
Michael J. Johnson
Amar Krishnaswamy
Percutaneous Structural Heart Disease Procedures
I.Introduction. The last decade witnessed a remarkable rise in the number of novel percutaneous, catheter-based procedures using new concepts and technologies for the treatment of structural heart disease. Most percutaneous therapies have largely evolved from concepts developed by surgeons and often incorporate the lessons learned from established surgical procedures. Currently, percutaneous catheter-based therapy is available for a number of valvular disorders, including aortic stenosis (AS), mitral stenosis (MS), mitral regurgitation (MR), and prosthetic paravalvular leaks (PVLs), as well as other structural cardiac disorders such as hypertrophic obstructive cardiomyopathy, patent foramen ovale (PFO), and atrial septal defects (ASDs). Whereas a comprehensive review of structural cardiac interventions is beyond the scope of this chapter, we provide an overview of the major devices and relevant clinical studies that have been performed.
II.Percutaneous Transcatheter Mitral Valve Repair (MVRe)
A.Background. MR is one of the most common valvular disorders, encountered in about 9% of the population aged >75 years. Surgical MVRe, when feasible, produces excellent outcomes, including lower operative mortality, improved long-term survival, and reduced incidence of endocarditis and thromboembolic complications, compared with mitral valve replacement (MVR). A number of percutaneous MVRe techniques have been developed that are analogous to surgical procedures. Percutaneous mitral annuloplasty leverages the close relationship of the coronary sinus (CS) to the posterior mitral annulus. The Carillon (Cardiac Dimensions, Kirkland, WA) device, which has been granted Conformité Européenne (CE Mark) approval, is deployed in the CS and serves to “cinch” the annulus, resulting in improved mitral valve (MV) leaflet coaptation. There are also numerous other devices in various stages of preclinical and clinical development.
The MitraClip (Abbott Vascular, Santa Clara, CA) remains the most widely tested system in humans and is currently the only device commercially available to treat degenerative MR in the United States. Over 25,000 MitraClips have been implanted worldwide since its inception. The technique is based loosely on the surgical Alfieri stitch, which brings the anterior and posterior leaflets in close apposition using a suture and creates an anatomic “double-orifice” MV. In the United States, transcatheter MVRe is indicated in symptomatic patients with severe degenerative MR who are considered to have prohibitive surgical risk. Patients with severe functional MR are currently being studied in the COAPT trial, which randomizes patients to MitraClip versus medical therapy. The MitraClip is available commercially for functional MR in Europe where this represents the majority indication of worldwide use.
B.Procedure. The MitraClip system uses a steerable 22F guide catheter that is introduced through the femoral vein and subsequently advanced to the left atrium via transseptal puncture (Fig. 64.1). Through this guiding catheter, a delivery system containing the V-shaped clip is introduced in the left atrium and positioned with the arms of the clip perpendicular to the MV line of coaptation using transesophageal echocardiography (TEE) guidance. The clip is advanced to the left ventricle in an open position and retracted to grasp the anterior and posterior MV leaflets at the desired location. After confirmation by TEE of clip position, the clip can be locked. If satisfactory (by TEE), the clip can be released; otherwise, it can be reopened and the process repeated. If necessary, multiple clips can be deployed to achieve a satisfactory result. Care must be taken in this situation to not impede forward flow across the valve and trade MR for MS.

FIGURE 64.1 Percutaneous transcatheter mitral valve repair. A: Right anterior oblique projection of the optimal positioning of the MitraClip device. B: Left anterior oblique projection of the optimal positioning of the MitraClip device. C: Right anterior oblique projection of MitraClip after its release from the delivery catheter. D: Left anterior oblique projection of MitraClip after its release from the delivery catheter.
C.Complications. Routine complications associated with vascular access like major or minor bleeding are the most commonly encountered complications. Recurrent MR and the requirement for MV surgery are the prime limitations of the current technique, especially in ischemic MR or in the presence of a significant annular calcification. Partial clip detachment is the most important mechanical problem encountered with the procedure and treatment with subsequent clip placement or surgical intervention is dependent on the mechanism of leaflet detachment. These are generally not symptomatic and are treatable with MV surgery or placement of an additional clip. Iatrogenic MS is a significant complication that may arise as a result of this procedure.
D.Outcomes. Data from the pivotal EVEREST II trial, which randomized 279 patients to MitraClip versus surgical repair in a 2:1 fashion, demonstrated freedom from the combined end point (death, MV surgery within 90 days, or MR > 2+ at 1 year) of 72.4% and 87.8% in the two groups, respectively, demonstrating noninferiority of the MitraClip to conventional surgical treatment. Nevertheless, the MitraClip arm had a higher incidence of residual significant MR and subsequent need for MV surgery and thus surgical MVRe remains the gold standard for patients able to undergo open heart surgery.
The initial studies of the MitraClip concentrated on patients with degenerative MR. However, the EVEREST II trial and the clinical experience in Europe (where the device received the CE Mark) have shown efficacy in a large number of patients with functional MR as well. Freedom from significant MR was enjoyed by more than 80% of high surgical risk patients, with resultant decreases in left ventricular (LV) volume, New York Heart Association class, congestive heart failure (CHF) hospitalizations, and improved quality of life.
III.Percutaneous Mitral Balloon Valvuloplasty (PMBV)
A.Background. Although there has been a significant reduction in the prevalence of rheumatic heart disease in western countries, it still represents a major public health concern in the developing world. MS is one of the most common presentations of rheumatic heart disease. It continues to represent a major clinical problem in the United States primarily because of outmigration from developing countries or the occurrence of restenosis after previous surgical commissurotomy. Stenosis of the valve may occur because of commissural fusion, leaflet thickening, and/or chordal shortening and fusion.
