Chapter 2
Ethical Considerations around using Animals in Research
History of the Use of Animals in Research
From ancient times, people have strived to understand the workings of nature and the universe. Animal use in science dates back to Eristratus and Herophilus in the third century BC and Galen in the second century AD1. Roger Bacon published Opus Majus in 1265, in which he noted the causes of error: authority, custom, popular prejudice and the concealment of ignorance with the pretence of knowledge. He pointed out that the two methods of acquiring knowledge are argument and experience. He asserted that argument alone is not enough, for ‘the strongest argument proves nothing so long as the conclusions are unverified by experience’. Bacon's insistence on the gathering of data is one of the hallmarks of modern science.
The birth of modern science dates to the year 1543 and the publication of Copernicus' De Revolutionibus Orbium Coelestium (On the Revolutions of the Heavenly Spheres) and Vesalius' De Humani Corporis Fabrica (On the Fabric of the Human Body), which challenged systems of belief dating back to the second century. Previously, medicine had been largely based on the teachings of Galen, whose knowledge of human anatomy was largely deduced from animal dissections (the dissection of human bodies was generally not accepted in ancient times), and whose treatment of disease was based upon the doctrine of the four bodily humours. Vesalius performed detailed dissections of the human body and spotted inaccuracies in Galen's descriptions. In challenging them he destroyed the foundation of medieval medical practice, which, like astronomy, was based upon ancient tradition and inherited knowledge. Ground-breaking experiments that demonstrated the circulation of the blood2 would not be countenanced today owing to their method in animal use, yet underpin modern cardiovascular physiology.
As the use of animals in research increased, so too did opposition to vivisection. In 1789, utilitarian philosopher Jeremy Bentham wrote of animals ‘the question is not, can they reason, nor can they talk, but can they suffer?’, opening the debate on the ethics of animal use in experiments. Legislation governing the protection of animals has been in existence for many centuries. In the UK laws were passed against cruelty to animals late in the 17th century. In 1822, Martin's Act (after its sponsor Richard Martin) was passed, and primarily protected cattle and horses (it did not include cats, dogs or birds). It was amended in 1835 to protect all domestic animals. A Society for the Prevention of Cruelty to Animals was founded in 1824. Princess Victoria extended her patronage to the society in 1835, and it became the Royal Society for the Prevention of Cruelty to Animals (RSPCA). The subsequent rise of the anti-vivisection movement encouraged the development of self-regulation. In 1831 British physiologists attempted self-regulation with the publication of Marshall Hall's five principles, which were:
- an experiment should never be performed if the necessary information could be obtained by observations,
- no experiment should be performed without a defined, obtainable, objective,
- scientists should be well-informed about the work of their predecessors and peers in order to avoid unnecessary repetition of an experiment,
- justifiable experiments should be carried out with the least possible infliction of suffering (often through the use of lower, less sentient animals),
- every experiment should be performed under circumstances that would provide the clearest possible results, thereby diminishing the need for repetition of experiments.
Hall also proposed that results should be made available for public scrutiny. These principles bear a great deal of similarity to the three Rs, published 128 years later, and to current legislation. In 1871 the British Association for the Advancement of Science developed a moral code of practice but regrettably neither this, nor Hall's proposal, were readily adopted. In August of 1874 the use of two dogs in an experimental demonstration of epilepsy created uproar at a meeting of the British Medical Association in Norwich. At the meeting there were vociferous protests against the experiments, which were performed by the French physiologist Eugene Magnan. At one stage the president of the Royal College of Surgeons of Ireland cut the bindings holding one of the dogs and released it. The RSPCA later took Magnan and the three Norwich doctors who had arranged the demonstration to court, accusing them of unnecessary cruelty to the animals. In this first prosecution against the use of animals in experiments, the Norwich men were found not guilty because they did not perform the demonstration, and Magnan had returned to France. Nevertheless, the magistrates granted that the RSPCA was justified in bringing the action, and declined to award defence costs.
