Chapter 11
Small Laboratory Animals
Rodents
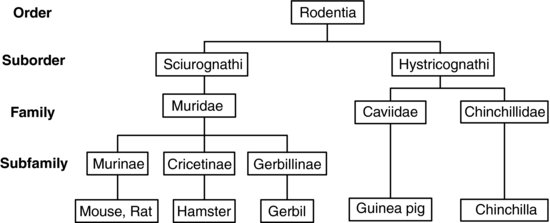
Rodents are a very successful order of mammals. They are found colonising almost all habitats, there are currently 2277 different species1, and new species are being discovered continually. As new techniques for genetic and phenotypic evaluation are developed, the classification of rodents changes, making this a dynamic and changing group2. Some studies have suggested that guinea pigs should be classed in a separate order, based on amino acid sequences3. More recent studies using wider sampling methods have restored guinea pigs to the order4,5, but within the order no single classification system has yet been universally adopted. Most classification systems divide rodents into the suborders Sciurognathi or Hystricognathi according to the shape of the lower jaw. One such classification system is illustrated in Figure 11.16,7.
FIGURE 11.1 Classification of rodents.
Rodents accounted for 81% of animals used in research in the UK in 20108, with mice comprising over 72% of animals used. Mice are used in studies including genetics, oncology, toxicology, immunology and developmental biology. Mice are used because they are small, have a high fertility rate and short gestation period, and are easy to maintain. They are also susceptible to many genetic diseases that afflict humans. Rats are used in studies including nutritional research and neuroscience.
Rodents have a number of distinguishing features. Despite their success, all members of the order are small: the largest rodent, the Capybara, weighs only 30–50 kg and is about the size of a small pig.
Dentition
The word rodent derives from Latin, meaning ‘to gnaw’. This apt description relates to the extremely sharp, chisel-like incisor teeth. Rodents have a single pair of incisors in upper and lower jaws. These are open-rooted, and grow throughout life. For example, an adult rat's upper incisors may grow 2.2 mm each week, (0.31–0.32 mm per day), and the lower incisors about 2.8 mm per week (0.4 mm per day)9. The teeth are worn away by chewing, and by bruxing (tooth grinding). The incisor teeth have enamel only on the labial (outer) surface, with softer dentine on the inner surface which wears away more quickly, ensuring the teeth always have a sharp edge. The jaw can move both sideways and forwards and backwards, to facilitate gnawing and bruxing.
Nutrition and digestion
Rodents have adapted to feed on a variety of foodstuffs, including seeds and grains, roots, leaves or fruits, as well as invertebrates and even small fish. Laboratory species eat mostly vegetable matter and cereals. Rodents have a simple stomach, with glandular and non-glandular parts, and cannot vomit. A fold of muscular tissue at the gastro-oesophageal junction forms a physical barrier, and they don't have the oesophageal muscle strength to overcome this barrier. Also they appear to lack the ability to coordinate the different muscles required for vomiting. Rodents, like other herbivores, require a microbial fermentation chamber in which digestion of cellulose from plant material can occur. This takes place in the caecum, located between the ileum and the colon. However, since this is at the end of the digestive tract, the digested matter has to be re-ingested in order for nutrients to be absorbed. During the dark period, pellets of digested material from the caecum are coated with mucus, and these caecotrophs are ingested directly from the anus, allowing the nutrients to be absorbed. This is known as coprophagy. Undigested material that has been through the digestive tract twice is expelled as dry pellets, which can be seen in the environment.
Rodents in the laboratory are usually fed ad libitum with a complete cereal-based pelleted diet, from hoppers suspended above the floor to prevent faecal contamination. These should be cleaned weekly, but the cleaning routine will depend on factors, such as the housing system, stocking density, or presence of newborn litters. Disease states and pregnancy affect the food requirements.
Water
Water is required for lubrication of the food as well as hydration, particularly in mice, and if insufficient fluid is available mice have difficulty eating. It may be supplied in automated systems or bottles. Water provided in bottles becomes contaminated by saliva and faecal matter, and frequent cleaning is required. For some strains, acidification or chlorination of the water may be needed to prevent disease arising in the animals from contaminated water. Automated systems ensure that there is a constant supply available, but when used with solid-bottomed cages there can be a risk of flooding, as animals may play with the sipper tubes or push bedding material into the tube, causing it to drip. When ill, mice drink very little and rapidly dehydrate. Medicines administered in the water may be ineffective, and care must be taken to ensure that adequate quantities of fluid are administered by other means.
Behaviour
Rodents are mostly social animals, living in groups and defending territories from intruders. A considerable amount of rodent behaviour is directed towards marking and maintaining their territory. Most rodents are crepuscular (active at dawn and dusk), or nocturnal. They often exhibit burrowing behaviour if presented with a suitable substrate, and this can be used to assess welfare10.
Senses and communication
Scent marking is particularly important in rodents. It helps maintain a familiar and secure environment, attracts mates and repels intruders, and helps the animal orientate itself. Removal of scent marks, such as occurs during cage cleaning, can lead to insecurity and increased aggression and leads to increased marking. Animals often form a dominance hierarchy, which helps to regulate conflict and reduce aggression and injury. However, fighting can and does occur, particularly between similarly matched individuals. If the territory comes under threat, rodents will mark and defend the territory, fighting if necessary. If an animal perceives the scent of an intruder but cannot then identify them, this can lead to stress.
Rodents have little or no colour vision, being nocturnal. They hear in the ultrasound range, mice detecting frequencies up 100 kHz (compared to about 20 kHz in humans), and are most sensitive in the 15–20 kHz range and around 50 kHz. They communicate both in the human audible range with squeaks (for long-distance warnings), and in the ultrasound range (for short-distance communication).
Housing
Rodents can be housed in conventional units where the pathogen status may be unknown, but more frequently are kept in barrier units with a defined and monitored health status. Cages are often of the shoebox type made of plastic. Rodent housing should have solid floors, with environmental enrichment, nesting and bedding material, and refuges, allowing them to exhibit a range of natural behaviours. Most species are sociable and can be housed in groups of compatible animals, although adult male mice may fight, and are sometimes housed alone, and hamsters are solitary by nature. Females of all species with litters will defend their young and are best separated while nursing.
Environment
To comply with the requirements of EU Directive 2010/6311, rodents require temperatures between 20 and 24°C, humidity between 45 and 65% and 12 h of daylight daily. The light intensity should be 350–400 lux, except for albino animals. For these, it should be less than 60 lux to avoid damage to the retina. Rodents also hear in the ultrasound range (the wild mouse has an upper frequency limit of 92 kHz)12, and care should be taken to monitor and reduce ultrasound in facilities, since many pieces of equipment and everyday activities such as running a tap may produce ultrasound.
Anaesthesia
Anaesthesia in small laboratory animals is associated with significant risk. Their small size makes access to blood vessels difficult and increases heat loss during anaesthesia, resulting in hypothermia. Anaesthesia should only be induced in healthy, stress free animals. Small animals need to be kept warm during and after anaesthesia, to prevent hypothermia, and may need supplementary fluids to prevent dehydration and encourage return to normal physiology in the post-anaesthetic period.
Anaesthesia can be induced by inhalation or injection, and inhalation is the preferred method for small-animal anaesthesia. It has a very high safety margin for all of the laboratory species and is rapidly excreted from the tissues via the lungs, which results in rapid recovery. There is virtually no biotransformation, making it the most suitable agent for studies that require maintenance of normal drug-metabolising ability.
Many anaesthetic agents are available (see Chapter 9). Drug doses are intended only as a guide and will have to be adjusted to take account of varying responses of different animals and strains.
Mouse
The mouse, Mus musculus, is the most commonly used laboratory animal. Many well-defined inbred strains and outbred stocks are available, for which the karyotypes are known. More is known about the genome of the mouse than any other species. An international project has undertaken to sequence the entire mouse genome, and there are numerous centres working on producing genetically modified mice that model human diseases. There are many types of genetically modified mouse available that are useful models for specific disease entities (see Chapter 6).
Behaviour
Mice are active, social animals, which naturally live in harmony in groups with one male and several females and their young once their hierarchy is established. They live in a variety of places near food sources, and construct nests from various soft materials. They exhibit a wide range of behaviours including exploring, foraging, climbing, grooming and resting. Mice usually run on all fours, but when eating, fighting or exploring they stand on their hind legs, supported by the tail. Mice are good jumpers, climbers and swimmers. Opportunities to perform as many of these behaviours as possible should be provided in the laboratory. Failure to provide an adequate variety of enrichment may lead to stress and abnormal behaviour, confounding experimental results.
Mice are territorial, and dominant males respect each other's territory, venturing into another's territory only if it is vacant. If two or more males are held together in a cage they will often show aggression unless they have been raised together from birth.
Mice are afraid of rats, which may kill and eat mice, so these species should not be held in the same air space.
Communication
Mice use pheromones to communicate, and it is vital to understand this communication in order to manage these animals successfully in the laboratory. Some of the effects of pheromones are listed below:
- stress in one mouse causes dispersal of other mice,
- female mice attract male mice, and vice versa,
- pheromones from lactating females attract the young,
- foreign females stimulate aggression by other females,
- foreign males provoke aggression from other male mice, and may cause recently mated females to abort (Bruce effect),
- group-housed females become anoestrus, and resume cycling if a male is introduced (Whitten effect),
- co-existing males emit pheromones to reduce aggression within the group, but which cause foreign males to avoid the territory.
Communication in mice through scent marking has been extensively studied. Two types of information are carried in scent marks: genetic information (fixed), and information on current status (variable; depending on diet etc.). Scents are detected by the main olfactory system, and via the vomeronasal organ, which detects involatile compounds. This is very finely tuned to specific compounds such as pheromomes.
Scents are composed of two components: airborne volatile compounds and small peptides, and major urinary proteins (MUPs), which are specialised communication proteins. Most MUPS are excreted in urine, but some are also excreted by other routes. MUPs in scent marks signal the genetic identity of the mouse to other mice, for example their sex and whether they are a relative. Individuals have different proteins, allowing animals to recognize each other and avoid mating with their relatives13.
Airborne volatiles allow the scent source to be identified from a distance. If the scent is known, the animal will tend to ignore it. If not, they will try to identify the source of the scent and investigate the MUP pattern, to determine the exact identity of the scent marker; that is, remote sensing via the airborne volatile compounds leads to contact sensing of the MUP pattern. The mouse then learns an association between the airborne volatile scent and the MUP pattern. The next time it encounters the airborne volatile it will be recognised and the MUP may not need to be investigated14.
