Chapter 9
Anaesthesia and Analgesia
Introduction
Scientific procedures carried out on animals may cause them to experience pain or distress. These impact on the welfare of animals and may adversely affect the quality of scientific data obtained. Steps must be taken to minimise these adverse effects, to safeguard the welfare of animals, to ensure scientific validity and to comply with legal requirements. EU Directive 2010/63 requires that procedures are carried out under general or local anaesthesia, unless anaesthesia is incompatible with the experiment or more traumatic than the procedure itself, and that analgesia or another method is used to ensure that pain, suffering and distress are kept to a minimum. Anaesthesia and analgesia in laboratory animals is a rapidly expanding discipline and regular review of anaesthetic and analgesic practices is required to ensure the most up-to-date methods are being employed.
Anaesthesia can be defined as a local or general loss of sensation, with or without the loss of consciousness, brought about by controlled reversible intoxication of parts of the nervous system.
- General anaesthesia is a state of total unconsciousness, resulting from the administration of anaesthetic drugs that depress the function of the brain.
- Local anaesthesia is a loss of sensation in a small area of the body, produced by injecting or applying local anesthetic drugs, which block nerve conduction.
- Regional anaesthesia is a loss of sensation in a region of the body produced by application of a local anaesthetic agent to all the nerves supplying that region; for example, epidural anaesthesia.
Clearly it is essential to induce and maintain general or local anaesthesia if a potentially painful procedure is to be carried out on an animal, and also occasionally it may be necessary to use sedation or anaesthesia to restrain an animal for a procedure which may not be painful, but which may cause distress to the conscious animal.
The main purpose of anaesthesia is to produce humane restraint and an absence of pain, but in addition anaesthesia must be safe for both animal and operator, produce consistent and repeatable results and be easy and practical to administer. However, successful anaesthesia is not achieved simply by administering a set drug or combination to an animal: the way an animal responds to anaesthesia can be unpredictable and depends on numerous factors, some of which are under the control of the operator, and some of which are not. These must be taken into account when designing an anaesthesia regime, otherwise the procedure may not be successful, resulting in unnecessary pain or distress for the animal, prolonged recovery times or even death of the animal.
Good anaesthesia requires more than just choosing the right agent(s). A good standard of animal care must be maintained, before during and after anaesthesia, to reduce stress and provide control of pain and distress. Lack of attention to care of the animal in the pre- and post-anaesthetic periods is a common failing of laboratory animal anaesthetic practice1. Problems, however, can be avoided by good pre-, intra- and post-anaesthesia care.
Pre-Anaesthetic Care and Evaluation
There are a number of factors to be considered in the pre-anaesthetic period.
Acclimatisation
It is important for animals to become acclimatised to their environment prior to the induction of anaesthesia. Animals become stressed during transport, and normal physiological responses to this stress will result in abnormal experimental measurements. Mice may need at least 48 h after transportation by road or aeroplane for high corticosterone levels to return to normal, and may need 1–2 weeks to acclimatise fully2. Even transporting animals between animal rooms can result in stress, requiring 4 days or more to resolve3. It is important to plan experiments such that animals have time to settle in before being subjected to procedures.
Training
Many animals can be trained to accept the handling required to facilitate procedures. Most animals can become accustomed to handling, and some can be trained to present themselves for injection or sampling. The temperaments of individual animals vary and some will be more suited to certain types of procedure than others, so choosing animals carefully to ensure they are suited to the procedure can reduce the stress placed on the animals.
Fasting
Fasting prior to anaesthesia is required in some species, to prevent vomiting on induction or recovery, or reflux of stomach contents into the oesophagus during anaesthesia. Many laboratory animals, including rodents and rabbits, cannot vomit, and are prone to the development of hypoglycaemia if deprived of food. These animals need only be fasted if there is an experimental reason for doing so, such as for upper gastrointestinal tract surgery, but in any case the stomach will only be empty if coprophagy is prevented. Birds have a high metabolic rate, and small birds should not be fasted for more than 2 h. Large birds (e.g. ducks or chickens) may be fasted for 6–12 h to reduce the risk of regurgitation. Fasting in reptiles and amphibians is generally not necessary.
Single-stomached animals, such as dogs, cats, ferrets, pigs and non-human primates, vomit readily, and these animals should be fasted for 8–12 h before anaesthesia. Fasting in ruminants has very little effect on the volume of food in the stomach, but can reduce the risk of bloat. Fish should be fasted for 24–48 h.
Handling
Careful and expert handling of the animals is important. Firm but sympathetic handling by a familiar handler can reduce the stress experienced by the animals in the pre-anaesthetic period.
Health status
It is essential to use animals that are healthy and disease-free. These are less likely to have problems during anaesthesia than those with overt or subclinical disease. Those with respiratory infections in particular should be avoided. It is best to use animals of known health status (see Chapter 6), but this may not be possible, and sometimes a previous experimental procedure may have altered the health of the animal. Either way, animals should be given a clinical examination prior to anaesthesia to rule out infectious and non-infectious diseases. The thoroughness of the examination depends on the species, the procedure and the likely duration of anaesthesia. As a minimum, the function of the respiratory and cardiovascular systems and food and water intake should be evaluated and veterinary advice sought in the event of any deviation from normal.
Local anaesthetics
When intravenous agents are to be used for induction, local anaesthetic creams can be applied topically over the site of venipuncture. These desensitise the skin, so the animal doesn't feel the needle. They should be applied at least 40–60 min before injection4.
Premedication
Pre-anaesthetic medication is often used in larger animals, where sedation aids induction of anaesthesia. However, pre-anaesthetic drugs may also be beneficial in small laboratory animals. The objectives of premedication include1:
- to decrease fear and apprehension, to aid in stress-free induction,
- to reduce the amounts of other anaesthetic agents required to induce general anaesthesia, so reducing their undesirable side effects,
- to assist a smooth recovery from anaesthesia,
- to reduce salivary and bronchial secretions,
- to block the vaso-vagal reflex, in which bradycardia occurs due to endotracheal intubation and handling of the viscera,
- to reduce post-operative pain.
There is evidence that production and release of stress-related substances produce pain, hypersensitise the nervous system to otherwise non-painful stimuli and promote inflammation, all of which delay wound healing and depress the immune response5. In addition, analgesics are more effective if given before pain is perceived6,7,8. Premedication therefore can reduce anxiety and reduce the perception of pain following surgery.
Pre-anaesthetic drugs fall into three main categories: sedatives and tranquillizers, analgesics and anticholinergics.
Tranquillizers and sedatives
Sedatives relieve anxiety and cause drowsiness. Tranquillizers remove anxiety without causing drowsiness, but animals may still be readily roused. However, many drugs fall into more than one classification, the difference depending on dose and the species. All of these drugs potentiate the action of anaesthetics and narcotic analgesics, so are useful in calming the animal before induction of anaesthesia and in reducing the dose of other drugs required to produce surgical anaesthesia.
Benzodiazepines
Examples of benzodiazepines include diazepam and midazolam. They cause sedation, with considerable species variation. In some species (e.g. rabbits, rodents)1 they cause marked sedation, together with good skeletal muscle relaxation, and they are potent anticonvulsants. They have minimal effects on the cardiovascular system. They potentiate the action of many anaesthetics and narcotic analgesics and are a valuable adjunct to many anaesthetic regimes.
α2-Agonists
Examples of α2-agonists include xylazine and medetomidine. These drugs act on α2-adrenoceptors and produce dose-dependent sedation. They are used alone for sedation, or for premedication, as they markedly reduce the quantity of anaesthetic required. Following administration there is initial hypertension, then marked bradycardia with moderate hypotension. Hyperglycaemia and polyuria also occur. Xylazine produces potent depression of the central nervous system (CNS), leading to sedation, narcosis, unconsciousness and eventually general anaesthesia with increasing doses. Its main use is in combination with ketamine to produce general anaesthesia. Medetomidine is a very potent and specific α2-agonist that produces sedation, analgesia and bradycardia. It also causes vasoconstriction in the periphery so blood pressure is maintained despite the fall in heart rate although it may decrease slightly after a few hours. Respiratory rate may also fall slightly. Medetomidine can cause heavy urination, so care must be taken to prevent the animal becoming wet, hypothermic or even dehydrated. Cyanosis may be apparent, with venous blood being very dark. Despite this, blood oxygen saturation remains high. Medetomidine is synergistic with ketamine and opioids, such as fentanyl, and it markedly reduces the dose of inhaled agents required. Medetomidine has a specific reversal agent, atipamezole. This is an α2-antagonist, which can also be used to reverse xylazine. The animal wakes up a few minutes after administration, avoiding potential problems from a prolonged recovery.
Ketamine
This is a dissociative analgesic agent which produces immobility in most species. However, it produces an increase in muscle tone and variable analgesia. Recovery can be prolonged and it may be associated with hallucinations9. For these reasons it is rarely used alone in most species. It can be used for premedication or sedation in Old World non-human primates.
Phenothiazines and butyrophenones
Examples of phenothiazines and butyrophenones include chlorpromazine and fluanisone respectively. These are potent sedative drugs with no analgesic properties which potentiate the action of anaesthetics. However, they produce hypotension and depress thermoregulatory mechanisms, leading to hypothermia. Therefore they are infrequently used alone. They are most frequently used as components of neuroleptanalgesia (see below).
Analgesics
Narcotic analgesics (opioids) are potent analgesic and sedative drugs which are used to provide sedation and analgesia prior to induction of anaesthesia, and also for post-surgical pain relief. Opioid analgesics interact at opioid receptors in the CNS and other tissues. There are three main receptor types: μ (mu) or OP3, κ (kappa) or OP2 and δ (delta) or OP1. Most of the clinically used opioids interact with the OP3 and OP2 receptors, producing analgesia and sedation, but also undesirable side effects such as respiratory depression, miosis (pupillary constriction), reduced gastrointestinal motility and euphoria. These drugs are potentially addictive, so most of them are controlled drugs under the UK Misuse of Drugs Regulations 2001. Lower doses cause depression of the CNS, but high doses cause excitement. Overdoses cause coma and death. Differential actions at each receptor type lead to different effects of the various analgesics. Agents may be pure agonists, activating the receptors (e.g. morphine, fentanyl and alfentanil, which are pure μ agonists), mixed agonist/antagonists (e.g. butorphanol, which antagonises μ receptors but is an agonist at κ receptors) or partial agonists (e.g. buprenorphine, which has analgesic effects at μ receptors but antagonises pure μ agonists). Increasing the dose of a pure agonist increases the analgesia produced, but also increases the undesirable effects. The respiratory depression produced by the partial agonists is limited and these drugs are less liable to abuse. Partial agonists can be used to antagonise the side effects of pure agonists, but maintain analgesia. This is sometimes known as sequential analgesia. Buprenorphine produces good sedation and analgesia, although it has a slow onset of action, but it lasts for 8 h. It is useful for producing sedation, alone or with phenothiazines, as part of premedication, but is used primarily for analgesia. It is the opioid analgesic of choice for most laboratory species.