With opinions polarising, increasing levels of violent protests, especially since the 1960s, reinforced a bunker mentality as laboratories using animals closed their doors to visitors. Some breeding centres were forced to close, with the unintended consequence of increased transport times for animals3. The principles of Reduction, Refinement and Replacement (the three Rs) in animal experiments, proposed by Russell and Burch4, took time to gain their now almost universal acceptance and for necessary improvements to follow, but the principles of the three Rs are implicit in the Animals (Scientific Procedures) Act 1986. All UK scientists are therefore legally obliged to use alternative approaches to the use of animals where possible, to use the minimum number of animals and to use protocols which cause the least pain, suffering or distress. The National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) was established in 2004 and is an independent scientific organisation, tasked by the UK Government with supporting the UK science base through the application of the three Rs. It is also the UK's largest funder of three Rs research. The NC3Rs works in partnership with bioscience research funders, academia, industry, regulators and animal welfare organisations, both in the UK and internationally, to advance the three Rs. However, the welfare of laboratory animals continues to be strongly debated. Most observers agree that constructive dialogue has increased the quality of both animal welfare and research output, whereas simple hostility has been counterproductive. Attitudes to laboratory animals will undoubtedly alter in the future as our moral and cultural perspectives change.
The Ethics of Using Animals in Experiments
The word ‘ethics’ is used in many contexts, often incorrectly, leaving the reader confused as to its meaning. For the purposes of this book, it is an examination of the acceptability of the motives that drive the behaviour of people5, whether they are scientists who use animals or people who stand up for animal rights. All use of animals for human benefit creates a dilemma. There is a spectrum of opinion on whether there is justification for using animals for our own ends: some people believe that animals have rights and it is wrong to use them, whether for food, in research, as beasts of burden, as pets or to kill them as vermin. Another group believes it is acceptable for humans to use animals in any way we think fit. Most people fall somewhere in between these two extremes.
The philosophical case for unimpeded use of animals for research is usually based around a concept of the superiority of humans over other species. When the research is for medical purposes the moral obligation of humans to prevent the suffering of other humans is also cited. Others argue that all living creatures have inherent value and hence moral rights. It would seem contradictory that a species could be similar enough to humans for experimental data to be useful, yet different enough for any suffering to be morally acceptable. Further, the moral status of a ‘subject of a life’ would include the right never to be treated merely as a means to the ends of others6. The justification for using animals in biomedical research depends on them being different from humans, with a reduced capacity to suffer (this in itself is the subject of much debate), whereas the validity of the results obtained depends on their physiological similarity to humans. The debate about animal experimentation has waxed and waned over the decades but it has never gone away, and some aspects of the debate are essentially unchanged from those used a century ago. An important factual point is whether or not animal experimentation has been essential in the development of important medical advances. Many abolitionist groups claim that animal research has never resulted in any medical benefits, that it has misled scientists, or that it is unnecessary because we have alternative non-animal techniques. Most doctors and scientists however, agree that animal research has played a major part in medical advances in the past and will continue to do so. Most researchers would like to see alternatives to animals developed, but most believe that animal research will continue to be necessary, at least for the foreseeable future. Other campaigners claim that animal research is cruel. There is no doubt that over 100 years ago it was cruel, before the advent of modern anaesthetic and analgesic drugs. But today things are very different: advances in veterinary science have made painless surgery possible, and everyone involved in animal research is very much aware of the need to maintain animal welfare. Research using animals can only be authorised provided the benefits outweigh the harms to the animals under a utilitarian framework with some additional restrictions. For example, work on developing cosmetic or tobacco products is not considered acceptable, no matter how much ‘benefit’ may be derived from the work. Similarly, work on great apes will not be authorised in the UK, for whatever purpose.
There is general agreement in the scientific community that progress through animal experimentation is expensive, bureaucratic and subject to animal-induced variability and that, aside from any ethical issues, it is rarely the method of choice. Extrapolation between species is a common cause of reducing confidence in experimental conclusions. However, these constraints alone are not enough to ensure that animals are only used when essential. Any justifiable use of animals must include an acceptance that animals are not merely tools. For many biomedical advances, animal research has indisputably been part of the pathway to progress, but that is not the same as saying that animal research was necessary for that progress. Indeed, some would say that advances have been made despite animal research. The crucial question is, how great are the benefits that only animal research can deliver?
Scientists who use animals would argue that an ability to balance or set aside the short-term price of agreeing to cause harm to animals for a long-term gain that is judged to be greater demonstrates moral responsibility, as they are acting as moral agents7. However, in order to perform statistically sound experiments animals usually need to be ‘grouped’ and this has an unintended effect of ‘depersonalising’ the group's members. In addition, when animals might suffer there is a natural tendency towards emotional detachment on the part of handlers and experimenters alike, for the protection of their own emotions. Both of these tendencies should be resisted in good laboratories by remembering the principle that ‘good welfare results in good science’ and must be based on understanding the individual as well as the group. Naturally, anti-vivisectionists find these arguments hard to accept.