Female mice can distinguish between dominant and weaker males by how fresh and dense a male scent mark is. Dominant individuals mark more than others, so leave more dense markings, and they also leave the most recent scent marks. Subordinate males scent mark to a lesser extent. Females are attracted to the airborne scent of familiar males and choose males with the most recent scent marks15.
When mice meet, the dominant male allows the intruder to sniff him before he attacks; this gives the intruder a chance to recognise him and run away, thus avoiding aggressive encounters.
Implications for laboratory mice
All laboratory mice have been shown by mitochondrial DNA to have come from a single female, and they therefore have very little variation in their MUP16. Thus they share common genetic signals: this reduces aggression between individuals. However, animals also recognise each other through non-genetic factors influencing the airborne volatiles, and changes in these airborne volatile signals can lead to aggression. Some experimental treatments may influence the airborne volatile signals, and can therefore increase aggressive encounters.
If mice are only exposed to airborne scents without finding the source for contact sensing, mice will eventually ignore it (this happens on racks of conventional cages). However, if they are then exposed to the contact scent of unfamiliar animals, this may lead to an aggressive response. Thus placing unfamiliar animals into shared equipment without cleaning between them can lead to aggressive behaviour and stress. Pheromones are used to maintain stability in the colony and if they are removed each time the cage is cleaned fighting will ensue and subordinate mice will be barbered (where the dominant mouse chews the whiskers of subordinates), or possibly injured (Figure 11.2).
FIGURE 11.2 Barbering in the mouse.

Keeping mice in compatible groups, providing environmental enrichment such as tubes, objects to climb on and refuges, and leaving a little of the soiled bedding in the new cage to reduce the need to keep re-establishing the dominance hierarchy and territory marking all help to reduce aggression and stress.
There are strain differences in behaviour. For example, male BALB/c mice are particularly aggressive, and fight wounds are common.
Feeding
Estimating the quantitative nutrient requirements for mice is difficult, since different strains have different requirements, and there is little published information on nutrient requirements for maintenance and production in mice17. Different environments also affect nutritional requirements: animals from conventional, specific-pathogen-free or germ-free environments have different intestinal flora, and these influence nutrient requirements. Temperature, diet composition, physiological status, microbiological status and genetic background influence maintenance energy requirements: obese mice have a lower energy requirement than lean mice, and females a lower requirement than males. The daily metabolisable energy (or ME) requirement for maintenance is approximately 160 kcal (670 kJ)/BWkg0.75/day in mice with no genetic or stress-induced abnormalities17. Diets for laboratory mice may be pelleted (compressed or expanded), powdered or in gel form. Diets for maintenance typically contain 4–5% lipid and 12–14% protein, and diets for growth and reproduction contain 7–11% lipid and 17–19% protein18. Mice will eat approximately 3.5–6.2 g of pelleted diet daily depending on strain and physiological status17.
Environment
Mice have a large surface area to volume ratio and lose heat rapidly, and so are sensitive to changes in ambient temperature. Much energy is expended in maintaining body temperature, and they cannot tolerate a reduction in room temperature. Mice are also susceptible to water loss. They cannot afford to sweat or pant to lose heat, as this would cause dehydration. In the wild they use behavioural mechanisms, such as burrowing, to keep cool. Therefore, maintenance of the correct environmental conditions and provision of bedding and shelters to allow the animals to manipulate their own microenvironment is vital.
Breeding
Male mice reach puberty at approximately 7 weeks, and females at 6 weeks. Females then cycle every 4–5 days. This is affected by photoperiod and the presence of others, a period of 12–14 h light daily is needed to maintain oestrous cycles18. Oestrus, mating and ovulation tend to occur during the dark phase of the light cycle. The Whitten effect can be used for synchronising oestrus or for timed matings. Mating results in the formation of a vaginal plug, which can be detected to confirm mating. Fetuses can be palpated from day 7–10. Gestation lasts 19–21 days and 1–15 pups may be born, depending on the strain. Females have a post-partum oestrus within 24 h of giving birth, and if kept with the male will be mated again at this time.
Mice may be bred using a harem system, with one male for two to six females, pregnant females being removed from the group to give birth, or may be kept together in a monogamous system. With the latter system, the young are removed before the next litter is born. Mice will breed until they are 12–18 months old, although the economic breeding life of most strains is around 6 months.
Growth
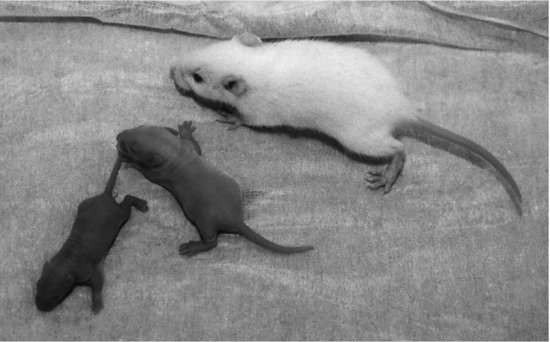
Mouse pups are dark purplish red at birth, and may have a visible milk spot in the abdomen. Their eyes and ears are closed and there is no hair coat. They become lighter in colour over the first few days, and in coloured mice pigment may be visible by day 3. By day 4 the ear flaps are starting to separate from the head, and this is complete by day 5. Hair appears from day 7 and covers them fully by day 10. From day 11, teeth begin to erupt and the eyes begin to open. These are fully open by day 12, and they start nibbling solid food19 (see Figure 11.3). They become increasingly active and increase their intake of solid food until weaning takes place at about 21 days. Weaning should only be carried out if the animals are large enough, and for slower growing strains this may take up to 28 days. Strains vary dramatically in their rate of growth, with some strains growing twice as fast as others17. In general, outbred stocks grow much faster than inbred strains.
FIGURE 11.3 Ageing the rodent. (From left to right.) At 4 days old, the pup is blind and deaf with no hair. At 8 days old, the pup is still blind and deaf but is covered with fine hair. At 18 days old, the eyes and ears have opened, the hair coat is well developed and the animal is increasingly independent.
Handling
Mice are generally easy to restrain, but their small size makes them vulnerable to physical injury. Some mice are also very active and may attempt to jump away from the handler. Movements must be quick and decisive. Handling mice for procedures is potentially a significant source of stress. The most common method used to capture and handle laboratory mice is to pick up and restrain the mouse by its tail; however, this induces a high level of anxiety, and mice do not habituate to handling by this method20. Picking mice up by either placing a tunnel in the home cage for the animal to crawl into or by cupping in the open hands causes less stress, and these methods should be used where possible. Approach the animal after first removing the lid of the cage. Otherwise, they may hide beneath the food hopper, which makes them harder to catch and also increases the risk of being bitten.
If it is necessary to restrain the animal for injection, grasp the base of the tail gently but firmly and lift the mouse. Place the mouse down on a non-slip surface, such as the top of the cage, without releasing the tail. The animal may then be sexed by lifting the tail to expose the perineum. The ano-genital distance in the male is approximately twice that seen in the female. Pull back slightly so the mouse grips the surface. Slide the thumb and index finger of the other hand up the animal's body and grasp the scruff of the neck to restrain the head. The animal is then secure and may be examined or injected safely. Extra restraint may be achieved by holding the tail with the fourth and fifth fingers (see Figure 11.4).
FIGURE 11.4 Restraining the mouse for injection. Photo: James and Steve.

To handle newborn mice, transfer the mother to a separate cage first, to prevent aggression from her. The pups can then be gently rolled into the palm of the hand. To avoid subsequent cannibalism by the mother rub the hands in soiled bedding material to acquire pheromones before handling the pups, and rub the young with nest material after handling before replacing the mother. From 10 days of age they can be handled as adults, but the mother should still be removed first.
Pain and stress recognition
Familiarity with normal appearance and behaviour is required in order to detect when all is not well. There will be normal variation between healthy animals, due to strain differences, age, diet or cyclical changes in the female due to oestrus, etc. Signs of normal appearance and behaviour include:
- alert, inquisitive and interacting with others in the group,
- well groomed with a glossy coat,
- relaxed with a normal gait: no lameness or ataxia, and not hunched or prone,
- bright and clear eyes,
- normal respiration, not shallow and fast, or deep and laboured,
- colour of the extremities (pink, pale or deep red),
- signs of eating and drinking,
- normal faeces and urine,
- teeth not overgrown and no malocclusion,
- moderate degree of body fat over the backbone,
- normal breeding performance with low neonatal mortality.
Signs of pain and distress include:
- increased sleep time,
- weight loss,
- piloerection and hunched appearance,
- isolated from the rest of the group,
- squeals on handling or pressure on affected area,
- may become more docile (or sometimes more aggressive),
- may eat bedding or neonates,
- abdominal writhing.
Common diseases and health monitoring
Conventionally housed mice may be carrying a number of commensal and potentially pathogenic organisms, and barrier-reared animals can develop infections if there is a breakdown in the barrier. Many infections do not produce clinical signs in adult animals. However, they may cause disease in immunocompromised animals, or interfere with research data. Respiratory infections increase the risks associated with anaesthesia and surgery. Regular health screens should be performed to ascertain the health status or the colony, and to ensure that there are no potentially problematic organisms in the colony (see Chapter 5). Table 11.1 lists some of the pathogens to be included in health-screening protocols. This list is not exhaustive, and more information can be found in reference 21.
