
I.EPIDEMIOLOGY AND ETIOLOGY OF STROKE
A.Annually, there are nearly 800,000 strokes in the United States and 15 million strokes worldwide. Stroke is the third leading cause of death in western societies and the leading cause of long-term disability in the United States.
B.Ischemic stroke accounts for approximately 85% of all strokes. Of these, 60% are embolic in nature whereas small vessel disease and large vessel atherothrombotic lesions account for 25% and 15%, respectively.
II.RISK FACTORS FOR CAROTID ATHEROSCLEROSIS
A.Smoking and age are the two most important risk factors for developing carotid atherosclerosis. The others, in order of importance, are hypertension, diabetes, gender (men more than women if younger than 75 years; women more than men if older than 75 years), and hyperlipidemia.
B.Between 30% and 60% of patients with peripheral arterial disease have carotid disease, and approximately 50% to 60% of patients with carotid disease have severe carotid artery disease (CAD). However, only 10% of patients with CAD have severe carotid disease.
III.PATHOPHYSIOLOGY
A.As with coronary disease, atherosclerotic carotid disease usually develops at branch points and bends, especially at the bifurcation of the common carotid artery and origin of the internal carotid artery (ICA).
B.The reasons that carotid stenoses become symptomatic are not completely understood, but there is a linear increase in the risk of stroke as the stenosis increases to >70%. Two hypotheses explain how carotid disease can cause stroke.
1.Carotid plaque is highly vascularized. Rupture of this vasculature or rupture of the plaque can result in plaque hemorrhage or ulceration, with subsequent in situ thrombus formation. This can lead to complete vessel obstruction or distal atherothromboembolism. This mechanism accounts for most cerebrovascular events caused by carotid disease.
2.Larger plaques can result in high-grade carotid stenosis or obstruction, with subsequent ischemic stroke because of a reduction in cerebral flow, in the setting of inadequate or absent collateral circulation.
IV.DIAGNOSIS
A.History and physical examination
1.Careful history can aid in the localization of neurologic symptoms. Hemispheric symptoms include unilateral weakness, numbness, difficulty with speech, and visual field defects, whereas vertebrobasilar symptoms can include cerebellar disturbances such as ataxia or brain stem symptoms including syncope, dysphagia, dysarthria, or diplopia. Amaurosis fugax is transient, unilateral vision loss ipsilateral to a carotid lesion.
2.An assessment for the presence of a cervical bruit is an important part of the physical examination but should not be relied on as the sole marker for the presence of carotid disease. In the North American Symptomatic Carotid Endarterectomy Trial (NASCET), the presence of a cervical bruit had an approximately 60% sensitivity and specificity for high-grade carotid stenosis. In the Framingham study, the presence of a carotid bruit in asymptomatic patients doubled the risk of stroke, but most of these strokes occurred in vascular beds different from those of the carotid bruit. The presence of a bruit may be a general marker for patients at higher risk for cerebrovascular and cardiovascular events.
3.In addition to auscultation for carotid bruits, a complete evaluation in a patient with symptoms includes a focused neurologic examination to correlate symptoms with neurologic territory, a fundoscopic examination to detect retinal embolization, and a cardiac examination to rule out potential cardioembolic sources for symptoms.
4.All patients should have an evaluation of the carotid arteries after a stroke or a transient ischemic attack (TIA). The risk of a second stroke is elevated for several years after the first stroke or TIA. Symptomatic patients with 70% or more stenosis have an 8% risk of stroke at 30 days and a 13% annual incidence of stroke. The risk of stroke in asymptomatic patients increases as the degree of carotid stenosis increases. Asymptomatic patients with 60% or more stenosis have a stroke risk of approximately 2% per year. Asymptomatic patients with 80% or more stenosis have a risk of approximately 5% per year.
B.Duplex ultrasound
1.Although carotid angiography is the gold standard, duplex ultrasound is the most widely used method for the detection and quantification of CAD. It has a sensitivity and specificity of >80% among patients with 70% to 99% stenoses and sensitivity and specificity of >95% among patients with complete carotid occlusion. Because of its high sensitivity and specificity in severe carotid disease as well as its noninvasive nature, duplex ultrasound should be the first study performed to assess for carotid disease.
