Chapter 15
Wild Animals
General Considerations
Research using wildlife species is often done for animal welfare and conservation reasons but field studies carry multiple risks for the animals involved and Refinement needs to be applied as rigorously as for other areas of research using animals1. There are some particular considerations when dealing with wild animals. The work is often unpredictable (such as the trapping of non-target species) and there should be appropriate contingency planning that identifies and mitigates the risks.
Since wild animals are not used to being handled they are likely to be more stressed by the presence of humans and by procedures than standard laboratory species. Captivity will induce particular stresses, and in many cases their exact biological needs will be unknown. All these features will contribute to the overall welfare burden on such animals and this should be taken into account when performing the harm/benefit analysis on the project licence application. Researchers should familiarise themselves in advance with the needs of the particular species, and techniques to minimise the distress caused to the animals during captivity. In addition to the physiological effects of handling stress on wild animals, they may also suffer from injuries during capture ranging from minor effects such as tooth and claw damage to severe injuries such as broken limbs or myopathy. The symptoms of myopathy have a delayed onset and so may not be seen at the time of capture but effect the animal after it has been released back to the wild. It is therefore very important to keep stress to the animal as low as possible by good handling and only brief periods of pursuit, capture and restraint.
The health status of animals in the wild will be largely unknown so adequate precautions should be taken to reduce the spread of infection and to protect personnel from the potential risk of exposure to zoonotic agents.
Legislation and Guidance
In the UK many wild species will be covered by other legislation in addition to the Animals (Scientific Procedures) Act 1986 (ASPA; see Chapter 2). Wild animals are protected by the Wildlife and Countryside Act 1981 (WCA). Part 1 of the WCA protects all wild birds and protected animals, which includes some mammals (all species of bat and species of dolphin, porpoise and otter), amphibians, reptiles and many species of insect. Under this Act it is an offence to take, kill, injure or sell a protected species, to disturb a protected species in its nest or place of shelter and to possess a protected species. To carry out such an activity therefore requires a licence issued by the appropriate authority. Some endangered or threatened species are covered by the Convention on International Trade in Endangered Species of Flora and Fauna (CITES; www.cites.org), which may affect the international movement of samples collected for scientific purposes. For work done in the UK it will also be necessary to take account of the following:
- the Animal Welfare Act 2006,
- Conservation (Natural Habitats etc.) Regulations 1994,
- Wild Mammals Protection Act 1996,
- and a range of species-specific legislation (e.g. the Protection of Badgers Act 1992, the Deer Act 1991)
Since there is such a wide variety of legislation that also varies between England and Wales, and Scotland and Northern Ireland according to devolved systems of government, a complete up-to-date guide is impossible to provide in this text. The UK statute law database (www.statutelaw.gov.uk) should be consulted for further guidance2. A useful summary of the relevant legislation covering keeping of wild animals in captivity can be found at www.defra.gov.uk/wildlife-pets/.
Relevant professional or local guidance must be followed at all times. This will include, as appropriate:
- guidance from expert bodies (such as the British Trust for Ornithology (www.bto.org) or Nature Conservancy),
- codes of practice set by professional bodies and learned societies (e.g. Association of the Study of Animal Behaviour (www.elsevier.com/framework_products/promis_misc/ASAB2006.pdf), Mammal Society, British Association for Shooting and Conservation (www.basc.org.uk) and English Nature (www.english-nature.org.uk),
- guidance from site owners/wardens,
- guidance from sponsors,
- health and safety advice,
- welfare guidance.
No one may work with animals in any capacity without having satisfactorily completed appropriate training. For example, if licences are required under the Animals (Scientific Procedures) Act, licensee training will be needed. In the case of research on wild birds training will be required in the netting and ringing of birds (from the British Trust for Ornithology), and for the trapping of mammals training from the Mammal Society may be needed, and training in relevant health and safety issues may be necessary. The supervisor of the programme must ensure competence in any necessary handling of the species, and the researcher must be sufficiently familiar with the habits and biology of the species to detect major departures from the normal state. Veterinary or animal welfare advice must be sought and taken in any case where the health or welfare of an animal gives rise to concern. Adequate time must be allocated to allow aptitude to be developed with adequate training, then assessed and the required standards achieved. For netting birds 6 months training is typically advised.