Table 11.1 Agents to be included in health-screening protocols for mice (FELASA).
| Type of infectious agent | Examples |
| Zoonoses | Hantaan virus, lymphocytic choriomeningitis (LCM) virus, leptospirosis |
| Disease-causing agents | Sendai virus, pneumonia virus of mice, Pasteurella pneumotropica, Staphylococcus aureus, mouse hepatitis virus (MHV), mouse norovirus, mouse rotavirus (epidemic diarrhoea of infant mice EDIM), Theiler's murine encephalomyelitis virus (TMEV), Clostridium piliformis (Tyzzer's disease), Mycoplasma spp., endoparasites (e.g. Syphacia obvelata, Aspicularis tetraptera, Tritrichomonas and coccidia) and ectoparasites (e.g. Myobia musculi and Mycoptes musculinus) |
| Diseases affecting research | Minute virus of mice (MVM), lactate dehydrogenase elevating virus (LDHV), Helicobacter spp. |
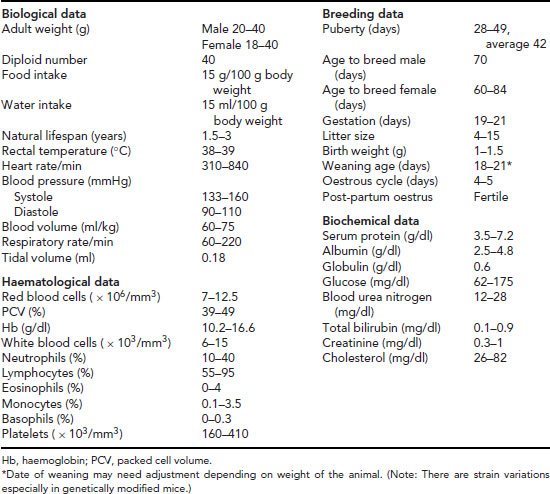
Biological data and useful reference data
Biological data and useful reference data are given in Table 11.2.
Table 11.2 Useful data: mouse.
Rat
Rats account for approximately 8% of animals used in research, and 293 905 rats were used in 2010 in the UK8. Rats used in research are mainly derived from the brown or Norwegian rat, Rattus norvegicus. Outbred and inbred strains are available. Commonly used outbred strains include the albino Wistar and Sprague–Dawley varieties, and the hooded Lister and Long–Evans strains. Inbred strains include the Lewis, Dark Agouti (DA) and Brown Norway strains. Some transgenic rat strains are also available.
Behaviour
Rats are social animals. In the wild, rats are territorial, and live in social groups in burrows. They usually live in groups of one dominant individual with several subordinates, and they establish and maintain their dominance hierarchies through agonistic behaviours, such as chasing and neck grooming. Usually the subordinate rat flees, and fighting is rare. Rats are usually friendly and easy to handle, although there are strain differences, and males tend to be friendlier than females. They become friendlier with more frequent handling. Rats are nocturnal or crepuscular, being active in the dark period. Their eyesight is poor, and blind rats behave as if perfectly normal. See www.ratlife.org for details of rat behaviour.
Housing
Rats are curious and intelligent creatures and need an enriched environment. They should be group housed in stable social groups, with solid floors and nesting material to allow them to create an appropriate microenvironment11. Tubes, houses, shelves or other structures allow the animals to divide their environment and make use of the three dimensional space in the cage. Paper-based commercial bedding, wood shavings or corn cobs may be used. Rats like to stand erect, and so cages with high lids are required. The minimum cage height specified in EU Directive 2010/63 is 18 cm, although the UK standard has been 20 cm and this is preferred.
Feeding
The rat is omnivorous, eating a wide variety of seeds, grains and other plant matter as well as invertebrates and small vertebrates22. The digestive tract is similar to other omnivorous rodents, and coprophagy occurs. Rats do not have a gall bladder. Nutritional requirements are better understood than for many laboratory rodents but still vary considerably with physiological status and developmental stage, strain and sex17. They can be fed ad libitum on a complete pelleted rodent diet, containing 20–27% protein and 5% fat. Higher protein levels than this may reduce reproduction efficiency. Rats are cautious eaters and will reject strange food. Rats will eat 5–10 g of feed per 100 g of body weight daily. This equates to 15 g/day for maintenance in young and adult rats, 15–20 g/day in pregnancy and 30–40 g in lactation. As rats age, it may be beneficial to restrict their food intake to 80% of ad libitum intake: this increases their life span and reduces the incidence of some types of neoplasia23.
Water
Water may be provided by sipper tubes or by automated watering systems. The water may need to be acidified or chlorinated to reduce contamination, particularly for immunocompromised rats. Rats drink approximately 5–10 ml of water per 100 g of body weight daily.
Environment
Rats should be kept between 20 and 24°C. Young rats have much brown fat to assist in thermogenesis, and this reduces with age. The humidity should be 45–65%. Rats are less sensitive to temperature changes than mice, but low humidity can lead to ringtail, in which an annular lesion appears around the tail, potentially leading to necrosis and sloughing of the tail. A 12 h light period is adequate for rats but bright light is deleterious, particularly for albino rats, and results in retinal degeneration. The level should be less than 400 lux, or 100 lux for albinos. Photoperiod affects the oestrous cycle, and 12–16 h light is best for optimal breeding. Ventilation is particularly important for rats, as many of their pathogens are aerosol-borne11.
Breeding
Rats become sexually mature at about 2 months, females maturing slightly earlier than males. Breeding is usually carried out from 3 months, when females weigh 250 g and males 300 g, and rats breed until they are 12–18 months old. Oestrus occurs every 4–5 days. The Whitten effect is less pronounced in rats than mice (synchronisation of oestrus in females by exposure to male pheromones), but does occur. Mating usually occurs at night, and a copulatory plug of gelatinous material is left in the vagina for 12–24 h, which then falls out and can be detected to confirm that mating has occurred. Gestation lasts 21–23 days, and can be detected from day 15 by palpation23. The female will start nest building in the later stages of gestation, so appropriate nest building material should be provided. A litter of 6–12 pups is born, often overnight. Pups are very sensitive to rearing conditions, which can influence physiological and psychological development23. In addition, if a female is disturbed during the post-partum period she may destroy her young, so it is very important to minimize disturbances and take extreme care cleaning cages during this time.
Polygamous mating systems are preferred. In these systems one male is housed with two to six females. Pregnant females are removed prior to parturition and returned after weaning. Females in this system produce more milk and have larger litters. Females produce 1–12 litters per year, and if held in a colony females may nurture their young collectively. Alternatively, with monogamous systems, males and females are housed in pairs. The female is mated at the post-partum oestrus, and the young are removed at weaning. This produces the maximum number of litters, but the male may interfere with the young. He can be removed at parturition, and returned to the female after the young are weaned. If the female is lactating during gestation then implantation can be delayed, leading to a 3–7 day increase in the length of gestation. In a variation on this system, a single male is moved between singly housed females, spending a week with each. One male is used for every seven females.
Growth and development
The pups are born pink and hairless, with eyes and ears closed. They begin to eat solid food from 11 days, and within 14 days the hair coat develops and the eyes open. Weaning occurs from 21 days. Rats grow rapidly in the first 3 months, reaching approximately 50 g at weaning. Adult weight is reached by about 15 weeks, although male rats exhibit prolonged growth, and bones do not become fully ossified until their second year. Male rats can become obese if fed ad libitum with insufficient space for exercise, reaching in excess of 500 g. Inbred and outbred rats differ slightly in their rates of growth, outbred strains tending to be larger.
After weaning, young rats should be group housed with plenty of space and enrichment to allow them to play, which has been shown to be essential in the development of normal adult behaviour23.
Handling
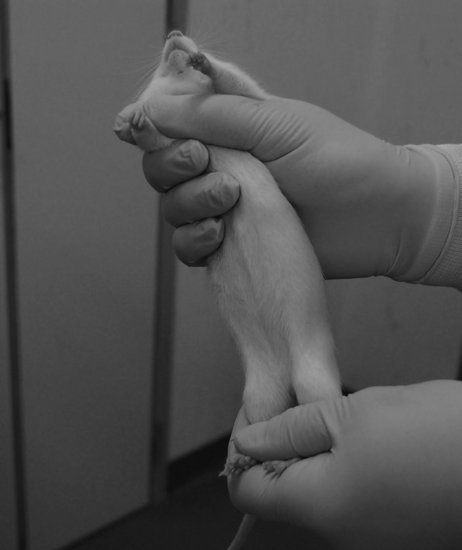
In general, rats are amenable animals, which rarely bite if approached correctly, particularly if they are routinely handled using appropriate techniques, although there are strain and sex differences. Approach the animal confidently and restrain it gently but firmly. Animals will typically only bite if stressed or in pain.
FIGURE 11.5 Handling the rat. Photo: James and Steve.
Pain and stress recognition
Rats are naturally curious, and will explore in any new situations. Failure to stand erect and take an interest in their surroundings is an indicator of poor health. They are generally docile but become more aggressive and resist handling during repeated stressful procedures. Acute pain or distress is accompanied by vocalisation and struggling. They will lick or guard a painful area and will sit crouched. Sleep patterns will be disturbed and increased. The Harderian gland, a modified tear gland, produces a red secretion that normally lubricates the eye. When the rat is stressed, this secretion overflows onto the face, producing a red ring around the eye, which is characteristic of stress. This is known as chromodacryorrhoea. Red staining may also appear at the nose, as the secretion flows down the nasolacrimal duct. Rats with abdominal pain may be reluctant to move, and show back-arching, twitching and a cat-like stretch (see www.digires.co.uk/product/pain-assessment-in-the-rat for more details).
Common diseases and health monitoring
Rats are susceptible to a number of diseases which may be zoonotic, have effects on the animals when under stress or affect research data. Some examples are listed in Table 11.3.
Table 11.3 Agents to be included in health-screening protocols for rats21.
| Type of infectious agent | Examples |
| Zoonoses | Hantaan virus, leptospirosis, Salmonella spp. |
| Disease-causing agents | Sendai virus, pneumonia virus of mice, rat coronavirus/sialodacryoadenitis virus (RCV/SDAV), Pasteurella pneumotropica, Streptococcus spp., Corynebacterium kutscheri, Mycoplasma pulmonis |
| Diseases affecting research | Kilham rat virus, rat parvovirus |
Biological data and useful reference data
Biological data and useful reference data are shown in Table 11.4.
Table 11.4 Useful data: rat.
Hamster
Several different species of hamster are used in the laboratory, and in 2010 4124 hamsters were used in research in the UK8. The commonest hamster used is the golden or Syrian hamster, Mesocricetus auratus. These are native to dry regions in Syria and Turkey. Syrian hamsters found in the laboratory are descended from a few animals captured in this region, so captive golden hamsters are highly inbred, and different strains are available. These hamsters are known for their cheek pouches, which they use to gather food for storage in deep burrows, particularly when photoperiod is decreasing. The pouches are unusual in having no lymph drainage, and therefore do not reject tissues transplanted to them. This is useful in immunological and other research. Golden hamsters also have a high incidence of dilated cardiomyopathy, and may be used as models for this condition. Syrian hamsters are often a golden brown colour with a pale underbelly.
