompressible veins after an initial event. False positives may occur when pelvic masses result in isolated noncompressibility of the common femoral veins.
d.d-Dimer. The sensitivity and negative predictive value of this test are high. The combination of a low pretest probability and a negative d-dimer has an extremely high negative predictive value for VTE (approximately 99%). A positive d-dimer, however, is nonspecific, and other diagnostic testing should be performed.
e.Other diagnostic tests less frequently used to detect DVT include magnetic resonance venous imaging and computed tomography (CT). These tests are mainly helpful in the diagnosis of DVT in the pelvic veins, inferior vena cava (IVC), or mesenteric veins.
5.Treatment
a.Anticoagulation. The main goals of treatment for DVT include relief of symptoms and prevention of PE, PTS, and recurrent VTE. Once the diagnosis of DVT is confirmed, anticoagulation should be started immediately unless there is a contraindication. Initial therapy should include heparin, low-molecular-weight heparin (LMWH), fondaparinux, or a direct oral anticoagulant (DOACs). Weight-based dosing of unfractionated heparin (UFH) (80 U/kg bolus followed by 18 U/kg/h intravenous [IV] infusion) has been shown to achieve a therapeutic activated partial thromboplastin time (aPTT) more rapidly than fixed-dose regimens. The aPTT should not be followed in patients with an abnormal baseline aPTT (e.g., in patients with lupus anticoagulant) and in patients who require unusually high doses of heparin such as in AT deficiency. In these situations, the anti-factor Xa assay should be used.
(1)LMWH is administered as a weight-based subcutaneous injection. Enoxaparin is given either as a once-daily injection (1.5 mg/kg/d) or twice per day (1 mg/kg every 12 hours). No monitoring is required except in obese, pediatric, or pregnant patients or patients with renal insufficiency. When necessary, the anti-Xa level using LMWH as a reference standard should be measured 4 hours after an injection. The therapeutic range is 0.5 to 1.0 IU/mL for the 12-hour regimen and ≥1.0 IU/mL for the daily dose.
(2)Once anticoagulation with UFH or LMWH is begun, a VKA may be initiated. The overlap with a VKA should be continued for a minimum of 5 days and until the international normalized ratio (INR) is within the target range of 2.0 to 3.0 for two consecutive days to permit adequate depletion of vitamin K–dependent coagulation factors.
(3)Fondaparinux, an indirect factor Xa inhibitor, is approved as treatment for acute DVT and PE when used in combination with a VKA. Fondaparinux is administered as a once-daily subcutaneous injection of 5, 7.5, or 10 mg based on body weight (<50, 50 to 100, and >100 kg, respectively) for the treatment of DVT or PE. Fondaparinux is contraindicated in patients with severe renal impairment (creatinine clearance < 30 mL/min) and bacterial endocarditis.