Considerations under the UK Animals (Scientific Procedures) Act 1986
Any scientific procedures in wild animals that may have potential to cause pain, suffering, distress, or lasting harm will be regulated under ASPA and may only be carried out as approved. The Home Office can advise on a case-by-case basis and must be consulted wherever a question arises as to whether or not a particular programme of work requires regulation under ASPA. Capture and handling are not in themselves regarded as coming within the definition of a regulated procedure but best practice is essential to ensure that trapping does not cause pain, suffering, distress or lasting harm. The netting and ringing of birds is also not regulated under ASPA, but is covered by controls drawn up by the British Trust for Ornithology on behalf of the Joint Nature Conservancy Council, English Nature and other bodies, under the WCA. Anyone wishing to work in an area which requires the capture of wild birds must be familiar with these controls and have received the necessary training.
It is important to bear in mind the potential consequences which interventions may have on animals other than those captured; for example on dependent young (lactating females should be promptly released from traps). Relevant issues should be discussed with the Home Office Inspectorate because regulation under ASPA may be required if a project has potential to cause distress to, for example, animals dependent on those captured.
Project Planning
Before beginning a project involving the use of wild species, there must be careful planning with attention paid to contingency and risk assessment. Consider the following points.
- Consult a statistician at an early stage to ensure that the minimum number of animals consistent with the aims of the project is used, and that animals are not wasted by having a sample that is too small. This is of course true for all projects involving animals, but is particularly important when dealing with endangered or threatened species.
- Review the literature, to become familiar with current techniques and husbandry methods. Ignorance of these may cause undue distress and is unacceptable.
- Ensure familiarity with the biology of the species involved. Avoid disturbances at times when interference may have a great impact on the species as a whole, such as breeding times, nesting, late pregnancy, parturition and neonatal periods.
- Avoid causing disturbances in the area to other species that are not being studied, and minimise damage to the habitat.
- Ensure maximum use of samples and material obtained from the study.
Catching and Trapping
The trapping of wild animals for use in research should be humane and may only be undertaken by competent personnel. The trap must keep the animal alive, uninjured and in a comfortable microenvironment. An open-sided wire mesh trap is unlikely to fulfil this requirement, since the animals will be uncomfortable and may feel exposed and vulnerable. Traps should be designed according to the biology of the species, and should avoid trapping non-target species. Best practice will include careful attention to the choice of trap, its layout, siting and positioning; and appropriate supply of food and bedding. Consider the animal's natural circadian activities: nocturnal animals should be held in darkness during daylight hours by covering the traps, and animals held in traps without food bait should be released so that they have sufficient time to gather food after release, or given supplementary food and water before release. Extra care will be needed in hot or cold weather or where traps may be in the presence of predators. Traps must be checked sufficiently frequently that stress to captured animals is minimised. Equal care is required on release to ensure animals have not been unduly compromised in their chances of survival. Consideration must be given to their ability to re-establish themselves within their own community and to find sources of food and water and that they are not unreasonably exposed to predators.
Handling Wild Animals
The amount of handling should be kept to the absolute minimum to reduce stress and also to minimise the risks to the handler. Wild animals are likely to bite, scratch or otherwise injure the handler, their health status is unknown and they may be carrying potentially zoonotic diseases (such as Leptospira, Crytosporidium)3, so appropriate protective clothing should be worn and risk assessments carried out. For many species, keeping the animal covered will aid handling and birds should be handled by appropriate restraint of the wings. All personnel working with wild animals should be appropriately trained in their care, and familiar with the action to be taken in the event of injuries, escape or disease. It is important to develop a strategy for dealing with injuries that are due to the procedure, or which the animals sustained prior to capture, so that decisions over whether or not to treat or euthanase such animals can be defended4,5.