2.The ultrasound diagnosis of carotid stenosis is based largely on peak systolic and end-diastolic velocities in the ICA. Duplex ultrasound criteria for carotid stenosis vary by institution, and each vascular laboratory must assess the accuracy of its criteria for stenosis in a quality assurance program. Compared with angiography, duplex ultrasound is noninvasive, is less expensive, and can be done at the bedside. Limitations include the inability to image intracranial disease, limited ability to assess collateral flow, occasional inaccuracy in distinguishing high-grade stenoses (“string sign”) from complete obstructions, and the need for an experienced sonographer. Conditions that may elevate intravascular flow velocities, such as common carotid disease, vessel tortuosity, contralateral carotid disease, or presence of a carotid stent, may result in an artificially high estimate of ICA stenosis. The ability of ultrasound to assess the posterior carotid circulation is limited.
C.Computed tomography angiography (CTA)
1.CTA offers high sensitivity and specificity for the identification of severe (>70%) CAD (sensitivity 75% to 100%, specificity 63% to 95%, negative predictive value up to 100%). CTA allows for visualization of the carotid artery lumen as well as adjacent bony and soft tissue structures. Advantages include high sensitivity (particularly for carotid artery occlusion), reproducibility, and the ability to visualize the entire carotid artery including the extracranial and intracranial portions. Disadvantages include cost and the need for contrast injection, which may be unsuitable for patients with chronic kidney disease or volume overload.
D.Magnetic resonance angiography
1.Contrast-enhanced magnetic resonance angiography (CEMRA) is rapidly gaining acceptance as a sensitive (91% to 95%) and specific (88% to 92%) test for severe carotid disease. Advantages include high sensitivity, reproducibility, and the ability to visualize the entire carotid artery, including the extracranial and intracranial portions. The use of a paramagnetic agent as a vascular contrast confers higher quality images less prone to artifact. Disadvantages include high cost and the inability to study critically ill patients, claustrophobic patients, or patients with ferromagnetic implants such as pacemakers.
2.The combination of CEMRA and Doppler ultrasound results in a lower number of misclassifications and higher sensitivity and specificity for the diagnosis of severe ICA stenosis. When there is concern regarding the accuracy of one study, it is justifiable to perform both.
E.Contrast angiography
1.Contrast angiography with digital subtraction angiography is the gold standard for assessment of carotid atherosclerosis. It allows the simultaneous assessment of the aortic arch, subclavian arteries, vertebral arteries, and intracranial circulation. Angiography enables the accurate assessment of collateral circulation. This is important because the presence of collateral circulation in medically treated patients with high-grade stenosis reduces the risk of ipsilateral stroke.
2.Two criteria are used to quantify carotid stenosis angiographically: the NASCET criteria and the European Carotid Surgery Trialists’ (ECST) Collaborative Group criteria (Fig 27A.1). According to the NASCET criteria, the normal reference internal carotid diameter is the maximum diameter of the ICA distal to the lesion and distal to the carotid bulb. According to the ECST criteria, however, the normal reference diameter is determined by the estimated position of the external wall of the carotid bulb. The same lesion has a higher percentage of stenosis using the NASCET criteria compared with the ECST criteria. In addition, the NASCET criteria are difficult to apply in subtotal occlusions with collapse of the distal ICA because of underfilling. However, the NASCET criteria inherently have less variability and are now recommended as the standard for reporting of angiographic carotid stenosis in Medicare physician quality initiatives.

FIGURE 27A.1 The North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the European Carotid Surgery Trialists’ (ECST) Collaborative Group criteria for determining the degree of carotid stenosis.