Naloxone is a pure opioid antagonist, having no sedative or analgesic action but capable of reversing the effects of an agonist.
Anticholinergics
Anticholinergic drugs were widely used in the past to reduce the production of salivary and bronchial secretions, reducing the risk of airway blockage. This was particularly important with irritant volatile agents such as ether. Modern volatile anaesthetic agents are not irritant, and the routine use of drying agents is no longer considered necessary10. They have a number of undesirable side effects, including increased heart rate, mydriasis (pupil enlargement) and reduced tear production. They reduce the watery component of saliva, rendering it more viscous, especially in ruminants, and decrease ciliary activity. They also reduce gut motility and sphincter tone. Their use may be appropriate if bradycardia is present, for example due to vaso-vagal reflexes triggered by handling of the viscera during surgery, or following the administration of vagomimetic drugs. Atropine is a natural alkaloid that crosses the blood–brain barrier and is eliminated mostly in urine. Rats, rabbits and possibly ruminants eliminate the drug rapidly. Cardiovascular effects last 40–90 min. Glycopyrrolate does not cross the blood–brain barrier and may be more appropriate for neurological studies, although it is slower in onset. Cardiovascular effects last 2–4 h.
General Anaesthesia
General principles
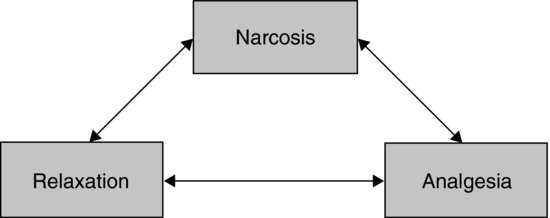
The primary reasons for giving an anaesthetic are to provide humane restraint. This requires modification of the animal's behaviour and level of consciousness by narcosis, providing sufficient muscle relaxation to facilitate procedures and, most importantly, sufficient analgesia to prevent the animal experiencing pain. These three elements make up the classical triad of anaesthesia (see Figure 9.1).
FIGURE 9.1 The triad of anaesthesia.
The ideal general anaesthetic agent has the following properties:
- provides humane restraint,
- is consistent and reliable, having the same effects each time it is used,
- has a wide safety margin, and is safe for both animal and user,
- does not interfere with the procedure or affect the scientific objectives,
- any alterations due to anaesthesia need to be minimal and consistent, to reduce any variability,
- keeps the animal physiologically stable,
- is cleared rapidly, so the animal recovers and returns to physiological normality quickly.
The production of analgesia is the single most important drug effect.
Balanced anaesthesia
No single agent alone will provide satisfactory narcosis, muscle relaxation and analgesia. This is therefore usually achieved by balanced anaesthesia, which is the administration of drugs in combinations, including premedicants, inhalation and injectable anaesthetics and analgesics, in order to produce stable anaesthesia and to reduce the potential side effects of any individual drug. Using several anaesthetics together can overcome many of the disadvantages encountered when using agents individually. Many anaesthetic drugs produce a synergistic (supra-additive) effect when combined, while decreasing the probability and seriousness of side effects.
Balanced anaesthetic regimes allow for minimal interference with the animal's physiology, and a smooth, rapid and pain-free recovery. Administering sedatives prior to induction or inducing anaesthesia with an injectable agent avoids the stress otherwise caused by induction with inhaled agents. Some injectable agents produce little analgesia alone, and addition of a low concentration of an inhaled agent can provide this. Similarly, when using inhalation anaesthesia, the concentration required to produce surgical anaesthesia can be reduced by administering a potent analgesic such as fentanyl. Induction of anaesthetic can be carried out using injectable combinations, followed by inhaled agents for maintenance. In large animals, the administration of an injectable anaesthetic to induce anaesthesia is essential, as it is difficult to restrain these animals for induction by a facemask. Maintenance of anaesthesia can then be satisfactorily achieved by intubation and the administration of a volatile anaesthetic.
The combination of drugs selected to produce ideal anaesthesia should include a narcotic, a behaviour modifier, a muscle relaxant, an analgesic and a stress reducer.
Choice of anaesthetic
The agents chosen to achieve balanced anaesthesia for a particular procedure will depend on a number of factors, including:
- the species,
- the duration of the procedure,
- the depth of anaesthesia required,
- the purpose of the investigation,
- the equipment available,
- the experience of the staff.
Anaesthetic regimes of different durations and depths will be required for different procedures, and some anaesthetic drugs will be contraindicated in certain protocols. Therefore, advantage should be taken of the wide variety of anaesthetic drugs and combinations available to find one which is best suited to the experimental protocol.
Surgical techniques and anaesthesia have effects on the physiology of the animal, which can be prolonged. Surgery results in a significant stress response with corticosteroid release, and levels can remain high for several days. This is designed to assist the animal in overcoming injury, but may be undesirable in the experimental subject. The physiological effects of anaesthesia may be quite minor compared with those produced by the experimental surgery, but nonetheless an anaesthetic regime with minimal potential for adverse effects should be selected. Good anaesthetic practice is an important aspect of animal welfare, and is also relevant to the scientific validity of any study that makes use of the animals, since poor anaesthetic practice can produce unwanted variability.
The anaesthetic should be chosen when all factors potentially influencing the protocol have been considered. While the ideal drug does not exist at present, new preparations are in development continually and new anaesthetic protocols are constantly being evaluated. It is important to keep reviewing available literature to identify possible refinements.
Administration of anaesthetics
There are two common methods by which anaesthetics are administered: by injection or by inhalation. Each method has advantages and disadvantages (see Table 9.1). These can be used individually or combined. Injectable agents are often used for induction, with inhaled agents used for maintenance of anaesthesia.
Table 9.1 Advantages and disadvantages of injectable and inhalation anaesthesia.
| Injectable | Inhalation | |
| Equipment required | Minimal | Considerable |
| Speed of induction and recovery | Variable | Rapid |
| Predictability | Moderate | High |
| Encumbrance during surgery | Minimal | Moderate |
| Ability to adjust depth of anaesthesia | Difficult | Easy |
| Safety margin | Moderate | High |
| Health and safety concerns from operator exposure to agents | Minimal | Moderate |
| Metabolism of agents and potential to interfere with the experiment | Moderate | None |
| Suitability for short procedures and for high frequency anaesthesia | Low | High |
| Operator skill required | i.v., high Other routes, minimal |
Moderate |
| Potential environmental impact | Minimal | Moderate |
| Ease of administration | i.v., difficult Other routes, simple |
Moderate |
| Risk of misadministration | High | Low |
| Ability to reverse overdose | Low | High |
| Stress on induction | i.v., Low/moderate Other routes, moderate/high |
Moderate |
| Legal category | Most are POM-V, some are controlled drugs | Most are POM-V |
| i.v., intravenous; POM-V, Prescription Only Medicine–Veterinarian. Entries in italic indicate disadvantages; those in normal type are advantages. |
||
Inhalation anaesthesia
Agents used for inhalation anaesthesia are volatile liquids, which are vaporized by passing a gas such as oxygen through or over them. The vapour/oxygen mixture is then inhaled. They are then absorbed from the lung alveoli into the bloodstream and are predominantly excreted the same way. This means that induction and recovery from anaesthesia are quicker with inhalation than injectable agents. This is important in regaining normal physiology, to control post-operative hypothermia and fluid or electrolyte imbalance. Recovery usually occurs within 1–10 min, although if animals have been maintained for an hour or more on inhalation anaesthesia, full recovery can take 20 min or longer.
The major advantage of inhalation anaesthesia is the ability to adjust the depth of anaesthesia. If the anaesthesia is too light, the concentration of vapour is simply increased, and within a few breaths the depth of anaesthesia will increase. Similarly, if the anaesthesia is too deep, the concentration can be reduced and the excess anaesthetic is breathed off until the depth of anaesthesia lightens. This makes inhalation anaesthesia relatively safe compared with injectable anaesthesia. Reversal of overdose of anaesthetic is quicker and usually more successful with inhalation agents than injectable ones, so reducing mortality. Recovery can be hastened if attention is paid to controlling the depth of anaesthesia during the procedure. As anaesthesia progresses the concentration of anaesthetic can usually be reduced slightly, and a further reduction in depth can be made during suturing of subcutaneous tissues and skin, so that recovery time following completion of surgery is reduced.
Some metabolic breakdown of inhaled agents does occur, although this is minimal with modern volatile agents such as isoflurane (<1%), and there is little or no induction of liver microsomal enzymes, which occurs with some injectable agents. This makes inhalation the anaesthetic method of choice where normal metabolism is required. Inhalation anaesthesia is particularly suited to short procedures, repeated anaesthesia and procedures where rapid return to normal metabolism is essential.
The disadvantages of inhalation anaesthesia are that a considerable amount of specialised equipment is required to deliver the anaesthetic vapour to the animal. Waste gases produced are a potential health and safety hazard to the user and have to be disposed of appropriately. Inhalation anaesthetics are greenhouse gases and globally contribute the equivalent of 1 million cars to global warming each year11.
Considerable skill is required to set up and operate an inhalation system. Despite these drawbacks, inhalation remains the anaesthetic method of choice for most procedures.
The volatile anaesthetic agents
Volatile agents are delivered to the animal from an anaesthesia machine using oxygen, or a mixture of oxygen and nitrous oxide, as the carrier. There are several volatile agents available. How these agents work remains incompletely understood.
The required concentration of volatile agent depends on the properties of the agent, not the weight of the animal. The dose an animal receives depends on the concentration of agent in the inhaled gas mixture, and its tidal volume. Therefore, heavier animals do not necessarily need a higher concentration of anaesthetic, but will receive a greater dose as they have a larger tidal volume. Each agent has a minimum alveolar concentration (MAC), which is the concentration at which anaesthesia is induced in 50% of animals (see Table 9.2).