Before the advent of PMBV, symptomatic MS was treated using surgical commissurotomy. Since the introduction of the percutaneous procedure in the early 1980s by Inoue and colleagues, PBMV has evolved to become the first line of therapy for appropriately selected patients with MS. The technique works similarly to commissurotomy, resulting in opening of the fused commissures.
B.Procedure. The Wilkins splitability score is the most widely used echocardiographic parameter to determine the safety and feasibility of PBMV, and this takes into consideration leaflet mobility, leaflet thickening, subvalvular thickening, and valve calcification (each scored 0 to 4 points). Multiple investigators have shown that patients with a score of 8 or less have the greatest freedom from death and valve surgery and the largest improvement in MV area with PMBV.
Selection of the appropriate balloon size is one of the most important steps for accomplishment of a successful PBMV. A good rule of thumb is to use the following formula to determine the maximum balloon dilation size: Balloon size (mm) = patient height (cm)/10 + 10 mm. Transseptal puncture is performed, most commonly with TEE guidance, via a sheath placed in the femoral vein. Once the sheath has entered the left atrium, it is exchanged for the Inoue balloon catheter.
The balloon catheter is slowly advanced into position in the left ventricle. The Inoue balloon consists of three portions with slightly different compliances. As pressure is added to the balloon, the distal portion inflates first followed by the proximal portion. As soon as the distal portion is inflated, the balloon is pulled until resistance is felt. On addition of more pressure, the proximal portion is inflated, which fixes the balloon in the middle waist portion of the valve. The middle waist has the least compliance and dilates only when substantial pressure is added to the balloon, thereby securing the balloon across the valve prior to the dilation of the annulus.
In our institution, we routinely use TEE to guide the valvuloplasty in order to assess the result of balloon inflation (in addition to simultaneous left atrial (LA)–LV gradient measurement) and, more importantly, to evaluate the degree of MR. Substantial increases in MR should preclude further inflation. In addition, the procedure should be aborted in the presence of left atrial appendage (LAA) clot.
C.Complications. The most common serious complications include hemopericardium or severe MR. Perforation of cardiac chambers, which occurs with a rate of 0% to 2%, may happen while manipulating the catheters in the heart. Whereas an increase in MR may routinely be noted after the PMBV, it rarely requires a surgical intervention.
D.Outcomes. Immediate postprocedural success with a final valve area >1.5 cm2 without moderate or severe MR is the best predictor of long-term outcome. The best results are obtained in young people with favorable anatomic characteristics. Randomized clinical trials have demonstrated that long-term results of PMBV in young patients are as good as open commissurotomy and are better than closed commissurotomy. In patients with optimal morphology, freedom from restenosis has been reported as 92% at 5 years, 85% at 10 years, and 65% at 15 years. Repeat PMBV has been recommended as first-line therapy in patients with symptomatic mitral restenosis after PMBV or commissurotomy in whom the mechanism of restenosis is commissural fusion.
IV.Balloon Aortic Valvuloplasty (BAV)
A.Background. Degenerative or calcific AS is one of the most common valvular disorders encountered in western countries. Surgical aortic valve replacement (SAVR) is the treatment of choice for patients who are safely able to undergo cardiac surgery, and transcatheter aortic valve replacement (TAVR) is emerging as a favorable option in patients at high risk for surgical complications. Balloon dilation of the calcified aortic valve results in stretching of the fused commissures. Because of rapid reversibility of these effects, there is an early loss of effectiveness in severe degenerative AS, and the valve returns to pre-BAV size in 3 to 6 months.
In patients with severe AS who are hemodynamically unstable and for whom urgent aortic valve replacement (AVR) is not feasible, BAV may serve as a “bridge” to valve replacement. Similarly, we have also seen significant functional improvement in patients after BAV so that patients unable to undergo AVR initially have improved to a point that TAVR or SAVR could be performed safely. In patients with symptomatic severe AS who require urgent noncardiac surgery, BAV may be considered as a temporizing measure in the hope of reducing the risks of perioperative hemodynamic changes associated with anesthesia.
A number of patients with severe AS have other comorbidities, such as chronic obstructive pulmonary disease or liver or kidney disease, that make it difficult to discern the degree to which AS contributes to their symptoms. In such cases, BAV may provide a therapeutic answer; improvement of symptoms points to AS as the driver of symptoms and may push for a more definitive valve replacement option. Finally, in patients without any option for either TAVR or SAVR, BAV may be considered as a palliative measure.
B.Procedure. The femoral retrograde approach is the most commonly utilized method for BAV, although in patients with severe iliofemoral disease, the procedure can be performed in an antegrade fashion via venous access and transseptal puncture. A Swan–Ganz catheter is placed in the pulmonary artery for continuous hemodynamic monitoring and assessment of cardiac output. A temporary pacemaker is placed in the right ventricle to perform rapid pacing (180 beats/min) during balloon inflation to reduce cardiac output and minimize balloon movement in the annulus. After crossing the aortic valve using a 5F AL-1 diagnostic catheter and a straight wire, a stiffer wire is inserted and positioned in the left ventricle. BAV is typically performed using balloons ranging from 18 to 25 mm in diameter. The balloon is sized based on the annulus diameter on transthoracic echocardiography (TTE); the maximum balloon size is 10% larger than the annulus, and we routinely begin dilation at smaller sizes and assess the hemodynamic result prior to increasing the balloon size. Procedural success with BAV is typically defined as a 50% reduction in mean aortic valve gradient and a 25% increase in aortic valve area (AVA); most patients usually experience almost a 50% increase in AVA.
