CHAPTER 1
1. Actually, rather than visible light, Compton used X-rays. This ultra-high-energy light had so much oomph that it easily knocked electrons out of atoms. To all intents and purposes, they reacted like free-floating electrons rather than ones tied to an atomic nucleus.
2. Remarkably, relativity could have been a natural and unsurprising outgrowth of sixteenth-century physics. As several people have realised since Einstein, relativity is actually an unavoidable consequence of two things. One is that the laws of physics look the same whatever your state of motion, as long as that motion is at constant velocity. For instance, a ball thrown between two people follows the same shaped trajectory whether they are standing in a field or on a train travelling at 100 kilometres per hour. And the second thing is that the laws of physics look the same no matter what your orientation in 3D space. It is not necessary to assume anything about the speed of light, as Einstein did. Galileo could have discovered relativity. See ‘The Theory of Relativity – Galileo’s Child’ by Mitchell Feigenbaum (http://xxx.lanl.gov/abs/0806.1234).
3. In fact, it has more than stood the test of time since it turns out that it is not only matter that is grainy but everything. This is the meaning of the word ‘quantum’ in quantum theory. A quantum is an indivisible grain of something. Matter comes in quanta. So does energy, electric charge, time, and so on. We live in a fundamentally grainy world.
4. It is always possible there is a deeper level of reality beneath quantum theory and that the probability of things happening is determined by factors operating at this fundamental level, just as the roll of a dice is determined by environmental factors. This possibility continues to be explored by some scientists, including the English physicist Antony Valentini and the Dutch Nobel Prize-winner Gerard t’Hooft. However, they are in a minority. The theory appears to work perfectly if the unpredictability is indeed nature’s fundamental, irreducible bedrock, so most physicists see no compelling reason to look any deeper.
5. Another irony is that, in 1900, the year Planck proposed the quantum, Lord Kelvin, one of the greatest physicists of his day, surveyed the achievements of his contemporaries and declared: ‘There is nothing new to be discovered in physics now. All that remains is more and more precise measurement.’ How wrong he was.
6. Young’s double-slit experiment is one of the pivotal experiments in the history of science. Today, however, you can prove that light is a wave with a £1 laser pointer and a £2 metal ruler. Simply shine the laser at a very shallow angle along the metal ruler so that its narrow beam spreads out enough to illuminate several of the most closely spaced gradations on the ruler. Each of the gradations will act as a secondary source of concentric light waves which, as they spread through space, will pass through each other. Where they reinforce, they will create bright spots, and these will show up if, for instance, there is a convenient white wall in the path of the light. Strictly speaking, the spots are a result of ‘diffraction’, a phenomenon closely related to interference, but an undeniable characteristic of waves nevertheless.
Further reading:
The Magic Furnace by Marcus Chown (Vintage, 2000).
CHAPTER 2
1. See Chapter 1.
2. Troublesome exceptions are elements such as chlorine, which weighs in at 35.5 times the weight of hydrogen. Prout did not know that chlorine comes in several types, each of which, individually, weighs an exact multiple of hydrogen’s weight but whose average is 35.5 times that of hydrogen.
3. Actually, on emission from radium, alpha rays were merely the cores, or ‘nuclei’, of helium atoms, but that is getting ahead of the story. But by the time Rutherford detected them, they had combined with electrons to make helium atoms.
4. In fact, there is a third type of ray that can be emitted by a radioactive substance. A ‘gamma ray’ is an ultra-high-energy form of light.
5. The term ‘nucleus’ was not used until 1912.
6. See Chapter 1.
7. The dark energy is invisible and fills all of space, and its repulsive gravity is speeding up the expansion of the Universe. Its energy density is a whopping 1 followed by 120 zeros smaller than predicted by quantum theory, our current best description of ultimate reality.
8. A word on this ‘scientific notation’ which is so commonly used by physicists. 1034 means 10 multiplied by itself 34 times. And 10–34 means 1/(10 multiplied by itself 34 times). So, for instance, 105 = 10 × 10 × 10 × 10 × 10 = 100,000. And 10–3 = 1/(10 × 10 × 10) = 1/1000 = 0.001.