Porter8 suggested that research scientists should follow Schweitzer's principle of ‘respect for all life’ and become anti-vivisectionists at heart. This apparent contradiction would act as an internal regulator of experimental design under the guidance of ethical tool kits, one of which he proposed. The three Rs of reduction, refinement and replacement have grown to become a popular shorthand against which to judge ethical tool kits, and are discussed in more detail below.
One consequence of the need for experimental planning is the creation of a convenient framework for assessment of likely harms and potential benefits. This has been seized upon by all sides of the debate, apart from abolitionists, as a means to judge the merits of any given proposal. The resulting mechanisms for practical ethical appraisal are more advanced than in most other spheres of veterinary work. For example, the ethical framework around using prosthetic limb replacements for companion animals is in an early stage of construction9.
The majority opinion today is a reluctant acceptance of the need to use animals in certain circumstances (although these differ markedly between individuals), and a wish for independent regulation such that scientific users of animals are answerable to society at large. The argument turns on whether society is prepared to set aside current and future benefits that may arise from animal research on the basis of a moral obligation to the species used. The philosophy that best describes this balance of public sentiment is utilitarianism. Crucially, utilitarianism does not rule out any harms as a matter of principle. However, parts of the regulatory framework of both the UK and EU override utilitarianism in an attempt to mirror majority public opinion of ethical ‘lines in the sand’. These elements arise from the philosophy of deontology which argues that some actions should be seen as moral duties to be adhered to whatever the consequences. A problem with mixing these two approaches is knowing when to apply each principle and in particular how to manage cases on the margins. This may be illustrated by the following examples.
- At what point in the phylogenetic tree should animals fall under ethical considerations and legal protection? EU Directive 2010/63, reflected in UK law, protects all living vertebrates and cephalopods. This suggests that these species matter whereas the remainder do not. Other species considered for inclusion during drafting of the Directive included decapod crustaceans, some spiders and insects such as honey bees. Complexity (number of synapses) of the brain is not always a helpful measure of sentience or suffering10 and the evolutionary tree has many examples of convergent as well as divergent evolution. Natural selection produces species that are best fitted to their environment, not those that are the most sentient. The idea that there is a link between degree of evolutionary development and capacity to suffer is a gross over-simplification, but is used as a basis for regulation. A purely ethical approach to regulation might be to confer the benefit of the doubt for any species where sentience is disputed.
- During the drafting of EU Directive 2010/63 calls were made for a ban on experiments involving severe or prolonged suffering, irrespective of the perceived need or justification. In effect, the harm/benefit balance would have deontological limits applied. Problems with this approach include how to define ‘severe and prolonged suffering’ and how to act in the unlikely event of overriding need, such as a lethal human pandemic. In Directive Article 15 it states that ‘a procedure is not performed if it involves severe pain, suffering or distress that is likely to be long-lasting and cannot be ameliorated’ but again this is subject to the force majeure arguments of Article 55 which allows the ‘line in the sand’ to be circumvented.
- Selling cosmetics that have been tested on animals was banned throughout the EU in 2009. The reduction in the use of animals for this purpose has been widely welcomed. However, a difficulty arises on the margins of defining a cosmetic. Substances such as botulinum toxin have a licence for only medical use but are also used for primarily cosmetic purposes; batches have to be tested for potency using animals, regardless of their end use. Should sunblocks, which reduce the risk of skin melanomas, be classed as cosmetics or medicines?
- Special protections are offered under UK law for research dogs, cats, equids and non-human primates. The reason is more to do with the special emotional attachment that humans have for these species than any scientific grounds. No special protections are offered for species with probably overlapping degrees of sentience such as pigs, sheep, dolphins, foxes or even crows. Should not all sentient species be protected equally?