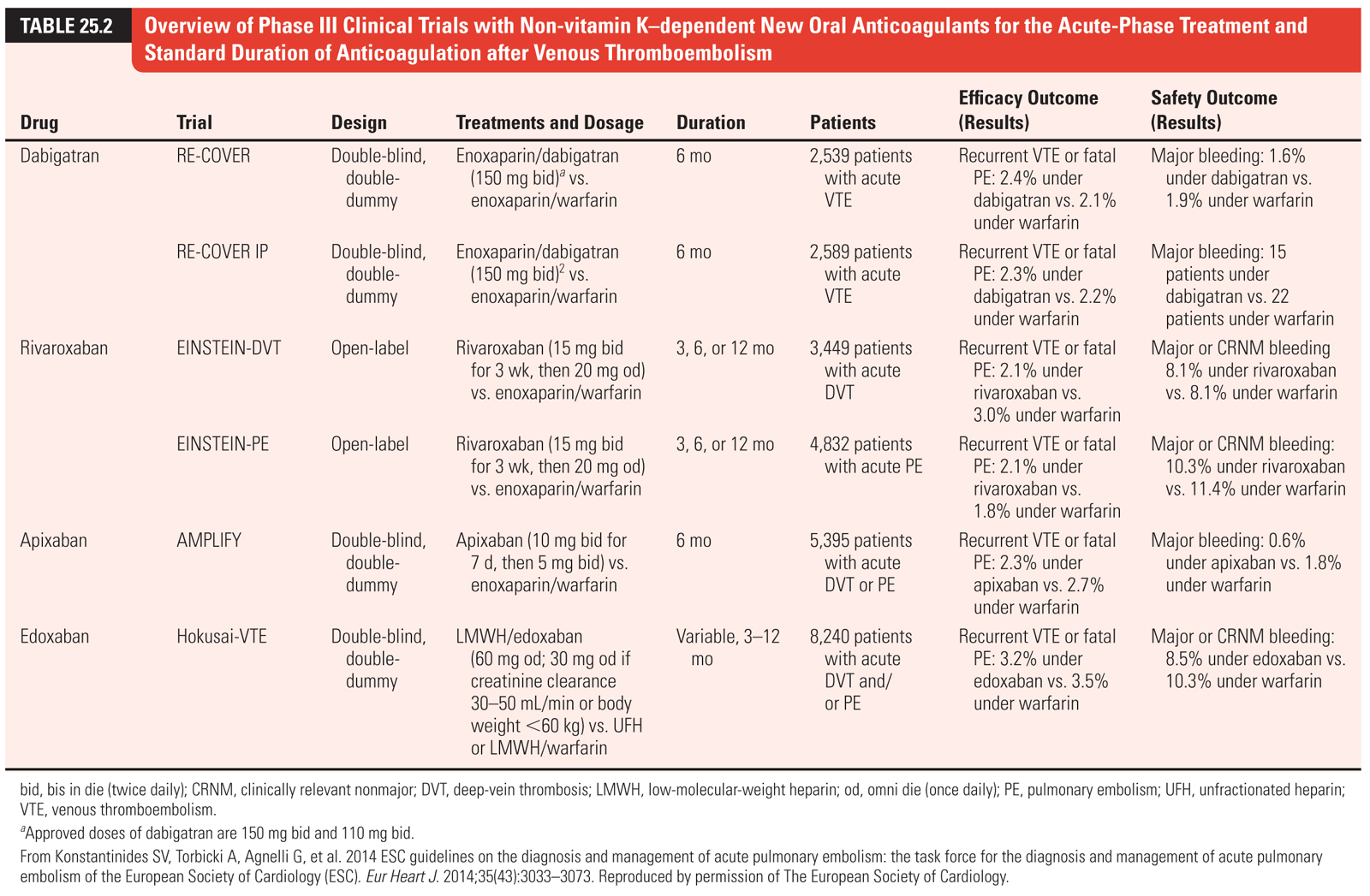
(4)Direct oral anticoagulants. Multiple new DOACs with different mechanisms of action have been evaluated in patients with VTE. These medications include rivaroxaban, apixaban, edoxaban (direct factor Xa inhibitors), and dabigatran (direct thrombin inhibitor [DTI]). The results of these trials are summarized in Table 25.2. Overall, DOACs have proven non-inferior to VKAs and are associated with fewer bleeding complications. Factor Xa inhibitors rivaroxaban and apixaban have a more rapid onset of action which obviates the need for parenteral anticoagulation in the initial treatment of VTE. Additionally, because these agents have stable pharmacodynamics (unlike warfarin), routine monitoring is not required. Current guidelines suggest DOACs over VKAs for the treatment of VTE, except in patients with active cancer in whom LMWH is the treatment of choice.
(5)Thrombolytic therapy for DVT may be beneficial in select individuals and is preferably administered locally via a catheter-directed approach. Systemic lysis is also an option if a catheter-directed approach is not available. Both routes carry an increased risk of systemic hemorrhage compared with standard anticoagulation alone. Current guidelines recommend against routine thrombolytic use in patients with DVT, except for those patients (without contraindication) with an extensive acute proximal DVT (ileofemoral DVT) or individuals at risk for limb gangrene secondary to venous occlusion. The recently published ATTRACT trial demonstrated among patients with acute proximal DVT that catheter-directed thrombolysis did not lower the risk of PTS and resulted in higher risk of major bleeding.
(6)Previous small trials suggested graduated compression stockings (GCS) may help prevent PTS; however, a large randomized trial reported no benefit. These trials have been criticized for methodologic flaws, but the routine use of GCS for the prevention of PTS has fallen out of favor. Early ambulation as tolerated after diagnosis is safe and should be encouraged.
(7)Vena caval interruption. Current guidelines recommend against the routine insertion of IVC filters for the treatment of DVT. Indications for placement of IVC filters are as follows: a contraindication to anticoagulation, a complication from anticoagulation, or recurrent thromboembolization despite adequate anticoagulant therapy. IVC filter alone is not an effective therapy for DVT, and resumption of anticoagulation as soon as risk of bleed is acceptable is recommended.