9. De Broglie thought matter waves were really waves of matter. But recall that the wave associated with a particle like an electron is actually more abstract than that. It’s a probability wave, which spreads according to the Schrödinger equation and whose height at any location – strictly speaking, the square of the height – is related to the chance, or probability, of finding the particle there.
10. Another popular explanation is that there is an infinite number of parallel realities stacked like the pages of a never-ending book. According to this ‘Many Worlds’ picture, when a particle is in a superposition which corresponds to being in two places at once, it is not actually at two places at once in one reality; it is at one place in one reality and another place in a neighbouring reality. In this view, a particle goes through only one slit in the opaque screen, but it interferes with a particle that went through the other slit in a neighbouring reality.
11. Here we are still talking about the ‘act of observation’, or interaction of the bullet with the wall, imparting some sideways jitter to the bullet. In other words, we are saying the uncertainty is not intrinsic to the bullet but caused by the act of observation. In fact, it is intrinsic. A better/complementary explanation is decoherence.
Further reading:
‘The Sun Likes Me’, Heart on the Left by Adrian Mitchell, p. 194.
CHAPTER 3
1. Mass here means ‘rest mass’. Some particles, such as photons, have no rest mass. They are born travelling at the speed of light and cannot exist at rest with respect to anything or anyone.
3. Of course, latitude and longitude are used to specify a location on the surface of a globe. But this is possible only because every point on the surface is at the same distance from the centre of the Earth.
4. What all this is emphasising is that quantum theory is just a theory about what we can know or measure – which, really, is what any scientific theory should be. If we can know only the outcome of an event such as the interaction between two identical particles, we have no right to ask how that outcome came about. In fact, the question is not a legitimate scientific question. It has no meaning. As Niels Bohr said: ‘It is wrong to think that the task of physics is to find out how nature is. Physics concerns what we can say about nature.’
5. Which I did not answer in my book Quantum Theory Cannot Hurt You (Faber, 2008).
6. Actually, all particles with half-integer spin – ½, 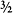 ,
, 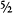 , and so on – are fermions, and all particles with integer spin – 0, 1, 2, and so on – are bosons.
, and so on – are fermions, and all particles with integer spin – 0, 1, 2, and so on – are bosons.
7. The proof that spin-½ particles (or, more generally, particles with half-integer spin) obey the Pauli exclusion principle was not straightforward. It was not until 1940 – 16 years after he had discovered the exclusion principle – that Pauli proved the so-called spin-statistics theorem.
8. It is the alignment of an electron’s spin in a magnetic field that gave the first hint of the existence of spin. When an electron in an atom jumps between one state and another – a so-called quantum jump – it spits out or absorbs light of energy equal to the difference in energy of the states. However, in a magnetic field, the light could have two slightly different energies – on either side of the expected energy. The explanation is that the electron’s spin can be aligned with the magnetic field or against it, and that each of the orientations corresponds to a slightly different energy. Peculiarly, Pauli discovered the exclusion principle before the discovery of spin by the Dutch-American physicists Samuel Goudsmit and George Uhlenbeck in 1925/26. Although Pauli did not know of spin, however, he nevertheless knew of the twofoldness, or zweideutigkeit, of the energy states of an electron in a magnetic field. See Pauli’s Exclusion Principle: The Origin and Validation of a Scientific Principle by Michela Massimi (Cambridge University Press, 2005).
9. See Chapter 2.
Further reading:
QED: The Strange Theory of Light and Matter by Richard Feynman (Penguin, 1990). This is the most brilliantly simple book on the most successful physical theory ever devised. I cannot praise it enough. Feynman was not only a genius physicist, he was a genius populariser too.
A Brief History of Time by Stephen Hawking (Bantam, 1995).
The Great Physicists from Galileo to Einstein by George Gamow (Dover Publications, New York, 1988).
CHAPTER 4
1. The only real influence the composition of a body has is on how easily heat escapes from it. This depends on the number of free electrons, since free electrons turn out to be good at ‘scattering’, or redirecting, radiant heat, hindering its progress outwards from the centre. An object predominantly made of hydrogen has a maximum of one free electron per atomic nucleus to dam up internal heat, whereas an object made of heavier atoms has more.