Once deontological exclusions have been applied, the remaining uses of laboratory animals are usually judged according to principles of utilitarianism, a development of consequentialist reasoning. Consequentialism argues that the value of an action is determined by its outcome. To go forward, the harms and benefits of any action need to be known and compared and the balance of benefit over harm should be greater than any other feasible option. Utilitarianism, espoused by philosophers such as Bentham, further argues that there is a moral duty to maximise the balance of benefits to harms7. Within laboratory animal use this has become the cornerstone of ethical judgements. The system has the perceived advantage of each proposal receiving case-by-case attention with no preconceptions.
An objective harm/benefit analysis must multiply the value of a hoped-for benefit by the likelihood of achieving it, before weighing that estimate against the predictable harms. However, a major problem for any utilitarian analysis is that the (anticipated) benefits and harms are measured in different units; the old problem of comparing apples and oranges. This is part of the problem of seeking to make ethics a matter of quantification. In the light of this difficulty the ethical review process in the UK requires the presence of a number of experts plus lay members to provide balance and increase public confidence11. This variety of experts can help a committee to appreciate the variety of harms and benefits involved, and offset any crude attempt at precise quantification. Potential benefits are easy to over-estimate. It is difficult for proponents of a particular project to remain objective, particularly when career progression is involved. Harms may be easier to predict but are difficult to measure. Limiting harms effectively is directly dependent on local standards of animal care put in place by management and the competence of staff to adhere to those standards.
The Local Ethical Review Process
Since 1 April 1999 all designated establishments have been required to implement a local ethical review process, or ERP. Under the UK Animals (Scientific Procedures) Act an establishment is required to have an Animal Welfare and Ethical Review Body. The aims of the process are to provide independent ethical advice to the holder of the Section 2C licence, to provide support to the animal care staff and designated veterinary surgeon and to use ethical analysis to increase awareness of animal welfare issues and develop initiatives to lead to the widest possible application of the three Rs. This makes sure that all use of animals at the establishment is carefully considered, that all possibilities for reduction, refinement or replacement are taken into account and that high standards of accommodation and care are achieved. The structure of the ERP should be designed to suit the size and nature of the establishment, and may consist of committees, e-mail discussion groups, project-refinement groups or other formats, all of which should encourage local consideration of ethical issues. In general, as many people as possible should be involved in the ERP, to widen the debate as much as possible. The animal care staff, designated veterinary surgeon and representatives of the users should also be included. The ERP should include people who do not use animals, and lay people from outside the institution may also be appropriate members. The local ERP provides a mechanism to help holders of a Section 2C licence to meet their responsibilities and encourage wider local involvement in addressing issues surrounding animal use. The inclusion of lay people from the local community among the membership when debating the issues may go some way toward satisfying the demand for public accountability of what research should be allowed to be performed on animals in the name of the public interest. The holder of the Section 2C licence has to decide not whether the proposed work can be done, but whether it should be done.
Measuring harms and benefits
Measuring benefits
Predicting beneficial outcomes of experiments before they have been conducted is fraught with difficulty. If outcomes were known in advance then experiments would be redundant. The nature of scientific enquiry depends more upon testing the reliability (or falsifiability) of a hypothesis than proving unequivocal truths12. However, scientific history is full of instances when progress in fundamental knowledge, using animals, has been shown after the event to have been crucial for advances in medicine and human quality of life. Treatments for life-threatening diseases such as polio, diabetes and smallpox are often cited. Some of this progress might have been achievable by other means, but we cannot know this.
The funding for research using laboratory animals arises from three main sources: government grants (national or international), charities and the biomedical and chemical industries. The first two categories usually require peer-reviewed grant applications, a process which examines claims of potential benefits. However, industry-driven research ultimately requires a return on investment, which to some extent skews the selection of research area as well as placing demands on efficiency.
Measuring harms
The modern concept of welfare, which is now promoted by the Farm Animal Welfare Council13, is that of a life worth living, from the animal's point of view, or, even better, a good life. A judgement of the quality of life of experimental animals against the benefits to other animals, or humans, is the basis of the harm/benefit analysis. It is much more than simply the prevention of cruelty: quality of life should be used for decision-making and to do this a quality-of-life balance sheet can be prepared14. Proper welfare assessment must be carried out. For more detail on this, see Chapter 4.
