Chapter 8
Humane Methods of Killing
Ethical Considerations in Killing Animals
Rearing animals for humane killing to provide tissues for studies in vitro represents the majority of laboratory animal usage. It can be argued that if death is not preceded by any premonition of the event, or any perception of pain, it does not affect welfare. On this basis, the reporting of statistics of animals used in experiments does not include animals reared, housed and killed by an approved method, providing no procedures were performed during life. This logic holds providing that (1) the quality of husbandry can be shown to be such that the animals have (enjoyed) a life worth living, (2) the animals' genetic background and phenotype are not intrinsically harmful and are compatible with their surroundings, (3) it is accepted that animals are not conscious of death or the potential for future life experiences and (4) death was indeed painless and free from fear.
Euthanasia, if carried out competently, may be easier for the animal than those parts of a procedure that may have caused pain, suffering or distress. On the other hand, performing euthanasia can be the most difficult part for the researcher and coping with death of research animals can take its toll on the emotions of those involved1,2. Killing an animal is never a pleasant task, but it does not have to be unpleasant for the animal, provided it is carried out competently and humanely. Decisions and actions on euthanasia must be made promptly if suffering is to be prevented. Collection of reliable data is essential since, without that, any suffering caused would be pointless and unethical, as the animal would have been used without purpose. For further discussion on the ethics of killing animals that have an inherent value to either themselves or others see the Nuffield Council on Bioethics (2005)3.
Legal constraints
Killing of animals for scientific purposes is controlled by Schedule 1 to the Animals (Scientific Procedures) Act 1986 and Annex IV to EU Directive 2010/63.
When to kill an animal
Well-designed experiments should detect early signs of distress, allowing the definition of a point at which adequate data have been obtained, but any animal suffering is minimised4. Ideally the maximum achievable information should be obtained from each animal while suffering and distress are kept to the minimum. The precise time at which to kill the animal must be based on accurate clinical judgement, assessing the degree of suffering against the potential loss of data. To go beyond what is required for a scientific outcome may cause unnecessary suffering and is therefore inhumane. By definition, procedures carried out under the Animals (Scientific Procedures) Act will have the potential to cause pain, suffering, distress or lasting harm, so it is necessary to address how these adverse effects might be controlled or, even better, prevented. However, many experiments will lead to the death of the animal and the continual refinement of the point of intervention by euthanasia should be sought and implemented. By defining a humane end point much potential suffering can be considerably reduced by killing the animal at an early point. The application of a defined humane end point will thus utilise a lower severity level than would be needed if the procedure were allowed to run its full course. Ideally the maximum achievable information should be obtained from each animal while suffering and distress are kept to the minimum. Utilising this concept of the humane end point will refine the procedure and reduce the costs to the animals, improving the justification and the harm/benefit equation for the experiment.
The idea behind the humane end point is to define a set intervention point which allows the collection of quality scientific data but limits the amount of suffering, either contingent or direct5, to which the animal may be subjected. The end point may be purely temporal; for example, the animal will be killed x hours/days/weeks after a particular technique is carried out when the adverse effects are not expected to increase but a time limit is set nonetheless, thus reducing the potential for contingent suffering. Or there may be a defined measurable point relating to a physiological parameter; for example, when the blood glucose level reaches x mmol/l, which indicates that the animal is in the required physiological state to gather the data, but not yet experiencing deteriorating clinical signs. Or, in some cases, the defined intervention point may be based on a collection of scores cumulatively measured, sometimes with weightings for more challenging clinical conditions, to ensure that any potential suffering is limited to the minimum consistent with obtaining satisfactory data. The use of a welfare assessment grid can be helpful in balancing some of the difficult decisions about the future of an animal. Such a grid can be used to give a schematic representation of parameters including clinical condition, psychological well-being, behaviour and environmental conditions6,7. It is unusual for laboratory animals to reach death in a laboratory as an expected consequence of a procedure without the intervention of euthanasia. For further detail on assessing welfare to aid with defining humane end points see Chapter 4 in this volume.
