CHAPTER 3
Federal Requirements
PTCE Knowledge Domain: Medications
12.5% of Exam
Knowledge Areas:
▪ Federal requirements for handling and disposal of nonhazardous, hazardous, and pharmaceutical substances and waste
▪ Federal requirements for controlled substance prescriptions (i.e., new, refill, transfer) and DEA controlled substance schedules
▪ Federal requirements (e.g., DEA, FDA) for controlled substances (i.e., receiving, storing, ordering, labeling, dispensing, reverse distribution, take-back programs, and loss or theft of)
▪ Federal requirements for restricted drug programs and related medication processing (e.g., pseudoephedrine, risk evaluation and mitigation strategies [REMS])
▪ FDA recall requirements (e.g., medications, devices, supplies, supplements, classifications)
After reading Chapter 3 you will be able to:
▪ Understand federal requirements for controlled substance prescriptions, including new prescriptions, refills, and transfers
▪ Describe each DEA controlled substance schedule and examples of each
▪ Explain federal requirements for receiving, storing, ordering, labeling, and dispensing controlled substances
▪ Describe the process for loss or theft of controlled substances
▪ Understand reverse distribution and take-back programs for controlled substances
▪ Describe FDA risk evaluation and mitigation strategies (REMS), and review REMS for specific medications
▪ Understand the Combat Methamphetamine Epidemic Act of 2005 and the impact of pseudoephedrine dispensing
▪ Differentiate between FDA recalls, including medications, devices, supplies, and supplements
▪ Recognize federal requirements for handling and disposing of nonhazardous and hazardous drugs, as well as pharmaceutical waste
Federal oversight has long had a place in pharmacy practice. Implementation of many laws and standards has helped protect the safety of both patients and pharmacy employees. This chapter focuses on federal requirements for controlled substances as well as restricted drug programs. It also highlights safe practices in drug handling and disposal.
Controlled Substances
The Comprehensive Drug Abuse Prevention and Control Act of 1970 was created to combat the growing drug problem in America. This act included the Controlled Substance Act (CSA), which consolidated several laws regulating the prescribing, distributing, and scheduling of controlled substances. The Drug Enforcement Administration (DEA) was established in 1973, and their main mission is to enforce controlled substances laws of the United States.
Schedules of Controlled Substances
Through the CSA, five schedules were created to group controlled substances based on potential for abuse and accepted medical use. The DEA determines the schedules for controlled substances. The definitions and example medications of each schedule follow.
Schedule I (C1)
• Has no accepted medical use
• Has high potential for abuse
Example Drugs
• 3,4-methylenedioxymethamphetamine (Ecstasy)
• heroin
• lysergic acid diethylamide (LSD)
• marijuana
• psilocybin (Magic Mushrooms)
Schedule II (C2)
• Has accepted medical use, but high potential for abuse
• May lead to physical or psychological dependence
Example Drugs
• amphetamine (Adderall)
• cocaine
• codeine
• fentanyl (Duragesic)
• hydrocodone (Hysingla, Norco—with acetaminophen)
• hydromorphone (Dilaudid)
• lisdexamfetamine (Vyvanse)
• meperidine (Demerol)
• methadone
• methylphenidate (Concerta, Ritalin)
• morphine (MS Contin)
• oxycodone (Oxycontin, Percocet—with acetaminophen)
Schedule III (C3)
• Low to moderate potential for abuse
• Less abuse potential than Schedule I or II
Example Drugs
• anabolic steroids (body-building drugs)
• buprenorphine
• butalbital (Fiorinal)
• codeine combination product (Tylenol with codeine)
• ketamine
Schedule IV (C4)
• Low potential for abuse or dependence
Example Drugs
• alprazolam (Xanax)
• carisoprodol (Soma)
• chlordiazepoxide (Librium)
• clonazepam (Klonopin)
• diazepam (Valium)
• lorazepam (Ativan)
• midazolam (Versed)
• phenobarbital (Luminal)
• temazepam (Restoril)
• zolpidem (Ambien)
Schedule V (C5)
• Low potential for abuse
• Many drugs that are schedule V are used for antitussive, anticonvulsant, or antidiarrheal purposes
Example Drugs
• diphenoxylate preparations (Lomotil)
• pregabalin (Lyrica)
Prescription Requirements
Prescriptions written for a controlled substance must follow DEA requirements before it can be filled by a pharmacy. The prescription must contain the following:
▪ Drug name
▪ Strength
▪ Dosage form
▪ Quantity prescribed (written out in word format—i.e., 10 tablets [ten])
▪ Directions for use
▪ Refills, if any authorized
▪ Date issued
▪ Patient’s full name
▪ Patient’s full address
▪ Prescriber’s full name
▪ Prescriber’s full address
▪ Prescriber DEA number
The prescription must also be manually signed on the date when it was issued (if not sent electronically). Electronic prescribing of controlled substances (EPCS) is not required by the DEA, but some states require all controlled substance prescriptions to be submitted electronically. Both the prescriber and pharmacy software must be certified by the DEA to send and receive controlled substance prescriptions electronically.