C.Complications. It should be noted that BAV carries considerable risk. The 30-day mortality associated with the procedure may be up to 10% and as high as 50% at a median follow-up period of 6 months, usually because of either aortic regurgitation (as a complication of the balloon procedure) or persistent heart failure. Other complications (occurring in up to 15% of patients) include stroke, peripheral vascular complications (because of the size of the devices used and concomitant incidence of peripheral arterial disease), coronary occlusion, need for permanent pacemaker implantation, renal failure, cardiac tamponade, and cardiac arrest.
D.Outcomes. Despite a modest improvement in valve area, a significant improvement in functional status may be noted after BAV. However, the benefit of BAV gradually disappears over the course of the next few months. The poor functional status of the patients, as well as only moderate, transient effects of the technique, is primarily responsible for the overall grim long-term results of stand-alone BAV. Extensive data indicate that stand-alone BAV does not change the natural course of AS, even after repeated procedures. Despite this, BAV holds an important place in the treatment of patients with severe AS.
V.Transcatheter Aortic Valve Replacement
A.Background. Up to a third of patients with severe symptomatic AS do not undergo surgical AVR, as they are deemed to have a high surgical risk because of age or multiple comorbidities. The interest in percutaneous aortic valve implantation began in the early 1990s. The first human experience was reported by Cribier et al. in 2002. Since then, rapid advancements in the design of the stented valve and delivery catheters and improved facility in implantation techniques have led to a consistent improvement in the postprocedural outcomes and have heralded a new era in the treatment of valvular AS.
The procedure involves implantation of a tissue pericardial valve that is mounted within a stent. The key to TAVR success is a careful selection of patient population for the procedure and a judicious use of preprocedural and intraprocedural imaging modalities, including fluoroscopy and aortic angiography.
Currently, the indications for TAVR include severe symptomatic AS with a valve area of ≤0.8 cm2, mean aortic valve gradient of ≥40 mm Hg, or a peak aortic jet velocity of ≥4.0 m/s with one or more of the following:
1.High risk for conventional AVR (Society of Thoracic Surgeons [STS] score > 10 or logistic EuroSCORE > 20%) (https://www.sts.org/resources/risk-calculators)
2.Contraindication to standard thoracotomy including prior multiple thoracotomies or radiation to the chest wall
3.Surgical factors not accounted for by the STS score resulting in high surgical risk (i.e., porcelain aorta, cirrhosis, frailty, etc.)
B.Procedure. There are a number of valves available worldwide for TAVR, but only two are currently available in the United States and have been studied within randomized control trials: the balloon-expandable Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA) and the self-expanding CoreValve ReValving system (Medtronic Inc., Minneapolis, MN) (Fig. 64.2). The largest human experience is with the Edwards Lifesciences series of balloon-expandable aortic valves. The valve consists of a tubular stainless steel stent with a fabric valve cuff and contains valve leaflets derived from bovine pericardial tissue. This valve is available in four sizes: 20, 23, 26, and 29 mm. Valve sizing takes into consideration the annular area measured using 3D imaging (computed tomography [CT] or TEE) as well as various aortic root characteristics. Although the valve was implanted initially using the transvenous access and subsequent transseptal puncture, it is now routinely implanted using either a retrograde transfemoral approach or an antegrade transapical approach. The newest iteration of the commercially available SAPIEN series, the S3, is delivered via a 14F (20-, 23-, 26-mm valves) or 16F (29-mm valve) expandable sheath.