2. Anaxagoras may have been this precise because he was the first to realise that the Moon is opaque and so casts a shadow on the Earth when it passes in front of the Sun. He was able to gauge the extent of the shadow from eye-witness reports, principally from sailors, during the annular eclipse of 478 BC. The shadow covered the Peloponnesian peninsula. So Anaxagoras concluded that the Moon was ‘as large as the Peloponnese’ and the Sun therefore ‘a little larger than Greece’.
3. See Chapter 3.
4. Initially, Herschel christened Uranus ‘George’ in honour of England’s monarch, George III. Not many people know this.
5. This estimate was made in 1658 by the Irish archbishop James Ussher.
6. The more sophisticated explanation of this uses the law of conservation of energy. In the act of pumping, the energy of motion of a piston is transformed into the heat energy of the air behind the piston – in other words, the random, frenzied motion of the air molecules.
7. See Chapter 2.
8. If the decay of an atom is unpredictable, then it implies that if you watch it for, say, ten minutes, then another ten minutes, and another ten, and so on, the atom will have the same chance of decaying in each interval. If it did not have the same chance and, say, was more likely to decay between 30 and 40 minutes than in any other interval, then clearly its behaviour would no longer be unpredictable – you would know it would be more likely to decay between 30 and 40 minutes. Now, say we have a sample containing a large number of radioactive atoms. And say the chance of it decaying in the first ten minutes is 1/2. After ten minutes, therefore, half the atoms will be undecayed. After 20 minutes, a half of what are left, which is a quarter, and so on. This trivial example shows how an equal chance of decay in each ten-minute interval leads to a decay law with a half-life of ten minutes. What is not so trivial is to realise that even if the chance of an atom decaying in any particular interval was 1/10 or 1/63 or 0.000023, the same kind of radioactive decay law characterised by a particular half-life would still result.
9. I tried to rectify the injustice that had been done to Cecilia Payne by writing an article about her work in 2003 in the magazine New Scientist (http://newscientist.com/article/mg18024205.300-the-star-who-unravelled-the-sun.html). Unfortunately, there was a cock-up at the printers and, when the article appeared, the photograph that accompanied the article, instead of showing Cecilia Payne, showed only the corner of her hat. Well, I tried. But perhaps some people are cursed, destined never to get the credit they deserve.
10. In fact, all that Atkinson and Houtermans did was turn on its head an idea originated by their colleague, George Gamow. He was the first to apply quantum theory to the atomic nucleus in an attempt to explain radioactive alpha decay, in which a nucleus of helium is ejected at ultra-high speed from an unstable heavy nucleus such as radium. The problem is that alpha particles have insufficient energy to escape their nuclear prison – they are trapped down a mine shaft – yet they still do escape, appearing spontaneously on the lower slopes of the nuclear hillside. The key to their Houdini-like behaviour, Gamow realised, is their quantum nature. Though they have insufficient energy to climb to the lip of the mine shaft, their spread-out waviness enables them to ‘tunnel’ through the hillside to freedom.
CHAPTER 5
1. It would turn out, however, that the lightest nuclei such as deuterium – heavy hydrogen – and helium were made in the fireball of the Big Bang. In fact, after the ten-minute fury of nuclear reactions, roughly 10 per cent of the nuclei have become helium, a proportion we see all over the Universe and the prediction of which is touted as one of the great triumphs of the Big Bang model.
2. Hoyle was one of those scientists who was often right when he was wrong. Although his mechanism for making red giants was incorrect, the cold, dense, dark clouds of hydrogen gas he postulated did exist. They are the places where new stars are born. Not only that, but ‘accretion’ – the process by which Hoyle envisioned stars gathering hydrogen gas about themselves – is one of the most important and ubiquitous processes in the Universe. Among other things, it feeds the monster ‘supermassive’ black holes that lurk at the heart of just about every galaxy, including our own Milky Way.
3. In fact, a star must have a mass of at least three times that of the Sun to ever reach a temperature of 100 million degrees.
CHAPTER 6
1. See Chapter 5.
2. Actually, the build-up of elements inside stars is a little more complicated than this. This is because there are often several alternative routes to building up a particular heavy nucleus. For instance, once carbon-12 and oxygen-16 become common in a star, heavy nuclei can be made by their direct fusion. Thus, two nuclei of carbon-12 can stick to make neon-20 plus a nucleus of helium-4. In practice, the direct fusion of carbon-12 and the direct fusion of oxygen-16 can leapfrog many of the nuclei made by the alpha process.