The Chinese hamster (Cricetulus griseus) originates from Mongolia and north China, where they are found in rocky plains and steppes. They used to be more common in the laboratory, but have been largely replaced by the mouse and rat. Some biological products are produced in vitro by inserting the relevant gene into Chinese hamster ovary cells. Chinese hamsters are grey-brown, with a black dorsal stripe along the length of the back and a white belly.
Female hamsters are usually larger than males. Syrian hamsters grow to 15–18 cm in length. Chinese hamsters are smaller, reaching 8–12 cm in length, and unlike other hamsters they have a tail 2–3 cm long.
Behaviour
Hamsters are terrestrial animals that live in burrows. In the wild they are crepuscular, but they tend to be nocturnal in the laboratory, sleeping during the day, waking at dusk and then being active through the night. Their sight is poor but their sense of smell and hearing are very acute and they rely heavily on these senses to recognise each other and their environment. Territory marking is done using a secretion from the flank glands, which are visible, particularly in the male Syrian hamster, as dark patches on each side. Hamsters may also be active for short periods during the day.
Housing
Hamsters are essentially solitary animals, particularly Syrian hamsters, which may attack each other. Females will attack males except for a brief period during oestrus, and often attack other females. Groups of same sex animals may be maintained, if they are put together at weaning or before puberty, but aggression may still occur. Chinese hamsters are more sociable, especially males, and will live in groups in the right environment in single sex or mixed sex pairs or groups if put together when young. However, they may still fight once sexual maturity is reached24. Male Chinese hamsters are easier to maintain in groups, but females can be intolerant of each other and may be better living individually.
In the dark period, hamsters are active, fast and agile and therefore require a large cage with plenty of space and enrichment in order to prevent behavioural problems developing. Chinese hamsters in particular are good climbers and will utilise three dimensional space. They can also jump quite high.
Hamsters are adept at chewing through cages, so tough plastic cages with solid bottoms are used, and the small size of Chinese hamsters allows them to escape between the bars of some cages. If they do manage to escape, unlike rats and mice they are unlikely to return to their cages. Deep-piled bedding is usually provided. Little waste is produced.
A breeding female must have deep bedding and soft nesting material, as an inadequate nest will lead to her abandoning or killing her young. To avoid disturbing a nursing mother, cleaning out may be done every 2 weeks.
Feeding
The exact nutritional requirements of hamsters have not been extensively studied. Their stomach consists of a non-glandular part, in which some microbial digestion takes place, and a glandular part. They also have a large caecum which is the main site for microbial digestion. They are coprophagic. In the wild hamsters are omnivorous, eating mostly grains but also shoots, fruit and insects, and hoard food for eating later. Hamsters should be fed a hamster-specific mix if available, although diets formulated for rats and mice may be adequate. These can be supplemented with fruit and vegetables. A diet with 16–20% protein, 5–7% fat and 60–65% carbohydrate appears to be sufficient. Complex carbohydrate (e.g. starch or cellulose) seems to be required and reduces the incidence of non-specific enteritis, known as wet tail17. Some hamsters benefit from the addition of small seeds such as millet, which also provides environmental enrichment. Hamsters eat approximately every 2 h during the active period, consuming 5–7 g of pelleted diet daily.
Syrian hamsters have blunt noses and therefore may have difficulty feeding from some wire-mesh-suspended hoppers, as the mesh is too small. They need hoppers with slots greater than 11 mm, so they can pull the food through onto the floor. Nursing females should be floor-fed, or they become preoccupied with pulling food from the hopper and neglect their young.
Water
Syrian hamsters drink 5–14 ml/100 g of body weight daily (10–20 ml total daily), and Chinese hamsters 11–13 ml/100 g of body weight daily (3–8 ml total)17. Lactating females may need more. Water bottles or automated systems can be used, but the sipper tubes must be stainless steel, as hamsters can bite through glass. For breeding animals, the sipper tubes should extend low into the cage so that neonates can reach them.
Environment
Hamsters originate from hot, dry countries, and burrow to avoid heat. If unable to burrow, they tolerate heat poorly. Cold may be tolerated better. Hamsters are usually kept between 20 and 24°C with 12–14 h light daily. Hamsters can enter a state of hibernation if the temperature and photoperiod reduce, provided they are alone, have nesting material and have sufficient stored food. If insufficient food is available then hibernation is delayed. Hamsters kept at a constant temperature between 21 and 22°C and minimum 14 h light show reduced seasonal variability, and this may eliminate hibernation25. Breeding animals may need to be held at a slightly higher temperature. Relative humidity should be 45–65%11.
Breeding
In Syrian hamsters puberty occurs from as early as 32–42 days, but breeding usually begins at 6–10 weeks for females and 10–14 weeks for males, when they weigh 90–130 g. The female exhibits oestrus every 4 days, and is receptive for a very short time. Fertility is lower in the winter. Gestation in Syrian hamsters lasts only 15.5–16 days, and between five and nine pups are born. Pregnant females should be handled as little as possible. Due to the short gestation period pups are small and immature, weighing just 2–3 g, and an adequate nest is essential to provide warmth and security. Weaning occurs from 21–25 days. Hand rearing of Syrian hamster pups from birth is difficult, so it is not possible to re-derive a colony by Caesarian section to control disease outbreaks. Cross fostering of slightly older pups has been done successfully25.
In Chinese hamsters puberty occurs from 48–100 days, and they are bred from 10–12 weeks. The oestrous cycle lasts 4 days. Gestation lasts 21 days, and 1–11 (average 5) pups weighing 1.5–2 g are born. These are weaned at 21 days, when they weigh 15–17 g.
Female Syrian hamsters are aggressive, and may not tolerate unfamiliar males unless in oestrus. Therefore males and females may be kept separately, and put together for a short period after dark for mating. If the female is not receptive and does not accept the male he should be removed at once. Otherwise he is removed at the end of the dark period, before the light part of the cycle begins. A system of monogamous pairs can be maintained if the male and female are put together at weaning and kept permanently together. Alternatively, females can be rotated through the male's cage at weekly intervals, one male being used for seven females, or a harem can be set up with several males and females together. The females are removed prior to parturition and returned after weaning. However, these systems can lead to fighting. Post-partum mating can occur but it is rare for conception to take place during lactation so this is generally infertile.
Hamsters commonly abandon or kill their young. This can be triggered by environmental disturbances, inadequate nesting material or early handling of mother and young. When disturbed, females may hide their young in their cheek pouches. This affords the young some protection, but it may also suffocate them. To avoid stressing a nursing mother sufficient food and bedding for at least 1 week should be provided just prior to parturition, so that she does not have to be disturbed for 7 days post-partum.
Hamster pups are altricial, being blind and hairless, but in all hamsters the incisors are erupted at birth. In Syrian hamsters the ears open from 4–5 days, the eyes open from 14–16 days and they begin eating solid food from 7–10 days. In Chinese hamsters both eyes and ears open from 10–14 days and they eat solid food from 12 days17.
Handling
Hamsters have a reputation for being vicious, but if handled carefully they are readily tamed and rarely bite unless startled or handled roughly. Males are more docile than females. Chinese hamsters are more easily handled, and they will cling to a finger with all four paws, like a harvest mouse.
Hamsters should be picked up gently but firmly by cupping the animal in the hand. If required, they can be restrained by grasping a large pinch of scruff to account for the cheek pouches, and turning over into the hand as for mice. If insufficient scruff is grasped, the hamster will turn and bite. Additional restraint may be achieved by grasping skin along the back between the fingers and palm.
There are no easily accessible veins for dosing and administration in the hamster. Small blood samples can be taken from the saphenous veins, and a technique has been described for cephalic venipuncture for administration of small volumes26.
Pain and stress recognition
Hamsters in distress show weight loss, extended sleep period and increased aggression or depression. Ocular discharge and diarrhoea may be associated with stress.
Common diseases and health monitoring
Hamsters suffer from few clinical diseases. They may get non-specific enteritis, or wet tail, which is sometimes associated with Salmonella or Campylobacter infection, both of which are potentially zoonotic. They can also carry lymphocytic choriomeningitis and Sendai viruses, and these should be included in the regular health-screening programme.
Biological data and useful reference data
Biological data and useful reference data are shown in Table 11.5.
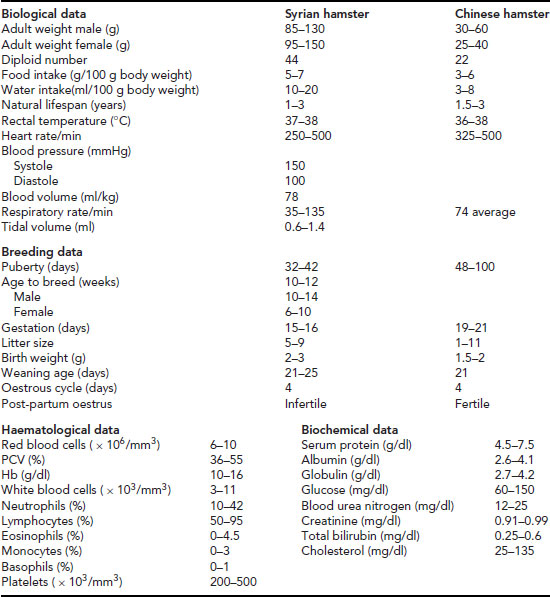
Table 11.5 Useful data: hamster.
Gerbil
There are over 100 species of gerbils recognized. They originate from Africa and Asia, and are are sometimes called desert rats. All species are adapted to dry habitats.
Gerbils have been used in research since the 1950s27, but are no longer common. Only 560 gerbils were used in the UK in 20108. The species used is usually the Mongolian gerbil, Meriones unguiculatus. These are hardy and easy to keep, with few diseases. Mongolian gerbils are typically 110–135 mm long, with a tail approximately 95–120 long. Adult gerbils weigh about 60–130 g, and males are larger than females28. The most common coat colours are agouti with a cream belly, black or spotted. Note: the ‘spotted’ gene is dominant and lethal if homozygous, so spotted gerbils should not be mated with each other to reduce in utero mortality of affected pups28.
Gerbils are used in a variety of research areas, including stroke research and studies into seizures. Seizure-prone and seizure-resistant strains of gerbils have been developed.
Gerbils produce a minimum of waste to conserve body fluids, thus they are very clean and have minimal odour.