b.Duration of treatment. The duration of treatment following the diagnosis of DVT is dependent on the risk of recurrence. Risk factors for recurrence include an idiopathic DVT, underlying hypercoagulable states (listed in subsequent text), and malignancy. In addition, placement of a permanent IVC filter, elevated d-dimer levels, advanced age, male sex, and increased BMI also place individuals at a higher risk for recurrence. The risk of recurrence is low while patients are on anticoagulation; however, clinicians must weigh the risk of bleeding against the risk of new thrombosis. Current guidelines recommend 3 months of anticoagulation for patients with a first episode of DVT secondary to a transient cause. Anticoagulation for at least 3 months is recommended for patients with a first episode of idiopathic DVT, with consideration given for indefinite anticoagulation in patients with a first unprovoked proximal DVT who are considered to be at low or moderate bleeding risk. Long-term (indefinite) anticoagulation is also recommended in patients with malignancy for as long as the cancer remains active and in patients who have unexplained recurrent DVTs. For patients with unprovoked proximal DVT who desire to stop anticoagulation, aspirin may be considered to prevent recurrent VTE. It should be noted that aspirin is much less effective than anticoagulation for VTE recurrence prevention and is not considered an alternative to anticoagulation.
c.Calf vein thrombosis. Current guidelines recommend anticoagulation for acute isolated distal DVT if there are severe symptoms or high-risk features for extension to the proximal veins. Risk factors for extension that favor anticoagulation over surveillance include positive d-dimer, extensive thrombosis (>5 cm in length, multiple veins, >7 mm maximal diameter), close proximity to proximal veins, no provoking factor, active cancer, history of VTE, or inpatient status. In patients who are managed with surveillance (serial imaging once or twice weekly for 2 to 3 weeks), anticoagulation is recommended if the thrombus extends further in the distal veins or to the proximal veins. The decision for anticoagulation or surveillance should also consider bleeding risk and patient preference.
d.Superficial venous thrombosis frequently occurs as a complication of an IV line in an upper extremity, but may occur spontaneously in the upper or lower extremities. Anticoagulation is not routinely administered, but may be considered for those at higher risk for extension to the proximal veins (>5 cm length, <5 cm from the sapheno-femoral or sapheno-popliteal junction). Patients with superficial venous thrombosis should undergo ultrasound to rule out DVT.
e.Upper extremity DVT. Upper extremity DVT is most often related to central venous catheter placement and/or pacemaker devices. Other less common causes include thoracic outlet syndrome, Paget–von Schröetter syndrome (also referred to as effort thrombosis), and hypercoagulable conditions including malignancy. Patients may be asymptomatic but more frequently complain of arm swelling and pain. Anticoagulation is indicated if there are no contraindications. Thrombolysis should be considered in younger patients with effort thrombosis, who have a low risk of bleeding and symptoms of acute onset. Determination of the length of anticoagulation should be decided using the same processes described for acute lower extremity DVTs.
B.Pulmonary embolism. It is difficult to approximate the true incidence of PE, but there are estimates that as many as 300,000 Americans have a fatal PE each year and as many as 34% of affected individuals present with sudden death. The majority of patients die because of a failure in diagnosis rather than inadequate therapy. In fact, the mortality rate for PE without treatment is approximately 30%, whereas it is only 2% to 8% with adequate treatment. PE remains the most common preventable cause of hospital death in the United States.
1.Pathophysiology and symptoms. Hemodynamic collapse related to PE results from the combination of vascular obstruction from thrombus and vasoconstriction caused by inflammatory mediators and hypoxia. Elevated pulmonary vascular resistance results in decreased right ventricular outflow, leading to a decrease in preload and cardiac output resulting in hypotension. Elevated right ventricular wall tension can lead to decreased right coronary artery flow and ischemia. Cardiopulmonary collapse from PE is more common in patients with coexisting coronary artery disease (CAD) or underlying cardiopulmonary disease.
PE may present as one of the following three syndromes: (a) acute cor pulmonale, (b) pulmonary infarction, or (c) dyspnea. Patients presenting with acute cor pulmonale, as manifested by the sudden development of dyspnea, cyanosis, shock, or syncope, usually have a massive PE leading to cardiovascular collapse. Patients with pulmonary infarction usually present with pleuritic chest pain, dyspnea, and hemoptysis, and an audible friction rub may be heard. The majority of patients present with generalized symptoms of chest pain, dyspnea, and malaise.
2.Diagnosis. Several pretest probability scores have also been developed for the diagnosis of PE similar to those for the diagnosis of DVT. In a validation study of the Wells clinical decision rule, only 0.5% of patients who were unlikely to have PE and had a negative d-dimer had subsequent nonfatal VTE.
a.Troponin. Cardiac troponins have been evaluated in the setting of acute PE, and elevated levels correlate with electrocardiographic and echocardiographic findings of right ventricular pressure overload. Troponin elevation can be seen in patients with and without CAD, but the overall mortality and inhospital complications are higher in patients with acute PE and elevated cardiac troponin than in patients without elevated cardiac troponin.
b.Brain natriuretic peptide elevation in the absence of renal dysfunction is also a marker of right ventricular dysfunction in patients with PE. Like elevated troponin levels, these elevated levels have also been shown to be predictors of adverse outcome in patients with acute PE.
