I.Introduction. Stress echocardiography (SE) is an effective method of evaluating for myocardial ischemia, based on the detection of stress-induced regional wall motion abnormalities (WMAs). Stressors include exercise, pharmacologic agents, and pacing. SE is used to screen for coronary artery disease (CAD), and it can help identify the coronary vessels involved. The accuracy of SE in the detection of significant coronary artery stenosis is 80% to 90%, which is superior to that of exercise electrocardiographic testing and comparable to that of nuclear stress imaging. In patients with left ventricular (LV) dysfunction and documented CAD, SE can differentiate viable myocardium from scarred myocardium, which may help predict whether LV function will improve after revascularization. As a diagnostic test for CAD, SE is safe and relatively inexpensive and can be rapidly performed. However, interpretation of SE images remains primarily subjective and requires a considerable learning curve. SE can also be used to assess the severity of valvular disease, hypertrophic cardiomyopathy, and exercise-induced pulmonary hypertension. In addition, it provides important prognostic information after myocardial infarction (MI) and prior to noncardiac surgery.
II.Pathophysiology
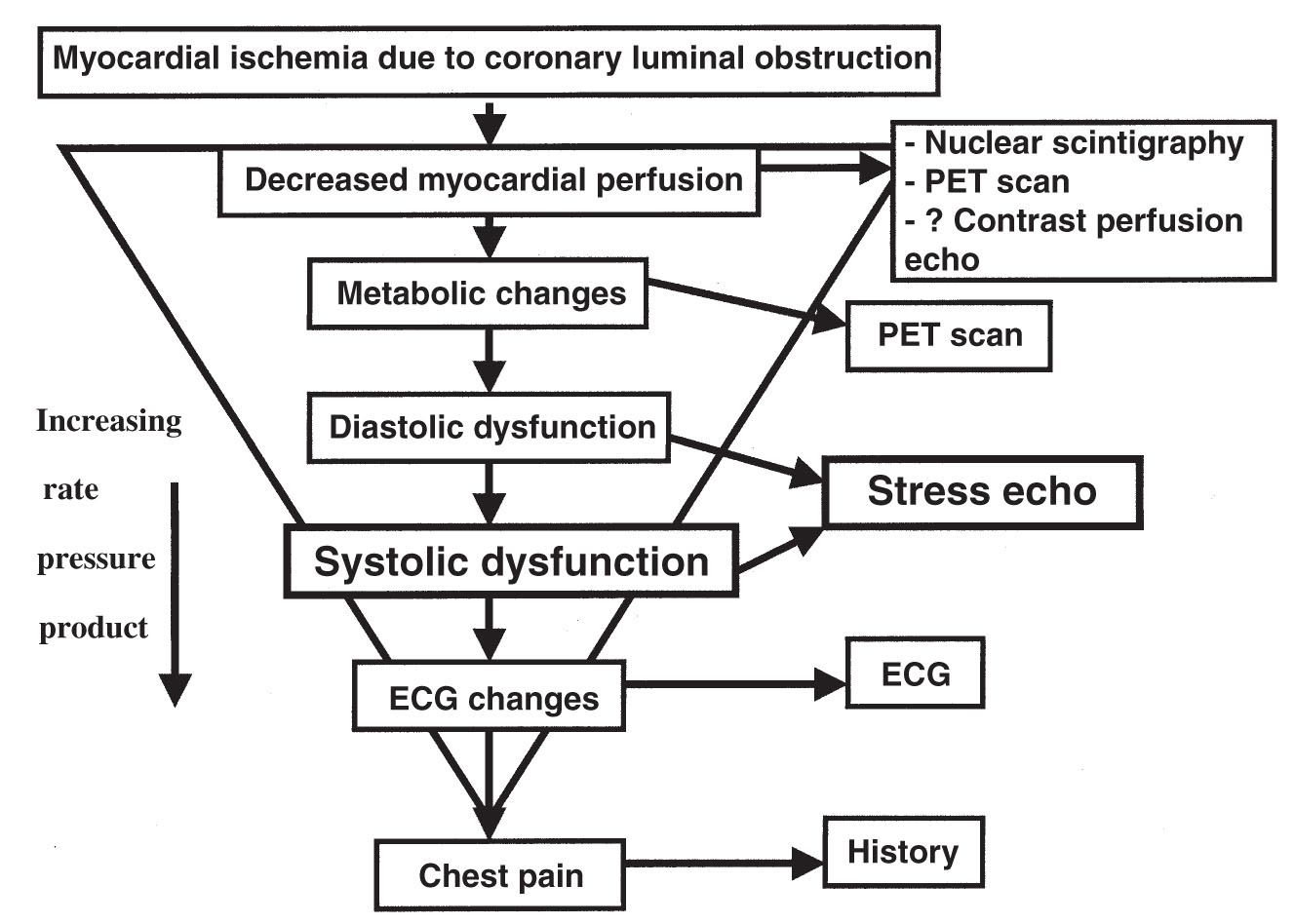
A.Exercise stress testing. Myocardial ischemia results from a mismatch between oxygen supply and demand. The ischemic cascade is illustrated in Figure 47.1. Echocardiography detects ischemia by identifying new or worsening WMAs earlier in the cascade than is detected by the electrocardiogram (ECG) or the onset of symptoms but usually after the onset of worsening diastolic function. Exercise can be performed with a treadmill or a bicycle.

FIGURE 47.1 Ischemic cascade. ECG, electrocardiogram; PET, positron emission tomography.
B.Pharmacologic stress testing. In patients who cannot exercise, pharmacologic stressors can be used. These drugs are sympathomimetic agents or vasodilators.