3. Baade’s great discovery was that the stars in the Milky Way fell into two distinct categories. Population I stars, found in the spiral arms of the Galaxy, are dominated by hot, blue stars. Population II stars, in the central ‘bulge’ of the Galaxy, are dominated by cool, red stars. Later, it would become clear that Population I stars are young and therefore dominated by newborn massive stars. Population II stars are old. All the hot, young stars have gone out, so they are dominated by old, red giant stars.
4. Though a brilliant and visionary astronomer, Zwicky was an eccentric and volatile character whose insistence on calling Baade a Nazi, which he wasn’t, eventually led to the shy, quiet Baade living in fear of his life. Zwicky classified people he did not like as bastards or spherical bastards, who were bastards whatever way you looked at them.
5. Every sugar-cube-sized volume would weigh as much as the entire human race. See Chapter 2.
6. The team was at Los Alamos in New Mexico.
7. In fact, the very lightest elements, principally helium, were forged in the Big Bang. The Big Bang model predicts that about 10 per cent of the atoms in the Universe should be helium, forged in the first ten minutes of the Universe’s existence. And this is exactly what is observed.
8. Actually, the alpha process makes iron-58 and nickel-62, both of which buck the trend and have slightly less mass per nucleon than iron-56. But the nucleus made by addition of a helium nucleus is zinc-60, and this does have more mass per nucleon.
9. Instead of 26 protons and 30 neutrons, which is the case for iron-56, nickel-56 contains equal numbers of protons and neutrons – 28 of each.
10. An iron meteorite is a natural alloy of stainless steel that contains nickel-56, chromium, magnesium and cobalt, all of which were synthesised in the furnace of a supernova.
11. How are elements heavier than iron made? Well, we know that big, highly charged nuclei like zirconium and uranium cannot be formed by fusing together smaller nuclei because, even if two nuclei hit each other at close to the speed of light – the cosmic speed limit – it would be insufficient to overcome their mutual electrical repulsion. This leaves only processes in which a nucleus captures neutrons, since they have no electrical charge. However, free neutrons disintegrate in about ten minutes. The only way a nucleus can accrue a lot of neutrons is if it is (a) exposed over a short period (less than ten minutes) to an intense burst of neutrons, or (b) exposed over a long period to a source of neutrons which is constantly replenished. The existence of uranium, which is neutron-rich, requires source (a). Such a source is believed to exist in supernovae when the nuclei in the imploding core come apart into neutrons, prior to making a neutron core. The existence of zirconium, which is not neutron-poor, requires (b). With a lot of time available, nuclei would have had time to beta decay, transforming a neutron in their nucleus into a proton. In fact, such a location exists inside normal but highly evolved stars. But iron nuclei will have to soak up a lot of neutrons to turn into nuclei of zirconium or uranium. This is not likely, which explains why zirconium and uranium are rare on Earth.
Further reading:
Home Is Where the Wind Blows by Fred Hoyle (University Science Books, Sausalito, California, 1994).
Supernovae and Nucleosynthesis by David Arnett (Princeton University Press, 1996).
CHAPTER 7
1. Basically, the intensity of starlight from a star drops off with the inverse square of its distance. So if it is twice as far away as a similar star, it is a quarter as bright; if it is three times as far away, it is a ninth as bright; and so on. On the other hand, the volume of a shell of space, which is directly related to the number of stars it contains, increases with the square of its distance. So if it is twice as far away, it contains four times as many stars; three times the distance, nine times as many; and so on. The two factors exactly compensate for each other (at least they do if space is not curved – but that’s another story).