Individual measures of welfare are most valuable when considered in conjunction with other welfare-related parameters. The welfare assessment matrix15,16 combines the clinical condition of the animal, behavioural deviations, the duration of the incident and the quality of the environment and shows how a grid can illustrate the temporal component of suffering. Since it is cumulative suffering that matters to the animal's quality of life, as much as single incidents, the points of suffering can be added up over a period of time to determine when a limit has been reached. The matrix includes contingent as well as direct suffering to truly reflect cumulative harms and allows an evaluation of the animal's quality of life. It can demonstrate the true welfare implications of research and the effect of refinements, both at the planning stage and when reviewing finished work, to ensure that harm/benefit scoring remains valid, and by the inclusion of a factor that reflects the justification can provide a weighting for the cumulative impact of the suffering incurred.
The Principles of the Three RS
The successful application of the three Rs has improved the use of laboratory animals and in some areas enabled progress without any animal use at all. EU Directive 2010/63 makes explicit reference to the three Rs and requires that ‘member States should contribute through research and by other means to the development and validation of alternative approaches’.
Regulated procedures using animals involves inflicting potential harm on them. The motive for this is usually to derive some benefit to humans or other animals. For research to be conducted in an ethical manner the researcher should analyse the motives behind the research: is it being done for a good enough reason, and could it be done any other way? Therefore before embarking on an experiment involving animals it is important to consider the questions in the flow chart in Figure 2.1, to apply the three Rs, and at all times throughout the experiment, consider what could be done to decrease the potential for suffering inflicted on the animals, and to maximise the likely benefit to be derived.
FIGURE 2.1 Application of the three Rs.
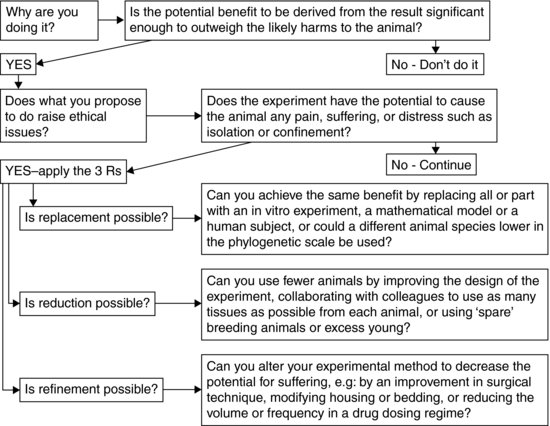
Replacement
Alternatives are often argued not to be ‘the real thing’, but neither are live animal models. In the field of toxicity testing, the US National Academy of Sciences17 has put forward a vision of replacement using high-throughput in vitro screening assays, tests in ‘lower’ organisms, systems biology, functional genomics and transcriptomics as well as predictive in silico approaches. Practical moves towards replacement among regulatory authorities include the Organisation for Economic Co-operation and Development (OECD) guidelines18 accepting the use of artificial human skin as a replacement for living skin in the testing of chemical irritancy. Groups such as the European Centre for the Validation of Alternative Methods (http://ecvam.jrc.it/), the European Partnership for Alternative Approaches (http://ec.europa.eu/enterprise/epaa/), the Centre for Alternatives to Animal Testing – Europe (http://caat.jhsph.edu/about/_includes/index_eu.html) and the In Vitro Testing Industrial Platform (www.ivtip.org/) have risen to prominence in recent years as impetus (and funding) has increased the rate of discovery and application of alternatives to chemical and drug safety testing.
Reduction
When animals cannot be replaced, proper planning of experiments is required in order to produce reliable data with the fewest and most suitable animals. The application of statistical methods such as power analysis and blocking, and the use of inbred strains of animal to reduce variation have been advocated for many years19 with some success. Yet it remains frustrating that surveys of published data continue to show improper designs or a lack of uptake of these ideas20.
Refinement
Refinement can improve welfare by acting on both direct and contingent suffering. The search for refinement must be continual to take advantage of technological advances.
Academic research tends to be very focused into narrow areas. Scientists are strongly driven to do high-quality research in their field, but may lack similar drive when evaluating the influence of methodology on the scientific quality of their results or on the welfare of animals used. The implementation of the three Rs is seen almost as a separate subject area, whereas it would be better if seen as a normal part of the research package and integrated into it. Within the pharmaceutical sector, strong corporate drivers for consistency and harmonisation within global organisations can be used to bring about refinement (e.g. housing improvements) and in an increasingly tough external environment, a strong focus on corporate reputation can also bring useful leverage to welfare initiatives.