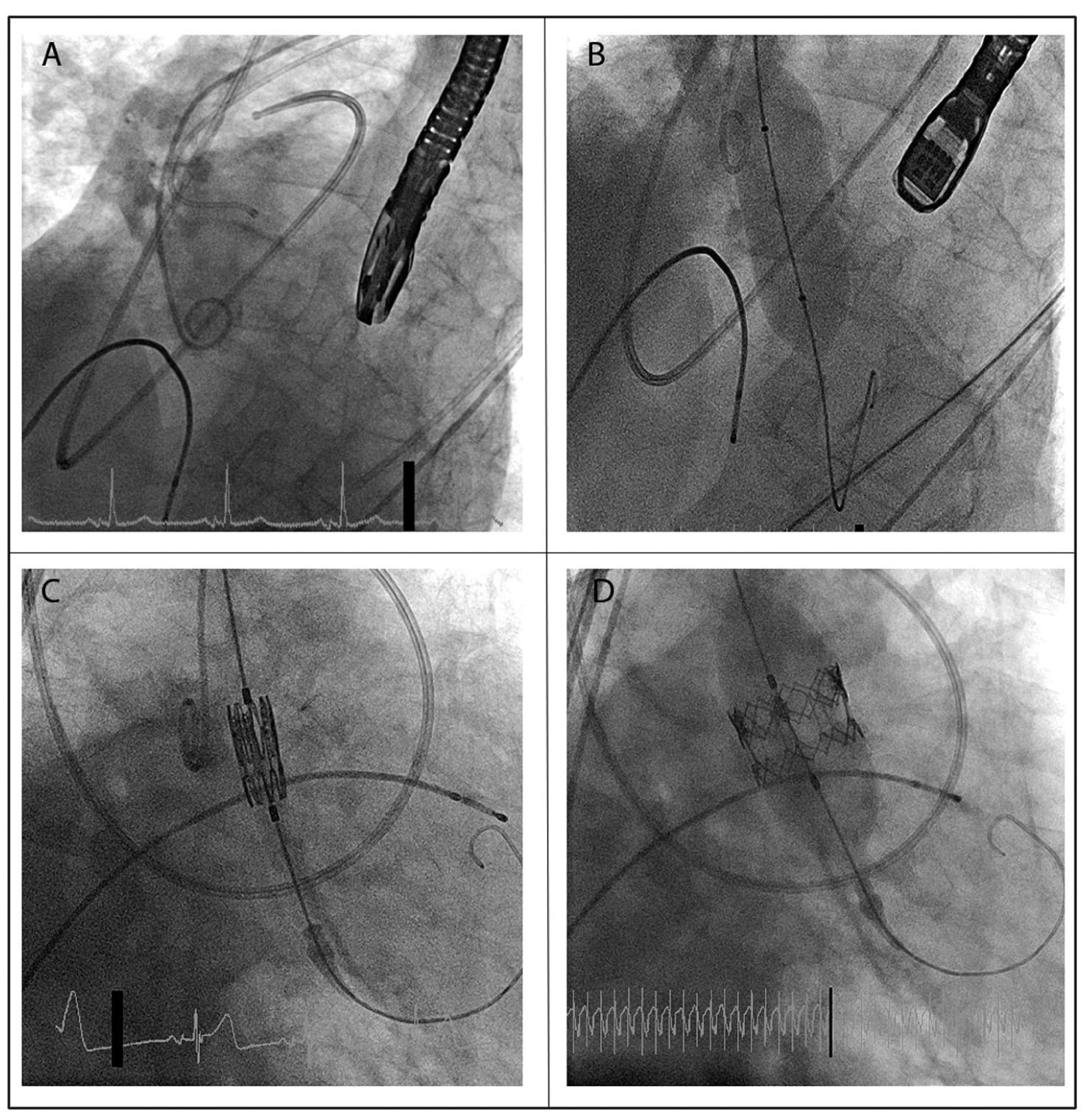
FIGURE 64.2 Transcatheter aortic valve replacement. A: Proper positioning of various catheters and devices for valve replacement: a transesophageal echocardiography probe in the esophagus, a Swan–Ganz catheter in the pulmonary artery, a pigtail catheter in the aortic root, an AL-1 diagnostic catheter in the ascending aorta (prior to valve crossing), and a temporary pacemaker wire in the right ventricle. B: Aortic valvuloplasty being performed using a standard balloon. C: Proper positioning of the crimped Edwards SAPIEN prosthesis across the aortic valve. D: Deployment of the valve using balloon inflation after initiation of rapid pacing.
The preprocedural assessment consists of TTE, iliofemoral contrast angiography or iliofemoral CT, 3D annulus imaging using CT angiography, TEE, or magnetic resonance imaging and coronary angiography. Appropriate patient selection, which includes the anatomic characteristics of the aortic annulus and iliofemoral system, is imperative to procedural success.
1.Transfemoral TAVR. Transfemoral TAVR is usually performed under general anesthesia or conscious sedation in a catheterization laboratory or hybrid operating room equipped with multimodality imaging equipment. Vascular sheaths are placed in a standard fashion in both femoral arteries and veins. A transvenous pacing wire is introduced into the right ventricle through the venous access port to enable rapid pacing (180 beats/min) in order to minimize cardiac output and valve movement during valve deployment. A BAV is often performed using standard technique, with a balloon that is slightly smaller than the size of the planned valve prosthesis to facilitate valve crossing.
Once the TAVR prosthesis is suitably positioned, the valve is deployed. This requires rapid pacing for the balloon-expandable valves, but is not necessary for self-expanding valves. The final positions of deployment as well as degree of paravalvular aortic regurgitation are assessed using echocardiography, hemodynamics, and aortography.
Inappropriate positioning may require a second valve deployment. Excessive paravalvular aortic regurgitation may be treated using further balloon dilation or deployment of a second valve. Echo and angiographic assessment for complications (including pericardial effusion, aortic root trauma, and coronary occlusion by the valve) are imperative prior to closure of the femoral access. Similarly, angiography of the iliofemoral system is necessary to evaluate for vascular trauma that might require endovascular or open surgical repair prior to completion of the procedure.
2.Alternate access for TAVR. Given the high incidence of peripheral arterial disease in patients with severe AS, several alternative access sites have been investigated and include the transapical, transcarotid, transsubclavian, transaortic, and transcaval approaches. The result of TAVR from these access sites is not as well studied as traditional transfemoral TAVR, and outcomes may not be as good.
C.Complications. Procedural success has ranged from 86% to 100% across the literature. After an initial learning curve, excellent procedural success rates with minimal intraprocedural mortality have been reported at major tertiary care centers performing TAVR. Vascular access complications are encountered more often (<10%) than with other interventional procedures because of the large sheath sizes used for TAVR. Complications associated with limited thoracotomy are expected after alternate access TAVR. Malposition of the aortic prosthesis may be encountered in about 1% to 3% of cases. Periprocedural strokes have been reported in 2% to 3% of patients undergoing TAVR with the newest generation Edwards S3 valve and about 3% with the CoreValve prosthesis. Major complications following CoreValve implantation include the need for permanent pacemaker implantation in up to one-third of patients.