Table 9.2 Induction and maintenance concentrations of volatile agents.
| Induction | Maintenance | |
| Anaesthetic | concentration (%) | concentration (%) |
| Sevoflurane | 5.0–8.0 | 3.3–4.0 |
| Isoflurane | ||
| Small animals | 3.0–5.0 | 1.25–3.0 |
| Large animals | 4.0–8.0 | 1.5–3.0 |
Isoflurane is a volatile liquid at room temperature which vapourises readily, with a boiling point of 48.5°C. It is completely nonflammable. Isoflurane produces very rapid induction and recovery from anaesthesia, allowing the depth of anaesthesia to be altered very readily. It reduces pain sensitivity and relaxes muscles, producing all three components of the anaesthetic triad to an acceptable degree. It can be used just with oxygen to produce anaesthesia suitable for most surgical procedures, and can be combined with opioid analgesics or nitrous oxide where additional analgesia is needed.
Isoflurane undergoes no metabolism and does not affect hepatic microsomal enzymes, so is suitable for studies requiring normal liver metabolism12. The maximum exposure limit for personnel is 50 ppm13.
Isoflurane causes some respiratory and cardiovascular depression, leading to a fall in blood pressure due to vasodilation. Although not irritant it has a pungent odour, which can cause some animals such as rabbits to breath-hold on induction. Although its use is declining in human medicine it is used commonly in veterinary anaesthesia.
Sevoflurane is a nonflammable liquid used for induction and maintenance of general anaesthesia. It has a boiling point of 58.6°C. Together with desflurane, it is replacing isoflurane in human anaesthesia. It is highly volatile, with a very fast induction and recovery. It is good where mask induction is needed as it is not irritant and is sweet-smelling. However, it produces profound respiratory depression in some species and there is a risk of accidental overdose due to the speed with which changes in inhaled concentration take effect. Sevoflurane is unstable in the presence of soda lime1 so should not be used in rebreathing anaesthetic circuits (see below). There is no workplace exposure limit defined for sevoflurane. The global warming potential of sevoflurane is lower than that of other inhalation agents.
Nitrous oxide is a colourless non-flammable gas with a slightly sweet odour. It is a weak general anaesthetic, produing euphoria on inhalation, but it is not potent enough for induction or maintenance of anaesthesia in most species. However, it has minimal cardiovascular and respiratory effects, and can be used together with a volatile agent to reduce the required concentration of that agent, reducing its side effects, while providing additional analgesia. It can be used 60/40 or 50/50 with oxygen to deliver the volatile agent for anaesthesia in larger species. It may also be beneficial in prolonged anaesthesia over 24 h, where administration of volatile agents in 100% oxygen can lead to oxygen toxicity. After prolonged anaesthesia, 100% oxygen should be given for 5–10 min, or oxygen can be displaced from the lungs by the nitrous oxide as it is breathed off, causing diffusion hypoxia, which can lead to suffocation. The occupational exposure limit for nitrous oxide is 100 ppm.
Anaesthetic machines
It is recommended that an anaesthetic machine with an appropriate vaporizer be used for inhalation anaesthesia. These allow precise control of the amount of volatile anaesthetic agent delivered to the animal, allowing precise control of the depth of anaesthesia. The components of an anaesthetic machine are:
- a source of oxygen (with or without nitrous oxide) as a carrier gas,
- a flowmeter to measure the oxygen flow,
- an anaesthetic vaporizer,
- a delivery system from the vaporizer to the animal,
- a mechanism to deal with waste gases.
Medical-grade oxygen and nitrous oxide can be provided from cylinders of compressed gas attached to the anaesthetic trolley, or piped into the facility. Where cylinders are used a reducing valve attaches to the cylinder to ensure that a constant low pressure of gas is delivered to the system. From the cylinder, the carrier gases pass through flowmeters, which allow volume of gas passing to the animal to be monitored and adjusted. The flowmeter consists of a graduated glass tube with a bobbin, which floats at a level determined by the amount of gas passing through the tube. The tube is calibrated such that the top of the bobbin is in line with the marking indicating the flow rate of the gas. The bobbin should rotate in the tube. Each flowmeter is calibrated specifically for a particular gas.
The carrier gases are then passed through a vaporizer, over the volatile agent. The vaporizer delivers a known concentration of the anaesthetic vapour over a given range of flow rates and temperatures. Each vaporizer is calibrated for a particular agent and should not be used with other agents.
All anaesthetic equipment must be serviced regularly to ensure the correct quantities of vapour are delivered. The machine must be checked before each anaesthetic is given to ensure the gas cylinders and vaporizer are full, there are spare cylinders, a key to turn them on is available and the flowmeters are working. The anaesthetic circuit, endotracheal tubes and scavenging system must be checked to make sure they are intact, correctly attached and functional. If any monitoring equipment is to be used it must be set up properly and in working order. Only once all these have been checked should the animal be anaesthetised.
Induction of anaesthesia
Inhalation anaesthesia can be induced in larger animals, such as pigs, using a face mask. Animals may need sedation for this, as many resent the handling required. In small animals they can be placed in an induction chamber, and oxygen containing anaesthetic vapour piped into the chamber. The chamber is a simple box with transparent sides so that the animal can be observed during the induction, and must be easy to clean. Absorbent paper can be placed on the floor of the chamber. The inlet pipe is at low level and the extract at high level, since the volatile agents are heavier than air. Anaesthesia is induced by piping in the induction concentration of the agent (see Table 9.2). As the animal breathes the anaesthetic vapour, it is absorbed in the lungs and begins to circulate. The concentration in the CNS starts low and gradually builds, until enough has been absorbed to induce anaesthesia. This can take several minutes and there is a time lag between inhaling the vapour and the onset of anaesthesia. The speed of onset depends on the concentration of vapour in the inspired gas and the particular agent used. To increase the speed of induction, the concentration of vapour in the inspired gas may be increased to a level which, if it were maintained, would cause excessive depression of the CNS and death. This is known as the induction concentration. Once anaesthesia is induced – the animal has lost its righting reflex, is immobile in the chamber and breathing regularly – the animal can be removed from the chamber. Anaesthesia will persist for a brief period (about 1 min), which may be sufficient for short procedures (such as ear punch). For longer procedures, anaesthesia can be maintained via a face mask or endotracheal or intranasal tube for as long as is required. The concentration of anaesthetic vapour must be reduced to a suitable maintenance concentration (Table 9.2). The concentration needed will vary with the individual animal, the nature of the procedure, and whether other drugs have been given to provide additional analgesia. The major advantage of inhalation anaesthesia is that the concentration can be adjusted to meet the exact needs of the individual animal.
After the procedure the chamber should be tipped to empty out any residual anaesthetic gas, and be cleaned between animals; otherwise pheromones left by previous occupants can cause stress to animals newly placed in the chamber.
Maintenance of anaesthesia: anaesthetic circuits
To maintain anaesthesia, the mixture of gases and volatile agent is delivered to the animal through an anaesthetic circuit. There are essentially two types of circuit: those where carbon dioxide is absorbed (rebreathing circuits) and those where it is not (non-rebreathing circuits). The particular one chosen depends on the size of the animal, the site of the operative field, whether intermittent positive pressure ventilation (IPPV) is to be used, and personal preference. The quantity of oxygen piped into the circuit depends on the needs of the animal (which depends on its body weight), and the type of circuit.
For non-rebreathing circuits, the expired air is not re-inhaled, and fresh gas from the anaesthetic machine is delivered continually to the animal. The exhaled gases are swept out of the open end of the circuit by the fresh gases flowing in during the expiratory phase. If the fresh gas flow rate is too low, rebreathing may occur, and carbon dioxide can build up to dangerous levels as there is no method of removing it. It is essential, therefore, to have a high-enough flow rate to remove the alveolar gas when it is expired so the animal does not rebreathe exhaled gases.
Most laboratory animals are small, and therefore have small tidal volumes. They also have limited capacity to continue breathing if there is resistance in the anaesthetic circuit. For these animals it is important that an anaesthetic circuit is used with low resistance to breathing and a low dead space.
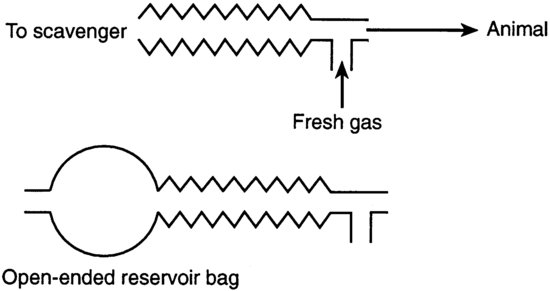
T-piece circuits have minimal resistance and little dead space and are the circuits of choice for animals up to 3 kg in body weight (Figure 9.2). The open tube acts as a reservoir of fresh gas. Exhaled gases are pushed out of the open end of the reservoir tube by fresh gases flowing in from the anaesthetic machine during the expiratory phase. T-piece circuits require a high gas flow rate, at least twice the animal's minute volume, and are therefore expensive to use for larger animals.
FIGURE 9.2 T-piece circuits.
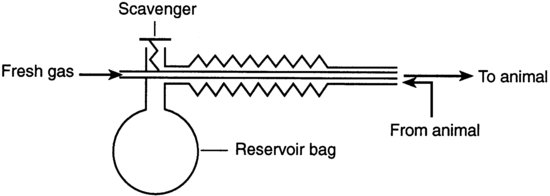
Co-axial circuits are a modification of the T-piece where the fresh gas inflow pipe runs inside the reservoir limb. During expiration the dead-space gases pass down the outer corrugated tubing to the scavenger. Some co-axial circuits, for example the Bain circuit (Figure 9.3), have a valve on the expiration arm. As the system fills, the pressure increases to a level at which the expiratory valve opens and then expired alveolar gas escapes through the valve. Since the valve increases resistance to breathing these circuits cannot be used with small rodents. They are suitable for animals of 3–10 kg, and a minimum flow rate of 100–300 ml/kg/min is needed to prevent rebreathing.
FIGURE 9.3 Bain co-axial circuit.
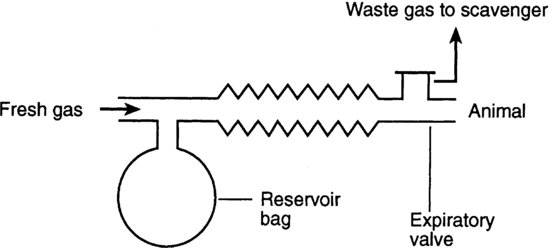
The Magill circuit (Figure 9.4) is suitable for animals between 10 and 40 kg. A fresh gas flow of 80–90% of minute volume (respiratory rate×tidal volume) is needed, making this economic to use in these larger animals. This circuit is only suitable for animals of 10 kg and over because the valve causes high resistance and it has a relatively high dead space.