Behaviour
Most gerbils are diurnal, although some exhibit crepuscular behaviour. They are small and easy to handle, since they are generally docile and rarely bite unless provoked. They are generally very active, and when approached they will resist being caught. Normally they exhibit exploratory behaviour in new surroundings, and if loose they do not hide but show curiosity and interest in the environment. In the wild they are crepuscular. Gerbils are sociable animals, and should be group housed, as breeding pairs with one or more generations of offspring, or in same-sex groups. They use their sense of smell to identify other individuals. Unfamiliar adults caged together will be aggressive. Stable groups may be established by putting animals together preferably at weaning, or by 7–8 weeks. Normal social behaviour will then be seen, in which animals wrestle and groom each other. If introducing unfamiliar gerbils it may be necessary to use a perforated divider in the tank to allow them to become familiar with each other's scent for 5–7 days before mixing them. If aggression is seen, animals should be separated. Aggression may sometimes occur suddenly in previously stable groups.
Gerbils may sit on their haunches to feed or observe their surroundings, and may stand on their toes using their tails for support. They rarely climb.
Housing
Housing designed for hamster or mice may not be suitable for gerbils, since they need access to a burrow system to dig tunnels. If this is not provided, development of normal behaviour can be impaired and they may exhibit abnormal stereotypic behaviours28. They should be provided with a dark nest chamber, accessed via a tunnel, to give the animals an opportunity to burrow. They should also be given material to gnaw, such as wood sticks or straw. Gerbils will also make use of sand baths if provided.
Gerbils can gnaw through plastic hamster and mouse cages, and plastic can cause problems if ingested. They need cages made from suitably strong material to resist attempts to escape. They prefer solid floors to mesh, and need at least 2 cm depth of bedding for nest building, which occurs even if the female is not pregnant. Sawdust or shavings made from pine should not be used, as the fur tends to become matted with these materials. Gerbils need at least 15 cm space between the top of the bedding and the roof of the cage, as they like to sit erect. Gerbils produce very little urine, and their faecal pellets are small and hard, so they are easy to keep clean.
Feeding
Gerbils have a simple stomach, and the caecum and colon are not especially well developed. Their natural diet consists of low-fibre foods such as seeds17. They are omnivorous, hoarding rodents, able to survive on a variety of foodstuffs including fresh and dried plant matter, tubers, bulbs, roots, seeds and insects depending on availability. In the wild they gather seeds and other foodstuffs from August onwards and store this in their burrows to last them through the winter. This enables them to thrive in dry habitats, where vegetation is sparse. They will gather food for as long as the weather is good, often continuing until winter. During winter gerbils may stay underground existing on their food stores.
Gerbils are coprophagic. Eating is spread throughout the day and night. Standard rodent diets with 22% protein are adequate, but a dietary fat level below 4% is recommended, to prevent obesity and high blood cholesterol. Obesity can lead to infertility in females due to fat deposition around the genital tract. Standard food hoppers are normally used, but supplementary food may be put onto the floor for young gerbils until they become used to the hoppers. Pellets can also be soaked. Gerbils consume 5–8 g/100 g of body weight pelleted food daily (5–6 g)17,28.
Water
Gerbils, being desert animals, can survive with very little drinking water, although older males and reproducing females need more than younger animals. They produce very concentrated urine, and are resistant to water loss. Nonetheless, plentiful drinking water should be provided in bottles or automated systems. Gerbils consume 4–7 ml/100 g of body weight water daily28.
Environment
Gerbils can tolerate a wide range of environmental conditions. At high temperatures they can regulate their body temperature by behavioural means if given the opportunity to burrow. They should be kept between 20 and 24°C, with humidity between 35 and 55%11: at higher humidities they develop matted fur. A period of 12 h light should be provided daily at 350–400 lux.
Breeding
In the wild, gerbils live in groups consisting of a breeding pair and several generations of offspring, and breeding is suppressed in the mature offspring. It is thought that by staying with their parents young gerbils develop parenting skills, improving their own reproductive performance subsequently28. Gerbils breed all year round in the laboratory, but are less-efficient breeders than other rodents. If a long-time partner dies, the remaining partner may not breed again. Puberty occurs from 6 weeks of age, and they are usually bred from 9–12 weeks. Successful breeding can be achieved by pairing a male and female between 60 and 90 days of age, and never separating them: if they are separated then reintroduced, they may fight. An alternative system is a harem system, with two or more females grouped with a single male. Oestrus occurs in the female every 4–6 days. Gestation lasts 24–26 days, unless the female is mated at the post-partum oestrus. In this case, lactation prolongs the gestation period to 27–48 days, resulting in an interbirth interval of 29–35 days. To avoid post-partum mating the male can be removed at parturition, but the separation should be for less than 2 weeks. Three to seven pups are born, weighing 2.5 g, and the male assists in nest building and caring for the pups. Neonatal mortality is high, up to 20%28. Pups are born blind, deaf and hairless. Hair appears from 5 to 7 days, ears open at 12–14 days, teeth erupt from 10 to 16 days and eyes open from 16 to 20 days. Pups begin to eat solid food from 16 days, and weaning takes place at a minimum of 21 days, when pups weigh 14–18 g.
Handling
Gerbils may be restrained using techniques as for mouse and rat. They readily run into a tube, or may be picked up in cupped hands. Great care must be taken if lifting by the tail: the body must be supported immediately, and if the tip of the tail is held the skin may slip off.
Pain and stress recognition
Healthy gerbils are inquisitive and active. If unwell, gerbils may show weight loss, piloerection with a characteristic scruffy appearance, and a hunched posture. Ocular discharges and diarrhoea may be associated with stress. Changes in behaviour, such as increased aggression or depression, increased respiratory rate, diarrhoea or constipation may also be seen.
Common diseases and health monitoring
Gerbils suffer from relatively few clinical diseases. They can develop Tyzzers disease, a frequently fatal enteric infection which also affects the liver, and can also carry lymphocytic choriomeningitis and Sendai viruses. Between 20 and 50% of gerbils may exhibit seizures. These may be triggered by fear, handling or a new environment. Seizures may be mild or severe, and animals may occasionally die. This trait can be used for studies of epilepsy. Should this occur, the best treatment is to leave the gerbil in a warm, dark, quiet place to recover. Frequent handling from an early age will reduce the frequency of seizures.
Biological data and useful reference data
See Table 11.6.
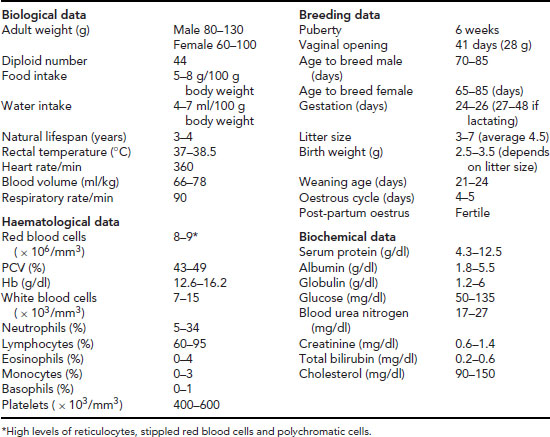
Table 11.6 Useful data: gerbil.
Guinea Pig
The guinea pig, Cavia porcellus, originates from South America, where they are still sometimes reared for their meat. The domestic guinea pig as such is not found in the wild, and is probably a descendent of a related species of cavy22,29. Guinea pigs have been extensively used as research subjects, but have now been largely replaced by rats and mice. In 2010, 13 586 guinea pigs were used in research in the UK8, mainly for applied studies, such as allergic responses, scurvy and tuberculosis. There are several different varieties of guinea pig available, including the short hair (English and American varieties), Abyssinian (which have hair in whorls) and Peruvian (which have long hair). Commonly used laboratory stocks are derived from the short-hair variety. The albino Dunkin–Hartley outbred stock is the most common type of guinea pig used. Strains 2 and 13 are inbred strains.
Behaviour
Guinea pigs are amenable animals which show little aggression and rarely bite. Naturally guinea pigs live in breeding groups consisting of one adult male and several females with their offspring. In the wild, male offspring leave the colony when mature, but in the laboratory they may become integrated into the group with little aggression30. They develop a stable dominance hierarchy, which is maintained mainly by olfactory cues, but with some barbering and chewing of subordinate males. Males mark females with secretion from their anal glands, and both sexes mark the environment with this secretion. Urine is also important in communication.
In small populations, linear dominance hierarchies develop in both sexes, the dominant male fathering most of the offspring. As the population increases above 10–15 individuals these split into subgroups consisting of one to four males and one to seven females, dominance hierarchies developing within each subgroup30. Each subgroup occupies a territory, with minimal overlap between the territories of adjacent subgroups. These groups are stable over time, and social stress and aggression are minimal in these circumstances, allowing guinea pigs to live in groups even at high stocking densities. Even unfamiliar animals can be grouped with minimal aggression, as a dominance hierarchy usually establishes quickly. Occasionally, fighting may ensue between unfamiliar adult males, particularly in cramped conditions or if oestrous females are present. If this is the case then the subordinate males should be removed.
Guinea pigs in the laboratory are active for periods throughout the day and night, with rest periods in between30. When startled, they either become immobile, or stampede and vocalize. This may lead to trampling of young and makes capture difficult. Providing bolt-holes and barriers within the pen and frequent handling to habituate the animals reduces this behaviour. The approach of a handler causes excitement, and guinea pigs should exhibit a scatter reaction as an attempt is made to pick them up. It is normal behaviour for a guinea pig to ‘resist arrest’ and vocalise strongly. If this does not occur it may indicate that there is a problem.
Guinea pigs are creatures of habit and become increasingly unable to cope with changes in routine as maturity approaches. If there are any changes in the type of food hopper or water bottle, or in the type of food or water, the guinea pig may be unable to adapt and cease eating and drinking. This is particularly disastrous with pregnant females. Similarly, if there are changes in the type of housing, problems may be encountered.
Housing
Guinea pigs are gregarious and should be housed in groups, consisting of a harem (one or two males with breeding females), pairs, or mixed-sex or all female groups. Solitary housing or single-sex groups of more than two males should be avoided.
They may be kept in floor pens, or large plastic or steel cages. Although guinea pigs rarely jump, cages should have sides at least 23 cm high to prevent escapes, and more height is required for open-topped floor pens (Figure 11.6). Guinea pigs thrive on solid floors with substrate. Mesh floors predispose to foot-pad ulcers and increased stress levels, and are not recommended.