DEA Number
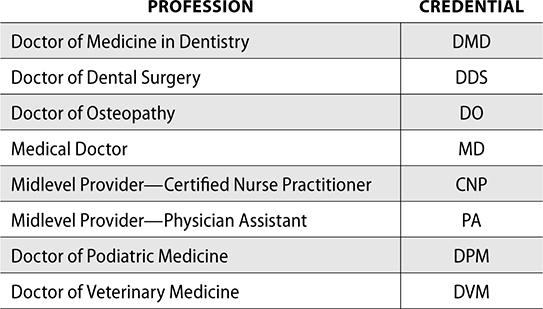
To prescribe a controlled substance, a provider must be registered with the DEA. A physician, dentist, podiatrist, veterinarian, or midlevel (physician assistant or nurse practitioner) can be authorized to prescribe controlled substances. For the prescription to be considered valid, the provider must be prescribing the controlled substance for a legitimate medical purpose in their field of practice. For example, a dermatologist could not prescribe a controlled substance to a patient for a toothache.
Before prescribing a controlled substance, a prescriber must obtain a DEA number. The DEA number consists of two letters: the first letter identifying the type of prescriber and the second letter being the first letter of the prescriber’s last name. The remaining DEA number consists of six digits and a seventh “check digit.” A DEA number can be validated by following these steps.
Dr. Rachel Durham, MD, has the DEA number CD6829343.
1. First, add together the 1st, 3rd, and 5th digits.
6 + 2 + 3 = 11
2. Next, add the 2nd, 4th, and 6th digits.
8 + 9 + 4 = 21
3. Double the answer from step 2.
21 × 2 = 42
4. Add this total to the total from step 1.
42 + 11 = 53
5. The second digit in this answer is the check digit.
53 = check digit = 3
Not all providers can write prescriptions for controlled substances. The following are providers who can write prescriptions for controlled substances and their credentials.
New, Refill, and Transfer Prescriptions for Controlled Substances
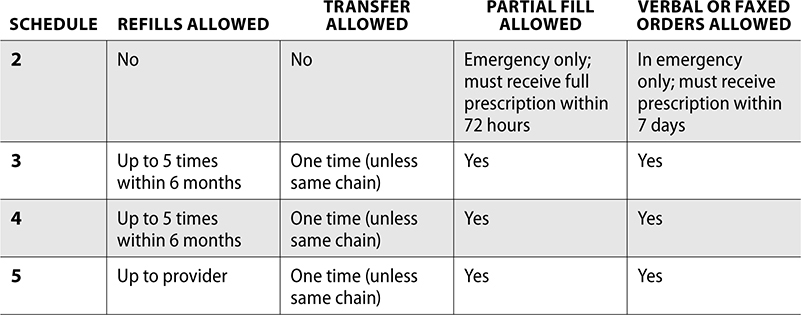
When prescribing a Schedule II controlled substance, the prescription must be handwritten or printed and signed by the prescriber. If the provider has a DEA certified electronic prescribing system, it may be sent electronically. The prescriber will validate identification with a two-factor authentication, which includes two of the following: something you know (such as a password), something you have (token or phone), and something you are (biometric—such as fingerprint).