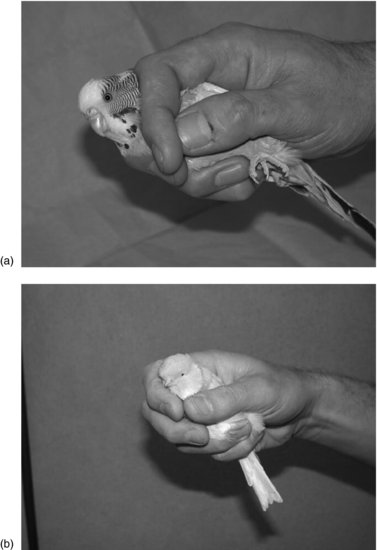
Birds are very susceptible to stress, particularly small species, and excessive exertion can result in respiratory collapse or hypoglycaemic episodes. These problems can be reduced by handling them slowly and deliberately, and by ensuring that movements of the sternum are not restricted. Injuries may occur as the birds flap their wings to try to escape, so gentle restraint of the wings is vital. Small birds can be held with the back against the palm of one hand. Place the thumb and index finger or index and middle fingers each side of the head to prevent the bird from turning and pecking the handler, and wrap the other fingers around the body to restrain the wings and legs (Figure 15.1). All birds are disoriented by sudden darkness, so handling can be facilitated by covering the head with a cloth or bag, or for a small bird with a yoghurt pot. Large birds should generally be approached from above, with the base of the wings held in a naturally folded position without flapping. Gloves, tissue or a cotton cloth should be used to prevent the feathers coming into contact with perspiration from the hand, since this can reduce waterproofing and interfere with insulation. Assistance may be required to restrain the heads of large birds with long necks6. Because of the potential health risks from handling birds, handlers should wear appropriate protective clothing, including gloves and a face mask as a minimum, to handle birds. Cuts and grazes should be protected, and hands and arms washed thoroughly in disinfectant afterwards.
FIGURE 15.1 Restraint of a small bird.
Anaesthesia of Wild Animals
General considerations
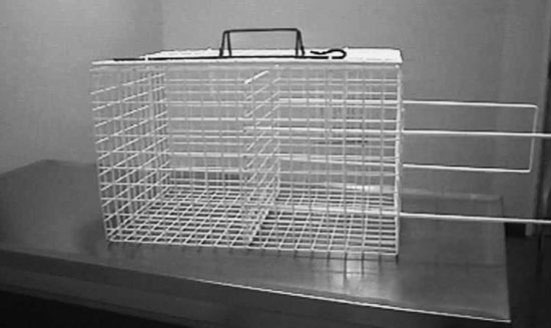
If pharmacological restraint is required it should be given under veterinary supervision. Anaesthetics and analgesics suitable for the species and procedure should be used, which allow the animal to make a rapid return to consciousness. Animals should only be released when they have recovered sufficiently from the procedures to be able to cope with hazards in the environment. Since there may not be the opportunity to check on the animals post-operatively, surgical procedures must be performed to a particularly high standard, using strict aseptic techniques and good surgical and suturing methods. A wide range of smaller wildlife species can be successfully lightly anaesthetised for restraint using isoflurane, either alone or in combination with injectable agents. For larger species such as badgers, otters and foxes induction of anaesthetic is generally by injectable agents. These are administered through the mesh of the trapping cage, such as a crush cage (see Figure 15.2), or by means of a blowpipe or dart gun. Use of these is controlled under the UK Firearms Act (1968) and requires permits. Many commonly used injectable combinations include non-reversible agents with associated prolonged recovery times. This is generally undesirable in the field where there are unlikely to be facilities to maintain adequately warm temperatures; it is difficult for personnel to monitor the recovery of more than a few individuals and animals are prevented from resuming normal activities (for example, hunting for food or protecting young). Well-controlled anaesthesia is important for the operator: an animal suddenly recovering from anaesthesia is likely to bite and so present a risk of injury and transfer of zoonotic disease.