D.Outcomes. The PARTNER 1 trial was the first multicenter randomized controlled trial of TAVR and provided two important randomized comparisons. The PARTNER 1B cohort showed a significant reduction in mortality (30% vs. 50% at 1 year and 72% vs. 94% at 5 years) in 358 inoperable patients with severe symptomatic AS after randomization to TAVR with balloon-expandable valve (Edwards SAPIEN) versus standard therapy including BAV. The PARTNER 1A cohort showed that outcomes of surgical AVR and balloon-expandable TAVR are comparable in high-risk patients, with no difference in 5-year rates of mortality and major clinical outcomes (including stroke, cardiac death, and myocardial infarction [MI]). A randomized control trial of self-expanding valves (CoreValve) showed superiority to surgical AVR in mortality at 1 year (14% vs. 19%), although the STS score was lower in this cohort compared with the PARTNER cohort (7.4% vs. 11.7%). The PARTNER 2 trial showed that balloon-expandable TAVR (Edwards Sapien XT) had comparable primary composite end points of all-cause death or disabling stroke (19% vs. 21%) at 2 years to surgical AVR in intermediate risk patients, with a suggestion that trans-femoral (TF)-TAVR patients fared better than SAVR. The S3 intermediate risk trial compared approximately 1,000 patients at intermediate surgical risk who underwent TAVR (88% TF approach) with a propensity-matched SAVR group from the PIIA trial. At 1 year, the TF-TAVR group demonstrated superiority over surgery with regard to the major end points of death (7.4% vs. 13.0%, p < 0.001) and stroke (4.6% vs. 8.2%, p = 0.004).
VI.PFO Closure
A.Background. PFO is a remnant of fetal cardiac circulation and is found in 27% to 33% of adults. Although generally believed to be an “innocent bystander,” PFO has been associated with stroke, migraines, platypnea-orthodeoxia, and decompression sickness among divers. PFO arises when the septum primum and septum secundum fail to fuse despite some degree of overlap. Atrial septal aneurysms and prominent Chiari networks have been associated with PFO. Percutaneous PFO closure is currently recommended in patients with platypnea-orthodeoxia and in divers with decompression sickness. Although several observational studies have shown the benefits of PFO closure in the prevention of migraine and cryptogenic strokes, these findings have not been confirmed in major randomized trials. Hence, controversy persists regarding definitive indications and settings for PFO closure for migraine and cryptogenic stroke prevention.
All PFO closure devices have a similar design, usually consisting of two atrial disks connected by a short neck. Under fluoroscopic and intracardiac echocardiography (ICE) guidance, the device is placed such that the two atrial disks lie on either side of the interatrial septum, with the neck positioned in the PFO tunnel. A large part of the atrial shunt is eliminated as a result of physical obstruction to flow. Most of the residual shunt is eliminated over the course of a few months after the procedure, as the device becomes endothelialized. Dual-antiplatelet therapy is generally recommended for the first 6 months after closure to minimize the risk of thromboembolism prior to device endothelialization.
B.Procedure. Several devices for transcatheter PFO closure have been tested in humans, including the Cardioform Septal Occluder device (WL Gore & Associates, Newark, DE) and the Amplatzer cribriform ASD occluder device (AGA Medical, Golden Valley, MN) (Fig. 64.3). Because these devices are Food and Drug Administration approved only for ASD closure, use for PFO closure is “off-label.” Sizing of the devices may vary based on the degree of associated atrial septal aneurysm; significant aneurysm generally requires a larger device.

FIGURE 64.3 Patent foramen ovale (PFO) closure using Amplatzer device. A: Balloon sizing of the PFO tunnel after crossing the PFO using a wire. The intracardiac echocardiography probe is visible next to the balloon. B: Deployment of the left atrial disk of the Amplatzer occluder device. C: Deployment of the right atrial disk of the Amplatzer occluder device. D: Device in place after its release from the delivery catheter.
Briefly, the procedure involves placement of an 8F to 12F vascular sheath (depending on device size) in one femoral vein and a 9F sheath in the contralateral femoral vein for ICE. The ICE catheter is parked in the right atrium for procedural visualization. The PFO is usually crossed using a wire and a Goodale-Lubin (GL) catheter under ICE and fluoroscopic guidance. Through the GL catheter parked in the left superior pulmonary vein, the wire is exchanged for a stiffer wire over which the closure device delivery system is advanced. The LA disk of the device is deployed and the system slowly retracted. This action facilitates apposition of the septum primum to the septum secundum and effectively closes the PFO. The right atrial disk is then deployed. After confirmation of the positioning and stability using ICE and fluoroscopy (and sometimes right atrial angiography), the device is released from the delivery catheter.
C.Complications. Transcatheter PFO closure is generally associated with a high procedural success rate and without significant risk of complication. Rare complications including device migration or embolization, symptomatic air embolism, and atrial fibrillation (AF) or chamber perforation with cardiac tamponade may occur intraprocedurally. Later complications associated with the procedure include thrombus formation (more common with CardioSEAL than Amplatzer), device erosion, or formation of a new ASD.