Verbal orders for Schedule II prescriptions are discouraged, but may be accepted in emergency situations. If an emergency fill is required, the pharmacy must write out the verbal order as a valid prescription and dispense a quantity sufficient required only for the emergency period (e.g., a fill for Saturday and Sunday until the prescriber’s office is open). The prescriber must then provide the pharmacy with a valid written prescription for this order within seven days. If a Schedule II prescription is faxed, it must only be used as a method to expedite prescription filling, and the original prescription must be presented upon final verification.
Schedule II prescriptions are not permitted to be refilled or transferred to another pharmacy. If a pharmacy does not have enough medication to fill the entire prescription, a partial fill may be dispensed if the remaining quantity can be given within 72 hours. After these 72 hours, if the prescription has not been picked up or is not available from the pharmacy, the patient must get a new prescription. Schedule II prescriptions may also be partially filled if needed if a prescription is written for a patient in a long-term-care facility or with a terminal illness.
Schedule III, IV, and V prescriptions may be called in verbally or faxed. Written and faxed prescriptions must be signed by the prescriber. These orders can also be sent through EPCS.
Schedule III–V prescriptions can be transferred to another pharmacy, though one time only. This rule is exempt if transferred within the same pharmacy chain. If a Schedule III–V prescription is transferred, it must meet requirements set from the DEA.
The following requirements must be met for a prescription to be transferred from a pharmacy:
▪ Write “VOID” on the prescription being transferred.
▪ Write the name, address, and DEA number of the pharmacy the prescription is being transferred to on the original prescription.
▪ Write the date and name of the pharmacist completing the transfer.
The following requirements must be met for a prescription to be transferred to a pharmacy:
▪ Date of issue of original prescription
▪ Original number of refills authorized
▪ Date of original dispensing
▪ Number of refills remaining and location of any previous refills
▪ Pharmacy name, address, and DEA number from which the prescription was transferred
▪ Name of the pharmacist transferring the prescription
Refills for Schedule III and IV prescriptions are permitted but limited to five refills within six months (or whichever occurs first). The following is a summary of requirements for all scheduled controlled substances.
All original controlled substance prescriptions must be filed and stored for a minimum of two years. There must be one file for Schedule II controlled substances dispensed, one file for Schedule III–V controlled substances, and a third file for noncontrolled substances dispensed. Electronic prescriptions must be retained electronically for a minimum of two years, unless the state board of pharmacy requires a longer period. All records must be readily retrievable, and if storage is limited in the pharmacy, approval must be obtained from the DEA for off-site storage.
Ordering and Receiving Controlled Substances
A new pharmacy must register with the DEA prior to ordering controlled substances. The pharmacy completes DEA Form 224, and this registration must be renewed every three years. The DEA certificate must be posted in the pharmacy.
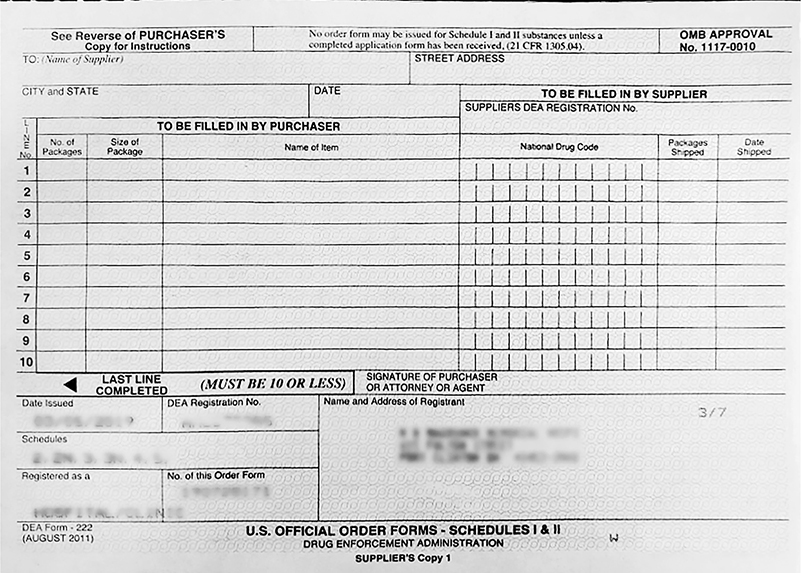
When ordering controlled substances, DEA Form 222 is used for Schedule I or II ordering. This paper form consists of a triplicate format, with one copy being for the DEA, one copy for the manufacturer or wholesaler, and the third copy for the purchaser. The wholesaler copy is mailed to the wholesaler and the copy for the DEA is mailed to the local DEA office. DEA 222 forms must be inventoried monthly to prevent diversion or abuse of ordering.