1.Sympathomimetic agents. Myocardial oxygen demand is determined by contractility (inotropy), heart rate (chronotropy), and wall stress (preload + afterload). Sympathomimetic agents produce stress by causing an increase in myocardial oxygen demand through increased inotropy, chronotropy, and blood pressure (BP) (afterload). Although a number of agents have been evaluated in combination with echocardiography, dobutamine is most widely used. Low-dose dobutamine has positive inotropic effects mediated through cardiac α1- and β1-receptors. At higher doses, it has positive chronotropic effects mediated through β2-receptors. The plasma half-life of dobutamine is 2 to 3 minutes. The normal response to dobutamine is an increase in heart rate and hyperdynamic wall motion, with only minimal effect on end-diastolic LV volume. It can be combined with atropine to achieve the usual target of at least 85% of age-predicted maximum heart rate (APMHR).
2.A vasodilator stress test is performed with dipyridamole or adenosine infusion. These agents result in perfusion abnormalities by causing blood to be preferentially shunted away from myocardial segments supplied by stenotic coronary arteries (i.e., coronary steal) and into more normal coronary vessels. This may lead to WMA in the perfusion territory of the stenotic coronary artery that is seen on echocardiography. These agents are less commonly used for SE. Adenosine has fewer side effects than dipyridamole, owing to a shorter half-life. However, because of the shorter duration of action of adenosine, the echocardiographic findings tend to be less pronounced and of shorter duration, resulting in a lower sensitivity.
3.The American Society of Echocardiography (ASE) guidelines recommend dobutamine as the first-line agent for pharmacologic SE. In addition, much of the data for preoperative risk stratification and viability assessment using SE were derived from pharmacologic studies using dobutamine.
C.Atrial pacing. Tachycardia induced by atrial pacing is an alternative to pharmacologic testing in patients that cannot exercise and in whom pharmacologic agents are contraindicated. In patients with a permanent pacemaker, stress is achieved by increasing the pacing rate until the target heart rate is reached. Transvenous and transesophageal pacing are options for patients without a permanent pacemaker but are rarely used in practice.
III.Indications and choice of stressor
A.The indications and contraindications for SE testing are similar to those used for exercise electrocardiographic stress testing. The addition of an imaging modality improves the sensitivity and specificity of exercise electrocardiographic stress testing. Table 47.1 lists factors that may limit the sensitivity of electrocardiographic stress testing to detect CAD; patients with these factors would benefit from a stress test utilizing an imaging modality (i.e., echocardiography, nuclear scintigraphy, or positron emission tomography [PET]).
TABLE 47.1 Factors Limiting the Sensitivity of Stress Electrocardiography to Detect Coronary Artery Disease |
Left bundle branch block or other intraventricular conduction delay abnormalities |
Paced rhythms |
Abnormal ST-segments at baseline: |
Digitalis effect |
Electrical left ventricular hypertrophy |
Previous evidence of myocardial infarction |
Nonspecific, abnormal ST-segment changes |
Women (higher rate of false-positive ST-segment changes) |
Left ventricular hypertrophy (even with normal-appearing electrocardiogram) |
B.Additional contraindications to SE occur with pharmacologic stress and depend on the underlying pharmacologic stressor. Patients with severe bronchospastic obstructive lung disease or high-grade atrioventricular (AV) block should avoid dipyridamole and adenosine. Patients with unstable ventricular arrhythmias should avoid dobutamine infusion. Relative contraindications to SE include unstable angina, severe baseline hypertension, uncontrolled arrhythmias, mobile LV thrombus, critical symptomatic aortic stenosis (AS), and decompensated heart failure.
C.Exercise stress is preferred over nonexercise stress because it more closely reproduces daily activity and is more sensitive in the detection of ischemia, provided the patient is able to achieve an adequate level of stress. No single exercise modality has been shown to have superior sensitivity, although the treadmill is more widely accepted among patients and physicians. Bicycle ergometry can be performed in the upright and supine positions. Images with treadmill stress testing must be obtained after exercise, whereas images may be obtained at peak exercise with bicycle ergometry while the patient continues to exercise. The sensitivity of treadmill testing to detect ischemia is reduced if images are not rapidly obtained (within 90 seconds) after exercise. However, the treadmill usually results in a greater level of stress than is associated with bicycle ergometry, which is more dependent on patient effort.
D.Up to 30% of patients referred for exercise echocardiography may not be able to achieve an adequate level of exercise stress because of peripheral vascular disease, chronic obstructive pulmonary disease, or musculoskeletal problems. Pharmacologic stress testing is usually indicated in these patients.
IV.Methodology
A.Patient preparation
1.Patients should avoid heavy food intake for several hours before the test.
2.Rate-slowing agents (particularly β-blockers) blunt the normal heart rate response to exercise and may limit the ability of the patient to achieve at least 85% of the APMHR. This may reduce the sensitivity of the test results. If possible, these agents should be withheld before the stress test, unless the aim of the test is to evaluate their effectiveness in preventing exercise-induced ischemia.
3.The standard connections for a 12-lead ECG may be used with minor modifications to allow imaging in the parasternal and apical windows without affecting the accuracy of the exercise electrocardiographic testing results.
B.Equipment. All SE studies are conducted with exercise electrocardiographic testing and standard hemodynamic monitoring equipment. An SE software package on the echocardiographic machine is necessary to acquire digital images and to allow side-by-side comparison of prestress images with peak stress or post–peak stress images. Resuscitation equipment and a defibrillator should be readily available.