Decisions on euthanasia must be reached swiftly and appropriate actions taken promptly if suffering is to be prevented. Euthanasia is defined by the Oxford English Dictionary as the bringing about of a gentle and easy death, so it should not in itself cause pain or suffering. Experimental animals may need to be killed for a variety of reasons. The Animals (Scientific Procedures) Act requires that at the end of a series of regulated procedures the animals used must be killed, except in particular circumstances. There are three possible end points to every experiment, as follows.
In addition, any animal that is suffering severe pain, which cannot be alleviated, must be humanely killed immediately. Animals may also be killed if their health gives cause for concern, if they have reached the end of their breeding life, if they are unwanted stock, or if tissues and blood are required.
Euthanasia is frequently the most difficult part of an experiment for the researcher, particularly if the animal has been used on a long-term project and is one of the higher species. By becoming a personal licence holder you must accept that you are responsible for taking that animal's life, and feeling compassion is a necessary part of that responsibility. Whichever method of humane killing is chosen, it is very important that the operator is confident and competent to carry it out swiftly and humanely. However, if there is any hesitancy, animals may experience unnecessary distress. No one should be expected to carry out a method of humane killing with which they are not comfortable. So, if in doubt, don't do it.
The definition of death is that an animal shall be regarded as continuing to live until the permanent cessation of the circulation or the destruction of the brain; therefore, any method of killing used must ensure that one or both of these criteria is met. Legislation imposes restrictions on the methods of killing which may be employed for experimental animals, or stock animals that are surplus to requirements. Schedule 1 to the Act lists standard methods of humane killing (see Table 8.1). Killing an experimental or a stock animal using a method listed in Schedule 1 for that particular type of animal is not a regulated procedure, so neither a project nor a personal licence is required to carry this out. However, the person carrying out the killing must be competent to do so without causing distress to the animals involved. Therefore training and competence must be ensured by local management and a register of those deemed competent should be kept and regularly updated. If a method of killing is used which is not in Schedule 1, or if one of the listed methods is used for an animal for which it is not deemed appropriate, then it becomes a regulated procedure, and must be authorised on appropriate licences.
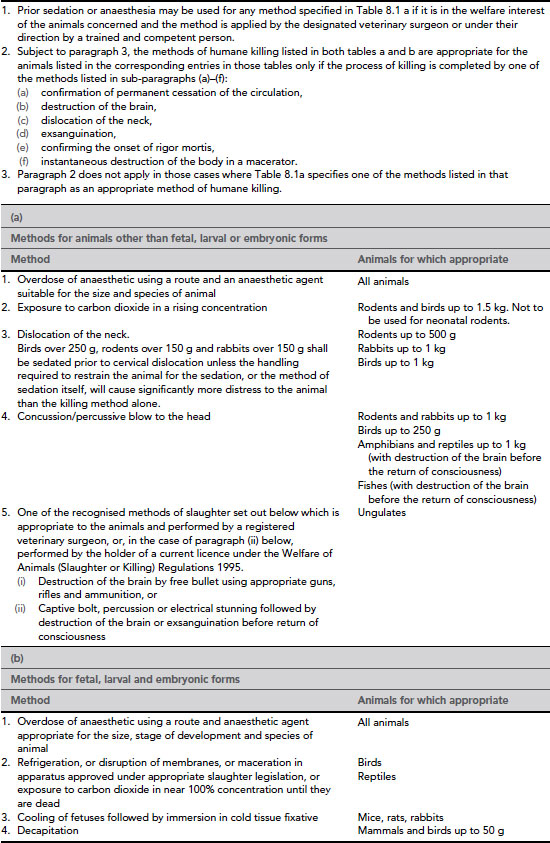
Table 8.1 Schedule 1: appropriate methods of humane killing.
A Section 2C licence includes conditions requiring the holder to keep a register of people who are competent to kill protected animals. In relation to each person named it must specify the description of animal and the methods of killing that the person is competent to use for that type of animal. A Section 2C licence also includes conditions for ensuring that a person is not registered unless (s)he has been adequately educated and trained in the killing of animals; that they are supervised when killing animals until they have demonstrated the required competence; and that at all times there is an adequate number of registered people present to enable the expeditious killing of any protected animal at that institute that needs to be killed.