Schedule I or II controlled substances must be ordered by a pharmacist. The form must be completed correctly for order processing. This includes only one item name per line, the number of packages and size of package for each item, and pharmacist signature. Not all pharmacists can order Schedule I and II medications upon hire. They must first be given power of attorney for ordering controlled substances before they are able to sign a DEA 222. Completed forms must be stored in the pharmacy or an approved location for a minimum of two years.
A DEA Form 224 follows.

A DEA Form 222 follows.
An alternative way to order Schedule I and II controlled substances is through the DEA Controlled Substance Ordering System (CSOS). CSOS reduces the number of ordering errors and increases accuracy. It is also more efficient, as it does not require time spent in the mail to the wholesaler and instead is submitted electronically for more timely and accurate validation by the supplier. This also helps pharmacies maintain a smaller inventory, because they can submit an electronic order and have the inventory replenished the next day. Records of electronic CSOS orders must also be maintained for two years.
Schedule III–V substances can be ordered be a pharmacist or pharmacy technician through the standard ordering process with a wholesaler or direct with a manufacturer. While CSOS or DEA Form 222 is required for ordering Schedule I or II controlled substances, CSOS may also be used for Schedule III–V substance ordering.
When an order for a Schedule II controlled substance is received in the pharmacy, the wholesaler invoice must be signed by the pharmacist. Each line of the CSOS receipt must also be signed by the pharmacist, indicating all controlled substances were received.
Orders for Schedules III–V can be opened and received by a pharmacist or pharmacy technician. A pharmacist is not required to sign off on these invoices. Schedule III–V invoices must be filed separate from Schedule II invoices and stored for a minimum of two years.
Storing, Labeling, and Dispensing Controlled Substances
After proper ordering and receiving is complete, the controlled substance can be put away and stored in an appropriate location. Schedule II controlled substances must be stored separately from the other noncontrolled medications. This includes within a vault or locked safe within the pharmacy. If an automated dispensing cabinet is used for storage in nursing units, the controlled substance must be in a locked cubby and not in an open matrix drawer. Retail pharmacies often have a locked cabinet for controlled substances, and only the pharmacist possesses the key to the cabinet.
Schedule III–V substances can be stored outside the locked storage of Schedule II substances or mixed into the noncontrolled inventory. Many pharmacies secure Schedule III–V with the Schedule II for additional security, though this is not a DEA requirement. It is at the discretion of the pharmacy to determine appropriate storage for Schedule III–V controlled substances.
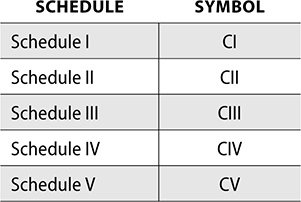
The DEA also has requirements for manufacturers related to labeling of controlled substances. Each commercial container must have a symbol printed designating the Schedule of the controlled substances.
The following symbols are used for this labeling:
In a pharmacy, when a controlled substance is dispensed to a patient, the label must contain requirements that are standard for all prescription labels, which includes:
▪ Pharmacy name
▪ Pharmacy address
▪ Pharmacy phone number
▪ Prescriber name
▪ Patient name
▪ Date prescription was filled
▪ Prescription number
▪ Medication and directions for use
▪ Cautionary statements (if any)
Cautionary statements for controlled substances may be applied through auxiliary labels, such as the following:
Controlled Substance
Dangerous unless used as prescribed
CAUTION: OPIOID
Risk of overdose and addiction
MAY CAUSE DROWSINESS OR DIZZINESS.
Alcohol may intensify this effect. Use care
when operating a car or machinery.