C.Performing the test
1.Exercise SE. Regardless of the exercise modality, a complete baseline echocardiographic scan is obtained for all patients. Resting images are obtained in the parasternal long- and short-axis and apical two- and four-chamber views and stored digitally. An apical long-axis view may be substituted for a parasternal long-axis view if the parasternal images are suboptimal. If endocardial definition is suboptimal, intravenous ultrasound contrast should be given to optimize the images.
a.Treadmill exercise is performed with standard protocols according to the functional status of the patient. Exercise is continued until at least 85% of the APMHR is reached, but it is preferably continued to the level of maximum exertion to maximize test sensitivity. APMHR equals 220 minus the patient’s age. Post–peak stress images are obtained as quickly as possible (in the left lateral decubitus position) after the patient transfers from the treadmill to the imaging table. Stress images in the same views as the baseline study are stored digitally and recorded on videotape. All post–peak stress images should be obtained within 90 seconds of completing exercise to maximize test sensitivity.
b.During upright bicycle echocardiography, baseline images are obtained in the standard left lateral position and are repeated with the patient in the upright position on the cycle ergometer. Adequate parasternal images may be recorded by having the patient lean forward. These images are recorded and digitized to allow comparable windows for the rest and peak stress images. Cycle ergometry is started at a workload of 25 W and increased by 25 to 50 W every 2 to 3 minutes until the patient reaches his or her level of perceived maximal effort. During upright bicycle echocardiography, images are obtained and digitized at rest, before peak, at peak, and after peak exercise.
c.With supine bicycle exercise, the entire study is performed while the patient is tilted 30° in the left lateral decubitus position, and images are obtained and digitized at rest, before peak, at peak, and after exercise. This exercise modality is not widely used.
d.Study end points for exercise SE include target heart rate (85% APMHR), severe electrocardiographic ischemia (ST-segment depression > 5 mm), intolerable symptoms (chest pain and dyspnea), severe hypertension (systolic BP > 220 mm Hg or diastolic BP > 110 mm Hg), hypotension (systolic BP < 90 mm Hg or a fall in systolic BP > 20 mm Hg from baseline), ventricular tachycardia or sustained supraventricular tachycardia, and the development of new WMAs in at least two contiguous segments.
2.Pharmacologic SE
a.Dobutamine SE
(1)Dobutamine infusion is started at 10 µg/kg/min and increased every 3 minutes to 20, 30, and 40 µg/kg/min. If the patient has not reached 85% of APMHR by the end of the 40 µg/kg/min dose, a 3-minute dosage of 50 µg/kg/min may be used. Infusion is begun at lower doses (5 µg/kg/min) if baseline LV function is abnormal and myocardial viability is being sought or if assessment of valvular lesions is being pursued. Images are digitized at rest and at low dosage (5 to 10 µg/kg/min), pre–peak dosage (30 µg/kg/min), and peak dosage.
(2)Atropine is used as needed to reach target heart rate >85% of APMHR if dobutamine alone is not effective. Atropine (0.25 to 0.5 mg) is given intravenously every minute, starting at the 40 µg/kg/min dobutamine dose level and continuing until an end point is reached or a total dose of 2 mg is given. Atropine should be used with caution in patients that have glaucoma or benign prostatic hypertrophy. Isometric handgrip may be performed at the peak infusion rate to help achieve target heart rate, as well.
(3)Study end points for dobutamine SE are the same as those used for exercise SE. If 85% APMHR has been achieved without any other end points, it is preferable to complete the protocol to the end of the 40 µg/kg/min infusion to increase the sensitivity of the test.
(4)Side effects. The most serious potential side effect of dobutamine is arrhythmia provocation. However, serious complications (e.g., arrhythmia, MI, and cardiac arrest) are rare, occurring in about 0.3% of studies in a large series of >5,000 patients. Less serious side effects include tremor, nervousness, and marked hypertensive and hypotensive responses. The most common minor complication is hypotension, which usually responds to supportive therapy including intravenous fluids. A hypotensive response with dobutamine may be caused by ischemia and dynamic outflow tract obstruction or may result from the vasodilatory effect of dobutamine in combination with a small hyperdynamic LV and a low stroke volume.
(5)If angina or severe side effects develop, the effects of dobutamine may be reversed with intravenous β-blockade (0.5 to 1 mg/kg esmolol given over 1 minute or 2 to 5 mg/kg metoprolol given every 2 to 5 minutes). Like dobutamine, esmolol has a very short half-life and, therefore, may be the preferred agent.
b.Dipyridamole or adenosine SE
(1)Patients with hypotension, AV block, or a history of severe bronchospasm should not undergo testing with these agents.
(2)Different protocols of dipyridamole infusion have been studied. The protocol recommended by the ASE is a low-dose, two-stage infusion. The first stage begins at 0.56 mg/kg dipyridamole over 4 minutes; if no adverse effect or clinical end points are reached, an additional 0.28 mg/kg is infused over 2 minutes. A high-dose regimen of 0.84 mg/kg given over 10 minutes has been developed to improve the sensitivity of the test relative to low-dose protocols.
(3)Adenosine is given as a continuous infusion because of its very short half-life. A typical protocol starts at a low dose of 80 µg/kg/min and is increased every 3 minutes by 30 µg/kg/min to a peak dose of 170 to 200 µg/kg/min.
(4)Regadenoson is an adenosine receptor agonist with a 2- to 3-minute half-life, as compared with adenosine’s 30-second half-life. Regadenoson is administered as one 0.4-mg dose over 10 seconds.
(5)Study end points for dipyridamole or adenosine SE are similar to those used for exercise SE. A notable exception is that patients are not stressed until the APMHR is achieved. Additional end points include third-degree AV block, severe hypotension, and intolerable side effects (e.g., bronchospasm). Symptoms usually start to resolve within 60 seconds after medication administration.
(6)If hypotension, bradycardia, or bronchospasm occurs, the effects of dipyridamole, adenosine, and regadenoson can be reversed with intravenous aminophylline 50 mg over 60 seconds.