FIGURE 15.2 Crush cage for use with small wild animals.
Although fieldwork presents particular challenges, many of the procedural refinements used in the laboratory or surgery can be transferred. A folding table (such as of the type used for pasting wallpaper), covered in thick polythene sheeting and disposable paper covers, functions well as an operating table which can readily be disinfected between animals (see Figure 15.3). Some of the problems encountered in the trapping and subsequent anaesthesia of small wild animals are due to their very small size and high metabolic rate. Their high ratio of surface area to body weight is such that they lose heat rapidly and hypothermia must be prevented. All animals should therefore be placed on bedding, over gel heat pads or hot-water bottles, during anaesthesia and recovery. They must be placed in sheltered positions, provided with food, and their holding cages covered with blankets or bubble wrap during recovery. Death rates in traps can be particularly high for shrews, possibly because of their very high metabolic rates and thermal requirements. Small birds also have a higher basal metabolic rate (BMR) than larger ones: a hummingbird weighing 3.5 g has a BMR of around 6720 kJ/kg per day, whereas a swan weighing 9 kg has a BMR of around 200 kJ/kg per day. This high metabolic rate renders birds prone to hypoglycaemia even if starved for only a few hours. The heart is relatively large and beats very fast, and birds will readily develop acute cardiovascular failure if stressed. Their body temperature is often 41°C or more, which allows the rapid metabolism required, but which also renders birds prone to hypothermia. Body temperature is regulated by physiological and behavioural means which varies between species depending on their natural habitat. Birds will fluff up their feathers, take shelter and shiver when it is cold, but they have limited capacity to lose heat if it is warm. They do not possess sweat glands, and therefore heat loss can only be effected by panting or gular fluttering, which is the rapid movement of the thin floor of the mouth and upper area of the throat. This must all be taken into account when they are anaesthetised.
FIGURE 15.3 An operating table that can readily be disinfected between animals.

Inhalational anaesthesia
Apparatus is available for inhalational anaesthesia in the field that is light enough to be portable in a simple backpack7. An isoflurane vaporizer, which weighs approximately 7 kg, is attached to a portable oxygen cylinder (see Figure 15.4). It is important to note even with a temperature-compensated model, prolonged exposure to temperatures below 10°C (for example, overnight storage in a vehicle in winter) will adversely affect the calibration for some time even after air temperatures have risen. This is due to the slow rate at which the vaporizer is designed to expand and contract. This problem can be overcome in the field by protecting the vaporizer from protracted exposure to low temperatures using an insulated box, if necessary supplied with hot-water bottles. The vaporizer is connected using standard circuitry to a portable 230 litre medical oxygen cylinder (weight 2.9 kg). The cylinder can be refilled from a standard-sized canister using a recharging adaptor. The cylinder should be fitted with a regulator permitting flow rates of 1–15 litre/min.
FIGURE 15.4 A vaporizer, which weighs approximately 7 kg, is attached to a portable oxygen cylinder.

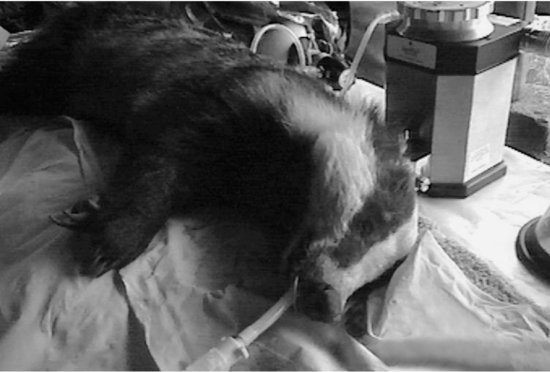
The protocol used for induction depends on the size of the animal. Small mammals caught in metal box Longworth traps can be gently tipped with their bedding into a transparent polythene bag. This bag then serves as an induction chamber, with the neck of the bag being held closed around the anaesthetic gas tube (see Figure 15.5). Larger species caught in wire mesh traps, such as squirrels, rats and rabbits, can be anaesthetised as above, except the whole trap is placed inside a polythene bag. Larger animals such as badgers and foxes are better given short-acting reversible injectable anaesthetic for induction (Figure 15.6). All species are then transferred to a face mask or intubated for maintenance (see Figures 15.7 and 15.8). Since fieldwork is usually conducted outside there may be no need for a scavenging system to be included in the circuit. The main advantage of this inhalation system is its safety for the animal, despite their unknown health status at induction. In addition, the animals recover very quickly from isoflurane: most are fit for release within 20 min of the end of anaesthesia and can be returned to the wild with less disruption to them and to their population than using injectable methods alone.