Additionally, the FDA requires that all controlled substances in Schedules II–IV must have a label saying: Caution: Federal law prohibits the transfer of this drug to any person other than the patient for whom it was prescribed.
After proper labeling is completed, the medication can be dispensed to a patient. Although state law may differ in requirements for pickup, the DEA requires the prescription be dispensed to the patient or a member of the patient’s household. Dispensing a controlled substance to anyone outside of this would be considered distribution and not dispensing.
Take-Back Programs and Reverse Distribution
If a pharmacy does not dispense all of a controlled substance, it may expire or outdate before it can be used. Outdated controlled substances (and all medications) must be quarantined from in-dated inventory. A pharmacy may either destroy this expired inventory or use a reverse distributor.
DEA Form 41 is submitted to the DEA if a controlled substance is destroyed. This form requires the name, strength, and dosage form of the medication destroyed, as well as the NDC of each drug. If a partial bottle is destroyed, the remaining count must be indicated. If a full bulk bottle is destroyed, the package size must also be documented and how many bottles were destroyed. The date, location, and method of destruction must be documented, and two employees must both sign the form as witnesses.
A DEA Form 41 follows.

A pharmacy may not be capable of, or desire to, destroy outdated controlled substances themselves. In this case, a pharmacy can dispose of expired controlled substances (and noncontrolled drugs) through a reverse distributor. A reverse distributor removes the expired inventory from a pharmacy and returns the drugs to the manufacturer. The manufacturer accepts the returns and may give credit back to the pharmacy. The reverse distributor must also be registered with the DEA. After the expired controlled substances are inventoried, a DEA form 222 must be completed. The reverse distributor then gives a copy of the triplicate form to the pharmacy, and one is mailed to the DEA for the transfer of the expired controlled substances. The reverse distributor must then complete a DEA Form 41 for any controlled substances that must be destroyed and not returned to the manufacturer.
If a patient has an outdated or unused controlled substance, it may become dangerous if these unused drugs end up in the wrong hands. Patients can dispose of these medications through take-back programs. These are designated days throughout the country that are sponsored by the DEA. A take-back day allows patients to dispose of unwanted medications safely and anonymously in a nearby location. Patients can clean out their medicine cabinets and help prevent drug abuse or misuse. Some pharmacies, hospitals, or police departments also offer medication drop boxes for year-round disposal.
Loss or Theft
In the event a pharmacy discovers there is a loss or theft of a controlled substance, they must notify the DEA within one business day of the discovery. In addition to notifying the DEA, a DEA Form 106 must also be completed. This can be completed online or downloaded and submitted to the local DEA office.
Information needed to complete a DEA Form 106 is:
▪ Name, address, and DEA number of the pharmacy
▪ Background information regarding the loss or theft (break-in, robbery, etc.)
▪ Estimated value of loss
▪ NDC and quantity of controlled substance
A DEA Form 106 follows.

To help remember the DEA forms, the following is a summary of some of the DEA forms mentioned in this chapter.

To prevent theft or loss, controlled substances must be counted at initial inventory and every two years thereafter. A complete count of all Schedule II substances must be completed, while the Schedule III–V may be estimated. These records of inventory must be kept for a minimum of two years, and the Schedule II inventory must be separated from III–V.
Restricted Drug Programs
Though medications must pass rigorous testing and clinical trials prior to FDA approval, some are approved or are later identified to require restrictions due to known patient safety issues. The FDA manages these through risk evaluation and mitigation strategies (REMS). The manufacturer is required to develop an REMS program to accompany the new drug approval or after approval if the FDA identifies a need. Without REMS, the medication could be withdrawn from the market or not approved for manufacturing. REMS are designed to reduce the frequency and severity of adverse events by informing patients of safety concerns or requiring specific practices for safe use.
One component of REMS may be the creation of an educational handout for patients to accompany the drug. This handout, known as a medication guide, contains FDA-approved information for patients in an easy-to-read format to help inform patients about the potential for serious adverse events. Medication guides are dispensed when the patient picks up a prescription.