D.Imaging techniques. Modern technology allows digital image acquisition of multiple cardiac cycles and side-by-side comparison in a split screen display, enabling easy comparison of regional wall motion at rest and peak stress or after stress. Detailed frame-by-frame evaluation of wall thickening or excursion is possible, which helps in the evaluation of regional myocardial function. Obesity and lung disease remain the primary reasons for poor-quality images. Harmonic imaging has improved endocardial definition, which can be further optimized with microbubble contrast agents.
1.Contrast echocardiography. Microbubble contrast agents provide improved echocardiographic resolution and allow real-time assessment of intracardiac blood flow. These agents are helpful when baseline SE images are suboptimal.
a.Intravenous agitated saline improves visualization of the right atrium and ventricle and enables visualization of intracardiac shunts. However, intravenous agitated saline is not able to cross the pulmonary circulation and opacify the left ventricle.
b.Second-generation microbubble contrast agents, such as Optison and Definity incorporate perfluoropropane gas encased in an albumin-based or phospholipid shell, are more durable and are able to cross the pulmonary circulation and opacify the left ventricle.
c.These agents are well tolerated and have a low complication rate. Absolute contraindications to administration include previous hypersensitivity reaction and fixed right-to-left, bidirectional, or transient right-to-left cardiac shunts. Intra-arterial injection is contraindicated. Administration is relatively contraindicated in patients who are pregnant or nursing, although data are limited in these populations, and guidelines indicate that contrast should be given if needed.
2.Real-time three-dimensional (3D) echocardiography. Significant advances have been made in 3D data acquisition without the need for off-line reconstruction. 3D imaging may shorten the acquisition period of postexercise images or peak exercise images, allowing improved sensitivity and minimizing the technical strains imposed on the technologist obtaining the images. Limitations include lower spatial resolution and lower frame rates; at this time, 3D SE is not routine in clinical use and remains under investigation.
V.Image Interpretation
A.Qualitative versus quantitative approach
1.Interpretation of SE findings is predominantly qualitative. Visual assessment of LV wall thickening and motion remains the standard method of interpretation of SE but is subject to interobserver and interinstitutional variability. Suggestions to optimize interpretation of SE images are outlined in Table 47.2. Each myocardial segment is visually assessed for wall thickening, rather than just wall motion, which may be influenced by myocardial tethering and translation. LV wall motion normally becomes hyperdynamic with stress. Worsening of WMAs or the development of new ones is the hallmark of stress-induced myocardial ischemia. SE responses and interpretation are summarized in Table 47.3.
TABLE 47.2 Suggestions to Optimize Interpretation of Stress Echocardiographic Images |
|
1. |
Ensure that prestress and poststress images are comparable views |
2. |
Ensure that the apex is not foreshortened, especially in two-chamber views |
3. |
True two-chamber views should not show any of the right ventricle |
4. |
Use ultrasound microbubble contrast agents when resting images are suboptimal |
5. |
Check that digital images are timed to begin at systole. If digital clips include diastole, there is an increased likelihood of calling a false-positive wall motion abnormality |
6. |
Check the heart rate for each poststress image. If images are obtained after the heart rate has returned toward normal, the sensitivity of the test will be reduced |
7. |
Compare the wall motion of individual segments from rest to stress in the four-screen display to define ischemia and infarction. Then compare segments in the poststress images to identify differences in contraction and in the development of “hinge points” |
8. |
Confirm any wall motion abnormality in a second view if possible |
9. |
Avoid overcalling ischemia in the basal inferior or basal septal segments |
10. |
Avoid calling a new wall motion abnormality if it is limited to only one myocardial segment; the abnormality should involve at least two contiguous segments |
TABLE 47.3 Stress Echocardiographic Responses and Interpretation |
|||
Resting or Baseline Function |
Response to Low-Dose Pharmacologic Stress |
Peak Stress and Poststress Function |
Interpretation |
Normal |
Normal |
Hyperdynamic |
Normal myocardium |
Normal |
Normal or new WMA |
New WMA or lack of hyperdynamic response; LV dilation or decreased EF (with exercise only) |
Ischemic myocardium |
WMA |
No change |
No change |
Infarcted myocardium |
WMA |
Improved |
Decreased (biphasic response) |
Viable (hibernating) myocardium |
WMA |
No change |
Improved |
Nonspecific |
EF, ejection fraction; LV, left ventricular; WMA, wall motion abnormality.
2.Quantitative methods of analysis improve the reproducibility of interpretation and enhance the detection of CAD, particularly by less experienced physicians. However, at this time, the ASE recommends further validation and simplification of quantitative analysis methods before they can be recommended for routine use. Examples of quantitative analysis methods include Doppler assessment of global systolic and diastolic function; automated endocardial border detection using integrated backscatter; and tissue Doppler assessment of myocardial displacement, velocity, strain, and strain rate.
a.Tissue Doppler assessment along the long axis using apical views allows quantification of regional longitudinal myocardial function. Tissue Doppler is thought to be a potentially sensitive marker of subendocardial ischemia because abnormalities in regional contraction occur earlier in longitudinal than radial segments.
b.Strain rate is a measure of the speed or velocity of regional myocardial contraction (time from QRS to the onset of regional myocardial relaxation). During dobutamine SE, strain rate increases (interval of time from QRS to myocardial relaxation decreases) in normal hearts and is reduced in areas of myocardial ischemia. The optimal cutoff for strain rate that gives the best sensitivity and specificity has been reported to be an increment of <0.6 per second. Strain rate imaging is a reliable predictor of coronary stenosis, is more specific than visually assessed wall motion scoring, and may allow readers to detect intermediate severity coronary stenosis that produces only subtle WMAs. It may be difficult to acquire technically adequate images at rest and especially at higher heart rates following stress, which limits its applicability.