Preparation for humane killing
As with any regulated procedure, humane killing requires a certain amount of preparation. Points to be considered are outlined here.
- Practice: any method of killing can cause distress if badly performed, so staff must be suitably trained and competent in the methods they will be using. Humane killing is included in accredited training courses (see Chapter 1).
- Handling: animals must be handled carefully and competently without causing them distress (see Chapter 5), until unconsciousness has occurred. Staff must be trained and competent to hold animals properly and securely. Some methods of killing may require two or more people to hold the animal, so make sure that there are sufficient people available to do the job properly. Animals will often be calmer if held by a person with whom they are familiar.
- Location: if an animal is frightened but conscious it may exhibit total immobility or it may show behavioural responses such as vocalisation, struggling, urination, defaecation, anal sac emptying and muscle tremor. Any fear or distress experienced can be communicated by sound or smell to other animals causing distress. Some of these responses may even be exhibited by unconscious animals before death occurs. It is most important that animals are removed to another room away from the group before they are killed.
- Equipment: some methods of killing require equipment such as anaesthetic-induction chambers, cylinders of gases, bottles of anaesthetic agents, needles and syringes, or firearms. It is essential to make sure that any equipment is clean, prepared and ready for use at the start, and that the operator is fully trained and competent to use it.
Methods of Euthanasia
There are a number of different methods of euthanasia available. There are a variety of guidelines that can provide assistance with the considerations relating to the carrying out of euthanasia8–14. The following points should be considered when choosing a method of humane killing15,16:
- death must occur without producing pain;
- the time required to produce loss of consciousness must be as short as possible;
- the time required to produce death must be as short as possible;
- the method must be reliable and non-reversible;
- there must be minimal psychological stress on the animal;
- there must be minimal psychological stress to the operators and any observers;
- it must be safe for personnel carrying out the procedure;
- it must be compatible with the requirements of the experiment;
- it must be compatible with any requirement to carry out histology on the tissues;
- any drugs used should be readily available and have minimum abuse potential;
- the method should be economically acceptable;
- the method should be simple to carry out with little room for error.
The different techniques are often divided into those using physical methods and those using chemicals administered by various routes. Some of the commonly used methods available are as follows.
Chemical methods of euthanasia
Overdose of anaesthetic agent
An overdose of anaesthetic, using a route and agent suitable for the species and size of animal, is considered to be suitable for all animals. With modern anaesthetic agents and combinations used to achieve balanced anaesthesia (see Chapter 9) there is generally a fairly wide safety margin, and indeed with some anaesthetics it is actually quite difficult to kill an animal using a reasonable volume or concentration. When carrying out euthanasia it is therefore important to select the agent carefully. The aim is to cause rapid loss of consciousness followed by respiratory depression, leading to hypoxia and death.
By injection
The drug of choice is pentobarbitone, which does not have a wide safety margin and so is unsuitable as an anaesthetic agent, but is suitable for euthanasia. It is usually presented as a 20% solution (200 mg/ml) and is administered at a dose of at least 140 mg/kg. It is preferable to give it intravenously for the most rapid action. If it is not possible to locate a vein in a conscious animal, it may be necessary to sedate it first with an agent administered by an easier route. When the sedative has taken effect and the animal can be handled more easily the pentobarbitone can be administered intravenously to kill it quickly and humanely. In the smaller species it may be administered intraperitoneally, using a suitably sized needle (see Chapter 7). The smaller the needle the better, as it will cause less pain on insertion, but pentobarbitone causes pain on injection and combination with a local anaesthetic agent will help to reduce this17. Pentobarbitone must not be given via the intramuscular or intrathoracic routes as it is very irritant to the tissue and will be painful. Intracardiac injection of pentobarbitone should only be attempted after the animal has been rendered unconscious.