Some medication guides must be dispensed with drug classes, such as SSRIs and NSAIDs. Additionally, medication guides are required to be issued with specific drugs if:
1. Specific information could be used to prevent serious adverse events.
2. The patient should be informed regarding potential for serious known side effects.
3. Patient compliance to the directions are essential for effectiveness.
The following are some examples of drugs that require a medication guide:

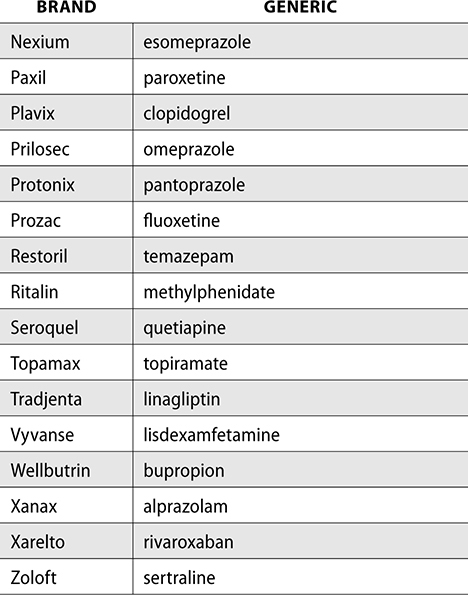
Although specific medications require medication guides, patient package inserts (PPI) contain information for patients on how to safely use a drug product. PPIs are developed by the manufacturer and are required to be dispensed with certain drug classes such as oral contraceptives or estrogen-containing products.
Requirements of a PPI include:
▪ Drug name
▪ Statement of risks versus benefits of use
▪ Contraindications and serious risks associated
▪ Warning regarding serious side effects
▪ Information on how to take the medication properly
▪ Manufacturer name and distributor
▪ Side effects
▪ Instructions on how to reduce risks
▪ Date of latest revision of PPI
REMS also include a goal for risk mitigation and activities or information that must be completed by the provider, patient, or pharmacist. The role for each caregiver or patient is specific to each medication. Some REMS are similar, but the key risk message and nature of the risk are different for each drug. For some medications, patients must sign an acknowledgment form that they understand the risks prior to taking the medication. Some REMS require lab testing prior to dispensing a prescription. Some REMS even require enrollment in a registry to ensure proper monitoring is completed and documentation of adverse events can occur. Additional requirements may include direct communication with the drug manufacturer and the health care provider, which includes pharmacists. Some REMS programs require completion of a certificate prior to dispensing, such as clozapine, isotretinoin, and thalidomide.
Next, the following examples of REMS are reviewed:
▪ clozapine
▪ isotretinoin
▪ opioid analgesic
▪ suboxone
▪ thalidomide
Clozapine
Clozapine (Clozaril) is a medication used for the treatment of schizophrenia and has been associated with causing severe neutropenia. Neutrophils are white blood cells that help with immunity. In severe neutropenia, the neutrophil count drops to less than 500µ/L (the normal range is between 1,500µ/L and 8,000µ/L). A significant drop in neutrophils can lead to the inability to fight infections.
REMS for clozapine must be completed by the patient, pharmacist, and provider prior to initiating therapy. The provider must complete and enroll patients in the REMS program in order to prescribe the medication. Once the patient has begun treatment, the prescriber must report the absolute neutrophil count (ANC) to the REMS program to continue therapy.
Pharmacies must also certify in the clozapine REMS to both order and dispense the medication. The pharmacy must also validate the provider has been enrolled in the program and review the ANC prior to dispensing. The patient must continue regular blood tests for ANC before receiving treatment.
Isotretinoin
Isotretinoin (Accutane) is a medication used for the treatment of severe nodular acne. There is an extremely high risk of birth defects if used by female patients during pregnancy or who may become pregnant. The REMS program for isotretinoin is known as iPLEDGE. The goal of this REMS to prevent fetal exposure and inform pharmacist, providers, and patients of the potential for teratogenic effects.
Prescribers of isotretinoin must first register with iPLEDGE and may only prescribe it to patients who have registered and met all requirements of the program. Patient requirements include:
1. Two negative pregnancy tests prior to first dose
2. Monthly pregnancy tests for female patients
3. Confirmation of two forms of contraception used
4. Completion of counseling
Isotretinoin cannot be dispensed until a patient has completed these requirements. Pharmacist must also be registered with iPLEDGE in order to purchase from a wholesaler. This certification requires annual renewal, and the wholesaler will not ship the medication without an active certification. Isotretinoin must not be automatically refilled each month, as a negative pregnancy test must first be taken prior to dispensing a refill. The prescription must also be picked up within seven days of the negative pregnancy test.