Some current examples of refinement include
- the almost universal application and continuous updating of anaesthesia and analgesia methods,
- a refined non-surgical technique involving trans-cervical instillation of genetically altered embryos into the uterus of surrogate recipients, potentially rendering laparotomies redundant for many thousands of mice21,
- clear evidence that the usual method of capturing and picking up mice (by the tail), results in anxiety compared with cupping, and may impinge on welfare22.
A key requirement for the three Rs is sharing accurate data. There remains a reluctance from scientists and publishers to use print space to report negative results or experimental ‘failures’, which, if overcome, would avoid needless repetition23. Sufficient detail on animal care for experiments to be reliably repeated without errors is often lacking24. The Animals in Research: Reporting In Vivo Experiments (ARRIVE) guidelines are a welcome initiative designed to correct this deficiency25.
Prospective, ongoing and retrospective review: reflective practice and the refinement loop
Refinement is an iterative process, which begins with a critical evaluation of practice, leading to recognition and assessment of poor or suboptimal welfare, identification of the causes of this, selection of improvement strategies and implementation of these strategies. Any changes then have to be evaluated for their efficacy; thus the process begins again, forming the refinement loop (see Figure 2.2)26.
FIGURE 2.2 The refinement loop26.
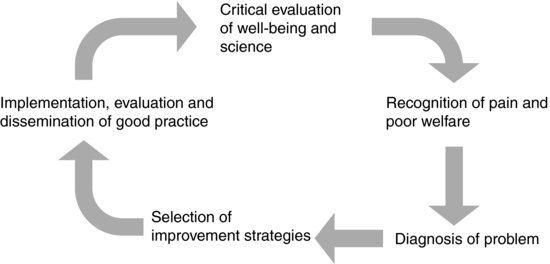
Critical evaluation of practice
Researchers have to look critically at what they do and identify whether there is a better way of conducting the research that will minimise welfare implications and maximise the scientific output, thus improving the harm/benefit balance and the justification for doing the work. It is particularly important that this is seen as an ongoing requirement: new developments in refinement may turn what was yesterday's best practice into today's outdated methodology.
Objective assessment of animal welfare and scientific quality
This requires first and foremost an understanding of well-being and what particular animals need. Only then is it possible to identify appropriate parameters to assess well-being and scientific quality in a meaningful manner. Sufficient resources have to be provided to perform the assessments effectively. This requires the researcher to schedule observations appropriately to maximise the likely detection of problems, to recognise where there is deviation from normal, taking into account species, strain and inter-animal differences and to be able to identify when there is room for improvement.
Evaluation of improvement strategies
Having implemented an improvement strategy it must be evaluated to determine whether there has been an improvement. It may be that the new method is found to be better, or that the current method is confirmed as being the best one available; either way, the information is valuable to others in the field. This information should then be disseminated to others working in the same area, ideally by publication in mainstream journals, but as a minimum it should be used for internal reference.
It is essential to coordinate the approach to refinement within an institution or organisation. It may be beneficial to identify someone with overall responsibility for this: a ‘refinement champion’ who is responsible for ensuring that refinements are actively implemented at the establishment. The institution needs to ensure that support is available for key people in developing a culture of care, and that the professional integrity of the experts in welfare assessment is recognised. The animal management team needs to communicate effectively with senior management, to make sure that adequate resources are provided to implement refinements appropriately. There should be a clear process for the implementation and reporting of problems, and the development of a culture of advice-seeking and support, not blame and isolation. Scientists need to recognise that there is a learning curve to all new methods, and accept that while inexperience may lead to new or increased problems in the short term, there will be longer-term benefits from perseverance. Refinements may be developed to enhance animal welfare, to improve scientific quality or sometimes simply as a defensive reaction to prevent criticism of old-fashioned methodologies. The underlying reason of why a procedure is done matters less than what is done, when, how and by whom.
Public Perceptions
The polling company Ipsos MORI has been asking the public in the UK about their views on animal research since 199927. Among their findings, as reported by the organisation Understanding Animal Research (www.understandinganimalresearch.org.uk/your_views), was that throughout this time more than 80% of respondents accepted the need for animal research provided that certain conditions were being met:
- that there is no unnecessary suffering,
- that the research is for serious medical or life-saving purposes,
- that there is no alternative.