By inhalation
Euthanasia can be carried out by exposing the animal to a high concentration of an inhaled anaesthetic agent, such as isoflurane. This is done in an induction chamber, with the agent piped in from an anaesthesia machine to ensure the even distribution of vapour. Depending on the agent chosen and the concentration used, unconsciousness usually occurs quite rapidly but death may take rather longer. It is important that the animal is physically separated from the liquid agent since volatile anaesthetics are very irritant to mucous membranes. While inhalation anaesthetics may be less aversive than carbon dioxide, they are still not particularly pleasant. Halothane has been found to be least aversive for rats and enflurane for mice18, although such agents should be used with appropriate scavenging systems to ensure compliance with health and safety regulations. It has therefore been suggested to use halothane (although this agent has been withdrawn from some markets) to induce loss of consciousness, then to infuse carbon dioxide to cause death. It is important to ensure that the animal is left in the chamber for long enough, and to confirm that it is dead, since it may recover if it is removed too soon and allowed to breathe room air. The chamber should be designed so that the animal can be easily observed, and so that it can be easily cleaned between batches of animals. Rodents communicate by pheromones in urine and faeces and placing the animals on disposable paper, which can be replaced each time, is a simple way of controlling this potential stress factor. Chambers of this type should always be used in an extraction cabinet or with a suitable ducting system to reduce exposure of staff to the volatile agent.
Similarly, as for injectable agents, modern inhalational anaesthetic agents are developed to have a wide safety margin and so it can be quite difficult to kill an animal with such agents. It may therefore be preferable to use them to induce unconsciousness and then kill the animal using carbon dioxide at 100%. This way the detection of pain from nociception will be avoided and death from hypoxia is rapid. Inhalation is suitable for small animals, but it can be difficult to restrain larger animals, and diving animals can hold their breath for long periods, making induction unacceptably slow. Newborn animals are resistant to hypoxia and can take an unacceptably long time to die by this method.
By immersion
Animals such as fishes, small amphibia and cephalopods may be euthanased by immersion in an agent such as tricaine methane sulphonate (MS222), which is then absorbed percutaneously. As for inhalation euthanasia in the rodent, care must be taken to ensure that the animal is left in the solution for an adequate length of time and that death has occurred when it is removed.
Exposure to carbon dioxide
The use of carbon dioxide for euthanasia, while very common, is controversial, leading to much debate over the most humane method of euthanasia19,20. It is now established that rats and mice find carbon dioxide aversive21 and its use as a euthanasia agent is considered unacceptable by many22. Carbon dioxide exposure causes immediate bradycardia due to nasal nociception activation and pain, and in humans carbon dioxide inhalation is painful at 50–100% exposure concentrations23. If rodents are exposed to a high concentration (>50%) of carbon dioxide, the time to unconsciousness and therefore the time for which the pain has to be endured is at least 10–15 s20. Many guidelines for euthanasia state that the concentration of carbon dioxide should be rising. However, if the chamber is filled slowly it can take around 2 min to reach unconsciousness at 35% CO224. While this avoids the acute pain seen with 100% CO2, there is significant dyspnoea and tachypnoea which may be very distressing to the animal even if it is not actually painful. Hawkins et al.20 state that carbon dioxide aversion in rats starts at a concentration of 10–15% and that this aversion is very high, not even being overcome by motivation of 24 h food deprivation. In humans, dyspnoea occurs at 7% carbon dioxide, is severe at 15–20% and said to be very unpleasant. Rats will show escape behaviour at 15–20% CO2. The choice with carbon dioxide would therefore appear to be between a high concentration initially, resulting in a faster more painful death, or a lower rising concentration of carbon dioxide, resulting in a slower death with less pain but more distress from dyspnoea. Neither of these is satisfactory.
One of the principal advantages of this method of euthanasia is that it can be carried out with the animal in its home cage and by placing the entire cage in a carbon dioxide cabinet. It has been shown that when killing entire cages of animals it is advantageous to kill them in the home cage as this causes much less stress25 than removing them to an unfamiliar location. The animal is not handled and the operator is able to remain detached from the process of killing.