FIGURE 11.6 Guinea pigs housed in floor pens.

Bedding materials provide comfort and a substrate for rooting behaviour. Materials such as wood shavings, paper-based bedding, ground corn cobs or sawdust may be used, together with hay. Fine shavings and sawdust alone may cling to moist areas, such as the perineum, and probably are best not given to breeding guinea pigs. Larger shavings are better for these animals. Guinea pigs are messy animals and will disperse opaque, creamy coloured urine and faecal pellets throughout the pen, requiring frequent cleaning.
Feeding and water
Guinea pigs are herbivores, their natural diet consisting mainly of grass. They are unique among non-primates in having a dietary requirement for vitamin C, and need a diet specifically formulated for guinea pigs, not one designed for any other species. They need approximately 10 mg vitamin C daily, and 20 mg if pregnant. This can be supplied in the food or water, or by giving cabbage, kale or oranges. Vitamin C in pelleted diet degrades within 90 days, so food must be stored correctly and used within the recommended shelf life to avoid deficiency. Supplements of hay may be given, and this also provides environmental enrichment. The food requirement is 6 g/100 g body weight daily. However, because much of the food is wasted more should be supplied. The food should contain 18–20% crude protein and 10–16% fibre. Coprophagy does occur in the guinea pig, but may not be essential. Guinea pigs are fastidious eaters and will reject unfamiliar food31.
Food and water bowls placed on the floor soon become soiled, so suspended hoppers are recommended. Guinea pigs also tend to play with drinkers, leading to wet bedding, and bottles quickly become empty. Automated watering systems ensure a constant water supply, but in solid-floored systems, care must be taken to prevent flooding. Any changes in watering system will upset the routine, and the guinea pig will need help to adapt. The water requirement is 10–14 ml/100 g body weight daily.
Environment
Guinea pigs thrive at temperatures between 18 and 24°C, with a humidity of 40 and 70%. They are better able to withstand cold than heat31.
Breeding
Guinea pigs can breed all year round, with peaks in spring. Female guinea pigs can reach puberty as early as 30 days, and males from 2–3 months. Pairing should take place when the female is 400 g (at 2–3 months), and the male 650 g (3–4 months). One boar can be housed with one to ten females. The oestrous cycle of the female lasts 15–17 days, and she is receptive for 6–11 h. The vagina is covered by an epithelial membrane, except during oestrus and parturition, both of which are signalled by perforation of the membrane. Gestation lasts 59–72 days (average 63–68 days), depending on litter size. In the last week, the pubic symphysis separates under the influence of the hormone relaxin, and once the gap reaches 15 mm parturition will take place within 48 h. Females should have their first litter before reaching 7–8 months of age, or the symphysis will be unable to separate sufficiently and dystocia (difficult birth) will result. In any case, there is a high incidence of abortion, dystocia and stillbirth. In smaller litters, pups are larger and dystocia is more common. Female guinea pigs can breed until they are 20 months old. Thereafter, the litter size tends to drop and dystocia is more common.
Growth
Between one and six pups are born. Neonatal guinea pigs are precocious, weighing 60–100 g, with hair, teeth, claws and partial eyesight. They are mobile at birth, and begin eating solid food within a few days, while continuing to suckle. Growth depends largely on the strain of guinea pig. Young guinea pigs should typically gain 2.5–3.5 g daily up to 60 days. Weaning takes place at 180 g (15–28 days), or 21 days (165–240 g). Weaned males intended for breeding need to be weaned late or group housed to allow development of normal adult reproductive behaviour.
Hand rearing is not difficult, making Caesarian re-derivation of colonies quite easy. Also the young may be hand reared to allow the sow to be mated at the post-partum oestrus. The young are not hungry until 12–24 h after birth, and can then be fed cows' milk or soaked guinea pig pellets.
Handling
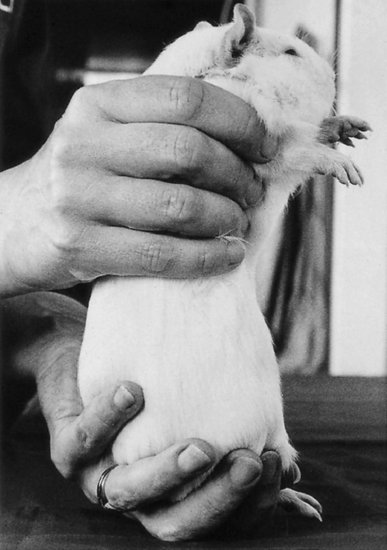
Guinea pigs are easily startled, and they vocalise and try to avoid capture when approached. The handler's movements should be rapid and smooth, to avoid frightening the animal. The animal should be grasped quickly and smoothly, placing the thumb and fingers of one hand on either side of the shoulders, then lifted and the free hand placed beneath the hindquarters to support the weight. The thumb can be placed under the jaw for additional restraint. The guinea pig can then be turned over for intraperitoneal injections or sexing. Holding the animal against the handler's body or positioning the thumb under the foreleg and beneath the chin as in the rat can provide additional restraint. Alternatively, one hand may be placed under the thorax and the other under the rear feet. It is particularly important to support pregnant females with two hands (see Figure 11.7).
FIGURE 11.7 Handling the guinea pig.
Recognition of pain and stress
Guinea pigs are alert, apprehensive animals that will try to avoid capture and restraint, and loud vocalization accompanies even minor procedures. They are stoical animals, and they mask signs of pain or distress until the disease process is advanced. It can be difficult to assess whether they are in distress. Any unusual sign of acceptance indicates the animal is unwell. They often appear sleepy when in pain and rarely show aggression.
Common diseases and health monitoring
Relatively few infectious diseases are seen in guinea pigs. Non-infectious diseases such as scurvy or diarrhoea may occasionally be seen if the diet is inadequate. Most infectious diseases seen in guinea pigs are bacterial, with abscesses and non-specific infections most commonly encountered. They may also develop respiratory infections. Guinea pigs can carry lymphocytic choriomeningitis virus (a zoonosis) and Sendai virus, and these two antigens should be included in regular screening programmes. External parasites (lice, mites, fungi) are rare.
Anaesthesia
These are probably the most difficult rodents in which to achieve safe and effective general anaesthesia. The response to injectable agents is variable and post-anaesthetic complications, such as respiratory infection, generalised depression, inappetance and digestive disturbances, may frequently be seen. Many of these problems may be avoided by careful selection of anaesthetic agents and high standards of pre-, intra- and post-anaesthetic care.
Biological data and useful reference data
See Table 11.7.
Table 11.7 Useful data: guinea pig.
Rabbit
The laboratory rabbit belongs to the order Lagomorpha (meaning ‘hairy ears’), and is derived from the domestic rabbit, Oryctolagus cuniculus. Many breeds of rabbit are available, ranging from the large giant chinchilla and Flemish giant, which may reach over 7 kg in weight, to the Dutch and Polish breeds which may reach no more than 0.9 kg. The commonest breed seen in the laboratory is the large outbred New Zealand white. Some inbred strains are available. Lagomorphs are characterized by having six incisors, whereas rodents have only four. An additional small pair is present behind the main upper incisors.
Rabbits are prey animals and have acute senses. Their large ears are highly vascular and are used for heat regulation and sound gathering. They have sensory hairs on the upper lips (vibrissae), a good sense of smell and their eyes are prominent and occupy a lateral position on the head, allowing them 360° vision. They have good vision in low light levels.
Their teeth are open rooted, and grow 10–12 cm each year. The skeleton of the rabbit is fragile, comprising only 8% of body weight. Their long bones and lumbar spine are particularly prone to fracture, especially if handling is poor32.
Behaviour
Wild rabbits are crepuscular or nocturnal, with clear daily patterns of activity. They emerge from their burrows at dusk, graze through the night and return to their burrows early in the morning. Their physiological parameters also show daily patterns: locomotor activity, food and water intake, expulsion of hard faeces and urine excretion all occur mainly in the dark phase. However, rabbits are very sensitive to external influences and their circadian rhythms may be entrained by light or feeding. Therefore, unless disturbances from husbandry or procedures are minimal, they often become diurnal, and periods of activity may be seen throughout the day and night33.
Wild rabbits live in social groups, the size of which changes depending on the season. In the winter they live in small, mixed-sex social groups of two to ten animals, defending small territories34. They may disperse during the summer months. Both males and females develop dominance hierarchies, although it is less obvious in females and may only be seen during competitive situations. In small groups, monogamous pairs are most common, whereas in larger burrows with multiple animals, promiscuity is often seen35.
Adolescent rabbits may leave the natal group at the end of breeding season and venture into different warrens36. Females may disperse for a chance of gaining a higher rank in a new territory, and males may disperse to find a mate. They are not forced out of their natal units due to aggression, but simply choose to leave.
Rabbits are very territorial, and they mark their territory through faeces, urine and glandular secretions from under their chins onto objects within their environment. They choose territories with plentiful food resources and ample cover, to provide protection from potential predators and high temperatures37.
Rabbits communicate mainly by smell. They mark their territory with secretions from their submandibular (chin) gland and anal glands, urine and dung hills38. These consist of large faecal pellets that are covered with secretion from the anal glands39. Males, both dominant and subordinate, visit the latrine sites more frequently than females, since their main duty is to guard the territory39. ‘Chinning’ involves rubbing their chin against an inanimate object while giving off scent from the chin gland. This is done for territory marking and to indicate whether they are willing to mate40. During breeding there is increased defence of the territory.
Housing
Rabbits are social animals, which are able to utilise a complex, three-dimensional environment. If given sufficient space, laboratory rabbits will exhibit the full range of behaviours seen in their wild ancestors, including standing on their hind legs, climbing up to a good vantage point, exploratory and tunnelling behaviour, and social activity. It is essential to provide sufficient space for the animals to perform these behaviours, to prevent boredom and skeletal problems. Cages should allow the rabbit to stretch out in at least one direction and be tall enough for the animal to stand, and include a box or shelf they can sit on. Environmental enrichment can be provided by giving hay, chew sticks or cardboard boxes to play with, and animals can be taken to exercise areas for short periods.