Much of the existing regulatory process is built around a desire to satisfy these caveats. The Ipsos MORI poll showed that, since 2005, a majority of the surveyed population ‘expect that the rules in Britain on animal experimentation are well enforced’. A significant, and often vocal, minority of the population are opposed to any use of animals in research and campaigning organisations such as the British Union for the Abolition of Vivisection (BUAV) argue that ‘harming animals in the name of science is morally indefensible’ (www.buav.org/humane-science).
For the most part, society accepts that the use of animals is necessary for medical progress, provided there are assurances over the purpose for which the work is carried out and the degree of suffering inflicted. Surveys have shown that people will accept a greater degree of suffering to animals in the pursuit of cures for devastating illnesses such as childhood leukaemia than for minor problems or for toxicity testing28. However, there is a significant sector in society which believes that however high the anticipated benefit no animal should undergo more than transient suffering, which may be at odds with the interests of science and industry. A majority of people seem to support the use of mice in fundamental biomedical research if they come to no harm, but begin to show disapproval as soon as pain, surgery or illness became involved. If animal suffering cannot be ruled out it may be hard to convince the public of the value of fundamental biological research. However, much research of this type is relatively benign, and so might win public support if measures to limit suffering were implemented effectively and described in detail. It is up to the scientist therefore to fully explain the nature of the work being undertaken, and to put it in context, such that the public can understand the motives for the work and gain a greater understanding of research.
In contrast to the many examples in which animals suffer pointlessly (it could be argued; such as over-exploitation of sporting animals in competitions, cosmetic mutilations or organised dog fighting) at the hands of people, usage of laboratory animals in experimental procedures is purposeful and is ethically scrutinised in advance. However, this process leads to the harms that might occur being premeditated, which may lead to accusations of scientists being unemotional, inhumane or even abusive29. Although animal research has historically received public criticism similar to activities such as hunting, fishing and factory farming, the moral case for carefully controlled research using animals is actually much more defensible. And yet, an individual animal's perception of pain and suffering is the same whatever the context or ‘reason’ for its life. A laboratory animal will not have the benefit of knowing that its harm is for a ‘good cause’; nor can it give permission. A system to compare life experiences of laboratory animals with farm, companion or wild animals has been proposed15.
Transparency and freedom of information
Modern society demands accountability in laboratory animal use. If this use is truly defensible and ethically robust, it should be more transparent. Labelling drugs and household substances to show whether animals were used during development could be part of this. This would build trust, assist those who do not wish to use products derived from experiments involving animals and increase uptake of the three Rs. However, some patients may refuse important treatment. Much of the information held in research institutes and by the funding agencies is now available under the UK Freedom of Information Act. This has caused some concern as researchers worry about the security implications of actions by animal rights protesters.
Campaigners in the UK have historically played a vital role in bringing about legislation to control animal research. Martin's Act of 1822 against cattle cruelty, the Cruelty to Animals Act 1876 and the Animals (Scientific Procedures) Act 1986 all owed an enormous amount to campaigners bringing the issues into the public arena. Activism has directly influenced the research of some groups30 and different parts of the scientific community have either adopted greater public openness or been driven to regrettable but understandable secrecy. Animal cruelty has no doubt occurred in the history of research and anti-vivisectionists have rightly called attention to it. Establishment in 1999 of the local ERP owes much to the importance placed on animal welfare and the ethics of animal research. There is now a degree of peer pressure in many establishments to ensure that the welfare of animals is paramount, both for its own sake and for the management of wider reputational risks. The modern research community generally puts the ethics of animal experimentation at the heart of all its work, and the aim for the future must be not only to maintain this high standard but to continue openly to seek improvement.
During a 2-day conference in Basel in 2010, more than 80 life science researchers from Switzerland, Germany, Sweden, France and Great Britain addressed the problems of animal research and adopted the Basel Declaration (www.basel-declaration.org/). The Declaration marks an unprecedented effort of the scientific community towards more trust, transparency and communication on animal research. The signatories commit to accepting greater responsibility in animal experiments and to intensive cooperation with the public, and also with national and international decision makers. They are actively seeking to show that science and animal welfare are not diametrically opposed and to make a constructive contribution to the dialogue taking place in society.