Exposure to a rising concentration of carbon dioxide causes animals to become unconscious due to a direct narcotic effect of the carbon dioxide then, as the concentration increases, they die from hypoxia. The concentration of carbon dioxide used for euthanasia must be rising and the animals should be placed in a chamber containing room air that is easy to clean and gives a clear view of the animals inside. It is important that the chamber is not overcrowded. The flow of carbon dioxide should then be turned on and gradually increased, displacing the air. After use the chamber must be inverted to tip out all the residual carbon dioxide since it is heavier than air and will sink to the bottom of the chamber. A controllable source of carbon dioxide should be used; it is not acceptable to use dry ice and preferably there should be a thermostatic mechanism to ensure that the gas reaching the animal from the cylinder is not uncomfortably cold.
Numerous studies in animals have indicated that carbon dioxide is aversive to breathe (see for example Gregory et al.26 and Leach et al.21), and animals killed by this method frequently exhibit epistaxis and damage to the nasal mucosa. If animals huddle together some animals may be held in pockets of air and avoid the gas, therefore remaining alive or suddenly being exposed to high concentrations. Unintended recovery after apparent death from carbon dioxide has also been reported, so death must be confirmed by exsanguination or dislocation of the neck. If carbon dioxide is to be used effectively and humanely it must be used properly. There should be standard operating procedures in place, which must be followed.
Neonatal animals and cold-blooded vertebrates are resistant to hypoxia, and diving birds and mammals can hold their breath for long periods, so the use of carbon dioxide is not recommended in these animals. The use of carbon dioxide for stunning or for euthanasia in farm animals has also been shown to be aversive whereas combination of the carbon dioxide with argon is less so in pigs26,27 and chicks28. The use of argon raises health and safety concerns since it is difficult to detect and rats and mice still show some aversive behaviour, despite it being less so than with carbon dioxide18.
The question is therefore whether there are any better methods that combine the advantages of not having to handle the animal and the aesthetic acceptability of being remote from the animal at the point of death, but which does not cause the animal respiratory distress or pain. While carbon dioxide can be a quick and simple method for the euthanasia of large numbers of animals, practical considerations should not take precedence over animal welfare, and alternative methods may be more humane.
Physical methods of euthanasia
Physical methods should cause immediate loss of consciousness through physical trauma to the brain. If carried out properly they are often more humane than some chemical methods, since unconsciousness is achieved very quickly. However, they may be distasteful to the person carrying them out, and this leads to a tendency to be rather hesitant. However, human feelings should not be allowed to influence the choice of the most humane techniques and manual dexterity and an ability to handle the animal confidently are essential to minimise any apprehension. It is essential to make careful observations of the method being carried out by a competent and experienced person, then to practise on dead animals before carrying out these methods on live animals. Death should be confirmed by exsanguination through the severing of major vessels or by destruction of the brain.
Dislocation of the neck
If carried out correctly dislocation of the neck causes extensive damage to the brainstem and instantaneous unconsciousness. Dislocation must be carried out quickly and with confidence, otherwise there might not be complete separation of the cervical vertebrae and the animal may experience distress or pain. It may be preferable to sedate or anaesthetise animals prior to cervical dislocation.
For small rodents, put the animal on a surface which it can grip, place a pencil or similar object firmly across the back of the neck, take a firm grasp around the hindquarters or the base of the tail and pull firmly. The neck will be dislocated and the animal will die instantly. For larger rodents and lagomorphs, hold the body firmly in one hand, the head in the other and pull firmly with a rotating action. For larger birds, hold the legs or body in one hand, and pull the neck firmly down and backwards with the other. It may be necessary to wrap the bird to prevent involuntary flapping of the wings, and to confirm death by severing the major vessels in the neck.