2. The speed of light is more than a million times faster than a passenger jet, so you have to admire anyone who finds a way to measure it. Ole Christensen Röemer’s idea was to time light crossing a known distance. Since light spanned terrestrial distances too quickly for clocks to measure, the seventeenth-century Danish astronomer looked to the heavens. Imagine there is a clock out in space that strikes midday when the Earth in its orbit around the Sun is closest to the clock. Six months later, when the Earth is at its furthest, the clock will be delayed in striking because the light will have to travel across the diameter of the Earth’s orbit. Röemer’s genius was to find a celestial ‘clock’ – Jupiter and its moons. Instead of the striking of midday, he used the instant at which the moon Io went behind Jupiter. In 1676, he found that such ‘eclipses’ were delayed by 22 minutes (the modern figure is 16 minutes 40 seconds). Combining this with an estimate of the diameter of the Earth’s orbit, he calculated the speed of light as 225,000 kilometres per hour. Röemer’s measurement was accepted only when confirmed by James Bradley in 1729. His idea was to measure the speed of light relative to something else fast: the speed of the Earth as it orbited the Sun, which he knew. The Earth’s motion changed the apparent direction at which light arrived from stars just as your running through rain changes its direction. Bradley measured the shift in position of stars and concluded light travelled at 298,000 kilometres per second, which is almost exactly right.
3. Light actually travels at about 300,000 kilometres per second, or a billion kilometres an hour. Click your fingers. In the time it took you to do that, a ray of light could have made the round trip between Europe and America about 30 times over.
4. The speed of light is only the cosmic speed limit in Einstein’s special theory of relativity of 1905. Ten years later, Einstein generalised the theory to deal not only with bodies moving at constant speed with respect to each other but with bodies changing their speed, or accelerating. In his general theory of relativity – which also turned out to be a theory of gravity – space is a backdrop to which the galaxies are effectively nailed. And that backdrop can expand at any speed it likes.
5. The expansion of the Universe also stretches space and, with it, the wavelength of light. Imagine a wiggly line scrawled on a balloon becoming stretched when the balloon is inflated. This ‘red shift’ – so-called because the stretching shifts visible light to longer, ‘redder’ wavelengths – results in lower-energy light. It therefore plays a small role in reducing the light energy raining down on the Earth, though it falls far short of explaining why the sky is dark at night.
6. This estimate was made before the 1998 discovery that the Universe’s expansion is speeding up. In an ever-growing universe, filling up space with light is like filling up a bath with water as the bath grows bigger at a faster and faster rate.
7. See Chapter 4.
8. The reason for this is that the Big Bang happened everywhere in the Universe at once. This is difficult to visualise because, of course, every terrestrial explosion – be it the detonation of a stick of dynamite or a volcano – has a centre. The Big Bang explosion had no centre.
9. The ‘peak’ emission of the cosmic background radiation is actually at about a millimetre in wavelength, which is not in the microwave ‘band’. However, people persist in calling it the cosmic microwave background radiation because the earliest measurements, by Penzias and Wilson and others, were at microwave wavelengths.
Further reading:
Cosmology by Edward Harrison (Cambridge University Press, 1991).
CHAPTER 8
1. It does this because, despite being the weakest force in nature by a very large factor, it has an infinite range and cannot be screened out. So the more matter there is, the greater its gravity. The gravitational force between a proton and an electron in an atom is 10,000 trillion trillion trillion times weaker than the electric force which keeps matter stiff. So gravity dominates in all bodies which have more than 10,000 trillion trillion trillion atoms, which corresponds to a body about 10 kilometres across. This, incidentally, is why all objects in the Solar System smaller than this are like irregular potatoes, while all objects bigger – like the Earth and the Moon – are crushed by gravity into neat spheres.
2. Quantum theory explains forces as due to the exchange of force-carrying particles. For instance, the electromagnetic force between charged particles arises from the exchange of photons. Think of two tennis players batting a tennis ball back and forth. A force is transmitted to each player by the impact of the tennis ball on their racquet.
3. See Chapter 7.
4. Something that increases exponentially – or is raised to the power of e (about 2.718281828 …) – doubles, then doubles in the same time again, and doubles in the same time again, and so on.
5. See Chapter 2.
Further reading:
‘The No-Boundary Measure of the Universe’ by James Hartle, Stephen Hawking and Thomas Hertog (http://arxiv.org/abs/0711.4630).