B.17-Segment model. Regional wall motion is assessed using a 17-segment model (Fig. 47.2), with results geographically represented on a circumferential polar plot (Fig. 47.3).

FIGURE 47.2 Diagram of vertical long-axis, horizontal long-axis, and short-axis planes showing the name, location, and anatomic landmarks for selection of the basal (tips of the mitral valve leaflets), mid-cavity (papillary muscles), and apical (beyond papillary muscles but before cavity ends) short-axis slices for the 17-segment system. (Reprinted with permission from Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542.)

FIGURE 47.3 Display, on a circumferential polar plot, of the 17 myocardial segments and the recommended nomenclature for tomographic imaging of the heart. (Reprinted with permission from Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542.)
1.The individual myocardial segments can be assigned to coronary artery territories, as illustrated in Figure 47.4. Of note, this approach is not always correct because of the anatomic variability. For instance, the left anterior descending coronary artery does not always supply the entire apex and the posterior wall is not always supplied by the left circumflex coronary artery. The system may also be problematic if multivessel disease is present, in which case the territory with the most ischemia is identified and less severe lesions may not be apparent.

FIGURE 47.4 Assignment of the 17 myocardial segments to coronary artery territories. LAD, left anterior descending; LCX, left circumflex coronary artery; RCA, right coronary artery. (Reprinted with permission from Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542.)
2.Wall motion is subjectively graded as normal, mildly hypokinetic, severely hypokinetic, akinetic, or dyskinetic and may be assigned a wall motion score of 1 to 4 (normal, hypokinetic, akinetic, or dyskinetic, respectively). Each myocardial segment in the rest and stress images is graded in this manner.
C.Exercise SE
1.A normal response to exercise stress includes a global increase in contractility, the development of hyperdynamic wall motion, and a gradual rise in the heart rate. This is manifested by increased wall thickness and increased endocardial excursion with stress.
2.Resting WMAs usually indicate prior MI, although regional variability may be seen in diffuse myopathic processes. Resting WMAs may be defined as hypokinetic, akinetic, or dyskinetic. Akinesia and dyskinesia usually indicate transmural infarction, whereas hypokinetic segments may be partially infarcted or viable.
3.An abnormal response to exercise is defined by the development or worsening of regional myocardial function. Regional myocardial dysfunction, as manifested by decreased endocardial excursion and wall thickening, is specific for myocardial ischemia. Decreased excursion alone is less specific and can occur with conduction abnormalities, with paced rhythms, and in the normal basal inferior myocardial segments.
4.Adjunctive diagnostic criteria for a positive SE examination include LV cavity dilation, a decrease in global systolic function, worsening diastolic function, and new or worsening mitral regurgitation (MR). However, these adjunctive diagnostic criteria are more specific for detecting severe CAD and may not be sensitive for detecting the presence of mild or moderate CAD.
5.False-positive findings may occur with left bundle branch block (septal WMA) and right ventricular (RV) pacing (apical WMA). A pathologic hypertensive response to exercise may also cause LV dilation and systolic dysfunction as can afterload mismatch in the setting of severe valvular lesions such as AS, MR, or aortic regurgitation.
6.False-negative findings may occur with a delay in capturing postexercise images, low workload, or inadequate heart rate response (i.e., with inadequate stress or the presence of β-blockers). Additional causes of false-positive and false-negative findings are outlined in Table 47.4.
TABLE 47.4 False-Positive and False-Negative Stress Echocardiographic Test Results |
|
Causes of Incorrect Stress Echocardiographic Interpretation |
Factors Reducing Specificity or Sensitivity |
|
False-Positive Results |
|
LBBB, prior cardiac surgery (e.g., myectomy) |
Reduced or abnormal septal excursion with normal septal thickness |
Right ventricular pacing |
Apical WMA |
Nonischemic cardiomyopathy |
Regional WMAs (exact cause unknown) |
Hypertensive response to exercise (SBP > 220 mm Hg, DBP > 110 mm Hg) |
Nonischemic WMAs and/or LV dilation |
Overinterpretation |
Observer bias may result in a lower threshold for calling a positive study; it is important to be blinded |
Basal inferior or septal WMA |
Areas most likely to be overcalled because of reduced excursion because of annular tethering effects |
Poor image quality |
|
|
False-Negative Results |
|
Single-vessel disease |
More likely to have subtle, rapidly resolving WMA than multivessel disease |
Inadequate level of stress (more likely with β-blockers) |
Important to stress maximally; reach at least 85% of age-predicted maximum heart rate |
LV cavity obliteration (more likely to occur with dobutamine) |
Makes segmental wall motion analysis difficult |
Left circumflex disease |
Lateral wall dropout; more likely to miss ischemia |
Delay in capturing images after maximal stress |
|
Poor image quality |
|
DBP, diastolic blood pressure; LBBB, left bundle branch block; LV, left ventricular; SBP, systolic blood pressure; WMA, wall motion abnormality.
D.Pharmacologic SE. With only a few exceptions, the principles of interpretation of pharmacologic SE findings are similar to those used for exercise echocardiography.
1.The typical ischemic response to dobutamine is characterized by normal resting wall motion and an initial hyperdynamic response at low doses followed by a decline in function at higher doses. Ischemia may also be identified on the basis of deterioration of normal wall motion without any transient hyperdynamic response.
2.LV cavity dilation and a decrease in global systolic function are not considered adjunctive diagnostic criteria in dobutamine SE. The LV cavity may not dilate, and global systolic function may improve with dobutamine despite new WMAs because of severe CAD.
3.Interpretation of results obtained from dipyridamole or adenosine SE requires detection of a new or worsening regional WMA during the infusion. There is only a mild increase in cardiac contractility during vasodilator stress.