FIGURE 9.4 Magill circuit.
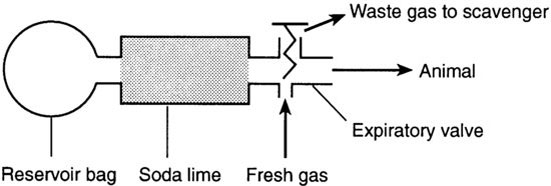
In rebreathing circuits, carbon dioxide in the exhaled gases is absorbed by soda lime so that the anaesthetic vapour can be re-circulated with the addition of fresh oxygen. The required gas flow rate is low, and re-use of expired anaesthetic vapour makes them very economical. Nitrous oxide should not be used with closed systems, since it is impossible to monitor the concentration in the recycled gas. One disadvantage is that the packed soda lime increases the resistance to respiration and dead space. This makes these systems unsuitable for animals under 15 kg in weight. The conservation of heat in the system may give rise to heat stroke in sheep. Sevoflurane should not be used with rebreathing systems.
In the To and fro system (Figure 9.5), the canister containing soda lime is placed between the animal and the reservoir bag to absorb the carbon dioxide. The dust from the soda lime may be inhaled and cause inflammation in the respiratory tract.
FIGURE 9.5 To and fro system.
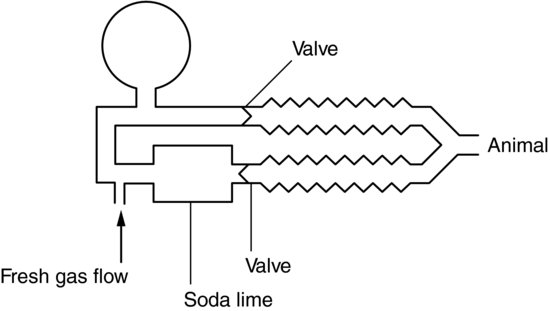
In Circle systems (Figure 9.6) there is a circular flow of gas controlled by two unidirectional valves. They are designed for large animals, as there is high resistance in the system.
FIGURE 9.6 Circle system.
Maintenance of anaesthesia: face masks and endotracheal tubes
The anaesthetic vapour and carrier gas are delivered from the circuit to the animal via a face mask or endotracheal tube. Face masks must fit snugly over the nose and mouth but not rub the eyes. Gas invariably leaks from around the mask, contributing to pollution of the atmosphere, so these should either be used in a flow hood, or a face mask incorporating a gas-scavenging system used. Face masks require a flow rate of three times the animal's minute volume.
Endotracheal tubes are rubber or plastic tubes inserted into the trachea. These are available in different sizes for different species, with or without an inflatable cuff, which is used to make an airtight seal in the trachea. The tube provides a patent airway so that artificial respiration may be given if required. Endotracheal intubation requires skill, and in some species the use of a laryngoscope. After induction of anaesthesia, the lubricated tube is passed over the tongue and epiglottis, and through the larynx. Once in situ, the cuff, if present, is inflated to seal the trachea and prevent the animal from breathing around the tube. Inflatable cuffs should not be used for tubes smaller than 5 mm diameter.
The size of tube used depends on the size of the animal, but as a rough guide adult beagles need a 9 or 10 mm tube with a cuff, cats need a 3–5 mm tube without a cuff and rats need a 16–12 G arterial cannula or modified urinary catheter. Dogs, cats and primates may be intubated while lying in left lateral recumbency (right-handed operator). An assistant holds the head and neck extended and lifts the upper jaw. The operator pulls the tongue forward and down, and usually the larynx can then be visualised. The tube is then passed gently through the glottis into the trachea. In cats, the larynx should be sprayed with local anaesthetic prior to passage of the tube to prevent laryngeal spasm.
Pigs have a particularly long larynx, and a laryngoscope is required to illuminate the larynx and hold down the tongue and epiglottis.
Small animals can be intubated via surgical implantation of a tube in the trachea in non-recovery procedures, or by passing a tube carefully into the mouth. For rats the animal is placed on its back and a laryngoscope or otoscope inserted into the mouth to visualise the larynx14. An alternative to a laryngoscope can be fashioned from a syringe barrel15. The tube can then be inserted through the glottis. For rabbits, the animal is placed in ventral recumbency and the head and neck extended by an assistant so the neck is straight. The tube is inserted in the mouth to one side of the incisors then straightened and advanced as far as the glottis. The tube is then advanced into the larynx during the inspiratory phase of respiration. Alternatively, an assistant stands in front of the prone rabbit holding its head up and pulling the tongue forward. The operator stands behind the rabbit, and places the tube as above16,17,18.
Endotracheal intubation in mice, gerbils and hamsters is difficult and requires skill and purpose-made apparatus1. This often has to be done following induction with injectable anaesthetics, because the animal may wake from inhalation anaesthesia in the time taken to place the tube. A method has been described in which intubation is facilitated, allowing for intubation after inhalation induction19.
An alternative to endotracheal intubation is the use of a nasal catheter. This is effective in many rodents as they are nasal breathers. Small nasogastric tubes, urinary catheters or intravenous catheters can be used. After induction of anaesthesia the catheter is inserted through the external nares and into the nasal passage, by applying gentle pressure and directing the catheter medially and ventrally. The typical gas flow rate required is three times the minute volume. Animals may sneeze if too lightly anaesthetised, and there may be mild epistaxis, which can be stopped by applying pressure to the side of the nose20.
Health and safety
Volatile anaesthetic agents pose some risk to human health. There may be alterations in cardiorespiratory function, hepatotoxicity occurs infrequently but has been reported and all modern agents can trigger malignant hyperthermia21. Prolonged exposure to some agents can cause abortion. Each volatile agent has a defined occupational exposure limit (OEL), which should not be exceeded, and so either an active or passive scavenger system must be used to extract the waste gases and dispose of them safely. A length of tubing attached to the expiratory port of the circuit can be used to duct waste gases to the outside, or into activated charcoal. Alternatively, the apparatus may be used in an extraction cabinet. Activated charcoal canisters must be weighed regularly and changed when they reach a specified weight. Activated charcoal will not remove nitrous oxide.
Injectable anaesthesia
Injectable agents are simple to administer with a minimum amount of equipment. This is useful when operating in the field or when equipment for administration of volatile anaesthetic agents is not available. With intravenous injection, anaesthesia is induced immediately, minimising stress. They are particularly useful for the induction of anaesthesia, which can then be maintained by inhalation, or for short-duration anaesthesia.
Modern injectable agents are available that are short-acting, and which can be given by intravenous infusion, allowing for improved control of the depth of anaesthesia and overcoming some of the disadvantages of using injectable agents.
When injectable agents are used it is still sometimes helpful to administer oxygen, to prevent development of hypoxia, for example during long-term anaesthesia lasting for more than 1 h, in which respiratory depression can lead to hypercapnia and acidosis. The ability to administer oxygen and control ventilation helps control many potential difficulties encountered in injectable anaesthesia.
Induction
Injectable agents are given either intravenously directly into the bloodstream, or via another parenteral route from which they are absorbed into the blood. Intravenous injections usually act very rapidly, producing loss of consciousness almost immediately. The calculated dose can be administered slowly, allowing time for the drug to circulate before deciding whether more is required. The drug can, therefore, be administered to effect and titrated to the exact requirements of the animal. Intravenous administration can be facilitated by applying local anaesthetic to the site of venipuncture prior to injection.
The small size or problems of restraint in many laboratory animals means that injectable agents are most frequently given via the intramuscular or intraperitoneal routes as a single dose. With this route, a large total dose is needed, and absorption can be slow and unpredictable, leading to variable induction. Giving the drug as a single bolus can result in overdosage, which is difficult to reverse, and the animal must be weighed first to ensure accurate dosing. Residual drug effects persist for long periods and full recovery can be prolonged. There is also the possibility of accidental injection into a blood vessel or major abdominal organ, which could have serious consequences. Ideally injectable anaesthetics should have a wide safety margin, and be able to be given in a small volume through a small needle.
A considerable amount of skill is required to perform intravenous injection. For all other routes less skill is required, but it is not possible to dose to effect, since the response to injection is slow. For injectable agents, more than inhaled agents, the level of anaesthesia achieved depends on the level of stimulation of the animal at the time. If an animal is stressed, for example due to poor handling or restraint in the pre-anaesthetic period, it will require a higher dose of anaesthetic agent, leading to a greater risk of overdosage and adverse effects. An animal that is given an injection and left undisturbed may be apparently deeply anaesthetised, but if stimulated it may respond by reflex movements of the limbs and an increase in the depth and rate of breathing. This does not necessarily mean that a further injection of anaesthetic is required: if more is given, and stimulation then stops, there is a risk that the level of anaesthesia will become dangerously deep. The depth and duration of anaesthesia produced is therefore variable.
Maintenance
Injectable anaesthetics produce variable periods of anaesthesia depending on the agent used. If maintenance is required this can be achieved by giving intermittent top-up injections, by continuous intravenous infusion, or by using an inhaled agent for maintenance. The response to injectable anaesthetic agents varies with the individual animal, being affected by sex, strain and age. This is true for all anaesthetics, but adjusting the depth of injectable anaesthesia to account for these differences is more difficult than for inhalation anaesthesia.
Intravenous injections allow dosing to be adjusted according to the individual animal's response, so there is less likelihood of over- or under-dosing. Many drugs and combinations can be given by constant intravenous infusion for prolonged anaesthesia.
Giving intermittent injections for maintenance inevitably results in variable depth of anaesthesia during the procedure. Monitoring the depth of anaesthesia is therefore particularly important, so that supplemental injections can be given in good time to prevent the animal from feeling pain, as there will be a time lag between the top-up injection and deepening of anaesthesia. It is not acceptable to give a further dose by injection as the animal starts to wake up. It is important to be familiar with the techniques required for the administration of injectable agents, and the type of anaesthesia obtained with particular anaesthetics in the type of animal to be used.
Recovery
Most injectable anaesthetics are excreted by the kidneys after metabolism in the liver. This process can be prolonged, leading to slow recovery from anaesthesia, but may also affect hepatic enzymes, interfering with some studies.