V.MANAGEMENT OF CAROTID DISEASE
A.Medical management
1.Risk factor modification. Aggressive cardiovascular risk factor modification is recommended to reduce the risk of stroke and prevent the progression of existing disease, regardless of whether or not revascularization is indicated. This includes careful attention to smoking cessation and optimal control of various cardiovascular comorbidities including hypertension, hyperlipidemia, and diabetes mellitus according to established guidelines.
2.Antiplatelet therapy
a.Aspirin is the most extensively studied antiplatelet drug for the prevention of stroke and should be initiated in all patients with evidence of carotid atherosclerosis. Current guidelines advocate initiation of aspirin 75 to 325 mg daily in all patients with extracranial carotid or vertebral atherosclerosis to reduce risk of MI and other ischemic cardiovascular events.
b.Clopidogrel 75 mg daily may be used in primary prevention settings when there is a contraindication to aspirin therapy. Following a stroke, it is reasonable to use clopidogrel as an alternative to aspirin in select clinical situations depending on the risk factor profile of the patient.
c.Dual-antiplatelet therapy (DAPT) is not routinely used in patients with carotid atherosclerosis in the absence of an endovascular intervention or alternate indication (i.e., coronary stenting). This is largely driven by increased bleeding risk with DAPT.
d.Low-dose aspirin (25 mg twice daily) plus dipyridamole (200 mg twice daily) has been found to be more beneficial than aspirin alone or dipyridamole alone for the secondary prevention of stroke in the European Stroke Prevention Study 2. Similarly, in the European/Australian Stroke Prevention in Reversible Ischemia Trial, extended-release dipyridamole administered with aspirin was superior to aspirin alone in the prevention of MI, stroke, or vascular death.
e.The Prevention Regimen for Effectively avoiding Second Strokes trial, which enrolled over 20,000 patients with noncardioembolic ischemic stroke, showed that clopidogrel monotherapy and aspirin plus extended-release dipyridamole have similar risks and benefits for secondary stroke prevention. There was no difference between treatment with aspirin plus extended-release dipyridamole or clopidogrel for the primary outcome of recurrent stroke or the composite secondary outcome of stroke, MI, or vascular death. However, despite the nearly identical event rates, the trial failed to meet the prespecified noninferiority criteria for treatment with aspirin and extended-release dipyridamole.
f.According to the American Heart Association (AHA)/American Stroke Association (ASA) Guidelines for the Prevention of Stroke in Patients with Ischemic Stroke or TIA, the accepted treatment strategies for the secondary prevention of noncardioembolic stroke include one of the following:
(1)Aspirin (75 to 325 mg daily)
(2)Aspirin (25 mg) and extended-release dipyridamole (200 mg) twice daily
(3)Clopidogrel (75 mg daily), especially for patients with aspirin allergy or resistance
3.Antihyperlipidemic agents
a.Epidemiologic data have shown higher stroke rates among patients with high LDL and low HDL cholesterol levels. Several studies have consistently shown carotid plaque regression in patients treated with statins, and clinical trials have shown a reduction in stroke among patients treated with statins. In the Scandinavian Simvastatin Survival Study, nonembolic strokes were significantly reduced in the statin arm. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels study demonstrated a 16% relative risk reduction in stroke with statin therapy in 4,731 patients with prior stroke or TIA randomized to atorvastatin versus placebo. In a meta-analysis of randomized trials that included a total of 165,792 patients with hyperlipidemia, each 39 mg/dL reduction in LDL cholesterol equates to a reduction in relative risk of stroke of 21.1%.
b.The beneficial effects of statins in reducing strokes are highest among patients at the highest risk for stroke. For primary prevention, all high-risk patients (e.g., diabetes, peripheral vascular disease, and CAD) should be treated with statins. For secondary prevention, all patients with a history of MI, TIA, and stroke should be on statin therapy.
4.Antihypertensive agents
a.Hypertension is the single most modifiable risk factor in the prevention of stroke, and epidemiologic data suggest that approximately 60% of all strokes are attributable to hypertension. Several randomized controlled trials (RCTs) have shown benefit of a variety of antihypertensive agents—angiotensin converting enzyme inhibitors (HOPE, PROGRESS), angiotensin receptor blockers (LIFE), and diuretics (ALLHAT) in particular.
b.Current updated guidelines recommend treatment of hypertension in patients with clinical cardiovascular disease or 10-year atherosclerotic cardiovascular disease risk >10% and blood pressure (BP) ≥130/80 mm Hg.