FIGURE 15.5 Induction chamber, with the neck of the bag being held closed around the anaesthetic gas tube.

FIGURE 15.6 Larger animals such as badgers and foxes are better given short-acting reversible injectable anaesthetic for induction.

FIGURE 15.7 All species are then transferred to a face mask or intubated for maintenance.

FIGURE 15.8 Badger with an endotracheal tube.
Injectable anaesthesia
Drug doses and body size
When administering drugs to wild animals it is common to extrapolate doses from other species where the dose rate is known. However, smaller animals have a relatively higher basal metabolic rate and so the dose required is not dependent on the body weight (W, kg), but on the metabolic body weight, which can be obtained by W0.75, and the energy group to which the animal belongs. Animals can be divided into five energy groups depending on the mean core temperature range (see Table 15.1)8. Hainsworth identified constants for each group, and when the metabolic body weight is multiplied by the Hainsworth constant the product can be used to set up ratios for scaling pharmacokinetic or physiological parameters from one species to another. This process is known as allometric scaling.
Table 15.1 Hainsworth's energy groups.
| Group | Constant (K) | Mean core temperature (°C) |
| A Passerine bird | 129 | 42 |
| B Non-passerine bird | 78 | 40 |
| C Placental mammal | 70 | 37 |
| D Marsupial mammal | 49 | 35 |
| E Reptile | 10 | 37* |
| *Requires body temperature to be held artificially at 37°C. | ||
To extrapolate dose rates or frequencies from one species to another the animal's specific minimum energy cost (SMEC) must be calculated. This is given by:
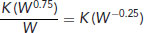
Then, calculate the test dose or frequency by extrapolating from a known dose rate or frequency:
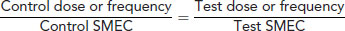
For example, if the dose of a drug for a 10 kg dog is 10 mg/kg twice daily, the dose D and frequency F for a 20 g mouse can be calculated by:
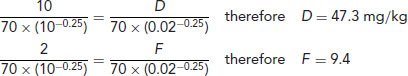
So, a mouse needs 47.3 mg/kg of the drug 9.4 times daily, or every 2.5 h.
These formulae can be used to calculate reasonable, effective dose regimes for any species of warm-blooded animal, ranging from the smallest birds to large ungulates.
Drug doses for wild animals
The following regimes may be used as guidelines for injectable anaesthesia of some particular species of wild animals9, pigeons and doves10 and European starling11.
Rats, mice, voles, rabbits
The doses described in Chapter 9 may be used, remembering that the health and condition of the animal may not be as robust as an animal in the laboratory.
Squirrel
Medetomidine 250 μg/kg plus ketamine 60 mg/kg administered together intramuscularly (i.m.). Reverse with atipamezole at 1 mg/kg subcutaneously.
Hare
Give medetomidine 0.25 mg/kg i.m. Adjust according to response. Reverse with atipamezole and adjust according to response. Try a low dose and give more if necessary after 3–5 min. Ketamine may be used if medetomidine does not prove successful.
Hedgehog
Ketamine at 20 mg/kg produces sedation with recovery in 30 min.