Concussion
Concussing the animal by striking the back of the head renders the animal unconscious, following which death must be ensured by dislocation of the neck or by exsanguination. As with dislocation of the neck, confidence in handling the animal and manual dexterity are required to carry out this method without causing distress to the animal. Support the animal by the hindquarters and swing the body downwards such that the back of the head comes sharply into contact with a hard surface such as a work bench. Considerable training and practice on dead animals is required to ensure competence with this method, and it is difficult to ensure that animals are stunned consistently. For amphibia, reptiles and fishes the brain must be destroyed immediately following concussion, as in these species the brain is very tolerant to hypoxia, and it cannot otherwise be guaranteed that concussion is irreversible or that unconsciousness will last until death.
Other methods of euthanasia
The physical methods described above are appropriate for small rodents, rabbits and birds and also for amphibians, reptiles and fishes. For ungulates, the animal may be killed by one of the standard methods of slaughter, such as use of a free bullet, a captive bolt or electrical stunning followed by destruction of the brain or exsanguination. However, these methods are controlled by other legislation, including control of firearms and will therefore only be available to those who have been appropriately trained and issued with the relevant licences.
For fetal, larval and embryonic forms there are other methods that can be used which take into account the degree of development of the nervous system in the various animals. Overdose of injectable anaesthetic may be used for all species. Refrigeration, disruption of membranes or maceration can be used for birds and reptiles, but the death of the reptile embryo must be ensured by overdose of anaesthetic, maceration or immersion in tissue fixative. Cooling of fetuses until movement has stopped followed by immersion in cold tissue fixative is appropriate for mouse, rat and rabbit fetuses. Decapitation using a sharp implement can be used for fetal mammals and birds up to 50 g in weight. In some jurisdictions decapitation for neonatal rodents is considered an appropriate method of euthanasia.
Other methods of killing may be employed occasionally if there is a scientific need. These will require authority on both personal and project licences. Microwaves can be used to fix brain metabolites without losing anatomical integrity29,30. Specialist apparatus is required, along with careful technique. The microwaves are focused on particular areas of the brain, producing death very rapidly. Electrical stunning may be used as in routine pre-slaughter stunning for ungulates and also for other species such as poultry or rabbits. It requires specialist equipment, and death must be confirmed using another method. Pithing may be carried out in unconscious fish, amphibia or reptiles. A sharp needle is inserted through the foramen magnum and agitated to destroy the brain. This requires technical skill to ensure rapid death, and must not be carried out in conscious animals. Rapid freezing using liquid nitrogen can be used in situ or following decapitation to freeze the brain. The animal must be rendered unconscious first, as it can take up to 90 s to freeze deep structures. Decapitation may not be humane in conscious animals, because of the length of time it takes for the decapitated head to lose consciousness, particularly in cold-blooded vertebrates. If this method is required for scientific reasons there must be particular justification, and consideration given to sedating or anaesthetising the animal first.
Schedule 1 methods
Schedule 1 is in two parts (see Table 8.1). Table 8.1a describes methods suitable for animals other than fetal, larval and embryonic forms, and Table 8.1b methods for fetal, larval and embryonic forms. Note that killing pregnant animals is usually considered to kill the fetuses, so killing a dam by a Schedule 1 method would not require licence authority. However, the subsequent use of any live fetuses more than two-thirds of the way through gestation for scientific study would require authority. Newborn animals fall under the first definition.
A: animals other than fetal, larval and embryonic forms
Overdose of anaesthetic by injection, inhalation or immersion
An overdose of anaesthetic, using a route and agent suitable for the species and size of animal, is considered to be suitable for all animals.
Exposure to carbon dioxide
Unconsciousness occurs more rapidly in smaller animals, so Schedule 1 restricts the use of carbon dioxide to the killing of rodents and birds up to 1.5 kg in weight.
Physical methods
Dislocation of the neck
This method is appropriate for rodents up to 500 g, rabbits up to 1 kg and birds up to 1 kg.
Concussion
Concussion may be used for rodents and rabbits up to 1 kg, birds up to 250 g and amphibians and reptiles up to 1 kg and fishes.
Recognised methods of slaughter
Ungulates may be killed under Schedule 1 by one of the standard methods of slaughter, such as use of a free bullet, a captive bolt or electrical stunning followed by destruction of the brain or exsanguination.