The action of some injectable anaesthetic agents can be reversed using specific antagonist drugs. Several antagonists are available commercially, and these can partially reverse some anaesthetic combinations. They have several major benefits.
- By reversing the sedation and reducing sleep time, animals become active more rapidly, and are less at risk of hypothermia.
- Many anaesthetic drugs depress respiration, and this depression can be promptly and effectively reversed with antagonist drugs.
- An additional advantage of sequential analgesia can be obtained when reversing pure agonist opioids, such as fentanyl, with partial agonists such as buprenorphine. The side effects produced by fentanyl are reversed, but the analgesia persists because of the action of the bupenorphine1.
Injectable agents
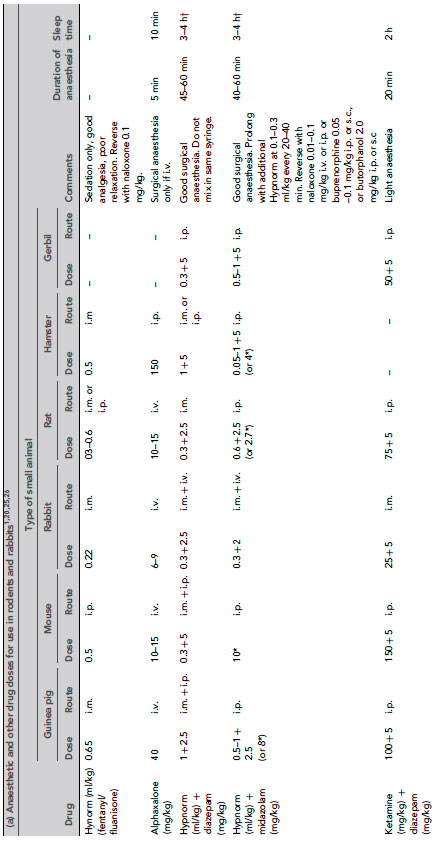
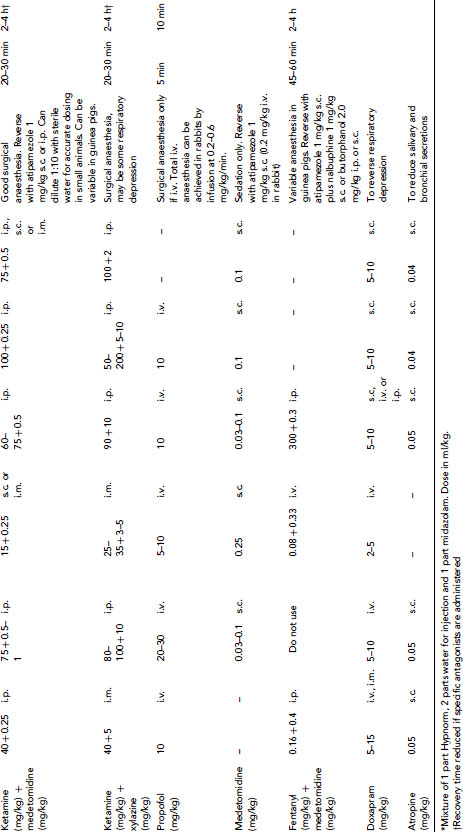
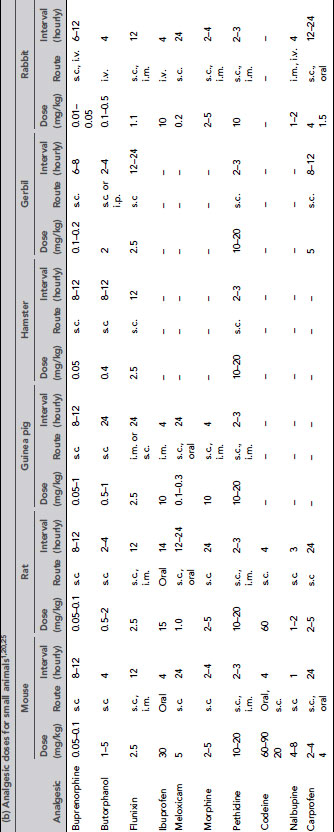
Many injectable agents are available. The quality of anaesthesia produced by different agents varies considerably. Very few anaesthetic agents can produce general anaesthesia when used alone, and most injectable agents are given in combinations. Even with agents that can induce general anaesthesia alone, reduction of anxiety with suitable premedicants, additional analgesia or use of an inhaled agent for maintenance may be required. Table 9.3 gives doses of anaesthetic drugs for small laboratory animals.
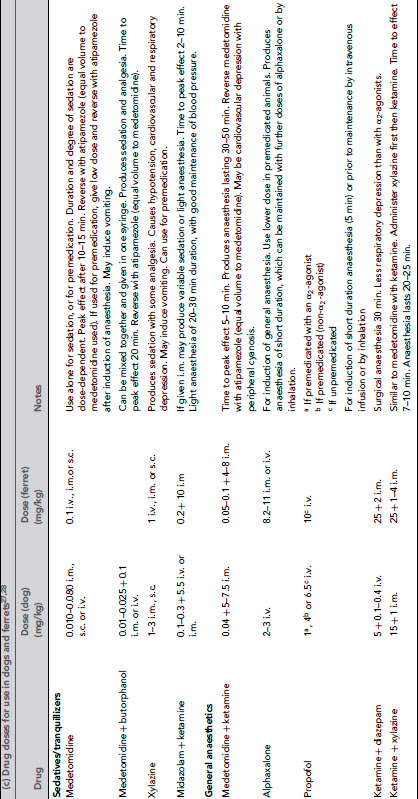
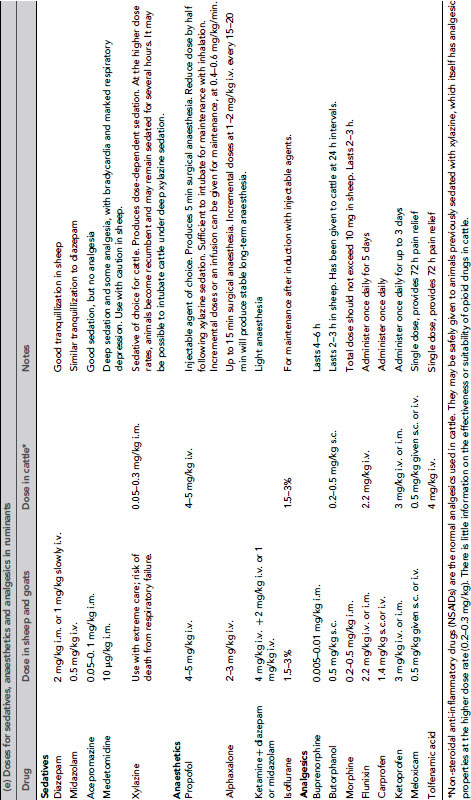
Table 9.3 (a) Anaesthetic and other drug dose rates for in rodents and rabbits1, 20, 25,26. (b) Analgesic doses for small animals1, 20,25. (c) Drug doses for use in dogs and ferrets 27,28. (d) Anaesthetic and analgesic dose rates for chickens. (e) Doses for sedatives, anaesthetics and analgesics in ruminants. (f) Sedative, anaesthetic and analgesic drug dose rates for pigs. (g) Dose rates for anaesthetics and analgesics in primates.




Propofol
This should be given by slow intravenous injection, as rapid administration may induce apnoea. It acts rapidly, inducing anaesthesia smoothly without excitatory side effects. It is ultra-short acting, and recovery is rapid and smooth. It can be used by continuous infusion for prolonged anaesthesia. It can be used safely in cats, dogs, monkeys, pigs and rabbits, and can be combined with a wide range of premedicants, analgesics and inhaled agents. Utility in small rodents is limited, however, due to the need for intravenous administration.
Alphaxalone
This is non-cumulative and produces good muscle relaxation with excellent cardiovascular stability and minimal respiratory depression except at high doses22. Intravenous administration rapidly results in anaesthesia, but the calculated dose should be given slowly, over 60 s, to avoid apnoea. It can be used for induction of anaesthesia prior to maintenance with inhalation agents, for short-duration anaesthesia or for long-term anaesthesia by intermittent injection or continuous infusion. Analgesia produced is limited, and additional analgesia may be required.
Recovery following anaesthesia is rapid, but it is preferable that animals are left undisturbed in the recovery period, otherwise paddling, minor muscle twitching or more violent movements may be seen.
At the time of writing, there has been limited use of alphaxalone in laboratory animals. However, it has been shown to produce dose-dependent pre-emptive analgesia in rats23, and has been used for anaesthesia in rats in studies requiring avoidance of significant respiratory and haemodynamic depression while facilitating mechanical ventilation24. It is likely that it will have a place in laboratory animal anaesthesia.
Pentobarbitone
This is a barbiturate that depresses the CNS and produces marked cardiovascular and respiratory depression. It has poor analgesic properties and very large doses are needed to reduce pain perception. It has a low therapeutic index: the lethal dose is only slightly above the clinical dose. Even after intravenous injection onset of anaesthesia is slow. Injections must be given very slowly to allow anaesthesia to deepen to its full extent before giving extra doses. If it is given intraperitoneally as a single bolus it has a poor safety margin, and mortality can be high. Recovery from anaesthesia is slow, and may be associated with convulsions and paddling. Its use is not recommended for recovery anaesthesia. In concentrated form, pentobarbitone is used for euthanasia.
Combination anaesthetics
Ketamine
This is an N-methyl-D-aspartate (NDMA)-receptor antagonist, which can be given by intramuscular or intravenous injection. It circulates rapidly after administration and produces analgesia but little muscle relaxation, and animals may exhibit tremors and remain responsive to external stimuli, particularly sound. Animals seem not to be aware of their surroundings, and analgesia varies with the species. It is the agent of choice for sedation of Old World monkeys. The corneal reflex is lost very quickly, and it is essential to protect the eyes with a bland ophthalmic ointment. The laryngeal and pharyngeal reflexes are maintained, but there is an increase in salivary secretions, which can cause airway obstruction, so ketamine is sometimes used with a drying agent such as atropine. There is good maintenance of blood pressure, but in most species the dose required to produce surgical anaesthesia causes severe respiratory depression and it is not used alone. It can be combined with xylazine, medetomidine, diazepam, midazolam or alphaxalone to produce general anaesthesia in many species (sheep, primates, pigs, rabbits and rodents). Using ketamine in combination with other agents also avoids side effects, such as muscle tremors.