5.Anticoagulation
a.Current guidelines recommend antiplatelet agents rather than anticoagulants in patients with extracranial carotid or vertebral atherosclerosis whether or not they have ischemic symptoms, unless there is an alternate indication for anticoagulation (i.e., mechanical heart valve, atrial fibrillation).
B.Surgical management: carotid endarterectomy (CEA). CEA is the standard of care for the reduction of stroke or TIA in patients with high-grade symptomatic or asymptomatic carotid stenosis. Several trials have firmly established the utility of CEA in preventing stroke in the presence of severe carotid stenosis as compared with medical therapy. It is important to keep in mind that high-risk patients were not enrolled in these trials. Because the risk of surgery among such patients probably would be higher than reported in these trials, extrapolation of these data to high-risk patients must be done with caution.
1.Major trials
a.See Tables 27A.1 and 27A.2.
2.Complications and management of patients after CEA
a.Major complications of CEA include MI, stroke, and death. Other complications include bleeding and wound hematoma, cranial nerve injury, wound infection, bradycardia, hyper- or hypotension, and, rarely, seizures and intracerebral hemorrhage. CEA should only be performed at institutions where the perioperative stroke and death rate is at the most <3% in asymptomatic patients and <6% in symptomatic patients.
b.After CEA and in the absence of contraindications, all patients should be treated with antiplatelet therapy.
3.The ASA/American College of Cardiology Foundation (ACCF)/AHA recommendations for CEA
a.Patients at average or low surgical risk who experience nondisabling ischemic stroke or transient cerebral ischemic symptoms, including hemispheric events or amaurosis fugax, within 6 months (symptomatic patients) should undergo CEA if the diameter of the lumen of the ipsilateral ICA is reduced >70% as documented by noninvasive imaging or >50% as documented by catheter angiography and the anticipated rate of perioperative stroke or mortality is <6%.
b.Selection of asymptomatic patients for carotid revascularization should be guided by an assessment of comorbid conditions, life expectancy, and other individual factors and should include a thorough discussion of the risks and benefits of the procedure, with an understanding of patient preferences. It is reasonable to perform CEA in asymptomatic patients who have >70% stenosis of the ICA if the risk of perioperative stroke, MI, and death is low.
c.Except in extraordinary circumstances, carotid revascularization is not recommended when atherosclerosis narrows the lumen by <50%.
C.Percutaneous carotid intervention
1.Since the first description of carotid angioplasty in 1980, a number of registry studies and trials have been published reporting high rates of procedural success. However, percutaneous carotid angioplasty is rarely performed as a stand-alone procedure because of unacceptably high rates of recoil, restenosis, and adverse procedural outcomes because of distal embolization. Carotid stenting with embolic protection device (EPD) use has become the standard of care for patients undergoing percutaneous carotid intervention.
2.Major trials
a.See Tables 27A.3 and 27A.4.