CHAPTER 9
1. Incidentally, it follows from the fact that a fly-by and its opposite are indistinguishable that the manoeuvre can never boost the velocity of a space probe relative to the Earth. After all, if it did, you would know which movie was the correct one – the one in which the space probe gained speed. Why, then, does NASA bother? Because while it is perfectly true that a space probe cannot be boosted relative to the Earth, the Earth is moving around the Sun. Consequently, it is possible to choose an ingoing trajectory so that the planet’s speed relative to the Sun either adds to or subtracts from the space probe’s speed relative to the Earth. Such fly-bys can therefore be used to boost a space probe’s speed to reach the planets of the outer Solar System, reduce its speed to reach the planets inside the Earth’s orbit, or merely to change its direction. Having said all this, the six spacecraft that have flown past the Earth since 1980 have shown velocity changes relative to the planet. The origin of these ‘fly-by anomalies’ is currently a mystery. See ‘Anomalous Orbital-Energy Changes Observed During Spacecraft Flybys of Earth’ by John Anderson et al. (Physical Review Letters, Vol. 100, 091102, 2008).
2. Even if you insisted that the laws holding the teacup together are quantum rather than Newtonian, the quantum laws are also time-symmetric. Actually, there is an intrinsic time asymmetry in the law governing nature’s weak nuclear force. However, the effect, known as CP violation, is extremely tiny. Also, it seems to have no bearing on processes like the shattering of teacups.
3. Boltzmann’s working definition of entropy – synonymous with disorder and randomness – is the ‘number of microscopic states possible for a given macroscopic state’. In other words, it is the number of possible ways that the components of an object can be arranged and still yield the object.
4. The light given out by warm bodies, including human bodies, is in the form not of visible photons but invisible ‘infrared’ photons. Some animals such as pit vipers have organs to ‘see’ heat, or infrared, enabling them to spot their warm-blooded prey even in the dead of night.
5. Actually, although in the long term the nuclear fuels that replace lost stellar heat become depleted and stars become cold, in the short term they get hotter. In fact, the Sun is about 30 per cent hotter than when it was born. This is because a star is a giant ball of gas. When the gas loses heat, it is no longer able to push outwards as hard against the gravity trying to crush it. The ball shrinks and, in shrinking, is squeezed and heats up. Lewis Carroll knew this. In Alice in Wonderland, Tweedledum and Tweedledee pose the riddle: ‘What gets hotter as it loses heat?’ Answer: a star. (Though I am sure I read this long ago, frustratingly I have not been able to find exactly where and confirm it.)
6. See Chapter 7.
7. Boltzmann, who had also come to the conclusion that the Universe must have been in a special state in the past, speculated that there had been some huge entropy-lowering statistical fluctuation and that our present arrow of time is a consequence of that. Recall that entropy is only overwhelmingly likely to increase. It is not ordained. In highly unlikely circumstances it can decrease. Boltzmann, however, was probably wrong about there being an entropy-lowering event in the past.
8. The nuclei were about 90 per cent hydrogen and about 10 per cent helium, with a tiny sprinkling of other light nuclei such as lithium. All were cooked in the era of ‘nucleosynthesis’, which took place between about one and ten minutes after the start of the Universe.
9. The Universe also contains invisible, ‘dark’ matter, which outweighs ordinary matter by a factor of six or seven. It does not interact with photons of light (which is why it is dark) and so is not thought to have been disrupted by their presence. Consequently, it is believed to have started clumping before ordinary matter.
10. A similar argument has recently been proposed by the Oxford mathematician Roger Penrose. It seems that Schulman’s idea is ‘in the air’.
11. A purist might dispute the connection between cups breaking and what the large-scale Universe is up to. After all, the Earth could have started out in a highly ordered state – the prerequisite for disorder to increase – simply by chance. Boltzmann’s explanation of why entropy increases is a statistical thing. It is not ordained that entropy will increase, only overwhelmingly probable. Consequently, an ordered Earth could have arisen as a highly unlikely local ‘statistical fluctuation’. But although this might be a plausible explanation for our local arrow of time, it fails to explain why the stars – a more cosmic phenomenon than teacups – are pumping starlight into space and thus continually boosting the entropy of the Universe. They can be doing this only if every one of them started off in a more ordered state, which implies the Universe started off in an ordered state. So here the local is explicitly connected to the cosmic.
Further reading:
Ludwig Boltzmann: The Man Who Trusted Atoms by Carlo Cercignani (Oxford University Press, 1998).
‘Sources of the Observed Thermodynamic Arrow’ by L. S. Schulman (http://xxx.lanl.gov/abs/0811.2787).