E.Reproducibility. The person who interprets the images must be well trained in order to develop an acceptable level of accuracy and must interpret an adequate number of studies on a regular basis to maintain accuracy. Concordance within centers is generally good; however, concordance between different centers may be <80%, particularly with technically difficult studies and studies of patients with mild CAD.
F.Limitations. The ability to interpret stress echocardiograms is mitigated by image quality, the presence of arrhythmias, conduction abnormalities, respiratory interference from hyperventilation, and difficulty in reproducing the translational and rotational motion of the heart.
VI.Diagnostic accuracy. The diagnostic accuracy of SE is superior to exercise electrocardiographic testing alone and similar to radionuclide perfusion techniques. Reported sensitivities and specificities (using coronary arteriography as the gold standard) vary between studies, depending on the prevalence of disease in the study population, the angiographic definition of significant disease, and the criteria used for a positive test. Clinical factors such as age, cardiac risk factors, and symptoms that influence the pretest likelihood of CAD also influence sensitivity and specificity. For the overall detection of patients with CAD, sensitivity ranges from 75% to 92%, depending on lesion severity, and specificity ranges from 64% to 100%. As with other imaging methods, the sensitivity is less for the detection of single-vessel disease and greater for the detection of multivessel disease.
A.Exercise SE
1.Comparison with exercise electrocardiographic testing. Exercise electrocardiographic testing remains the first-line diagnostic test for CAD. However, SE has greater diagnostic sensitivity and specificity, which is predictable on the basis of the earlier occurrence of a systolic WMA before electrocardiographic changes or symptoms in the ischemic cascade (Fig. 47.1). Many factors limit the sensitivity of electrocardiographic testing alone to detect CAD (Table 47.1), and these subgroups should be considered for exercise electrocardiographic testing with an imaging modality.
2.Comparison with myocardial perfusion scintigraphy
a.Myocardial perfusion scintigraphy is based on the detection of a perfusion defect during maximal hyperemia, with reduced perfusion of areas subtended by significant coronary artery stenosis (>50% stenosis). Perfusion abnormalities occur at an earlier stage in the ischemic cascade than do systolic WMAs, and nuclear scintigraphy should, theoretically, have a higher sensitivity than SE for CAD.
b.Studies using single-photon emission computed tomography (SPECT) myocardial perfusion scintigraphy have demonstrated a sensitivity of >90%, slightly higher than that for SE. However, the specificity of SE is superior to that of SPECT, especially in patients with LV hypertrophy or left bundle branch block. The overall accuracy of SPECT and SE has been found to be similar in meta-analyses; the superior sensitivity of SPECT is balanced by the superior specificity of SE. The exception may be in women, where SE may be more accurate than SPECT, owing to less artifact from breast attenuation.
c.SE is convenient and provides information on cardiac structure and function, and the results can be interpreted immediately, with rapid feedback to the patient and referring physician. SE also avoids exposure to radioactive tracers and is substantially less expensive than SPECT.
d.SPECT allows for more objective interpretation, with quantification of perfusion abnormalities. It may also be slightly superior for patients on antianginal therapy when it is necessary to induce ischemia. SPECT appears to be more sensitive in the detection of single-vessel disease and may be superior in the detection of ischemia in the setting of resting WMAs, in which the recognition of worsening wall motion may be difficult. SPECT may also be superior in patients that have poor acoustic windows, for example, those with chronic obstructive pulmonary disease. Local expertise, cost, exposure to radiation, and patient selection are all important factors in determining which imaging modality to use.
B.Pharmacologic SE
1.Dobutamine SE has a sensitivity ranging from 68% to 96% and a specificity of 80% to 85%, similar to the values for exercise SE. Vasodilator SE has a sensitivity of 52% to 92% and a specificity of 80% to 100%. In general, the specificity of vasodilator SE is superior to that of other echocardiographic stress techniques. However, single-vessel disease is more difficult to detect with this technique.
2.Myocardial perfusion scintigraphy. Compared with dipyridamole SPECT, dipyridamole SE is believed to be less sensitive but more specific; however, few studies have compared the two tests in the same patients. As with exercise SE, dobutamine SE appears to be slightly less sensitive but more specific than SPECT.
VII.Assessment Of Viability
A.Myocardial contractility ceases when 20% or more of the transmural thickness is ischemic or infarcted. Dobutamine SE can be used to detect viable myocardium, whether stunned or hibernating. Myocardial stunning after MI is common, and it is characterized by viable nonischemic noncontracting myocardium. Patients with chronic ischemia may experience myocardial hibernation. Hibernating myocardium is characterized by viable, chronically ischemic noncontracting myocardium.
B.Dobutamine infusion may result in augmentation of regional myocardial function predictive of recovery of function after revascularization. This is important prognostically, because revascularization of hypoperfused but viable myocardium improves survival. A contractile response to dobutamine requires that at least 50% of the myocytes in a given segment are viable.
C.Demonstration of a biphasic response to low-dose (5 to 10 µg/kg/min) dobutamine strongly suggests viable myocardium. A biphasic response is present when a resting WMA improves in response to low-dose dobutamine and decreases in function at peak stress or poststress. The initial improvement reflects recruitment of contractile reserve and hence viability. Higher doses lead to subendocardial ischemia and worsened WMA. A biphasic response predicts eventual functional recovery of the myocardium after revascularization. A uniphasic response is less predictive of recovery, and a classic ischemic response is not predictive of the recovery of resting function. Because the biphasic response is the most reliable finding, the preference is to induce ischemia whenever possible by proceeding to maximal stress (40 µg/kg/min).
D.Myocardial wall thickness is also an important marker of myocardial viability. When the wall thickness is <6 mm, there is a low likelihood of recovery of function.