c.Arterial blood gas. PE can result in significant hypoxia, but in the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) study, 26% of patients with angiographically proven PE had Pao2 >80 mm Hg. Similarly, a normal alveolar–arterial gradient does not preclude the diagnosis of PE. Therefore, a normal Pao2 cannot rule out PE; however, hypoxia in the absence of cardiopulmonary disease should raise the suspicion for this diagnosis. In patients with cardiopulmonary collapse, a normal Pao2 suggests that PE is an unlikely cause.
d.Chest radiography may be more helpful in establishing other diagnoses. When present, findings are nonspecific and include pleural effusion, atelectasis, and consolidation. The classic signs, including the Westermark sign (regional oligemia), Hampton hump (pleural-based, wedge-shaped shadow), and Palla sign (enlarged right inferior pulmonary artery), are uncommon.
e.Electrocardiography. Like chest radiography, the major utility of the electrocardiogram (ECG) in the diagnosis of PE is to rule out other major diagnoses, such as acute myocardial infarction (MI). The most specific finding on an ECG is the classic S1Q3T3 pattern, but the most common findings are nonspecific ST-segment and T-wave changes. Other commonly reported findings include sinus tachycardia, atrial fibrillation, and right bundle branch block.
f.Echocardiography. More than 50% of hemodynamically stable patients with PE will not have evidence of right ventricular dysfunction on transthoracic echocardiography (TTE). Patients with hemodynamic collapse, however, will generally have severe right ventricle dysfunction, and TTE can provide rapid bedside assessment in these critically ill patients. TTE findings include right ventricular dilation, right ventricular hypokinesis, tricuspid regurgitation, septal flattening, paradoxical septal motion, diastolic left ventricular impairment secondary to septal displacement, pulmonary artery hypertension, lack of inspiratory collapse of the IVC, and, rarely, direct visualization of the thrombus. In patients with large PE, it has been observed that despite moderate or severe right ventricular free wall hypokinesis there is relative sparing of the apex. This finding is referred to as McConnell sign and has a specificity of 94% and a positive predictive value of 71% for PE. McConnell’s sign may be useful in discriminating right ventricular dysfunction because of PE versus other causes.
g.Ventilation–perfusion (V/Q) scanning. V/Q scanning is now considered a second-line imaging method for the diagnosis of PE. V/Q scans are helpful in patients who have normal chest radiography or who are unable to have CT scanning, such as those with renal insufficiency, contrast allergy, or pregnancy. The results of PIOPED suggest that V/Q scanning is helpful if the scan is normal or at high probability for PE (87% of patients with high-probability scans had PE, but only 4% of patients with normal scans had PE). Intermediate-or low-probability scans are the most common finding, however, occurring in approximately 70% of patients in the PIOPED study. In addition, patients who had a high or intermediate clinical suspicion for PE but a low-probability scan still had a 40% and 16% rate, respectively, of PE diagnosed by pulmonary angiography. Hence, it is currently advised that patients with a high or intermediate clinical suspicion for PE but a low-probability V/Q scan have additional tests to confirm or refute the diagnosis.
h.Computed tomography pulmonary angiography (CTPA). Because of its wide availability and the ability to directly visualize thrombus, CT imaging has become the standard imaging technique for the diagnosis of acute PE. CTPA not only allows direct visualization of the thrombus but also has great value in excluding other diseases, including aortic dissection, pneumonia, or malignancy. It is especially useful in evaluating central PE (thrombus is seen as an intraluminal filling defect), and although the diagnostic yield for peripheral or subsegmental PE was low initially, the use of multidetector CTPA has greatly increased its sensitivity and specificity for the diagnosis of small peripheral or subsegmental PEs. The major disadvantages of CT are radiation exposure and the possibility of contrast-induced nephrotoxicity. A meta-analysis of 23 studies including 4,657 patients who were suspected of having PE but had normal CT scans found that only 1.4% developed VTE and 0.51% developed fatal PE by 3 months. These rates are similar to those in other studies involving patients who had suspected PE but were found to have normal pulmonary angiograms.
i.Pulmonary angiography remains the gold standard diagnostic test for PE, but it is used infrequently because of the advent of CT scanning. In some situations where a lung scan shows perfusion abnormalities and is nondiagnostic for PE, selective angiography of the abnormal area may be considered so as to limit the amount of contrast needed. In experienced centers, associated morbidity and mortality are low.
j.Magnetic resonance angiography (MRA) may be an alternative to CT for the diagnosis of PE in patients who have contrast allergy or for whom avoidance of radiation exposure is desired. At the current time, an MRA should be considered only at those centers with experience with this modality and only for patients for whom standard tests are contraindicated.