D.Outcomes. Several large observational studies have demonstrated the benefit of PFO closure in secondary prevention of migraine and prevention of recurrent neurologic thromboembolism in patients with cryptogenic stroke or transient ischemic attack. However, these findings have not been ratified in randomized clinical trials. The Migraine Intervention with StarFlex Technology study failed to demonstrate any significant difference in migraine cessation between the closure and the “sham” arms. The Closure of Patent Foramen Ovale versus Medical Therapy after Cryptogenic Stroke (RESPECT) trial randomized 980 patients with cryptogenic stroke to PFO closure versus medical therapy alone. The initial results of the RESPECT trial showed no difference in the primary composite end point of recurrent nonfatal ischemic stroke, fatal ischemic stroke, or early death on the primary intention-to-treat analysis, but there was statistical evidence of benefit to PFO closure in the secondary as-treated and prespecified per-protocol analysis with a reduction in event rate of 0.43 versus 1.38 events per 100 patient-years. Long-term results of the RESPECT study demonstrated a significant decrease in risk of recurrent cryptogenic stroke of 4.3% versus 1.5% with PFO closure.
VII.ASD Closure
A.Background. ASD is one of the most common adult congenital heart diseases encountered in the U.S. population. Although ASDs generally remain asymptomatic till early adulthood, they are associated with several clinical presentations, including right ventricular failure, atrial arrhythmias, pulmonary hypertension, and paradoxical embolism.
Patients with hemodynamically significant ASD should undergo elective closure either surgically or with the transcatheter technique, although in recent years, the transcatheter approach has become the first line of therapy in patients with appropriate secundum ASD anatomy. The hemodynamic significance of an ASD is determined by the presence of right ventricular enlargement in the presence of defect diameter >1 cm or the presence of significant left-to-right shunting with an elevated shunt ratio (Qp:Qs > 1.5).
The principle of ASD closure is similar to that of PFO closure, entailing implantation of an occlusion device with atrial disks lying on either side of the interatrial septum and the central waist residing in the ASD. Because of significant variations in size and anatomy of ASD, transcatheter ASD closure is technically more challenging than PFO closure.
B.Procedure. Several devices have been used for percutaneous ASD closure. The Amplatzer atrial septal occluder (AGA Medical, Golden Valley, MN) has the largest reported human experience. The other major device available is the Cardioform (WL Gore & Associates, Newark, DE).
The procedural approach to percutaneous ASD closure is similar to the percutaneous PFO closure approach as described above. A few important differences exist that are worth considering.
1.Because of a significant variation in the size of ASD and the shape of the defect, proper invasive balloon sizing (by ICE and fluoroscopy) is needed. Both oversizing and undersizing of the device may be hazardous and may lead to complications like device erosion or device embolization.
2.Device deployment and orientation during transcatheter ASD closure poses greater technical challenges than during PFO closure. The technical complexities are more frequent when ASDs are associated with other structural anomalies like atrial septal aneurysms or in cases of inadequate rim capture. It is imperative to demonstrate an adequate rim surrounding the defect for percutaneous closure.
3.Multiperforated septa demonstrate a particularly greater challenge to complete closure. In these cases, specific devices like the Amplatzer cribriform occluder or multiple devices may be needed to achieve a good clinical result.
4.The Amplatzer device is sized based on the defect size (i.e., 16-mm device for a 16-mm ASD) and can be used to close defects 2 to 38 mm in diameter. The Cardioform device is sized 1.7 times the size of the defect (i.e., 30-mm device for a 15-mm ASD) and can be used to close defects 2 to 17 mm in diameter.
C.Complications. As in transcatheter PFO closure, procedural success in experienced hands is high and complications are rare. Potential complications include device embolization, perforation as a result of device erosion into the atrial wall, residual shunting, or atrial arrhythmias. ASD device mismatch is encountered in 2% to 5% of cases. Other rare complications include vascular access complications, sizing balloon rupture, and entrapment of right atrial structures. Chest discomfort or new onset of arrhythmia may be clues to device erosion or embolization and should prompt urgent echocardiographic evaluation.
D.Outcomes. Most patients demonstrate improved functional class and exercise capacity after a successful closure. Several observational studies have demonstrated an improvement in overall long-term survival. Timely ASD closure prevents the development of right heart failure and pulmonary hypertension. There has been evidence of reduction in the size of enlarged cardiac chambers with normalization of intracardiac pressures after successful ASD closure.
VIII.Ventricular Septal Defect (VSD) Closure
A.Background. Although isolated VSDs are a relatively common form of adult congenital heart disease, congenital VSDs of hemodynamic significance are rare, with a prevalence of 0.03% in the adult U.S. population. More commonly, VSDs are acquired as a result of complication of MI, cardiovascular surgery, or chest trauma. Postinfarction ventricular septal rupture (VSR) is associated with >90% mortality if left untreated. The postinfarction or traumatic VSRs may be located in the posterobasal (inferior infarct) or apical (anterior infarct) portions of the muscular ventricular septum and are typically irregular with multiple ruptures in necrotic myocardium that are prone to rupture or expansion. This presents significant challenges with respect to surgical as well as transcatheter repair.
Percutaneous VSD/VSR closure involves devices that are similar to those described for ASD closure. The device is positioned under fluoroscopy and echocardiographic guidance (TEE and/or ICE as needed) such that the two disks lie on either side of the ventricular septum and the waist lies in the septal defect. The width of the connecting waist determines device sizing.
B.Procedure. The only available device that is approved for closure of congenital muscular VSDs is the Amplatzer muscular VSD occluder device (AGA Medical, Golden Valley, MN). There is a specially designed Amplatzer postinfarction muscular VSR occluder device, which is currently under investigation in the United States. It is similar to its congenital counterpart, except for a longer waist, large disk diameter, and a larger waist diameter. In patients with postinfarct VSR, it may be necessary to use an atrial septal occluder, as the distance between the disks of the VSD occluder device may be too large. Again, this would be considered an “off-label” usage.