E.The negative predictive value of dobutamine SE for determining viability is lower than that of thallium stress-redistribution-reinjection SPECT and fluorodeoxyglucose-PET scanning. However, the positive predictive value is greater. Concurrent use of β-blockers can reduce the number of viable segments detected and the sensitivity of testing.
F.Assessment of myocardial perfusion with echocardiography. Second-generation microbubble contrast agents are small in diameter and reliably traverse the myocardial microvasculature. The microbubbles are destroyed with ultrasound energy, and the rate of microbubble replenishment represents mean red blood cell velocity and myocardial perfusion. Although subject to extensive research, this technology has had limited utilization in clinical practice and is not used routinely in most echocardiography laboratories.
VIII.Prognostic Role Of SE
A.Suspected or known chronic CAD. The major determinants of prognosis in patients with chronic CAD are LV function and the anatomic extent and severity of myocardial ischemia. SE is an excellent modality for the evaluation of both.
1.Negative test result. Perhaps the most important aspect of the prognostic literature is that a negative test result portends an extremely low risk of subsequent cardiovascular events, as evidenced by an event rate of <1% per year for the subsequent 4 to 5 years. However, the risk is slightly higher in patients with diabetes or chronic kidney disease.
2.Presence of ischemia. Abnormal findings during SE indicate elevated risk for future cardiac events. Patients at intermediate risk for CAD who have abnormal SE findings have a 1-year cardiac event (i.e., MI, percutaneous coronary intervention, coronary artery bypass grafting, or death) rate of 10% to 30%. However, this information needs to be integrated with other stress data (i.e., exercise capacity, hemodynamic response to exercise, heart rate recovery, chronotropic index, Duke treadmill score, and the type and extent of WMA). Electrocardiographic changes and hypotension are relatively insensitive measures of ischemia during dobutamine SE. However, from the prognostic standpoint, the development of echocardiographic evidence of ischemia with dobutamine is analogous to its development during exercise.
3.Presence of nonviable myocardium. In patients with the same pretest probability of disease, those with evidence of nonviable myocardium during SE have higher rates of cardiac events than those with normal SE findings, but they have fewer events than those with evidence of ischemia during SE. Heart failure is a more common end point among the group of patients with nonviable myocardium.
B.Post–myocardial infarction. High-risk patients after acute MI are routinely identified by age, recurrent angina, LV failure, and shock. In addition, echocardiographic features predicting outcome after MI include LV ejection fraction, the extent of resting WMAs, inducible ischemia (detected as stress-induced WMA), and the amount of viable myocardium. All of these may be identified using SE, and several large studies (most with pharmacologic stressors) have gathered prognostic data using SE in patients post-MI.
C.Noncardiac surgery
1.Preoperative evaluation studies have been predominantly conducted with pharmacologic stress agents, primarily dobutamine. However, exercise SE should be considered if possible. A low ischemic threshold during stress (ischemia at heart rate < 70% APMHR) is the strongest predictor of perioperative cardiac events.
2.The predictive value of a positive test ranges from 7% to 25% for hard events (i.e., MI or death). The negative predictive value ranges from 93% to 100%. Only a few studies have compared SE and SPECT for the prediction of perioperative cardiac events. A meta-analysis concluded that the tests had comparable levels of accuracy, but the cost features weighted in favor of SE.
D.Cardiac transplantation. Transplant vasculopathy is a major cause of mortality after cardiac transplantation. SE appears to lack both sufficient sensitivity and specificity to be a viable alternative to routine angiography as a screening method for vasculopathy.
E.Reading beyond wall motion. Important prognostic information can be obtained beyond traditional wall motion analysis. Ischemic heart disease may cause subclinical diastolic dysfunction. Left atrial enlargement correlates with the chronicity and severity of diastolic dysfunction. A normal resting left atrial volume index (<28 mL/m2) is strongly predictive of a normal stress echocardiogram. RV dysfunction is a significant predictor of events, independent of LV ischemia or ejection fraction.
IX.Diastolic Stress Echocardiography
A.In many patients, “diastolic” heart failure is the dominant form of dysfunction, without any detectable systolic dysfunction at rest or during stress. The transmitral peak early diastolic velocity (E ) and the mitral annulus early diastolic velocity (e′) are utilized to assess the diastolic dysfunction. In the presence of normal LV systolic function and volumes, an E/e′ ratio >15 suggests elevated LV filling pressure and diastolic dysfunction, whereas a ratio <8 excludes diastolic dysfunction. The primary utility of diastolic SE is to evaluate patients in whom diastolic dysfunction is suspected but the resting echocardiogram is indeterminate (i.e., E/e′ 8 to 15). Assessment for diastolic dysfunction should be completed during routine SE, because its presence and severity add to the negative prognostic value of resting or stress-induced systolic dysfunction.
B.Exercise or adrenergic stress normally results in improved myocardial lusitropy (relaxation) to allow for better filling in a shorter amount of time. The tachycardia associated with exercise results in an abbreviated diastolic filling period and an increase in the transmitral peak E velocity. In healthy patients, both the transmitral peak E velocity and the mitral annulus early diastolic velocity increase with exercise, and the E/e′ ratio is not changed. However, in patients with diastolic dysfunction, the mitral annulus early diastolic velocity is minimally affected by the change in preload caused by exercise and the E/e′ ratio increases.
C.Assessment of diastolic dysfunction can be difficult at rest and is even more so with stress. When diastolic function is the major indication for testing, exercise SE is optimally performed using supine bicycle ergometry, because it allows for the acquisition of Doppler recordings during exercise. However, evaluation is routinely performed using treadmill exercise or dobutamine. Tachycardia may result in fusion of the transmitral E and A velocities at peak stress, making the tracings impossible to interpret. Therefore, Doppler assessment of the mitral inflow velocities should be assessed at rest, during exercise, and in recovery, if possible.