3.Treatment. All patients with PE are treated with anticoagulation and supportive care. Risk stratification using a combination of hemodynamic stability, biomarkers, and echocardiographic criteria is utilized to determine the use of catheter-directed therapies and systemic lysis.
a.Risk stratification and management algorithm. The pulmonary embolism severity index (PESI) is a validated clinical tool to assess prognosis for patients presenting with acute PE (Table 25.3). In normotensive patients, this tool can reliably distinguish between intermediate and low risk and therefore identify patients who may require further evaluation. Additionally, there is a growing body of evidence supporting early discharge or home therapy for patients at low risk. Current guidelines incorporate risk stratification utilizing the PESI score into an algorithm for managing patients with acute PE (Fig. 25.1).

FIGURE 25.1 Risk-adjusted management strategies in acute PE. A/C, anticoagulation; CT, computed tomographic pulmonary angiography; PE, pulmonary embolism; PESI, pulmonary embolism severity index; RV, right ventricular; sPESI, simplified pulmonary embolism severity index. aIf echocardiography has already been performed during diagnostic work-up for PE, and RV dysfunction detected, or if the CT already performed for diagnostic work-up has shown RV enlargement (RV/left ventricular [LV] ratio ≥0.9), a cardiac troponin test should be performed except for cases in which primary reperfusion is not a therapeutic option (e.g., because of severe comorbidity or limited life expectancy of the patient). bMarkers of myocardial injury (e.g., elevated cardiac troponin I or T concentrations in plasma) or of heart failure as a result of (right) ventricular dysfunction (elevated natriuretic peptide concentrations in plasma). If a laboratory test for a cardiac biomarker has already been performed during initial diagnostic work-up (e.g., in the chest pain unit) and was positive, then an echocardiogram should be considered to assess RV function, or RV size should be (re)assessed on CT. cPatients in the PESI class I–II, or sPESI of 0, and elevated cardiac biomarkers or signs of RV dysfunction on imaging tests are also to be classified into the intermediate–low-risk category. This might apply to situations in which imaging or biomarker results become available for the calculation of the clinical severity index. These patients are probably not candidates for home treatment. dThrombolysis, if (and as soon as) clinical signs of hemodynamic decompensation appear; surgical pulmonary embolectomy and percutaneous catheter-directed treatment may be considered as alternative options to systemic thrombolysis, particularly if the bleeding risk is high. eMonitoring should be considered for patients with confirmed PE and a positive troponin test, even if there is no evidence of RV dysfunction on echocardiography or CT. f The simplified version of the PESI has not been validated in prospective home treatment trials; inclusion criteria other than the PESI were used in two single-armed (nonrandomized) management studies. From Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(43):3033–3073. Reproduced by permission of The European Society of Cardiology.
b.Anticoagulation and supportive care have remained the standard of care in the management of acute PE. Current guidelines recommend initial treatment with anticoagulants for patients with a high clinical suspicion for PE while awaiting the results of diagnostic testing. Traditionally, patients with acute non-massive PE have been treated with parenteral anticoagulation (IV UFH, LMWH, fondaparinux) followed by the initiation of a VKA. The advent of DOACs has revolutionized the management of VTE.
c.DOACs discussed previously, are outlined in Table 25.2. As with DVT, current guidelines suggest DOACs over VKAs for the treatment of VTE, except in patients with active cancer in whom LMWH is the treatment of choice.
d.Systemic thrombolysis for the treatment of acute PE is highly individualized, as there have been no clearly established short-term mortality effects. Because of favorable outcomes with prompt recognition and anticoagulation for PE, thrombolysis should be reserved for hemodynamically unstable patients with acute massive PE and a low risk of bleeding. The recent PEITHO trial investigated the role of systemic thrombolysis among patients with acute PE at intermediate risk, defined as hemodynamic stability but with positive cardiac troponin and evidence of right ventricular dysfunction by either CT or echocardiography. This double-blinded trial randomized >1,000 patients to receive IV tenecteplase single bolus (30 to 50 mg based on body weight) or placebo in addition to anticoagulation. Overall, mortality was <2% at 7 days with no significant difference between groups. The group receiving thrombolytics was less likely to develop hemodynamic decompensation; however, this was at the cost of significantly increased risk of intracranial and other major bleeding. Based on these results, routine use of systemic thrombolysis is not recommended in normotensive patients at intermediate risk.
e.Catheter-based therapies have gained interest in recent years given the significant bleeding risk associated with systemic thrombolysis. These procedures include percutaneous catheter-delivered intrapulmonary thrombolysis, mechanical fragmentation, aspiration thrombectomy, rheolytic thrombectomy, and ultrasound-assisted thrombolysis (utilizing the EKOS catheter). Data are limited to observational studies and small clinical trials with surrogate endpoints, but suggest these therapies may be an effective option with acceptable safety profile. Currently, catheter-based therapies are only recommended at experienced centers for high-risk patients with contraindications to systemic thrombolysis or failed thrombolysis.
f.Surgical pulmonary embolectomy was the first definitive therapy to be performed for PE. There have been no randomized trials evaluating embolectomy, and the primary use of this procedure is in patients with shock and a contraindication to thrombolysis or failed thrombolysis.