B: fetal, larval and embryonic forms
The Animals (Scientific Procedures) Act 1986 covers immature forms from particular stages of development. For mammals, birds and reptiles this is two-thirds of the way through gestation or incubation, and for amphibia, fish and cephalopods the point at which they become capable of independent feeding. Schedule 1 describes methods of humane killing that can be used for these forms, which take into account the degree of development of the nervous system in the various animals. Some species are highly developed at birth and therefore require special consideration during the last few days of gestation or incubation, whereas others are less well developed and are less able to perceive pain at this stage.
Overdose of anaesthetic
This may be used for all animals. An anaesthetic agent appropriate for the size and stage of development of the animal should be chosen. In large fetuses or embryos anaesthetics may be given by the intravenous or intraperitoneal routes; smaller animals and larvae may be immersed in anaesthetic.
Refrigeration, disruption of membranes, maceration or exposure to 100% CO2
These methods can be used for birds and reptiles. In birds, cooling to below 4°C is effective, but reptile embryos are resistant to hypothermia and hypoxia, so death must be ensured by overdose of anaesthetic, maceration or immersion in tissue fixative.
Cooling followed by immersion in fixative
Mouse, rat and rabbit fetuses may be chilled until movement has stopped, then immersed in cold (4°C) tissue fixative.
Decapitation
This is a humane and quick method that can be used for mammals and birds up to 50 g in weight which are not well developed, using a sharp implement. Note: this method cannot be used under Schedule 1 for neonatal mammals.
Confirmation of death and disposal of carcasses
After carrying out any method of euthanasia it is vital to check that the animal is really dead before disposing of the carcass. Thus the process of killing must be completed by one of the following six methods:
All animal carcasses are classed as clinical waste and as such must be disposed of correctly either by maceration or in clinical waste bags that are then incinerated. There will be local rules in the work area relating to the method of disposal and these must be followed.
References
1. Pekow CA (1994). Suggestions from research workers for coping with research animal death. Lab Animal 23: 28–29
2. Halpern-Lewis JG (1996). Understanding the emotional experiences of animal research personnel. Contemporary Topics 35: 58–60
3. Nuffield Council on Bioethics (2005). The Ethics of Research Involving Animals. London: Nuffield Council on Bioethics
4. Morton DB (2000). A systematic approach for establishing humane endpoints. ILAR Journal 41: 80–86
5. Russell WMS and Birch RL (1959; republished 1992). The Principles of Humane Experimental Technique, special edition. Wheathampstead: Universities Federation for Animal Welfare
6. Wolfensohn S and Honess P (2007). Laboratory animal, pet animal, farm animal, wild animal: who gets the best deal? UFAW Symposium: Quality of Life: The Heart of the Matter. Animal Welfare 16 (suppl. 1): 117–123
7. Honess P and Wolfensohn S (2010). A matrix for the assessment of welfare and cumulative suffering in experimental animals. Alternatives to Laboratory Animals 38(3): 205–212
8. Canadian Council on Animal Care (1980). Guide to the Care and Use of Experimental Animals, vol. 1. Ottawa: Canadian Council on Animal Care
9. Close B, Banister K, Baumans V, Bernoth E-M, Bromage N, Bunyan J, Erhardt W, Flecknell P, Gregory N, Hackbarth H et al. (1996). Recommendations for euthanasia of experimental animals. Part 1 Report of a Working Party. Laboratory Animals 30: 293–316
10. Close B, Banister K, Baumans V, Bernoth, E.-M., Bromage N, Bunyan J, Erhardt W, Flecknell P, Gregory N, Hackbarth H, Morton D and Warwick C (1997). Recommendations for euthanasia of experimental animals Part 2 Report of a Working Party. Laboratory Animals 31: 1–32