VSD closure can be achieved via the retrograde approach from the left ventricle or via the femoral or jugular vein. The VSD is crossed using a diagnostic catheter and wire, with the wire then externalized to the artery (if approached from the right ventricle) or vein (if approached from the left ventricle) to create a continuous arteriovenous (AV) loop. A delivery catheter containing the device is subsequently delivered over the loop. Under fluoroscopic and echocardiographic guidance, proper alignment and position are confirmed and the device is subsequently deployed.
C.Complications. The major complications of transcatheter VSD closure include device embolization, air embolism, residual shunting, defect enlargement, complete heart block requiring pacemaker implantation, arrhythmias, valvular regurgitation because of impingement, and intravascular hemolysis.
D.Outcomes. The results of transcatheter closure of congenital VSDs are encouraging; however, the closure of postinfarction VSR has yielded disappointing results in several studies. Although surgery has been recommended as the gold standard for the repair of postinfarction VSD, emergent surgical repair carries an operative mortality of about 50%. This often leads surgeons to recommend delaying the surgery for at least 2 weeks after the initial ischemic event to improve the tissue integrity for sutures/patches and results in a lesser operative mortality and improves the chance of success. Because of this, several centers have attempted transcatheter VSR closure in patients who are either not surgical candidates or too unstable to survive the “waiting period.” Data from observational studies have reported variable success, with mortality rates ranging from 20% to 100%.
IX.LEFT ATRIAL APPENDAGE Occlusion (LAAO)
A.Background. AF is associated with 15% of all ischemic strokes. Autopsy studies have shown that up to 90% of the thrombi in nonvalvular AF originate in the LAA. Although warfarin has been demonstrated to reduce the rate of stroke in patients with AF, there are several limitations, including risk of bleeding, pharmacologic interactions, and need for prothrombin time (international normalized ratio) monitoring. In several patients, the use of warfarin may be contraindicated, necessitating another method of stroke prevention.
Surgical amputation and exclusion of the LAA have been demonstrated to reduce the risk of stroke after MV surgery, without any significant impact on the rate of postoperative AF. Given the favorable location of the LAA for percutaneous closure, a number of methods have been developed.
B.Procedure. The two devices with the most significant human experience available for LAAO are the Amplatzer Cardiac Plug (ACP) (AGA Medical, Golden Valley, MN) and the WATCHMAN device (Altritech Inc., Plymouth, MN). The WATCHMAN and ACP devices have CE Mark approval, whereas only the WATCHMAN is commercially available in the United States. The size and the shape of the LAA is most commonly assessed using TEE, although modified discrete cosine transform analysis is often helpful in sizing and understanding the morphology.
The devices are generally implanted using 14F femoral venous access. A transseptal puncture is performed under fluoroscopic and TEE guidance. The device delivery system allows for collapse, repositioning, or removal of the device in case of unsatisfactory result. Adequacy of the LAAO (defined as mild to absent leak) is confirmed using radiopaque contrast injection into the LA cavity.
C.Complications. The complications of the procedure include pericardial effusion, cardiac tamponade, residual leakage, or major vascular complications requiring transfusion.
D.Outcomes. The two major randomized trials of LAAO versus anticoagulation therapy are the PROTECT-AF and PREVAIL studies. In totality, the trials demonstrate at least equivalence of the two strategies with respect to stroke outcomes. Long-term (4-year) outcomes from PROTECT-AF demonstrated superiority in the primary efficacy outcome of stroke/death/systemic embolism in comparison with warfarin. There have been no major randomized trials of the novel oral anticoagulants compared with the WATCHMAN. In clinical practice, and based on the current trials, the WATCHMAN represents a good alternative to anticoagulation for patients who are intolerant of anticoagulation or at high bleeding risk.
X.Alcohol Septal Ablation (ASA). Septal myectomy (SM) is considered the historic gold standard for the treatment of symptomatic left ventricular outflow tract (LVOT) obstruction in patients with hypertrophic cardiomyopathy. ASA has emerged as an attractive, less invasive alternative to myectomy that is applicable to those patients with favorable anatomy for ASA who cannot or do not want surgery. Over the last 15 years, the number of ASA procedures done worldwide has surpassed the total number of myectomies performed in the last 50 years. ASA may be considered in patients who have clear evidence of septal hypertrophy (>18 mm) projecting into the LVOT, patients who have dynamic LVOT obstruction (gradient >50 mm Hg at rest or upon provocation), and patients who have been refractory to medical therapy. Patients with coexistent abnormalities of the mitral apparatus are best treated by surgery rather than ASA.
ASA entails creating a controlled myocardial necrosis by injecting alcohol into the septal perforator that perfuses the area of obstruction-inducing hypertrophy. Postablation, the LVOT gradient demonstrates a triphasic response. There is an immediate reduction in the LVOT gradient after a successful procedure, attributable to the loss of septal contractility and associated systolic anterior motion of the MV. Over the next few days, there may be an increase in the LVOT gradient because of recovery of the stunned myocardium and edema of the tissue. The more permanent decrease in LVOT gradient happens over the course of the next few months secondary to myocardial thinning and septal remodeling.
A.Procedure. ASA is performed via dual arterial access. Because transient heart block is common during the procedure, an active-fixation temporary pacemaker is inserted via the internal jugular vein and left in place for 48 to 72 hours during inpatient observation for conduction deficits. A pigtail catheter is placed in the LV to allow LV, aorta gradient measurement during the procedure. A guiding catheter is introduced into the left main coronary artery and a guidewire advanced to the septal perforator of interest. Subsequently, a short “over the wire” (OTW) balloon is advanced to the septal perforator and inflated to obstruct backflow of alcohol from the septal to the left anterior descending artery. Angiographic contrast is injected through the balloon to ensure that there is absolutely no backflow into the LAD.