C.VTE prophylaxis. Although PE is the third most common cause of hospital-related death in the United States, less than half of all hospitalized patients at risk for VTE receive adequate prophylactic treatment. Most hospitalized patients have at least one or more risk factors for VTE, and without prophylaxis, the incidence of hospital-acquired DVT is 10% to 20% among medical patients and even higher among surgical patients (15% to 40%).
There are two major forms of prophylaxis, mechanical and pharmacologic. Those who cannot receive prophylactic anticoagulation should be prescribed mechanical modalities such as intermittent pneumatic compression devices. Pharmacologic prophylaxis can be achieved by a number of agents, including UFH, LMWH, fondaparinux, a VKA, and rivaroxaban, which is approved for VTE prophylaxis after hip and knee replacement surgery. Other new oral anticoagulant agents (dabigatran and apixaban) are available outside the United States for prophylaxis and will likely be available sometime in the near future in the United States. In high-risk populations such as those with hip or knee replacement, a combination of mechanical and pharmacologic therapies should be considered. In select surgical procedures, extended prophylaxis is recommended. For example, extended prophylaxis for up to 28 to 35 days is recommended for patients who have had a hip fracture or who undergo total hip replacement surgery.
II.Hypercoagulable Conditions. Conditions that predispose persons to an increased risk of thrombosis are referred to as hypercoagulable states or thrombophilia. These conditions are being identified more frequently and may be classified as inherited or acquired. Hypercoagulability testing should be considered in individuals with idiopathic VTE, family history of clotting, a first thrombotic event before the age of 50 years, thrombosis at unusual locations, resistance to anticoagulation, and those with recurrent thromboses.
A.Factor V Leiden and prothrombin gene mutation. The FVL mutation is more prevalent in persons of European and Scandinavian ancestry. The prothrombin gene mutation G20210A (PT G20210A) is also inherited as an autosomal dominant mutation and may lead to a higher plasma level of prothrombin. It is also more common in those of Caucasian ancestry and confers a 2.8-fold increased risk of VTE. The role of FVL and PT G20210A mutations in arterial thrombosis is unclear. There is only a modest association between inherited thrombophilias and major arterial thromboses such as MI, stroke, or peripheral arterial disease. Therefore, routine screening for these mutations is not warranted in most patients with arterial thrombosis. FVL and PT G20210A are associated with VTE during pregnancy, OCP use, and HRT. There are no clear evidence-based guidelines for managing patients with thrombosis in the setting of these thrombophilias. In general, acute thrombosis should be managed in a standard fashion, but the duration of therapy is less clear, and the benefits of long-term anticoagulation must be weighed against the risks of bleeding.
B.Defects in the natural anticoagulants (protein C, protein S, and antithrombin). Deficiency of any of the three natural anticoagulants is associated with an increased risk of venous thrombosis. All are inherited as autosomal dominant defects and are further subclassified based on reduction in their levels or defective quality of the protein. Levels of PS and PC are lower in conditions such as disseminated intravascular coagulation, inflammatory states, nephrotic syndrome, acute thrombosis, and liver disease. Pregnancy and OCP use can also decrease the levels of PS. Both PC and PS levels are lowered by warfarin therapy, and, therefore, these tests should not be assayed in patients who are receiving VKAs. Similarly, initiation of warfarin therapy without concomitant anticoagulation in the setting of acute VTE may lead to warfarin-induced skin necrosis (manifested as painful necrosis of the skin, primarily in fatty areas including the breast, buttocks, and thighs).
C.Homocysteine. Hyperhomocysteinemia is a risk factor for venous and arterial thromboses. It may be inherited, and genetic defects causing a deficiency of cystathionine β-synthase or a mutation in methylenetetrahydrofolate reductase have been reported. Acquired causes include deficiencies in vitamin B12, B6, or folate; smoking; and liver or renal failure. Treatment with folate in doses between 0.5 and 5 mg is usually effective in reducing the levels of homocysteine; however, this does not reduce the risk of major cardiovascular events, symptomatic venous thrombosis, or recurrent venous thrombosis.