CHAPTER 10
1. The complexity of the everyday world is indeed due to the fact that there are 92 types of atomic building blocks rather than just one, as pointed out in Chapter 3. However, as with many things in science, there is a deeper level of explanation. And that is what we are talking about here – the ultimate source of the complexity of the Universe.
2. Notice the switch from talking about the information needed to describe the Universe to the information contained in the Universe. The two statements are equivalent. They reflect the growing suspicion among physicists that information is a fundamental ‘thing’, underpinning all of physics.
3. Binary was invented by the seventeenth-century mathematician Gottfried Leibniz. It is a way of representing numbers as a string of 0s and 1s. Usually, we use decimal, or base 10. The right-hand digit represents the 1s, the next digit the 10s, the next the 10 × 10s, and so on. So, for instance, 9217 means 7 + 1 × 10 + 2 × (10 × 10) + 9 × (10 × 10 × 10). In binary, or base 2, the right-hand digit represents the 1s, the next digit the 2s, the next the 2 × 2s, and so on. So, for instance, 1101 means 1 + 0 × 2 + 1 × (2 × 2) + 1 × (2 × 2 × 2), which in decimal is 13.
4. Usually, the number of distinct arrangements of the subcomponents of a system is defined as e(information content) rather than 2(information content), with e, one of the most famous constants in maths, being 2.718281828 … The difference is not important since all the figures in this chapter are rough, ‘order of magnitude’ estimates.
5. Actually, it is a bit more complicated than this and the number of possible arrangements of N thermal photons is eN. But eN is approximately the same as 2N.
6. The reason our 13.7 billion-year-old Universe is 84 billion light years across and not 13.7 × 2 billion light years across, as might naively be expected, is that during inflation, it expanded faster than the speed of light. See Chapter 7.
7. The conservation of information is behind the black hole ‘information paradox’, highlighted by Stephen Hawking in 1976. A black hole, it turns out, is not completely black but shines with ‘Hawking radiation’, which allows it to ‘evaporate’ and eventually disappear. A paradox arises because the Hawking radiation can carry no information about the interior of the black hole, since, by definition, nothing can escape from one. So when the black hole has gone, there remains the puzzle of what happened to the information that described the dying star whose catastrophic shrinkage led to the creation of the black hole in the first place. The strong suspicion now is that it goes into creating myriad tiny bumps on the ‘event horizon’, the imaginary surface – or point of no return for in-falling matter – that surrounds the black hole. This means that the information that described the precursor star – a three-dimensional body – is encoded in the two-dimensional event horizon. The event horizon is like a hologram. The implications of this for the Universe are fascinating because it too is surrounded by a horizon – a horizon in time rather than space, but a horizon nonetheless. It appears that the three-dimensional Universe can contain no more information than can be impressed on the two-dimensional surface that surrounds it. Remarkably, it seems we are living in a giant cosmic hologram.
8.2(10^89) is 2 multiplied by itself (10 × 10 × 10 …) times, where there are 89 tens inside those brackets. Or, to put it another way, 2(10^89) is approximately (103)89 = 10267.
9. To some extent this was anticipated by the great British physicist Paul Dirac in 1939. Although he knew nothing of inflation and how ridiculously small the Universe had been at the outset, he nevertheless realised that if the Universe was expanding, as the observations of galaxies indicated, it would have been far smaller in the past, which meant it would have been too simple to seed the complexity we see around us today. At least it would be too simple if classical physics described the Universe. Dirac realised, however, that quantum theory might come to the rescue and that unpredictable quantum jumps in the early Universe might be the origin of the Universe’s complexity. By recognising the role of quantum theory in the origin of the Universe, Dirac anticipated by four decades the field of quantum cosmology. See The Strangest Man: The Hidden Life of Paul Dirac, Quantum Genius by Graham Farmelo (Faber & Faber, 2009).
10. See Chapter 8.
11. Bizarrely, the discovery of the ‘dark energy’ in 1998 shows that in the past few billion years, the normal vacuum has changed back into an inflationary-type vacuum, though with a tiny, tiny fraction of the energy that drove inflation. Dark energy, like the inflationary vacuum, is speeding up the expansion of the Universe. Nobody knows whether there is any connection between the inflationary phase and the current dark-energy-driven phase, though if there is, two mysteries would be reduced to one.