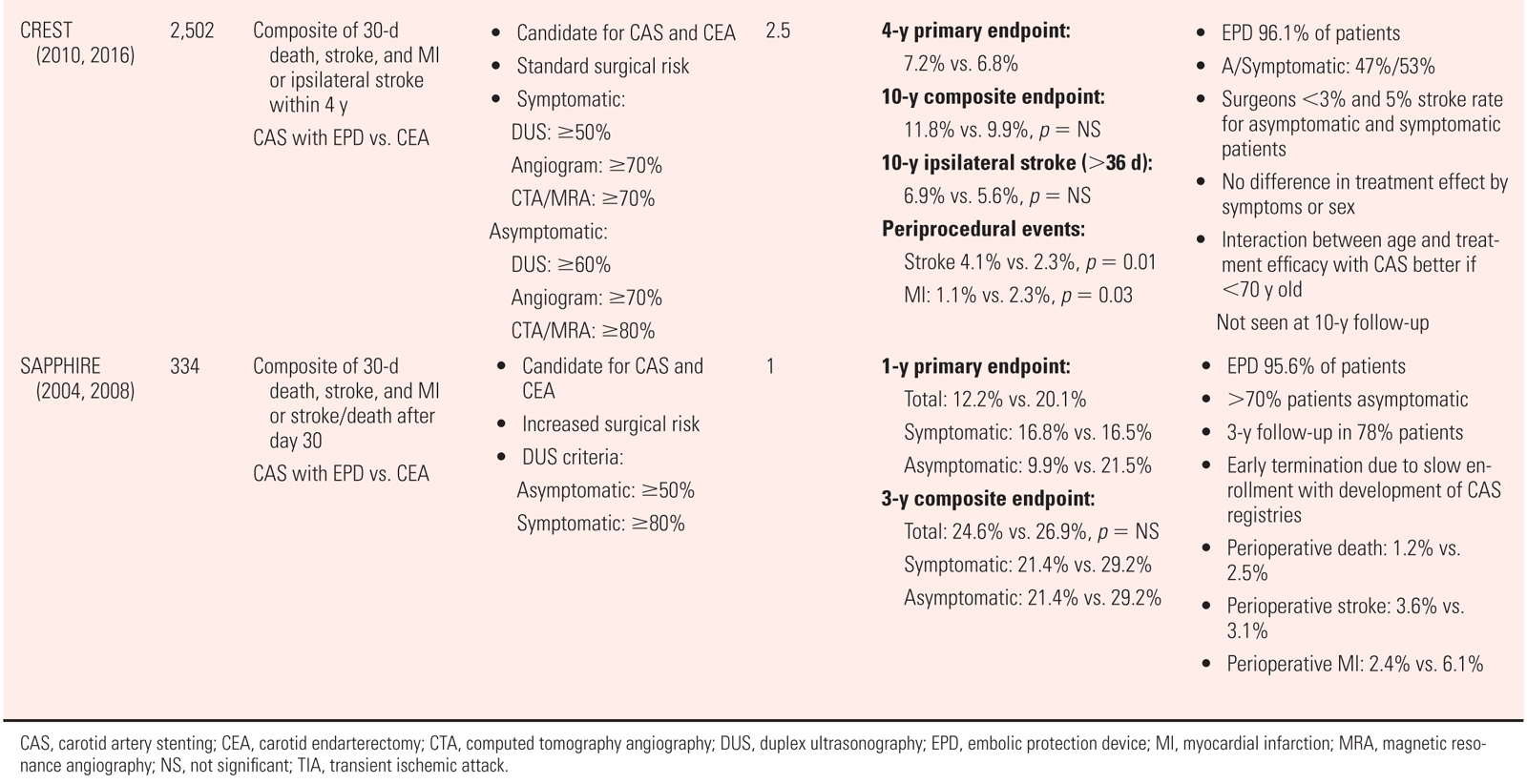
3.Complications and postprocedure management
a.The major periprocedural complications during carotid stenting are TIA/stroke, MI, and death. Other complications include access site issues (i.e., bleeding, pseudoaneurysm, atrioventricular fistula, dissection), renal dysfunction, bradycardia, hypotension, cerebral hyperperfusion, seizures, and intracranial hemorrhage. Advanced age and long or multiple stenoses have been found to be independent predictors of periprocedural stroke.
b.Periprocedural cerebrovascular events occur largely because of embolization of plaque debris and thrombus into the cerebral circulation during manipulation of the carotid plaque. Alternate mechanisms of TIA/stroke include embolization of debris from the aortic arch, iatrogenic introduction of air or thrombus, hypoperfusion in the setting of periprocedural bradycardia/hypotension, hyperperfusion syndrome, and thrombus formation on stent. In addition to employing fastidious procedural technique to minimize risk of introducing air or thrombus into system, routine use of EPDs, adequate procedural anticoagulation, and preloading with DAPT are important considerations with regard to minimizing stroke risk. All patients should undergo a thorough and well-documented neurologic examination before and after the procedure. Standardized stroke scales are often utilized (e.g., National Institutes of Health Stroke Scale, Barthel, and modified Rankin). Nursing staff should also be instructed to perform routine neurologic assessments in the first 24 hours according to established protocols and to alert the responding clinician with any changes in neurologic status.