11. American Veterinary Medical Association (2001). American Veterinary Medical Association 2000 Report of the AMVA panel on euthanasia. Journal of the American Veterinary Medical Association 218(5): 669–696
12. Reilly JS (ed.) (2001). Euthanasia of Animals Used for Scientific Purposes. Australian and New Zealand Council for the Care of Animals in Research and Teaching. Adelaide: Adelaide University
13. American Association of Zoo Veterinarians (2006). American Association of Zoo Veterinarians Guidelines for Euthanasia of Non-domestic Animals. Yulee, FL: American Association of Zoo Veterinarians
14. Office International des Epizooties (2007). Guidelines for the slaughter of animals. In Terrestrial Animal Health Code, 16th edn, Appendix 3.7.5. Paris: OIE World Organisation for Animal Health. http://www.oie.int/
15. Carbone L, Baumans V and Morton DB (2004). Report of the Workshop on Euthanasia Guidelines and Practices. Alternatives to Laboratory Animals 32 (suppl. 1): 445–446
16. Wolfensohn S (2010). Euthanasia and other fates for laboratory animals. In UFAW Handbook on the Care and Management of Laboratory and Other Research Animals, R Hubrecht and J Kirkwood (eds), pp. 219–226. Oxford: Wiley-Blackwell
17. Ambrose N, Wadham J and Morton D (2000). Refinement of Euthanasia. Progress in the Reduction, Refinement and Replacement of Animal Experimentation. Amsterdam: Elsevier Science
18. Leach MC, Bowell VA, Allan TF and Morton DB (2002b). Degrees of aversion shown by rats and mice to different concentrations of inhalational anaesthetics. Veterinary Record 150: 808–815
19. Conlee KM, Stephens ML, Rowan AN and King LA (2005). Carbon dioxide for euthanasia: concerns regarding pain and distress, with special reference to mice and rats. Laboratory Animals 39: 137–161
20. Hawkins P, Playle L, Golledge H, Leach M, Banzett R, Coenen A, Cooper J, Danneman P, Flecknell P, Kirkden R et al. (2006). Newcastle Consensus Meeting on Carbon Dioxide Euthanasia of Laboratory Animals. 27–28 February 2006, Newcastle upon Tyne, www.lal.org.uk/
21. Leach MC, Bowell VA, Allan TF and Morton DB (2002). Aversion to gaseous euthanasia agents in rats and mice. Comparative Medicine 52(3): 249–257
22. Leach MC, Bowell VA, Allan TF and Morton DB (2004). Measurement of aversion to determine humane methods of anaesthesia and euthanasia. Animal Welfare 13: S77–S86
23. Danneman PJ, Stein S and Walshaw SO (1997). Humane and practical implications of using carbon dioxide mixed with oxygen for anaesthesia and euthanasia of rats. Laboratory Animal Science 47: 376–385
24. Golledge H, Roughan J, Niel L, Richardson C, Wright-Williamson S and Flecknell P (2005). Carbon dioxide euthanasia in rats – behavioural and autonomic system responses to exposure. Abstract, SECAL-ESLAV 2005 International Congress, Elche, Spain
25. Hackbarth H, Küppers N and Bohnet W (2000). Euthanasia of rats with carbon dioxide – animal welfare spects. Laboratory Animals 34: 91–96
26. Gregory NG, Moss BW and Leeson RH (1987). An assessment of carbon dioxide stunning in pigs. Veterinary Record 121: 517–518
27. Raj ABM (1999). Behaviour of pigs exposed to mixtures of gases and the time required to stun and kill them: welfare implications. Veterinary Record 144: 165
28. Raj ABM and Whittington PE (2003). Euthanasia of day old chicks with carbon dioxide and argon. Veterinary Record 136: 292
29. Ikarashi Y, Maruyama Y and Stavinoha WB (1984). Study of the use of the microwave magnetic field inactivation for the rapid inactivation of brain enzymes. Japanese Journal of Pharmacology 35: 371–387
30. Stavinoha WB, Frazer JW and Modak AT (1978). Microwave fixation for the study of acetylcholine metabolism. In Cholinergic Mechanisms and Psychopharmacology, DJ Jenden (ed.), pp. 169–179. New York: Plenum Publishing