12. For a long time the vacuum energy was exactly zero, so it contained no energy to dump into other forms even if it were possible for it to decay. However, in the past few billion years, with the arrival on the cosmic stage of the dark energy, everything has changed. Since nobody knows why the dark energy switched on in the first place, it is always possible that one day it will switch off. However, since the dark energy is so small compared to the vacuum energy that drove inflation, the information injected into the Universe by such a decay will be correspondingly smaller.
Further reading:
‘Information, Information Processing and Gravity’ by Stephen Hsu (http://arxiv.org/abs/0704.1154).
CHAPTER 11
1. Plutonium-239 is one of two heavy nuclei that split, or ‘fission’, when struck by a neutron, liberating a large amount of energy. Since further free neutrons are created, more nuclei can be fissioned, causing a runaway nuclear ‘chain reaction’ and the unleashing of a dam burst of energy. This is an ‘atomic bomb’. The other nucleus that can that support a runaway chain reaction is uranium-235.
2. A more trivial ‘Fermi problem’, typical of the kind Fermi used to challenge his students, was: ‘How many piano tuners are there in Chicago?’ A rough estimate can be obtained by the following reasoning. Chicago has a population of about 10 million. Pianos tend to be owned by families, not individuals (assuming pianos owned by schools, concert halls and so on account for only a small minority). If an average family has five members, then that makes 2 million families in Chicago. If one in 20 families owns a piano, then there must be 100,000 pianos. Say each piano requires tuning just once a year. That makes 100,000 tunings a year. And say a piano tuner can tune two pianos a day and works about 200 days per year, thus tuning 400 pianos a year. Since there are 100,000 pianos in Chicago, the city must have about 250 piano tuners.
3. The concept of self-reproducing machines would later be explored in detail by the Hungarian-American physicist John von Neumann, famous for inventing the modern computer program. Such probes are therefore often called von Neumann probes.
4. The first person to spell out this argument involving von Neumann self-reproducing space probes was the American physicist Frank Tipler in 1981.
5. Pluto is nowadays not considered a planet but as just one among maybe 100,000 icy ‘Kuiper Belt’ objects in the outer Solar System. So, essentially, Earth is the only planet with a moon comparable in size to itself.
6. In 1952, Stanley Miller and Harold Urey of the University of Chicago famously took a mixture of gases thought to have existed on the primordial Earth and subjected it to electrical discharges and ultraviolet light. Their experiment yielded aldehydes, carboxylic acids and amino acids, precursors of life. But things stalled there.
7. See my book The Universe Next Door (Headline, 2002).
8. See my book The Never-Ending Days of Being Dead (Faber & Faber, 2008).
Further reading:
‘Explanation of the Code “6EQUJ5” on the Wow! Computer Printout’ by Jerry Ehman (http://www.bigear.org/6equj5.htm).
‘Scintillation-Induced Intermittency in SETI’ by James Cordes, Joseph Lazio and Carl Sagan (Astrophysical Journal, Vol. 487, p. 782, 1997).
‘When Will We Detect the Extraterrestrials?’ by Seth Shostak (Acta Astronautica, Vol. 55, p. 753, 2004).
Where Is Everybody? Fifty Solutions to the Fermi Paradox and the Problem of Extraterrestrial Life by Stephen Webb (Praxis Books, New York, 2002).
‘Possibility of Life-Sustaining Interstellar Planets’ by David Stevenson (Nature, Vol. 400, p. 32, 1999).
The Cosmic Connection by Carl Sagan (Cambridge University Press, 2000).
‘An Explanation for the Absence of Extraterrestrials on Earth’ by Michael Hart (Quarterly Journal of the Royal Astronomical Society, Vol. 16, p. 16, 1975).
‘The “Great Silence”: The Controversy Concerning Extraterrestrial Intelligent Life’ by David Brin (Quarterly Journal of the Royal Astronomical Society, fall 1983, Vol. 24, p. 283).
‘Five or Six Step Scenario for Evolution?’ by Brandon Carter (http://arxiv.org/abs/0711.1985).
A New Kind of Science by Stephen Wolfram (http://www.wolframscience.com/nksonline/toc.html)