References
1. Schiller J (1967). Claude Bernard and vivisection. Journal of the History of Medicine 22: 246
2. Harvey W (1628). Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibus. Translated at www.fordham.edu/halsall/mod/1628harvey-blood.html
3. Wolfensohn S and Maguire M (2010). What has the animal rights movement done for animal welfare? Biologist 57: 22–7
4. Russell WMS and Burch RL (1959). The Principles of Humane Experimental Technique. London: Methuen
5. Dolan K (1999). Ethics, Animals and Science. Oxford: Blackwell Science
6. Regan T (2004). The Case for Animal Rights. Berkeley: University of California Press
7. Nuffield Council on Bioethics (2005). The Ethics of Research Involving Animals. London: Nuffield Council on Bioethics
8. Porter DG (1992). Ethical scores for animal experiments. Nature 356: 101–2
9. McCulloch S (2011). The Bionic Vet: we don't know where to draw the line. Veterinary Times 41(4): 14
10. Hubrecht R (2013). Species choice and animal welfare. In Animal Welfare and Ethics of Animal Use in Research. Oxford: Wiley-Blackwell (in press)
11. RSPCA and LASA (2010). Guiding Principles on Good Practice for Ethical Review Processes, 2nd edn, M. Jennings (ed). A report by the RSPCA Research Animals Department and LASA Education, Training and Ethics Section. www.lasa.co.uk/GP%20ERP%20July%202010%20print%20FINAL.pdf
12. Popper K (1963). Conjectures and Refutations. London: Routledge and Keagan Paul
13. Farm Animal Welfare Council (2009). Farm Animal Welfare in Great Britain: Past, Present and Future. London: Farm Animal Welfare Council. www.fawc.org.uk/pdf/ppf-report091012.pdf
14. Wathes C (2010). Lives worth living? Veterinary Record 166: 468–9
15. Wolfensohn S and Honess P (2007). Laboratory animal, pet animal, farm animal, wild animal: which gets the best deal? Animal Welfare 16(S): 117–23
16. Honess P and Wolfensohn S (2010). A matrix for the assessment of welfare and cumulative suffering in experimental animals. Alternatives to Laboratory Animals 38(3): 205–12
17. US National Academy of Sciences (2007). Committee on Toxicity Testing and Assessment of Environmental Agents, National Research Council. Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington DC: National Academies Press
18. Organisation for Economic Co-operation and Development (2010). In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method. OECD Guideline for the Testing of Chemicals no. 439. Paris: OECD. http://iccvam.niehs.nih.gov/SuppDocs/FedDocs/OECD/OECD-TG439.pdf
19. Festing MFW, Overend P, Das RG, Borja MC and Berdoy M (2002). The Design of Animal Experiments. Laboratory Animal Handbook no. 14. London: Royal Society of Medicine Press
20. Kilkenny C, Parsons N, Kadyszewski E, Festing MFW, Cuthill IC, Fry D, Hutton J, and Altman DG (2009). Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS ONE 4(11): e7824
21. Green MA, Bass S and Spear BT (2009). A device for the simple and rapid transcervical transfer of mouse embryos eliminates the need for surgery and potential post-operative complications. BioTechniques 47(5): 919–24
22. Hurst JL and West RS (2010). Taming anxiety in laboratory mice. Nature Methods 7(10): 825–8
23. Magalhaes-Sant'Ana M, Sandoe P and Olsson IAS (2009). Painful dilemmas: the ethics of animal-based pain research. Animal Welfare 18: 49–63
24. Kilkenny C and Altman DG (2010). Improving bioscience research reporting: ARRIV-ing at a solution. Laboratory Animals 44: 377–8
25. Kilkenny C, Browne WJ, Cuthill IC, Emerson M and Altman DG (2010). Improving bioscience research: the ARRIVE guidelines for reporting animal research. PLoS Biology 8(6): e1000412
26. Lloyd MH, Foden BW and Wolfensohn SE (2008). Refinement: promoting the 3Rs in practice. Laboratory Animals 42: 284–93
27. Ipsos MORI (2010). Views on Animal Experimentation. www.ipsos-mori.com/DownloadPublication/1343_sri-views-on-animal-experimentation-2010.pdf
28. Aldhous P, Coghlan A and Copley J (1999). Let the people speak. New Scientist 162: 26
29. Gilbert CL and Wolfensohn S (2012). Veterinary ethics and the use of animals in research: are they compatible? In Veterinary and Animal Ethics, C Wathes (ed.). Oxford: Wiley-Blackwell
30. Cressey D (2011). Battle scars. Nature 470: 452–3