Narcotic analgesics (opioid analgesics)
Opioids such as fentanyl, alfentanil and sufentanil are useful as premedicants, components of balanced anaesthetic regimes and in neuroleptanalgesia (see below). They are potent analgesics but have significant side effects such as respiratory depression. Fentanyl is a potent analgesic that lasts for 15–20 min. In rats, dogs and primates it has sedative effects, but in mice, cats and horses it causes excitement. Alfentanil is less potent than fentanyl but the onset of activity is more rapid. Sufentanil is 10 times more potent than fentanyl, but has not been used extensively in laboratory animals.
Neuroleptanalgesia
Combinations of opioid analgesics and sedatives produce neuroleptanalgesia, which is similar to a light plane of anaesthesia. The animal no longer responds to stimuli, but is not totally unconscious. Neuroleptanalgesics produce some respiratory depression and poor muscle relaxation, but when combined with benzodiazepines these problems are markedly reduced and a state of neuroleptanaesthesia may be produced, under which surgical procedures may be carried out. Combinations of fentanyl/fluanisone with either midazolam or diazepam can provide good anaesthesia for rodents and rabbits. Midazolam is water-soluble and can be administered in the same syringe as the fentanyl/fluanisone. It can also be diluted with sterile water for accurate dosing in small rodents.
To speed recovery and counteract side effects, the opioid component of neuroleptanalgesics can be reversed at the end of surgical procedures by either narcotic antagonists (such as naloxone) or partial agonists (such as buprenorphine or butorphanol), which also provide post-operative analgesia. Naloxone reverses analgesia as well as any side effects, so it must not be used unless surgery has been completed and adequate analgesia provided by some other means. Reversal is not always required, as most neuroleptanalgesics will wear off by themselves, but recovery without the use of antagonists can be prolonged.
Medetomidine and atipamezole
Medetomidine can be given by intravenous, intramuscular or subcutaneous injection. Animals should be left undisturbed for 10–15 min after administration for the drug to reach maximum effect, preferably in a quiet environment, as they may still react to noise. Medetomidine does not induce general anaesthesia when used alone, but is excellent when used in combinations. If used as a premedicant it reduces the requirement for inhaled anaesthetics markedly, and can be used with fentanyl/fluanisone to produce good anaesthesia and analgesia. It may also be used successfully with ketamine in many species. The biggest advantage of medetomidine is that it can be reversed very rapidly with the specific antagonist atipamezole. See also the section on premedication, above.
Long-term and non-recovery anaesthesia
Long-term anaesthesia may be required for some procedures. This can be achieved with intermittent top-up injections by the intramuscular or intraperitoneal routes, although the depth of anaesthesia can vary markedly. If the top up is not administered in time the animal may experience pain, so good anaesthetic monitoring is essential. Alternatively an anaesthetic can be given by continuous intravenous infusion (e.g. alphaxalone, propofol or fentanyl/midazolam). Short-acting agents enable the plasma concentration and depth of anaesthesia to be adjusted quickly, and lead to a rapid recovery. Many different types of infusion pump are available to facilitate administration. Long-term anaesthesia can also be achieved by the use of inhaled agents for maintenance, after induction with inhaled or injectable agents.
Many experimental protocols require terminal anaesthesia. There is little difference between recovery and non-recovery anaesthesia in terms of animal care and management, although some more invasive surgical techniques such as surgical exposure of a blood vessel for catheterisation and tracheostomy creation are more often used if the animal is not to be allowed to recover from anaesthesia.
Some anaesthetic agents are only suitable for non-recovery experiments, e.g. alpha-chloralose results in a very prolonged recovery period associated with considerable involuntary excitement, and urethane can produce severe peritoneal irritation and is carcinogenic. Chloral hydrate is a very poor analgesic in many species, and can cause paralytic ileus in the rat.
Recovery and post-anaesthetic care
At the conclusion of a procedure it is important that the animal wakes up as soon as possible, so that its physiology returns to normal. It is better to have a rapid recovery and provide adequate post-operative analgesia than to have prolonged anaesthesia. With inhaled agents the vaporizer can simply be switched off and the animal allowed to breathe oxygen for a few minutes until the anaesthetic gas has been exhaled, resulting in recovery within a few minutes. Injectable agents usually have to be metabolised by the liver and excreted by the kidneys. This process can be prolonged, and recovery from injectable anaesthetics usually takes longer than for inhaled agents. This increases the risk of hypothermia or dehydration. Some injectable drugs have specific reversal agents, the administration of which will lead to a return to consciousness within minutes. Reversal agents can also be used to reverse any respiratory depression, which develops in the post-operative period. Some injectable agents are ‘ultra-short-acting’, and are metabolised very quickly. Use of these newer drugs has overcome some of the problems previously associated with injectable agents.
The quality of care following recovery from an anesthetic can have a dramatic effect on the speed of recovery. If not cared for properly, semi-conscious animals may lie in their urine or faeces and develop skin lesions, or get bedding material in their eyes and noses. Their cage mates may attack them, and they may remain inappetant for long periods. Many of the monitoring procedures described below should be continued in the immediate recovery period. This helps to ensure that optimal attention is given to the animal, and that all the measures needed to ensure its comfort and well-being are undertaken promptly and effectively.
Since all animals will require some degree of special attention in the recovery period, it is preferable to provide a separate recovery area. This not only enables more appropriate environmental conditions to be maintained but also encourages individual attention and special nursing. Individual housing will often be indicated anyway to prevent problems such as injury of semi-conscious animals by their cage mates.
The recovery area should be stocked with a range of emergency drugs and equipment. Additional apparatus such as monitoring equipment may be needed after major surgery and/or prolonged anaesthesia.
The prevention and management of pain following a surgical procedure is a particularly important aspect of care in the recovery period. Pain can produce physiological changes that may alter the rate of recovery from surgical procedures, and that these changes may also affect the experimental results. Acute pain can be alleviated by the use of centrally or peripherally acting analgesic drugs administered systemically, by local anaesthetics or by the application of supporting bandages or other means of protecting and immobilising damaged tissues. The selection of a particular treatment will vary, depending upon the nature of the pain, its cause and its estimated severity and duration. For mild to moderate pain, non-steroidal anti-inflammatory drugs (NSAIDs) such as meloxicam, carprofen or flunixin may be sufficient. For moderate to severe pain, opioid analgesics such as buprenorphine may be needed.
The degree of personal attention given to animals will depend upon the species. Some animals may respond well to personal contact, whereas this may be stressful for others. Recovering animals should be left in a warm environment with subdued lighting, and be disturbed as little as possible. The animal should be on comfortable bedding and kept clean and dry. Animals may need additional fluid, given by injection or orally, and it may be appropriate to offer palatable food. Urine and faecal output should be monitored. All clinical observations and drugs administered must be recorded.
Local anaesthetics
Local anaesthetics act directly on nerves to block the conduction of impulses, thus eliminating pain and other sensations. Both local and regional anaesthesia are widely used in the management of pain in humans, and there are also well-established techniques in clinical veterinary practice. They are still relatively under-used in the smaller species of laboratory animals, principally because although pain sensation may be abolished there still remains the problem of restraint in these species.
Local anaesthetics may be applied topically, by injection, locally into the skin and/or underlying tissues in a specific area, or as a nerve block, for example epidural or paravertebral block, which results in desensitisation of a large area, known as regional anaesthesia. Local anaesthetics have an important role in providing anaesthesia and pain relief for large animal species. When combined with a sedative or hypnotic to provide humane restraint they can be used for major surgery and remove any of the risks associated with general anaesthesia.
Topical application
Some agents are available for direct administration to tissues. Drops can be used to desensitise the cornea and conjunctiva. An aerosol may be used for the larynx prior to intubation to prevent laryngospasm. For the skin, cream (e.g. EMLA, AstraZeneca) can be used to facilitate venipuncture.
Infiltration of the skin and underlying tissues
This method is useful for minor techniques, such as skin biopsies or suturing minor wounds. Injectable local anaesthetic is administered using a very fine needle. Agents such as lignocaine or bupivacaine can be infiltrated around the surgical site to provide analgesia during surgery or post-operatively. This method can be very useful for provision of post-operative analgesia, since there are no systemic side effects. Lignocaine preparations typically last 1–2 h, and bupivacaine can provide analgesia for up to 8 h20. Some preparations of lignocaine contain adrenaline to prolong efficacy: care should be taken not to overdose with these as this may delay wound healing.
Regional anaesthesia
Injectable local anaesthetic can be injected around a major nerve to desensitise a particular area. The use of nerve blocks for regional anaesthesia requires a detailed knowledge of anatomy and specific training in the techniques. The technique can be used for specific nerve blocks, such as the pudendal nerve for work in the pudendal region, and the cornual nerve block for dehorning or foot blocks using specific nerves to regions of the feet in horses. Epidural or spinal blocks involve injection of local anaesthetic into the epidural space or directly into the spinal cord to produce desensitisation of the region posterior to the site. This is a useful technique to provide anaesthesia of the pelvic region in cattle and sheep, and may be used post-operatively to provide continuing analgesia. A paravertebral block involves injecting local anesthetic around several lumbar spinal nerves as they emerge from the vertebral column to produce desensitisation of the flank. This method is useful in ruminants.
Although local anaesthetics may be very successfully used in the post-operative period as an adjunct to other methods of pain control, to provide adequate restraint and to reduce stress in most laboratory animals it is usually preferable to use general anaesthetics.
For doses of anaesthetic agents for each species, see Table 9.3.
Anaesthetic Management
All anaesthetic drugs will, if given in sufficient quantity, cause death. On the other hand, if an insufficient amount is given, the animal will feel pain. To maintain the animal between these two extremes, care must be taken in the maintenance of physiological stability, particularly airway patency, respiratory function, circulatory function and body temperature, and in monitoring the depth of anaesthesia, to ensure that the parameters of unconsciousness, analgesia and muscle relaxation are being met. Poor animal care during anaesthesia can result in prolonged recovery times, or in extreme cases, even death. The animal should be monitored frequently, at least every 5 min, or more often if stability has not been established, to ensure the animal is physiologically stable, and to determine the depth of anaesthesia. Records should be kept of the measurements to enable adverse trends to be spotted easily and corrective action taken. It also allows a series of anaesthetics to be compared. Anaesthetic monitoring depends on watching the animal closely and recording the measurements. An example of an anaesthetic record chart is shown at Figure 9.7.
FIGURE 9.7 Record of general anaesthetic.