Badger
Ketamine at 15–20 mg/kg i.m. produces immobilisation, and at 30 mg/kg produces deep tranquillisation. This lasts for about 45 min but will take much longer to work if the environmental temperature is low. For general anaesthesia 20 μg/kg medetomidine with 4 mg/kg ketamine plus 0.8 mg/kg butorphanol is an excellent combination. Alternatively, use 2.0 mg/kg xylazine with 7.0 mg/kg ketamine i.m. or 70–80 μg/kg medetomidine with 3.5–4 mg/kg ketamine i.m. Reverse with 350–400 μg/kg atipamezole i.m.
Otter
Medetomidine 40 μg/kg plus ketamine 4 mg/kg administered via a blow pipe to produce heavy sedation, then 30 μg/kg medetomidine plus 3 mg/kg ketamine given i.m. will produce 40 min of anaesthesia and can be reversed with 350 μg/kg atipamezole.
Birds
Ketamine combinations: for wildfowl, 15–50 mg/kg will give light surgical anaesthesia. It may be given in incremental doses of 5 mg/kg at 5 min intervals and can be used to maintain light anaesthesia/sedation for several hours. For small birds mix 0.1 ml of ketamine (100 mg/ml) in 0.9 ml of sterile water. Give 1 mg (0.1 ml) per bird i.m. This will produce sedation in 3–4 min and recovery in about 20 min. At 2 mg/bird there is light surgical anaesthesia for 5–12 min and recovery in 30 min. At 3 mg/bird there is 5–20 min of anaesthesia and recovery in 60 min. Ketamine may be used alone for induction prior to using isoflurane for longer procedures, but is also used in combination with other drugs. For deeper anaesthesia in surgical procedures where some relaxation of the muscles is required, ketamine at 20 mg/kg i.m. may be combined with midazolam (4 mg/kg i.m.) or diazepam (1.5 mg/kg i.m.).
Identification of Wild Animals
The ringing, tagging or marking of an animal for the primary purpose of identification is not regulated under ASPA if it causes only momentary distress and no lasting harm. However, best practice must be followed – as prescribed, for example, by the British Trust for Ornithology – and account taken of the ways in which identification techniques may compromise an animal's ability to function. Individual animals may be identified by their unique features, or by methods as used in other laboratory species. A variety of attachment devices are used with telemetric and GPS devices. These should be as light as possible and less that 5% of the animal's own body weight. Collars or harnesses should be prepared to allow for potentially wide variation in body size within the population and fitted to allow enough room for growth.
Leg rings are the most common method for birds. Tags in the ear or wing can be used in some species but care must be taken in use of colour so that they do not affect camouflage, and they should be positioned so they do not get caught on vegetation leading to tissue damage, especially in species that burrow12. Fur clipping can be used as a temporary marking method or tattooing (under general anaesthesia) as a permanent method. Microchips are also suitable, as is use of individual identification based on natural markings in some species. Methods that involve physical alteration of the animal such as ear notching or toe clipping are not recommended, since they can affect the animal's behaviour or ability to survive once released back into the wild.
Release of Animals Back to the Wild
Animals that have been taken into captivity and subjected to scientific procedures may need to be released back into the wild at the end of or during the procedures. The release of animals under these circumstances, however mild the procedures, requires the prior consent of the Home Office. ASPA requires that animals on which regulated procedures have been performed are killed at the conclusion of the series of regulated procedures, unless a veterinary surgeon or other competent person determines that they are not suffering or likely to suffer as a result of the procedures. In addition, if the animal is to be released at a place which is not an establishment with a Section 2C licence, a veterinary surgeon may need to verify that the animal will not suffer if it is released in that place. However, such work may be undertaken at remote sites (e.g. nature reserves), where it is not practicable for a veterinary surgeon to be in constant attendance. In such situations it may be necessary to rely on observance, by suitably trained and qualified persons, of a written protocol, which is not expected to cause lasting harm if followed correctly. The veterinary surgeon may then be assured that an animal will be fit, if the protocol is carefully followed by these trained and competent individuals. As part of conservation projects sometimes animals that have been bred in captivity are released into the wild and these also need to be assessed for fitness before release13.