X.Stress Echocardiography in Nonischemic Cardiac Disease. SE can be used to evaluate the functional significance of a variety of valvular lesions as well as hypertrophic cardiomyopathy. SE is especially helpful when there is a discrepancy between clinical symptoms and the assessment of valve severity at rest. In addition to the effect of exercise on wall motion, ventricular size and function, other parameters are assessed when valve disease or hypertrophic cardiomyopathy is the prime focus of the stress echocardiogram. Functional capacity, change in pressure gradients across individual stenotic valves of the LV outflow tract (LVOT), change in severity of regurgitation, and the effect of exercise on estimated RV systolic pressure may all be important in understanding the hemodynamic consequences of the lesion under study. It is important to discuss with the sonographer performing the test in what order individual valve or myocardial parameters should be assessed at peak stress to optimize the value of the study. Thus, in mitral stenosis, for instance, peak mitral gradients followed by peak TR velocity and then wall motion and LV size is the appropriate order of peak acquisition of data.
A.Aortic stenosis
1.Exercise testing is contraindicated (American College of Cardiology/American Heart Association [ACC/AHA] class III, level of evidence [LOE] B recommendation) in patients with symptomatic AS. In asymptomatic patients with a calcified aortic valve and a peak aortic velocity over 4 m/s or a mean pressure gradient over 40 mm Hg, exercise testing may be considered (ACC/AHA class IIa, LOE B recommendation) to elicit exercise-induced symptoms and abnormal BP responses. Reduced functional capacity for age and gender is associated with worse outcomes in severe AS, especially if valve replacement is not promptly performed.
2.Dobutamine SE is reasonable (ACC/AHA class IIa, LOE B recommendation) in the diagnostic evaluation of patients with low-flow/low-gradient AS, defined as Doppler-derived aortic valve area < 1 cm2, LVEF < 50%, and mean pressure gradients < 40 mm Hg or peak aortic velocity < 4 m/s. In these patients, dobutamine is used to assess both the severity of AS and the presence of contractile reserve.
3.In severe AS, low-dose (20 µg/kg/min) dobutamine infusion results in increased cardiac output with a parallel rise in the mean transvalvular gradient. Provided the calculated aortic valve area remains <1 cm2, an increase in the mean transvalvular gradient to a value >40 mm Hg or velocity >4 m/s is consistent with severe AS. If dobutamine infusion results in an increase in the valve area (typically to >1 cm2) with little change in the gradient, it is likely that LV dysfunction rather than AS is the primary problem, and aortic valve replacement is unlikely to be beneficial.
4.Dobutamine SE is also used to identify contractile reserve in patients with low-flow/low-gradient AS. Contractile reserve is defined as >20% increase in stroke volume with dobutamine infusion. Lack of contractile reserve is associated with poorer prognosis with either medical or surgical therapy.
B.Mitral regurgitation. In asymptomatic patients with severe MR, exercise SE is reasonable (AHA/ACC class IIa, LOE C recommendation) to assess exercise tolerance and the effects of exercise on pulmonary artery pressure and severity of MR. SE can help predict latent LV dysfunction in patients with normal baseline LV systolic function, severe MR, and minimal or no symptoms. An increase in the LV cavity size or decrease in LV ejection fraction at peak stress suggests latent LV dysfunction and an increased risk of LV dysfunction after valve repair. Inability to achieve a functional response expected for age and gender is indicative of poorer outcomes in those not undergoing early surgical intervention for severe MR.
In symptomatic patients with severe MR, when there is a discrepancy between symptom severity and the severity of MR, exercise testing is reasonable (AHA/ACC class IIa, LOE B recommendation).
C.Mitral stenosis. Exercise SE should be performed (ACC/AHA class I, LOE C recommendation) to assess the hemodynamic response of the mean gradient and pulmonary artery pressure in patients with mitral stenosis when there is a discrepancy among resting Doppler echocardiographic findings, clinical findings, symptoms, and signs. An increase in the mean transmitral pressure gradient >15 mm Hg or pulmonary artery systolic wedge pressure >60 mm Hg may be indications to consider percutaneous or surgical intervention.
D.Hypertrophic cardiomyopathy. In patients with hypertrophic cardiomyopathy and high resting LVOT gradients, routine exercise testing is not performed owing to increased risks of arrhythmias and hypotension. Exercise SE provides valuable information, including exercise hemodynamics, exercise capacity, worsening of MR, and provocable gradients in patients that are asymptomatic at rest. Although these patients may have only mild to moderately elevated resting LVOT gradients, using SE to identify elevated provocable gradients may help explain their exertional symptoms and quantify their exercise tolerance. Additionally, more recent data indicate that asymptomatic or minimally symptomatic hypertrophic cardiomyopathy patients may be accurately risk stratified by their exercise capacity, regardless of their gradients with exercise.
ACKNOWLEDGMENTS: The author thanks Drs. Matthew Deedy, Patrick Nash, Ryan Daly, and Michael Brunner for their contributions to prior editions of this chapter.
Guidelines
Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J Am Soc Echocardiogr. 2011;24:229-–267.
Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2215-–2245.
Nishimura RA, Otto CM, Bonow RO, et al. ACC/AHA 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440-–2492.
Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63:380-–406.
Reviews
Gentry Iii JL, Phelan D, Desai MY, et al. The role of stress echocardiography in valvular heart disease: a current appraisal. Cardiology. 2017;137(3):137–150.
Lancellotti P, Pellikka PA, Budts W, et al. The clinical use of stress echocardiography in non-ischemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2016;17:1191–1229.