D.Heparin-induced thrombocytopenia. HIT is a common, underrecognized but potentially devastating condition in patients who receive heparin or LMWH. The reported incidence is between 3% and 5% in patients exposed to UFH and lower (<1%) in patients exposed to LMWH. The pathogenesis of HIT involves the formation of antibodies (usually immunoglobulin G [IgG]) against a heparin–platelet factor 4 (PF4) complex. The HIT antibodies then trigger procoagulant effects through platelet and endothelial cell activation, as well as thrombin generation leading to both microvascular and macrovascular thromboses. The clinical spectrum of HIT ranges from isolated thrombocytopenia without thrombosis (referred to as isolated HIT) to HIT(T), which is associated with both arterial and venous thromboses. Other manifestations of HIT may include hypotension from adrenal hemorrhage secondary to adrenal vein thrombosis and ensuing infarction, skin necrosis at injection sites, or venous limb gangrene. HIT should be suspected in any patient who develops thrombocytopenia while receiving heparin or LMWH; any patient who develops a >50% decline in platelet count after the initiation of either anticoagulant; or any patient who develops new thrombosis or extension of an existing thrombosis while receiving either of these agents. In patients with HIT and de novo exposure to heparin, thrombocytopenia (platelet count < 150,000 per µL) usually occurs between days 5 and 14 (with day of heparin exposure being day 0). In patients with a recent exposure to either agent (generally within the last 100 days), HIT may develop sooner and is referred to as rapid-onset HIT. This complication may also develop 9 to 40 days after heparin or LMWH has been discontinued and is known as delayed-onset HIT.
Laboratory tests to aid in the diagnosis of HIT include functional assays such as heparin-induced platelet aggregation and serotonin release assays (SRAs), and antigen assays (immunoassays) which detect IgG, IgM, or IgA antibodies that bind UFH to PF4. The SRA has the highest sensitivity and specificity for the diagnosis of HIT.
The first step in the treatment of HIT is the prompt discontinuation of all sources of heparin or LMWH, including heparin flushes, heparin-coated catheters, any intermittent use of heparin during dialysis, and total parenteral nutrition or angiography. However, approximately 20% to 53% of patients with HIT will develop thrombosis (many within the first month) when heparin is discontinued or treated with LWMH alone. Therefore, the initiation of an alternative anticoagulant, unless contraindicated, is recommended. DTIs, including lepirudin and argatroban (both approved by the Food and Drug Administration), may be used initially. Once platelet counts are more than 100,000 to 150,000 mm3, warfarin may be started at a low dose (2.5 to 5 mg preferred). Early introduction or higher doses of warfarin may lead to venous limb gangrene or warfarin-induced skin necrosis. Overlapping the DTI with the VKA should be continued for at least 5 days and not discontinued until the INR is therapeutic for two consecutive days. Argatroban falsely elevates the INR; therefore, it should not be discontinued until INR >4, as recommended by the manufacturer. After cessation of argatroban, the INR should be rechecked within a few hours to confirm that it is between 2 and 3. The duration of anticoagulation for patients with HIT is generally determined by the location and type of thrombosis. In patients without thrombosis (isolated HIT), the duration of anticoagulation is less clear, but given the high incidence of thrombosis within the first month, it is reasonable to continue anticoagulation for at least a month in the absence of contraindications.
E.Antiphospholipid antibodies are a heterogeneous group of autoantibodies that, if present in a patient with thrombosis, lead to the antiphospholipid syndrome. Antiphospholipid antibodies can be divided into three groups: (a) anticardiolipin antibodies, (b) lupus anticoagulants, and (c) β2-glycoproteins. They are often associated with other autoimmune conditions and can cause recurrent pregnancy loss, as well as arterial or venous thrombosis. Thrombocytopenia is also an occasional feature of this syndrome. Anticardiolipin antibodies are detected and quantified using an enzyme-linked immunosorbent assay and may be IgG, IgM, or IgA. IgG titers have been correlated more often with thrombosis. Lupus anticoagulants prolong phospholipid-dependent blood clotting times, and it has been reported that there is about a fivefold increased risk of thrombosis in patients with this finding. Once a thrombotic event occurs, long-term therapy with warfarin must be considered. A higher target INR had been considered necessary in the past (approximately ≥3.0), but is no longer considered necessary as data have suggested that most patients can be maintained with a target INR of 2.0 to 3.0. In those individuals that are suspected of failing adequate therapy, one strategy is to correlate the INR to factor II and factor X levels (of ≤20% to 30%) to ensure adequate anticoagulation.
F.Malignancy. Many malignancies induce a hypercoagulable state, and in patients with idiopathic VTE, a search for age- and gender-specific malignancies may be necessary.