Echocardiographic contrast is then injected through the OTW balloon in order to map the myocardium that would infarct as a result of ASA. This is an important step, because it helps determine the appropriateness of the procedure and helps in selecting the optimal branch for alcohol injection. Subsequently, 1 to 2 mL of alcohol is injected slowly through the balloon based upon the degree of gradient reduction. The balloon is then deflated and removed.
B.Complications. Besides the routine vascular complications that may arise in any interventional procedure, new-onset right bundle branch block is a significant complication of this procedure and is reported in up to 50% of patients in some series. Complete heart block requiring a need for a permanent pacemaker may occur in about 10% of patients undergoing ASA. The other potential long-term complication includes ventricular arrhythmias, hypothesized to arise as a result of creation of arrhythmogenic myocardial scar. Recent meta-analyses have failed to demonstrate significant differences in ventricular arrhythmias between myectomy and ASA.
C.Outcome. Although there are no randomized comparisons between SM and ASA, large meta-analyses of observational data indicate no significant differences between the two modalities in terms of mortality and improvement in functional status postprocedure. The caveat of ASA is an increased incidence of conduction abnormalities requiring permanent pacemaker implantation, which is necessary in approximately 10% to 15% (a two- to threefold higher rate than patients undergoing SM). Whereas residual gradients are slightly higher with ASA, functional benefit remains similar. Ultimately, patient selection for ASA requires confirmation of appropriate LVOT and coronary anatomy and a conversation among interventionalists, cardiac surgeons, and patients regarding the relative risks and benefit of ASA versus SM for a given patient.
XI.Percutaneous Paravalvular Leak Closure
A.Background. PVL is a rare but serious complication that may arise after surgical MVR or more rarely after SAVR. Although most PVLs are small and remain relatively asymptomatic, more than 10% of patients undergoing MVR or SAVR may develop large symptomatic PVLs with serious clinical consequences such as heart failure, endocarditis, or hemolytic anemia. Many PVLs become clinically apparent within 1 year after valve surgery, but a significant proportion may either present later or not require any definitive treatment till later. Several risk factors, including reoperation (on the same valve), extensive annular calcification, evidence of endocarditis, large atria, renal failure, and older age, have been identified that may predispose a valve to develop PVL postsurgery. Unfortunately, reoperation for PVL is associated with increased morbidity and mortality. Transcatheter closure of symptomatic PVL in a relatively small number of high-risk patients has been attempted in several centers over the last two decades. The devices approved for closure of other intracardiac defects have been utilized in an off-label fashion for transcatheter PVL closure.
B.Procedure. In order to deploy an occlusion device across a PVL, it is first necessary to cross the defect with a delivery catheter. Aortic PVLs are generally crossed in a retrograde fashion from the femoral artery. The mitral PVL is most commonly crossed in the antegrade fashion by advancing a catheter from the right atrium into the left atrium via transseptal puncture, sometimes via direct transapical access, and rarely in a retrograde manner via the femoral artery. Similar to percutaneous VSD/VSR closure, creation of an AV loop is helpful to form a supportive rail for the passage of equipment, but may not be necessary if the delivery sheath passes easily with the aid of a stiff wire either in the LV or advanced to the descending aorta. Once the delivery sheath is advanced across the defect, the plugging device is unsheathed into place. Multiple plugs may be placed until adequate closure is demonstrated by adjunctive ICE and/or TEE imaging.
C.Complications. Immediate and delayed device-related complications have been described as a result of technical failure. The early technical failure happens because of device impingement on nearby critical structures, and the delayed technical failure happens as a result of device embolization. Persistent PVL and/or its associated sequelae (i.e., CHF and hemolytic anemia) may be encountered after PVL closure. Although no procedure-related deaths have been described in any series, rare instances of strokes, dysrhythmias, and cardiac perforation have been described.
D.Outcomes. Transcatheter PVL closure is one of the most challenging structural heart disease interventions. The long-term outcomes after transcatheter PVL closure largely depend upon surmounting the limitations imposed by the current nonspecific devices for PVL closure, as well as challenges in imaging the area of interest. Over the long-term follow-up described in a few relatively large case series, resolution of hemolysis was reported in 60% to 83% of patients, improvement in CHF symptoms was reported in 50% to 100% of the patients, and repeat surgery was required in only 4% to 18% of the individuals. The incidence of long-term mortality has ranged from 25% to 30% over 3- to 36-month of follow-up across various studies.
ACKNOWLEDGMENTS: The authors thank Dr. Shikhar Agarwal for his contributions to earlier editions of this chapter.
SUGGESTED READING
Agarwal S, Tuzcu EM, Desai MY, et al. Updated meta-analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;55:823–834.
Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–100.
Feldman T, Young A. Percutaneous approaches to valve repair for mitral regurgitation. J Am Coll Cardiol. 2014;63:2057–2068.
Leon MB, Smith CR, Mack M, et al. Transcatheter or surgical aortic valve replacement in intermediate risk patients. N Engl J Med. 2016;374:1609–1620.
Nobuyoshi M, Arita T, Shirai S, et al. Percutaneous balloon mitral valvuloplasty: a review. Circulation. 2009;119:e211–e219.
Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation. JAMA. 2014;312(19):1988–1998.