Overeating, leading to obesity, and overgrooming, leading to the formation of gastric hairballs, may be stereotypic behaviours, and are often associated with boredom. If there is insufficient opportunity for exercise, there will be prolonged periods of inactivity and increased stereotypic behaviour. There may be hypoplasia of bone tissue and osteoporosis, and an increased risk of fractures and nerve damage leading to cage paralysis. These problems are seldom seen in floor-housed rabbits. Single caging should be used only where group housing is inappropriate, such as for adult males, or if an animal needs to be isolated for measurement of food and water intake. Pair housing in large cages may be an acceptable alternative in many circumstances.
Aggressive behaviour may be seen if incompatible animals are housed together. They may chase each other, tail flag, urinate, squeal and fight. The incidence of aggressive behaviour depends on many factors, including strain (Dutch rabbits are more aggressive than New Zealand Whites), sex, age and weight, pen size and construction, the relatedness of the individuals and the proximity of other rabbits of the opposite sex. Aggressive behaviour is seen most in breeding and pubertal animals, and adult males. Males are most aggressive when competing for food, territory or females. It is common for them to be separated from 10 weeks of age to prevent fighting.
Rabbits should always be housed where they can see other rabbits. Solid floors with bedding are preferred: grid floors predispose to sore hocks (pododermatitis), and high ammonia levels can trigger the development of respiratory disease.
Groups of compatible rabbits, such as breeding females, a doe and litter, single-sex groups of newly weaned animals and stable groups of animals on procedure, can be housed in floor pens. Animals to be group housed should be of similar weights, and it is important to provide ample dividers, cardboard boxes or large tubes within the pen so nervous or frightened animals have bolt-holes where they can hide from aggressive conspecifics. Continued monitoring of newly formed groups for excessive aggression is required.
Group housing in pens has many advantages over single cages (see Figure 11.8). Group-housed animals generally have improved physical and psychological well-being. Animals can exhibit natural behaviour and interact socially, which reduces stereotypy; they tend to be calmer and more docile, the increased opportunity for exercise reduces osteoporosis and cage paralysis, and improved ventilation leads to fewer respiratory problems. However, there may be increased aggressive behaviour and stress in unstable and incompatible groups, identification and treatment of individuals is more difficult and exposure of staff to soiled bedding may increase the risk of allergy. Females group housed after puberty may develop pseudopregnancy, which can become a problem in breeding colonies. This will usually resolve once the animal leaves the group.
FIGURE 11.8 Group-housed rabbits in floor pens.

Rabbits feel secure and confident if surrounded by their own smell, and are disturbed by excessive cleaning or the use of strong smelling disinfectants. Over-frequent cleaning simply results in increased territory marking and upsets the animals' confidence. Floor pens should be deeply lined with bedding materials, which do not have a strong smell. The rabbits will urinate and defaecate in latrine areas, but faecal matter will become spread throughout the pen.
Rabbits produce copious, turbid urine, which may be yellow to dark red, due to the presence of a varying quantity of porphyrins.
Feeding
Rabbits are coprophagic. The digestive system includes a large glandular stomach, a long small intestine and an enlarged caecum, which contains complex microbial flora and is the main site of cellulose digestion. Food remains in the stomach for several hours before being gradually introduced into the small intestine. Undigested particles pass into the caecum, where microbes continue digestion. Caecal contents gradually enter the colon, where they become surrounded by mucus and form into a string of soft pellets known as caecotrophes. These are excreted during the dark phase and are swallowed directly from the anus. The now digested pellets pass through the small intestine again, where nutrients are absorbed. Any waste matter remaining is then expelled as hard faecal pellets that are seen in the environment. It is rare to see caecotrophs: these may mean that the diet is unsuitable or that the rabbit is over-eating. The digestive process can last around 24 h from beginning to end. Rabbits naturally feed mainly on roughage, and this should form the main component of the diet. If the fibre level is inadequate, this may lead to gut problems such as mucoid enteritis. Fibre should comprise as much as 22.5% especially for long-term maintenance: this allows feeding ad libitum without risk of obesity, and also reduces the risk of hairballs or diarrhoea. Lower fibre levels may be acceptable for breeding and growth. A diet consisting mainly of good-quality roughage (straw or hay) is adequate. If a rabbit is fed on pellets or mixed cereals, good-quality hay should supplement the daily ration. Otherwise, unless the amount is restricted the animal can overeat and become overweight41.
Roughage keeps the gut working efficiently and wears down the constantly-growing teeth. Ad libitum nibbling of hay prevents boredom and behavioural problems. Small quantities of concentrates (cereals, seeds, etc.) and fresh vegetable matter may also be given for variety. Grass may be suitable for this purpose: it has very high fibre level (around 20%) and around 14% protein.
A diet with 12–22% fibre, and 12% protein for maintenance or 15–17% protein for growth is recommended. Rabbits need 5 g of high-energy food per 100 g body weight daily (100–300 g diet daily).
As gut flora play an important part in digestion, changes in diet should be done gradually, over a 4–5-day period, to allow the flora to adapt. Failure to do this will result in diarrhoea or anorexia. Newly acquired animals should be fed on the diet that they are used to and change should be gradual. Rabbits can be very fussy and may even starve rather than accept a different product.
Water
Rabbits consume about 10 ml water per 100 g body weight daily (except in lactating does, which will consume up to 90 ml/100 g body weight, sometimes more than 1 litre per day). Water should be supplied ad libitum. Rabbits have a tendency to play with water bottles, so they should be checked frequently to ensure they are not empty, and that the floor has not become wet.
Environment
Rabbits require temperatures between 15 and 21°C. Neonates cannot maintain their body temperatures until they are 7 days old, so they must be kept in a warm environment. Humidity should be kept between 45 and 65%. Females require 14–16 h of light daily, and males 8–10 h, with or without periods of twilight. Shorter light cycles may result in reduced sexual activity in the autumn. Low-intensity light should be provided for albino animals. Rabbits are sensitive to high-pitched sounds, hearing from 75 to 50 000 Hz42, so care should be taken not to expose them to excessive ultrasound. Background noise can help to prevent the animals from being startled by sudden noises. Ventilation is particularly important for rabbits. Poor ventilation, allowing a build-up of ammonia, or low relative humidity levels predispose to respiratory diseases.
Breeding
Puberty occurs from 3–4 months in dwarf breeds, later in large breeds. Females reach puberty earlier than males: females begin breeding at 4–9 months, males at 6–10 months. Rabbits are induced ovulators, and have no oestrous cycle as such. They are receptive for 7–10 days, then inactive for 1–2 days. When receptive, the vulva may be red and swollen and the doe stands to be mounted. Does are also receptive at intervals during pregnancy and lactation. There is some seasonal effect, and ovarian activity diminishes as days shorten43. If day length is maintained at 16 h/8 h or 14 h/10 h light/dark, with a high calorie intake and elevated environmental temperature, the seasonal effect will be suppressed. Does may breed until they are 3 years old or more. Breeding problems reduce if doe is less than 6 months old when first bred. A doe can produce eight litters/year, but breeding efficiency falls after 7–11 litters, or 3–4 years.
Various mating systems exist. Group-housed does can be taken individually to the buck, or a buck may be taken to a group of two to five does, and removed after mating or after a few minutes if mating does not occur. Coitus induces ovulation approximately 10 h post-mating, and results in pregnancy lasting 31–32 days in 75% of does. Care should be taken in handling the doe during gestation, as the pregnancy is easily aborted.
The doe must have a clean nest box in the last week of gestation. The doe will pull fur from her flank to line her nest. Does usually kitten in the morning, and between four and ten kits are born, although this varies with the breed. Parturition is very rapid, taking 10 min or less. Dystocia is rare but some young may be stillborn or rejected from the nest. Litters retained more than 34 days are usually stillborn. Rarely, the kits may be eaten by the doe if there is a deformity or they are dead, but it may also happen if the doe is inexperienced or if there is some environmental disturbance. A doe will scatter her young if the nest is dirty, or if it smells of disinfectant. Does may be rebred shortly after parturition, but more usually are mated after weaning.
Growth
Newborn rabbit kits are altricial, naked with closed eyes and ears, and receive very little care from their parents. They are only suckled for 5–10 min once daily, in the morning. This is sufficient because rabbit milk has a high nutritive value. Kits can miss a feed and survive 48 h, and older kits can survive two missed feeds. Kits will drink from any lactating doe and at any time of day or night so fostering is relatively easy.
The doe does not brood the kits, clean them or retrieve them if they stray from the nest. The kits remain quiet in the nest, covered with nest material between feeds. Then, 1–2 h before feeding, their activity gradually increases, they become exposed, and they become very sensitive to disturbances. When the doe arrives, the kits lift their heads up and search for nipples. This allows for very rapid feeding: they can consume 25% of their body weight in a few minutes.
Kits respond to auditory stimuli from day 7. The eyes open at day 9–10, and they begin to leave the nest from 13–18 days. They start to eat dry food from 3 weeks, and should be weaned at 6–8 weeks. Hand rearing of rabbits is relatively easy.
Handling
Rabbits should always be approached in a calm and confident manner. They are generally placid and amenable, and can be readily trained. They rarely bite, but can inflict painful wounds with their sharp claws. They can become accustomed to frequent handling, which facilitates the performance of procedures. There are a number of particular considerations when handling rabbits. The animal should never be lifted by the ears, and the back must be supported at all times, otherwise contractions of the strong spinal muscles during struggling can result in injuries to the spine. Rabbits have powerful hind legs, and the handler must be careful to avoid being kicked or scratched. Before removing a rabbit from a cage or floor pen, orientate the rabbit so it is facing you. Grasp the animal by the scruff, avoiding the ears, and lift the front end. Then either slide the free hand beneath the rabbit, placing the thumb and little finger in front of the hind legs to extend them and lift the animal, or place the free hand behind the animal's hindquarters and scoop the animal towards you. Once the animal has been lifted clear of the cage or pen, it should be carried held head uppermost against the chest, or resting on the arm with its head tucked under the armpit, with the scruff securely held and the back and hindquarters supported (see Figure 11.9).
FIGURE 11.9 Handling the rabbit. The rabbit is held with its head tucked under the handler's arm and with the back and hindquarters supported by the handler's forearms.