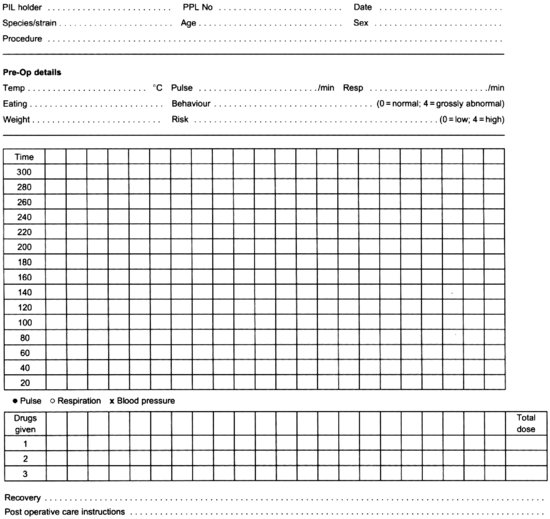
Personnel carrying out prolonged procedures may become fatigued, and steps should be taken to ensure that this does not impact on the care of the animal. There should be sufficient staff available to ensure that monitoring animals under anaesthesia is carried out effectively and diligently.
Physiological stability
There must be maintenance of adequate tissue oxygenation and removal of waste carbon dioxide during anaesthesia. The amount of oxygen reaching the tissues depends on several factors, as demonstrated by the oxygen flux equation:

where Q is cardiac output (depends on the efficacy of the heart) in millilitres per minute, arterial saturation (of the blood) depends on efficacy of the lungs, Hb is the haemoglobin level (g/ml) and K is a constant equal to 1.36.
Minor changes to one part of the equation may have little effect, but if there are changes in several parts the overall effect is magnified. Health problems can affect any or all parts of the equation, leading to a reduction in the quantity of oxygen available to the tissues. As general anaesthesia will reduce both cardiac output and lung efficiency, the combined effects of disease and anaesthesia can cause a severe reduction in tissue oxygen levels leading to hypoxia or even death. Animals must be monitored to ensure that there is adequate maintenance of tissue oxygenation. If anaesthesia is too deep the animal may be in danger of dying from respiratory and cardiovascular failure. The animal may exhibit slow shallow breathing, or deep gasping breaths, blue coloration of the mucous membranes and a fall in blood pressure. There may be a fixed, staring eye. Monitoring of the vital signs will detect these changes early on, and enable action to be taken to prevent death.
When monitoring animals under anaesthesia, it helps to remember A for Airway, B for Breathing and C for Circulation. The airway should be checked to ensure the animal can breathe and that the trachea is not blocked. For inhalation anaesthesia this should include checking that the anaesthetic machine is set up properly and that there is sufficient oxygen and anaesthetic vapour.
Breathing
Breathing is easily monitored, and assessments should be made of the rate, depth and pattern of breathing. This is done by watching the movement of the chest wall, or reservoir bag, or in larger animals by the use of an oesophageal stethoscope. An apnoea alarm can be useful. The effectiveness of pulmonary gas exchange can be assessed by the colour of the mucous membranes, and of blood being shed at the site of surgery. Virtually all anaesthetics cause some respiratory depression, leading to hypoxia and hypercapnia. Hypoxia may be indicated if the colour of blood or mucous membranes changes, but hypercapnia is not. Providing the animal with oxygen intra-operatively helps to prevent the development of hypoxia. Carbon dioxide concentration can be assessed by measuring the end-tidal CO2, or by direct arterial blood-gas analysis. Transcutaneous O2- and CO2-monitoring equipment can be used in some species.
The respiratory rate usually falls during anaesthesia, and a fall of up to 50% from the normal is acceptable. If the rate continues to drop, there may be a problem. If the animal is not breathing the anaesthesia may be too light and the animal holding its breath, or conversely the anaesthesia may be too deep. This demonstrates the need to monitor more than one variable. Apnoea on induction of anaesthetic is common, particularly with the use of certain anaesthetic agents. If there is genuine apnoea, first ensure that the airway is clear: the pharynx or trachea may be blocked with mucus or blood, particularly in small animals, or the endotracheal tube may be kinked. Make sure that the thoracic movements are not restricted by the position of the animal, or by limbs being tied down too tightly. Check that undue pressure is not being placed on the chest wall from instruments and take care not to lean on the animal.
Respiration can be stimulated by moving the endotracheal tube, artificial ventilation, needle stimulation of the nasal philtrum or by respiratory stimulants (such as doxapram). For some anaesthetic combinations antagonists are available and these can be given if surgery has finished. It is advantageous if the animal has been intubated, or in non-recovery cases a tracheotomy tube has been placed, to enable a clear airway to be maintained, or to facilitate intermittent positive pressure ventilation (IPPV).
Circulation
Some anaesthetics cause a fall in cardiac output, and hypotension. Hypoxia and hypercapnia, resulting from respiratory depression, cause cardiac dysrhythmias. Loss of blood or body fluids can cause hypovolaemic shock and cardiac arrest. Circulatory function should be assessed frequently during anaesthesia. The simplest method of monitoring is to assess the rate, rhythm and quality of the pulse, using a superficial artery depending on the species. In larger species a stethoscope or oesophageal stethoscope can be used to indicate heart rate and rhythm. An electrocardiogram (ECG) gives an indication of cardiac function, but it only indicates the electrical activity of the heart. It is possible to have a normal ECG with a cardiac output of zero for a short while. A pulse oximeter indicates the blood oxygenation level and the pulse rate. Capillary refill time indicates tissue perfusion. This can be tested by applying pressure briefly to a mucous membrane to blanch it, then measuring how long it takes for the pink colour to return. It should take less than 2–3 s. Cardiac failure may occur suddenly, but more usually it is gradual in onset, and frequent monitoring can spot declining cardiovascular function allowing intervention to prevent a disaster.
It may be necessary to give warmed isotonic fluids such as saline or Hartmann's solution to support the circulation. Blood can be given in larger animals: transfusion reactions are rarely encountered on a first transfusion and never if another animal of the same inbred strain is used. A secure venous line should be established for infusion of fluids. This allows easy administration of analgesics and anaesthetics, and facilitates dosing with emergency drugs. If intravenous access is not available, fluids can be given subcutaneously.
Body temperature
It is essential to monitor this, particularly in small animals. All anaesthetics affect thermoregulation, and body temperature will fall unless measures are taken to prevent this. This is exacerbated by the flow of cold air from the anaesthetic machine, shaving the animal, use of cold skin preps, placing on a cold operating table, exposing viscera during surgery and administering cold fluids. Smaller animals have a larger surface area to volume ratio, so are particularly susceptible to heat loss. Animals less than 1 kg in weight will require supplementary heating to prevent a drop in body temperature.
Temperature can be monitored by feeling the animals' paws and ears, and by using a rectal or oesophageal probe, and core temperature can be compared with skin surface temperature. Ideally the difference should be less than 2–3°C. To reduce heat loss, any shaving should be over a minimal area, the fur should not be wetted excessively and dry drapes should be used. The environment should be warm, and ideally the operating table should be heated. Fluids to be administered should first be heated to 38°C. Heat loss can also be minimised by insulation with cotton wool, bedding, foil or bubble wrap. Additional heating can be provided with heat lamps, heating blankets, instant heat pouches or hot water bottles. Thermostatically controlled heating devices are available. A temporary heat source can be made simply by filling a glove with warm water. Care must be taken not to burn the animal.
Measures to prevent hypothermia must be continued throughout the recovery period. This can be achieved in small animals by using incubators. For adults, the temperature should be 25–30°C, and for neonates 35–37°C. Bedding also helps provide insulation. Sawdust, however, should not be used as this will stick to the animal's nose and mouth, or to wound surfaces. Hypothermia is the commonest cause of mortality in small rodents and slows recovery in larger species, so monitoring of body temperature and taking steps to prevent hypothermia are vitally important.
Other considerations
Care must be taken that the cornea does not dry out during anaesthesia, as the animal will not blink while asleep, and the flow of gases from the anaesthetic machine may exacerbate corneal drying. A bland ophthalmic ointment should be applied to protect the corneas and prevent drying.
Animals should be placed on a warm and comfortable surface during anaesthesia. For large animals in particular it is important to pad bony areas to prevent the development of pressure sores during long procedures. In some animals it may be necessary to empty the bladder during long procedures, and in any case the animal may pass urine so should be placed on absorbent material.
Depth of anaesthesia
Historically, anaesthesia in humans has been described as having four stages, with stage three (surgical anaesthesia) being divided into three planes. This classification was devised for use with a single volatile anaesthetic, and relies on assessment of cardiovascular and respiratory signs. In animals, with different species and combinations of drugs, this becomes unworkable. There exists a spectrum of levels of consciousness/depth of anaesthesia and it is essential to know approximately where in that spectrum the animal is at any time.
Anaesthesia aims to produce narcosis, relaxation and analgesia. Consciousness must be lost to a sufficient degree to prevent distress. Muscle relaxation can be assessed simply by evaluating muscle tone in the limbs or tail in smaller animals, or lower jaw in larger animals.
There must be sufficient analgesia to prevent the animal perceiving pain. This can be judged by the presence or absence of certain reflexes. The pedal withdrawal reflex is the most commonly used reflex. This involves extending one hind limb and pinching the toe-web (or toe base in small rodents), and assessing whether there is any withdrawal of the limb or evidence of conscious response. The tail or ear (e.g. in rabbits) may also be pinched. These responses are abolished if surgical anaesthesia has been reached.
Ocular reflexes can be useful in some circumstances but are variable indicators of the depth of anaesthesia. The palpebral reflex is lost at variable times depending on the species. In rodents it is hard to assess, and in rabbits it may not be lost until anaesthesia is very deep1. If ketamine is used the reflex is abolished very early. The corneal reflex is lost later and may be seen when drops are applied to moisturise the cornea. Observation of the position of the eyeball, the degree of pupillary dilatation, and whether nystagmus is occurring are all useful indicators once experience is gained with one species and a particular anaesthetic regime.
Since anaesthetics cause dose-dependent depression of cardiovascular and respiratory systems, changes in respiratory and heart rates, blood pressure can be used in conjunction with assessment of reflexes to determine the depth of anaesthesia. However, the way this depression manifests varies with different anaesthetics and it is difficult to generalise about the effects. It is usual for there to be a slight increase in heart and respiratory rates under anaesthesia in response to surgical stimulation, but there should be no pedal withdrawal reflex.