G.Other conditions. Elevated factor VIII levels and the dysfibrinogenemias have also been associated with thrombosis; however, the role of deficiencies of plasminogen, tissue plasminogen activator (of the fibrinolytic system), and factor XIII polymorphisms as emerging risk factors for hypercoagulability is less clear.
ACKNOWLEDGMENTS: The authors acknowledge the contributions of Dr. Firas Al Solaiman, Dr. Esther S.H. Kim, Dr. Vijay Nambi, and Dr. John Bartholomew to earlier editions of this chapter.
Suggested Readings
Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510.
Birdwell BG, Raskob GE, Whitsett TL, et al. Predictive value of compression ultrasonography for deep vein thrombosis in symptomatic outpatients: clinical implications of the site of vein noncompressibility. Arch Intern Med. 2000;160:309–313.
Buller HR, Davidson BL, Decousus H, et al. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med. 2004;140:867–873.
Buller HR, Davidson BL, Decousus H, et al. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med. 2003;349:1695–702.
Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med. 2003;349:1133–1138.
de Moerloose P, Boehlen F. Inherited thrombophilia in arterial disease: a selective review. Semin Hematol. 2007;44:106–613.
Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775.
Eriksson BI, Dahl OE, Büller HR, et al. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005;3:103.
Goldhaber SZ. Echocardiography in the management of pulmonary embolism. Ann Intern Med. 2002;136:691–700.
Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3:1611–1617.
Kaufman JA, Kinney TB, Streiff MB, et al. Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol. 2006;17:449–459.
Kearon C, Ginsberg JS, Julian JA, et al. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA. 2006;296:935–942.
Konstantinides S, Geibel A, Olschewski M, et al. Importance of cardiac troponins I and T in risk stratification of patients with acute pulmonary embolism. Circulation. 2002;106:1263–1268.
Lassen MR, Gallus A, Raskob GE, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487–2498.
Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement: a randomized double-blind trial. Lancet. 2010;375:807–815.
Meyer G, Vacaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370(15):1402–1411.
Moores LK, Jackson WL Jr, Shorr AF, et al. Meta-analysis: outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography. Ann Intern Med. 2004;141:866–874.
Tamariz LJ, Eng J, Segal JB, et al. Usefulness of clinical prediction rules for the diagnosis of venous thromboembolism: a systematic review. Am J Med. 2004;117:676–684.
van Belle A, Buller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, d-dimer testing, and computed tomography. JAMA. 2006;295:172–179.
Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED d-dimer. Thromb Haemost. 2000;83:416–420.
Wells PS, Anderson DR, Rodger M, et al. Evaluation of d-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227–1235.
Wells PS, Owen C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA. 2006;295:199–207.
Wolde M, Tulevski II, Mulder JW, et al. Brain natriuretic peptide as a predictor of adverse outcome in patients with pulmonary embolism. Circulation. 2003;107:2082–2084.
Landmark Articles
Benotti JR, Dalen JE. The natural history of pulmonary embolism. Clin Chest Med. 1984;5:403–410.
Bratzler DW, Raskob GE, Murray CK, et al. Underuse of venous thromboembolism prophylaxis for general surgery patients: physician practices in the community hospital setting. Arch Intern Med. 1998;158:1909–1912.
Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326:1240–1245.
Lensing AW, Prandoni P, Brandjes D, et al. Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N Engl J Med. 1989;320:342–345.
Mattos MA, Londrey GL, Leutz DW, et al. Color-flow duplex scanning for the surveillance and diagnosis of acute deep venous thrombosis. J Vasc Surg. 1992;15:366–375; discussion 375–376.
Moser KM, LeMoine JR. Is embolic risk conditioned by location of deep venous thrombosis? Ann Intern Med. 1981;94:439–444.
PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED). JAMA. 1990;263:2753–2759.
Ribeiro A, Lindmarker P, Juhlin-Dannfelt A. Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J. 1997;134:479–487.
Sandler DA, Martin JF, Duncan JS, et al. Diagnosis of deep-vein thrombosis: comparison of clinical evaluation, ultrasound, plethysmography, and venoscan with X-ray venogram. Lancet. 1984;2:716–719.
Sreeram N, Cheriex EC, Smeets JL. Value of the 12-lead electrocardiogram at hospital admission in the diagnosis of pulmonary embolism. Am J Cardiol. 1994;73:298–303.
Stein PD, Goldhaber SZ, Henry JW. Alveolar-arterial oxygen gradient in the assessment of acute pulmonary embolism. Chest. 1995;107:139–143.
Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest. 1991;100:598–603.
Vandenbroucke JP, Koster T, Briet E, et al. Increased risk of venous thrombosis in oral-contraceptive users who are carriers of factor V Leiden mutation. Lancet. 1994;344:1453–1457.
Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795–1798.