c.Bradycardia and hypotension occur often during carotid stenting because of instrumentation and stretching of the carotid sinus baroreceptors. These hemodynamic effects are usually transient but can persist for up to 24 hours after intervention. When not immediately reversible with balloon deflation, management options include intravenous crystalloid infusion, atropine administration, and/or a low-dose vasopressor infusion (i.e., dopamine or phenylephrine). In some cases, the hemodynamic derangement can persist into the postprocedure setting, in which case continued vasopressor infusion or use of pseudoephedrine may be temporarily required. Unless the patient is hypertensive, antihypertensive and negative inotropic medications are usually withheld immediately preprocedure and postprocedure. In all cases, telemetry monitoring should be continued for 24 hours.
d.Hyperperfusion syndrome is an uncommon complication that can occur because of the rapid return of flow to a chronically underperfused cerebral vascular bed with resultant disordered autoregulation. Risk factors include severe hypertension, critical carotid stenosis, and contralateral carotid occlusion. The initial manifestation is often an ipsilateral headache with or without focal neurologic symptoms. This may be followed by seizures, cerebral edema, and/or intracerebral hemorrhage. This syndrome often occurs in patients with postprocedure hypertension, highlighting the importance of strict blood pressure control (systolic BP < 150 mm Hg) in patients following carotid stenting.
e.Postprocedure antiplatelet management. Patients undergoing carotid stenting should be preloaded with aspirin and clopidogrel at least 2 days prior to the procedure if possible. After the procedure, lifelong aspirin therapy should be instituted, and clopidogrel (75 mg daily) should be continued for at least 6 weeks. For patients with recurrent symptoms or a history of neck irradiation, clopidogrel should be continued indefinitely. The incidence of restenosis after carotid stenting is lower than after coronary stenting and ranges between 1% and 6% per year. Patients should be followed in the outpatient setting with routine clinical assessment as well as duplex US monitoring after carotid stent implantation.
4.The ASA/ACCF/AHA recommendations for carotid stenting
a.Carotid stenting is an indicated alternative to CEA for symptomatic patients at average or low risk of complications associated with endovascular intervention when the diameter of the lumen of the ICA is reduced by >70% by noninvasive imaging or >50% by catheter angiography and the anticipated rate of periprocedural stroke or mortality is <6%.
b.It is reasonable to choose carotid artery stenting over CEA when revascularization is indicated in patients with neck anatomy unfavorable for arterial surgery.
c.Prophylactic carotid artery stenting might be considered in highly selected patients with asymptomatic carotid stenosis (minimum 60% by angiography and 70% by validated Doppler ultrasound), but its effectiveness compared with medical therapy alone in this situation is not well established.
D.Patient selection for carotid stenting versus CEA
1.There is an abundance of RCT data demonstrating similar safety and efficacy of both carotid stenting and CEA for the treatment of severe atherosclerotic disease involving the carotid arteries. Whichever approach is chosen, it is important to recognize the importance of proper patient selection as well as adherence to guideline-recommended medical therapy. Furthermore, given the potential for devastating complications with either procedure, both should be performed only by experienced operators within the field.
2.Factors that may favor carotid stenting over CEA include difficult surgical anatomy (i.e., a high carotid bifurcation), prior neck irradiation, restenosis after CEA, as well as significant comorbidities that may increase surgical risk.