If animals are to be released to the wild in the course of regulated procedures – for example in studies where individuals are captured, measurements are made, then the animals are released and recaptured at intervals – additional safeguards must be put in place. If regulated procedures are applied to animals taken from the wild the holder of the licence authorising the work must ensure that the animals are captured by a competent person using a method which does not cause the animal avoidable pain, suffering, distress or lasting harm. The licence holder is also responsible for ensuring that if an animal taken from the wild is found to be injured or in poor health it is not subjected to a regulated procedure unless it has been examined by a veterinary surgeon or other competent person and action has been taken to minimise the suffering of the animal, unless an exception to this has been agreed in the conditions of the licence.
In addition to ensuring that the requirements of other relevant legislation have been met it is equally important to ensure the well-being of animals released into the wild after capture, even if they have been used for purposes not regulated under ASPA. In the case of research on farms or other partially protected sites it may be possible to exercise a degree of supervision after their release. Where possible there should be appropriate monitoring of the animal for unexpected adverse effects. The animals should not find themselves at a biological disadvantage as a result of the procedures performed or of its captivity. Regard should also be had to the welfare of other individuals (e.g. the offspring of those captured). The following considerations should apply:
- animals should not be released in such a way as unreasonably to expose them to risk (e.g. of predation, thirst or hunger),
- animals should be released at a suitable site, normally within their home range,
- the timing of the release should take account of the animal's normal daily and seasonal activity patterns and other relevant factors,
- where animals have been injured or found to be injured or unwell, account must be taken of relevant veterinary advice and welfare considerations,
- the duration of capture should be the minimum consistent with both the object of the experiment and humane release,
- consideration should be given to the consequences of the release of animals into the food chain.
References
1. Inglis JR, Mathews F and Hudson A (2010). Wild mammals. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals, 8th edn, R Hubrecht and J Kirkwood (eds), pp. 231–45. Chichester: Wiley-Blackwell
2. Mitchell-Jones AJ, Marnell F, Matthews JE and Raynor R (2008) Mammals and the law. In Mammals of the British Isles Handbook, 4th edn, S Harris and DW Yalden (eds). Southampton: Mammal Society
3. Chalmers RM, Robinson G and Elwin K (2009). Cryptosporidium sp Rabbit Genotype, a newly identified human pathogen. Emerging Infectious Diseases 15: 829–30
4. Lane JM and McDonald RA (2010). Welfare and ‘best practice’ in field studies of wildlife. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals, 8th edn, R Hubrecht and J Kirkwood (eds), pp. 92–106. Chichester: Wiley-Blackwell
5. American Association of Zoo Veterinarians (2006). American Association of Zoo Veterinarians Guidelines for Euthanasia of Non-Domestic Animals. www.aazv.org/displaycommon.cfm?an=1&subarticlenbr=441
6. BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement (2001). Laboratory birds: refinements in husbandry and procedures. Laboratory Animals 35 (suppl. 1)
7. Mathews F, Honess P and Wolfensohn S (2001). Refinements in wildlife anaesthesia. Veterinary Record 150: 785–7
8. Fowler ME and Miller RE (2008). Zoo and Wild Animal Medicine: Current Therapy, vol. 6. St Louis, MO: Saunders Elsevier
9. Osofsky SA and Hirsch K.J (2000). Chemical restraint of endangered mammals for conservation purposes: a practical primer. Oryx 34: 27–33
10. McGregor A and Haselgrove M (2010). Pigeons and doves. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals, 8th edn, R Hubrecht and J Kirkwood (eds), pp. 686–96. Chichester: Wiley-Blackwell
11. Bateson M and Asher L (2010). The European starling. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals, 8th edn, R Hubrecht and J Kirkwood (eds), pp. 697–705. Chichester: Wiley-Blackwell
12. Redfern C (2001). The Ringers Manual. Thetford: BTO
13. Mathews F, McLaren GW and Gelling M (2004). Keeping fit on the ark: assessing the suitability of captive bred animals for release. Biological Conservation 121: 569–77