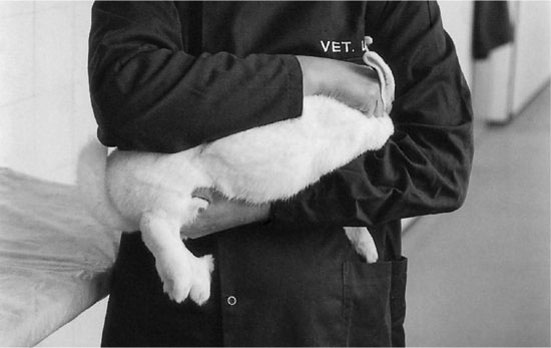
Pain and stress recognition
Rabbits often react to painful procedures with stoic acceptance. This may relate to feral behaviour where concealment is important for survival. A rabbit in pain may be isolated from the group. There may be reduced food and water intake and limited movement. Abdominal pain may cause twitching and writhing, but this is often very subtle. There may be apparent photosensitivity and ocular discharge with protrusion of the third eyelid. Faecal staining of the coat, digestive disturbances and dehydration may also be seen.
Common diseases and health monitoring
The most significant problems seen in rabbits are husbandry-related. It is very important that the diet, housing, environment and general management are of a high standard. Obesity and hairballs are associated with boredom, cage paralysis can occur if there is inadequate exercise, and digestive disturbances can result from lack of fibre or sudden changes in diet.
Rabbits suffer from very few viral diseases, but myxomatosis and viral haemorrhagic disease of rabbits are both potentially fatal. Myxomatosis is common in wild and pet rabbits, and can be brought unwittingly into the laboratory by personnel who have contacted the virus outside or by insect vectors. Viral haemorrhagic disease of rabbits is often characterised by sudden death. It is currently rare in laboratory-reared rabbits.
Rabbits suffer from a number of bacterial infections, the most important of which is Pasteurella multocida. This is mainly a respiratory pathogen, which can be carried subclinically in the upper respiratory tract, causing problems when the animal is stressed. Treatment may reduce the symptoms of Pasteurella, but it will not eradicate it. If this organism is present, it is essential to maintain adequate ventilation, particularly humidity, ensure meticulous hygiene, keep the stocking density low and avoid stress.
Many rabbit populations are infected with Encephalitozoon cuniculi, a protozoan parasite. This is often subclinical, or may be associated with a variety of clinical signs including ill- thrift and neurological signs. It is transmitted in urine.
Diarrhoea is also common. Causes include sudden diet changes, infestation with intestinal parasites such as coccidia and other infectious causes. Mucoid enteropathy occurs in growing rabbits. It seems to be associated with an inadequate level of dietary fibre and stress. Inappetance is another commonly encountered problem, associated with environment or diet changes, hairballs or subclinical diseases. This can cause a rapid loss of condition and should be treated promptly.
Acquisition of rabbits from disease-free stock, an appropriate barrier including quarantine periods and appropriate protective clothing, and a good standard of hygiene are essential to prevent these diseases.
Biological data and useful reference data
See Table 11.8.
Table 11.8 Useful data: rabbit44.

Anaesthesia in the rabbit
Rabbits have a small lung volume, and are susceptible to respiratory depression. Stress prior to induction of general anaesthesia can lead to cardiac and respiratory arrest. It is therefore very important to keep stress to the absolute minimum, before and after anaesthesia. Rabbits may develop gut stasis after anaesthesia and may need treatment with probiotics or drugs to encourage the restoration of gastrointestinal motility.
References
1. Wilson DE and Reeder DM (eds) (2005). Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd edn. Baltimore, MD: Johns Hopkins University Press
2. Keeble E (2009). Rodents: biology and husbandry. In BSAVA Manual of Rodents and Ferrets, E Keeble and A Meredith (eds), pp. 1–17. Gloucester: BSAVA
3. Graur D, Hide W and Li W (1991). Is the guinea-pig a rodent? Nature 351: 649–52
4. Lin Y-H, McLenahan PA, Gore AR, Phillips MJ, Ota R, Hendy MD and Penny D (2002). Four new mitochondrial genomes and the increased stability of evolutionary trees of mammals from improved taxon sampling. Molecular Biology and Evolution 19: 2060–70
5. Carleton MD and Musser GG (2005). Order Rodentia. In Mammal Species of the World, 3rd edn, DE Wilson and DM Reeder (eds), pp. 745–52. Baltimore, MD: Johns Hopkins University Press
6. Wilson DE and Reeder DM (1993). Mammal Species of the World: A Taxonomic and Geographic Reference. Washington DC: Smithsonian Institution
7. Wilson DE and Cole FR (2000). Common Names of Mammals of the World. Washington DC: Smithsonian Institution
8. Home Office (2011). Statistics of Scientific Procedures on Living Animals: Great Britain 2010. London: The Stationery Office. www.official-documents.gov.uk/document/hc1012/hc12/1263/1263.asp
9. Addison WHF and Appleton JL (1915). The structure and growth of the incisor teeth of the albino rat. Journal of Morphology 26: 42–96
10. Deacon RM (2009). Burrowing: a sensitive behavioural assay, tested in five species of laboratory rodents. Behavioural Brain Research 200(1): 128–33
11. Federation of Laboratory Animal Science Associations (2007). Euroguide on the Accommodation and Care of Animals used for Experimental and Other Scientific Purposes. London: RSM Press
12. Heffner RS, Koay G and Heffner HE (2001). Audiograms of five species of rodents: implications for the evolution of hearing and the encoding of pitch. Hearing Research 157: 138–52
13. Cheetham SA, Thom MD, Jury F, Ollier WER, Beynon RJ and Hurst JL (2007); The genetic basis of individual recognition signals in the mouse. Current Biology 17: 1771–7
14. Hurst J (2009). What the nose knows. Presentation given at the LAVA Spring Meeting. University of Birmingham, 31 March 2009
15. Ramm SA, Cheetham SA and Hurst JL (2008) Encoding choosiness: female attraction requires prior physical contact with individual male scents in mice. Proceedings of the Royal Society Series B 275: 1727–35
16. Cheetham SA, Smith AL, Armstrong SD, Beynon RJ and Hurst JL (2009). Limited variation in the Major Urinary Proteins of laboratory mice. Physiology & Behaviour 96: 253–61
17. National Research Council (1995). Nutrient Requirements of Laboratory Animals, Fourth Revised Edition. Washington DC: National Academies Press
18. Baumans V (2010). The laboratory mouse. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals, 8th edn, R Hubrecht and J Kirkwood (eds), pp. 276–310. Chichester: Wiley-Blackwell
19. Jackson Laboratory (2012). JAX Mice Pup Appearance by Age, poster. http://jaxmice.jax.org/literature/posters.html
20. Hurst JL and West RS (2010). Taming anxiety in laboratory mice. Nature Methods 7(10): 825–6
21. Federation of Laboratory Animal Science Associations (2002). Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Report of the FELASA Working Group on Health Monitoring of Rodent and Rabbit Colonies. Laboratory Animals 36: 20–42
22. Nowak RM (1999). Walker's Mammals of the World, 6th edn. Baltimore, MD: Johns Hopkins University Press
23. Koolhaas JM (2010). The laboratory rat. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals, 8th edn, R Hubrecht and J Kirkwood (eds), pp. 311–26. Chichester: Wiley-Blackwell
24. Eames A. (2008). Chinese Hamster. http://foreverhamsters.webs.com/chinesehamsters.htm
25. Whittaker D (2010). The Syrian hamster. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals, 8th edn, R Hubrecht and J Kirkwood (eds), pp. 348–58. Chichester: Wiley-Blackwell
26. Ransom JH (1984). Intravenous injection of unanaesthetised hamsters (Mesocricetus auratus). Laboratory Animal Science 34: 200–201
27. Schwentker V (1963). The gerbil. A new laboratory animal. Illinois Veterinarian 6: 5–9
28. Waiblinger E (2010). The laboratory gerbil. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals, 8th edn, R Hubrecht and J Kirkwood (eds), pp. 327–47. Chichester: Wiley-Blackwell
29. Weir BJ (1974). Notes on the origin of the domestic guinea-pig. In The Biology of Hystricomorph Rodents, IW Rowlands and BJ Weir (eds), pp. 437–46. New York: Academic Press
30. Kaiser S, Kruger C and Sachser N (2010). The guinea pig. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals, 8th edn, R Hubrecht and J Kirkwood (eds), pp. 380–98. Chichester: Wiley-Blackwell
31. Wagner JE and Manning PJ (1976). The Biology of the Guinea Pig. New York: Academic Press
32. Weisbroth SH, Flatt RE and Kraus AL (eds) (1974) The Biology of the Laboratory Rabbit. Academic Press. New York
33. Jilge B and Hudson R (2001). Diversity and development of circadian rhythms in the European rabbit. Chronobiology International 18(1): 1–26
34. Kunkele J and von Holst D (1996). Natal dispersal in the European wild rabbit. Animal Behaviour 51: 1047–59
35. Roberts SC (1988). Social influences on vigilance in rabbits. Animal Behaviour 36: 905–13
36. Daly JC (1981). Effects of social organization and environmental diversity on determining the genetic structure of a population of the wild rabbit, Oryctolagus cuniculus. Evolution 35(4): 689–706
37. Vertebrate Pest Research Section, Forrestfield (2003). Department of Agriculture Farmnote: European rabbit, no. 39/2003. www.agric.wa.gov.au/objtwr/imported_assets/content/pw/vp/rab/fn039_2003.pdf
38. Mykytowycz R and Dudzinski ML (1972). Aggressive and protective behaviour of adult rabbits Oryctolagus Cuniculus (L.) towards juveniles. Behaviour 43: 97–120
39. Sneddon IA (1991). Latrine use by the European rabbit (Oryctolagus cuniculus). Journal of Mammology 72(4): 769–75
40. Gonzalez-Mariscal G, Albonetti ME, Cuamatzi E and Beyer C (1997). Transitory inhibition of scent marking by copulation in male and female rabbits. Animal Behavior 53: 323–33
41. Meredith A (2010). The Importance of Diet in Rabbits. British Rabbit Council. www.thebrc.org/diet.htm
42. Lidfors L and Estrom T (2010). The laboratory rabbit. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals, 8th edn, R Hubrecht and J Kirkwood (eds), pp. 399–417. Chichester: Wiley-Blackwell
43. Boyd IL and Myhill DG. (1987). Seasonal change in condition, reproduction and fecundity in the wild European rabbit (Oryctolagus cuniculus). The Zoological Society of London 212: 223–33
44. Jenkins JR (2008). Rabbit diagnostic testing. Journal of Exotic Pet Medicine 17: 4–15