Equipment monitoring
Aside from monitoring vital signs, it is essential to monitor the function of any equipment used. Anaesthesia machines and infusion pumps may be fitted with alarms, which sound when the machines fail, or are empty.
Anaesthetic Emergencies
Monitoring the parameters discussed above should allow for early detection of impending problems and appropriate corrective action before the development of an emergency. If an animal goes into circulatory arrest, and the cerebral circulation is not restored within 3 min, irreversible brain damage will occur. In a research setting, an animal which has experienced anaesthetic problems may no longer be a suitable experimental subject, and it may be appropriate to humanely kill the animal in such circumstances. If it is appropriate to try to resuscitate an experimental animal following an anaesthetic emergency, steps must be taken to maintain tissue oxygen levels, by ensuring the blood contains sufficient oxygen and is circulating properly.
Monitoring and resuscitation attempts are directed towards:
- A for airway,
- B for breathing,
- C for circulation.
First, check the airway is patent and that oxygen is available. Ensure the head and neck are extended and there is no blockage from mucus or blood. Then ensure the animal is breathing. In rodents and rabbits, a fall in respiratory rate to less than 40% of pre-anaesthetic value indicates impending respiratory failure1. If a volatile anaesthetic is used, reduce the inhaled concentration, and stop infusions of injectable agents or administer antagonists if available. Implement artificial ventilation if there is an endotracheal tube in place. If not, begin intermittent gentle compressions of the chest wall. It may be possible to stimulate breathing by stimulating the philtrum, or by use of respiratory stimulant drugs such as doxapram. Administer oxygen either directly via an anaesthetic machine or by placing the animal in an enriched oxygen atmosphere.
If there is evidence of circulatory failure, administration of fluids by the intravenous route may be sufficient to restore blood pressure. External cardiac massage can be achieved by placing the animal on its (right) side and compressing the chest wall firmly just behind the left elbow.
Hypothermia should be considered as a possible cause if recovery from anaesthetic is slow, particularly in small animals. Supplementary heating should be given as outlined above to raise the animal's core body temperature. Shivering to increase heat production results in an increase in oxygen demand; therefore, oxygen should be given to the hypothermic animal.
Most of the means for correcting cardiac failure used in humans such as defibrillation and drug treatments can be used in animals, but these are specialist techniques. If surgery is to be undertaken where there is a high risk of the development of an anaesthetic emergency then expert advice should be sought.
Muscle Relaxation During Anaesthesia
Relaxation is part of the triad of anaesthesia (narcosis, analgesia and relaxation). Muscle relaxation facilitates access to body cavities and to allow limbs to be moved easily. This reduces any damage caused to the muscles by using excess traction when trying to overcome muscle tone.
The abolition of muscle tone can be brought about in three ways, as follows.
Neuromuscular blockers have no narcotic or analgesic effects. Their administration abolishes all ability to respond to pain but does not abolish the ability to feel pain. It is essential to ensure complete unconsciousness of all animals to which they are administered. The use of neuromuscular blocking agents is strictly controlled in many countries. In the UK, specific permission must be obtained from the Secretary of State at the Home Office and it must be clearly written into the Project Licence. Guidelines on the use of neuromuscular blocking agents are available on the Home Office website.
Monitoring anaesthesia under neuromuscular blocking agents
Many of the normal reflexes used to judge the depth of anaesthesia are abolished with neuromuscular blockers (e.g. palpebral reflex, pedal-withdrawal reflex) so other methods need to be employed to monitor the depth of anaesthesia under these conditions. Before the procedure to be carried out under neuromuscular block is performed it is essential to be familiar with the anesthetic regime to be employed to make sure that it provides a sufficient depth of anaesthesia for the procedure to be carried out. Then, under neuromuscular block, the following parameters can be used to monitor depth of anaesthesia:
- blood pressure,
- heart rate,
- twitching of muscles in response to surgical stimulation: this indicates that the depth of anaesthesia or neuromuscular block is inadequate,
- electroencephalogram (EEG): however, the interpretation of the EEG is often open to question and there is not always direct correlation with the level of narcosis. The reliability of this method is currently a matter of some discussion.
It is essential to use an endotracheal tube when using neuromuscular blockers to maintain the airway. An endotracheal tube facilitates IPPV and prevents air from being pushed into the stomach and intestines during forced breathing.
To measure the degree of neuromuscular blockade, a peripheral nerve stimulator can be used to determine muscle responses (the so-called train of four response). Alternatively the presence or absence of respiratory efforts can be evaluated. The intercostal muscles and diaphragm are generally the last muscles to become paralysed by neuromuscular blockers and the first to recover. However, monitoring these alone is inadequate to monitor the depth of anaesthesia.
Neuromuscular blocking agents
Sometimes simply called muscle relaxants, these produce paralysis of the skeletal muscles by blocking transmission of impulses from nerve endings across the neuromuscular junction (NMJ). There are two main types of neuromuscular blocking agent: depolarising agents/blockers and non-depolarising agents/blockers.
Depolarising agents
These act by mimicking the action of the normal transmitter (acetylcholine, ACh) at the neuromuscular junction. As a result these agents cause initial generalised muscle twitches, followed by complete skeletal paralysis. The main example is suxamethonium. Depolarising blockers are usually short-acting and are allowed to wear off naturally.
Non-depolarising agents
These are sometimes called competitive blockers since they work by competing with ACh for its receptors at the neuromuscular junction, reducing the degree of depolarisation of the membrane in response to nerve impulses. Examples include pancuronium, alcuronium, atracurium, vecuronium and gallamine. Their effects can be reversed by the administration of anticholinesterase drugs, which block the activity of enzymes that break ACh down, for example neostigmine, edrophonium or pyridostigmine.
References
1. Flecknell PA (1996). Laboratory Animal Anaesthesia, 2nd edn. London: Academic Press
2. Landi MS, Kreider JW and Lang CM (1982). Effects of shipping on the immune function in mice. American Joumal of Veterinary Research 43: 1654–7
3. Tuli JS, Smith JA and Morton DB (1995). Stress measurements in mice after transportation. Laboratory Animals 29(2): 132–8
4. Flecknell PA, Liles JH and Williamson HA (1990). The use of lignocaine-prilocaine local anaesthetic cream for pain-free venipuncture in laboratory animals. Laboratory Animals 24: 142–6
5. Burchiel K (ed.) (2002). Surgical Management of Pain. New York: Thieme Medical Publishers
6. Lascelles BDX, Cripps PJ, Jones A and Waterman-Pearson A (1997). Post-operative central hypersensitivity and pain: the pre-emptive value of pethidine for ovarohysterectomy. Pain 73(3): 461–71
7. Lascelles BDX, Cripps PJ, Jones A and Waterman-Pearson A (1998). Efficacy and kinetics of carprofen, administered preoperatively or postoperatively, for the prevention of pain in dogs undergoing ovarohysterectomy. Veterinary Surgery 27: 568–82
8. Welsh EM, Nolan AM and Reid J (1997). Beneficial effects of administering carprofen before surgery in dogs. Veterinary Record 141: 251–3
9. Wright M (1982). Pharmacological effects of ketamine and its use in veterinary medicine. Journal of the American Veterinary Medical Association 180: 1462–71
10. Dugdale A (2010). Veterinary Anaesthesia: Principles to Practice. Chichester: Wiley-Blackwell
11. Sulbaek Andersen MP, Sander SP, Nielsen OJ, Wagner DS, Sanford Jr TJ and Wallington TJ (2010). Inhalation anaesthetics and climate change. British Journal of Anaesthesia 105(6): 760–61
12. Eger EI (1981). Isoflurane: a review. Anesthesiology 55(5): 559–76
13. Health and Safety Executive (2011). EH40/2005 Workplace Exposure Limits. Containing the list of workplace exposure limits for use with the Control of Substances Hazardous to Health Regulations (as amended). www.hse.gov.uk/pubns/priced/eh40.pdf
14. Costa DL, Lehmann JR, Harold WM and Drew RT (1986). Transoral tracheal intubation of rodents using a fibreoptic laryngoscope. Laboratory Animal Science 36: 256–61
15. Jou IM, Tsai YT, Tsai CL, Wu MH, Chang HY and Wang NS (2000). Simplified rat intubation using a new oropharyngeal intubation wedge. Journal of Applied Physiology 89(5): 1766–70
16. Alexander DJ and Clark GC (1980). A simple method of oral endotracheal intubation in rabbits. Laboratory Animal Science 30: 871–3
17. Conlon KC, Corbally MT, Bading JR and Brennan MF (1990). Atraumatic endotracheal intubation in small rabbits. Laboratory Animal Science 40: 221–2
18. Davies A, Dallak M and Moores C (1996). Oral endotracheal intubation of rabbits (Oryctolagus cuniculus). Laboratory Animals 30: 182–3
19. Spoelstra EN, Ince C, Koeman A, Emons VM, Brouwer LA, van Luyn MJA, Westerink BHC and Remie R (2007). A novel and simple method for endotracheal intubation of mice. Laboratory Animals 41: 128–35
20. Richardson C and Flecknell PA (2009). Rodents: anaesthesia and analgesia. In BSAVA Manual of Rodents and Ferrets, E Keeble and A Meredith (eds), pp. 63–72. Gloucester: BSAVA
21. Goble EA and Ruhnke AB (2009). Adverse effects of the volatile anaesthetics. Adverse Drug Reaction Bulletin 259: 995–8
22. Bryant S (ed.) (2010). Anesthesia for Veterinary Technicians. Ames, IA: Wiley-Blackwell
23. Gilron I and Coderre T (1996). Preemptive analgesic effects of steroid anesthesia with alphaxalone in the rat formalin test: evidence for differential GABA-A receptor modulation in persistent nociception. Anesthesiology 84(3): 572–9
24. Reynolds PS, Tamariz FJ and Barbee RW (2010). Strategies of experiment standardization and response optimization in a rat model of hemorrhagic shock and chronic hypertension. Shock 33(4): 442–9
25. Flecknell P and Waterman-Pearson A (2000). Pain Management in Animals. London: WB Saunders
26. Flecknell PA (2000). Anaesthesia. In BSAVA Manual of Rabbit Medicine and Surgery, PA Flecknell (ed.), pp. 103–15. Gloucester: BSAVA Publications
27. National Office of Animal Health (2011). Compendium of Data Sheets for Animal Medicines. www.noahcompendium.co.uk/compendium/overview/
28. Ramsay I (ed) (2011). BSAVA Small Animal Formulary, 7th edn. Chichester: John Wiley and Sons