3.Given the potential for higher stroke risk and worse procedural outcomes with increasing age, CEA may be a better option in older patients. Also, anatomic considerations such as difficult aortic or carotid anatomy (i.e., type 3 aortic arch, significant tortuosity, circumferentially calcified lesions, thrombotic lesions) may lend themselves better to CEA rather than carotid stenting. It is important to note that when the best approach is in doubt, it is best to consult with the patient, vascular medicine specialists, and vascular surgeons so that the most appropriate decision for the patient can be made.
4.Despite the well-established efficacy of both carotid stenting and CEA, the importance of aggressive medical management cannot be understated. Many of the aforementioned studies were conducted prior to the advent of the most current established medical regimens leading many to question the impact this may have on the role of carotid revascularization. Indeed, despite increasingly complex patients with carotid disease, stroke rates have continued to decline in patients with severe asymptomatic carotid disease who are being medically managed. On this basis, CREST-2 will set out to address the impact of modern aggressive medical therapy with or without carotid revascularization (carotid stenting or CEA) on the rates of stroke and survival both in the periprocedural period and in long-term follow-up in patients with asymptomatic severe (≥70%) carotid stenoses. The results of these trials are eagerly anticipated.
E.Combined CEA and coronary artery bypass grafting (CABG)
1.Patients with severe coronary disease may have severe carotid disease, and surgery in this population is a high-risk procedure. The optimal treatment strategy remains controversial. In this setting, options include simultaneous CABG and carotid intervention, staged procedures, or CABG without carotid intervention.
2.In a single center study conducted at our institution, 350 patients who underwent carotid revascularization within 90 days prior to open heart surgery (OHS) were analyzed with respect to rates of a composite of all-cause death, stroke, or MI. The population included 45 staged CEA-OHS procedures, 195 combined CEA-OHS procedures, and 110 staged CAS-OHS procedures. All patients who underwent carotid stenting were required to complete a 3- to 4-week course of DAPT prior to OHS. In this cohort, patients who underwent staged CEA-OHS experienced the highest rates of the primary outcome in the short term, primarily driven by the occurrence of MI. The early event rates were similar between the combined CEA-OHS and staged CAS-OHS groups. However, after 1 year, the patients in the CAS-OHS group experienced fewer events than both the staged and combined CEA-CABG groups. Thus, provided that concomitant carotid revascularization is required and OHS can be delayed 3 to 4 weeks to allow for a course of DAPT to be completed, CAS followed by OHS may be the most attractive strategy.
3.Although in ideal circumstances, OHS would be delayed when carotid stenting is performed, this is not always feasible. In a small, single center study comprising 20 patients who underwent CAS prior to CABG, CABG could be performed safely at a mean of 6.4 days post stent implantation. Despite the use of DAPT, there were no significant bleeding complications, and no strokes or deaths were seen at a mean follow-up of 486 days. Although perhaps not generalizable to all patients, this study does suggest that in carefully selected patients in whom carotid revascularization is needed and CEA is significantly high risk, early CABG following carotid stenting could be an option.
4.It is important to note that not all patients with severe carotid stenoses who are scheduled for OHS require carotid revascularization. Patients who are at low risk from a neurologic perspective can have their carotid disease medically managed and undergo their OHS without concomitant carotid intervention. High-risk patients, including symptomatic individuals and those with significant contralateral disease, may benefit from either staged CAS-OHS or combined CEA-OHS, depending on the specific patient and procedural characteristics.
ACKNOWLEDGMENTS: The author thanks Drs. Hemal Gada, Adnan Chhatriwalla, and Christopher Bajzer for their contributions to earlier editions of this chapter.
Key Reviews
Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009;8:453–463.
Bates ER, Babb JD, Casey DE, et al. ACCF/SCAI/SVMB/ASITN 2007 Clinical Expert Consensus Document on carotid stenting. J Am Coll Cardiol. 2007;49:126–170.
Blauw GJ, Lagaay AM, Smelt AH, et al. Stroke, statins, and cholesterol. A meta-analysis of randomized, placebo-controlled, double-blind trials with HMG-CoA reductase inhibitors. Stroke. 1997;28:946–950.
Bonati LH, Dobson J, Featherstone RL, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomized trial. Lancet. 2015;385:529–538.
Brott TG, Halperin, JL, Abbara S, et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS 2011 guideline on the management of patients with extracranial carotid and vertebral artery disease. J Am Coll Cardiol. 2011;57:1002–1044.
Cho L, Yadav JS. Embolization in atherosclerosis. Neuroimaging Clin N Am. 2002;12:365–372.
Daniel, GK. Update of carotid stent trials. Catheter Cardiovasc Interv. 2006;68:803–811.
Fayed AM, White CJ, Ramee SR, et al. Carotid and cerebral angiography performed by cardiologists: cerebrovascular complications. Catheter Cardiovasc Interv. 2002;55:277–280.
Furie KL, Kasner SE, Adams RJ, et al. Guidelines for prevention of stroke in patients with ischemic stroke of transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276.
Mathur A, Roubin GS, Iyer SS, et al. Predictors of stroke complicating carotid artery stenting. Circulation. 1998;97:1239–1245.
Mohr JP, Albers GW, Amarenco P, et al. American Heart Association Prevention Conference. IV. Prevention and rehabilitation of stroke. Etiology of stroke. Stroke. 1997;28:1501–1506.
Moore WS, Barnett HJ, Beebe HG, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Circulation. 1995;91:566–579.
Rogers VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209.
Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107–116.
White CJ. Carotid artery stent placement. JACC Cardiovasc Interv. 2010;3:467–474.
Wolf PA, Kannel WB, Sorlie P, et al. Asymptomatic carotid bruit and risk of stroke. The Framingham study. JAMA. 1981;245:1442–1445.
Landmark Articles
Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy. I. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106.
Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–1425.
Barnett HJM, Taylor DW, Haynes RB, et al; North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453.
Bhatt DL, Fox KA, Hacke W, et al; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717.
Brott TG, Hobson RW II, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23.
CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329–1339.
CAVATAS Investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357:1729–1737.
Char D, Cuadra S, Ricotta J, et al. Combined coronary artery bypass and carotid endarterectomy: long-term results. Cardiovasc Surg. 2002;10:111–115.
Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316.
Corti R, Fuster V, Fayad ZA, et al. Lipid lowering by simvastatin induces regression of human atherosclerotic lesions: two years’ follow-up by high-resolution noninvasive magnetic resonance imaging. Circulation. 2002;106:2884–2887.
Diener HC, Cunha L, Forbes C, et al. European stroke prevention study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13.
Diethrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg. 1996;3:42–62.
Ederle J, Dobson J, Featherstone RL, et al; International Carotid Stenting Study Investigators. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomized controlled trial. Lancet. 2010;375:985–997.
Hass WK, Easton JD, Adams HP Jr, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med. 1989;321:501–507.
Henderson RD, Eliasziw M, Fox AJ, et al. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke. 2000;31:128–132.
Hobson RW II, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med. 1993;328:221–227.
Kapadia SR, Bajzer CT, Ziada KM, et al. Initial experience of platelet glycoprotein IIb/IIIa inhibition with abciximab during carotid stenting: a safe and effective adjunctive therapy. Stroke. 2001;32:2328–2332.
Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671.
Mayberg MR, Wilson SE, Yatsu F, et al. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA. 1991;266:3289–3294.
Mohr JP, Thompson JL, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–1451.
Nederkoorn PJ, Mali WP, Eikelboom BC, et al. Preoperative diagnosis of carotid artery stenosis: accuracy of noninvasive testing. Stroke. 2002;33:2003–2008.
O’Holleran LW, Kennelly MM, McClurken M, et al. Natural history of asymptomatic carotid plaque. Five year follow-up study. Am J Surg. 1987;154:659–662.
Ringleb PA, Allenberg J, Brückmann H, et al; The SPACE Collaborative Group. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomized non-inferiority trial. Lancet. 2006;368:1239–1247.
Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. PRoFESS Study Group. N Engl J Med. 2008;359:1238–1251.
Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389.
Walker MD, Marler JR, Goldstein M, et al. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–1428.
Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus carotid endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–1501.