Opioid Analgesic
In response to the opioid epidemic, a REMS was created by the FDA for the use of opioid analgesics in the treatment of pain. The goal of the opioid analgesic REMS is to educate providers, including pharmacists, on the treatment and monitoring of patients with pain. With an increase in education, providers will have a better understanding of pain management and the role of opioid analgesics in treatment, along with nonpharmacological treatment methods. This education also helps identify the risks of opioids, including the risk of addiction, unintentional overdose, and death.
To complete the opioid analgesic REMS, providers must complete an FDA-REMS compliant continuing education course. Patients must also be counseled on the safe use of opioid analgesics, as well as the appropriate storage and disposal methods and risks associated with use.
Suboxone
Suboxone is a combination of buprenorphine and naloxone. Buprenorphine is an opioid and naloxone blocks the effects of the opioid, including those that may lead to dependence. Suboxone is indicated to treat opioid addiction. Misuse of suboxone may lead to accidental overdose and death.
Prescribers of suboxone must first verify the patient meets appropriate criteria for treatment. Suboxone is prescribed as a limited amount and only enough to last until the next visit. Patients must have appointments scheduled to accommodate this dosing. A prescriber must also be certified to treat opioid dependence, and if prescribing suboxone for this purpose, must have an “X” number issued by the DEA.
Pharmacies must verify the prescription is written by a prescriber who is eligible for prescribing suboxone for opioid addiction. State prescription drug monitoring programs (PDMPs) must also be reviewed to identify potential behaviors of abuse and review medications for appropriate co-prescribing. Patients must be counseled and pharmacies should be aware of potential for fraudulent prescriptions or prescribing from multiple providers.
Thalidomide
Thalidomide (Thalomid) is used for the treatment of multiple myeloma. Thalidomide was once used as an antinausea agent for pregnant women and prevention of morning sickness. As a result, thousands of babies were born with severe birth defects, including deformed limbs. Due to the potential for teratogenic effects, a REMS is required for all thalidomide prescribing. The goal of this REMS is to inform patients, pharmacists, and prescribers of the potential for serious risks of thalidomide therapy.
Prescribers must first become certified and enroll in the thalomid REMS program. Patients must be counseled on benefits and risks, as well as contraception and emergency contraception. Female patients must have a negative pregnancy test before initiating therapy and before each new prescription. Providers cannot prescribe more than a 28-day supply or refills over the phone. The manufacturer must approve the provider to write prescriptions of thalidomide.
For a pharmacy to dispense thalidomide, staff training must be completed, which is provided by the manufacturer. Patients must be counseled and the prescription supplied for no more than 28 days without refills. Records must be maintained with each prescription dispensed and a completed education checklist with each patient. A pharmacy must be certified by REMS to order and dispense thalidomide.
Pseudoephedrine
The Combat Methamphetamine Epidemic Act (CMEA) of 2005 regulated retail sales of the following drugs:
▪ Pseudoephedrine
▪ Ephedrine
▪ Phenylpropanolamine
Each of these three medications is a common ingredient in cough and cold medications and can also be used as precursors to produce methamphetamine.
Requirements and restrictions of the CMEA include:
▪ Daily sales not to exceed 3.6 grams
▪ 30-day purchase limit of no more than 9 grams
▪ Photo ID or proof of identity of any purchaser
▪ Retrievable records of all purchases to be kept for a minimum of two years
▪ Limit access to customers by selling behind the counter
In addition to these restrictions, the pharmacy must self-certify with the DEA each year. By completing the certification with the DEA, the pharmacy is attesting that all employees have been trained on the CMEA requirements and that the pharmacy is following all restrictions on sales, following record-keeping requirements, and tracking all purchases in a logbook.
FDA Recalls
The previous drugs are all examples of medications that have known adverse effects and how best to mitigate or prevent them. If a medication or product has been found to be defective, it may prompt a manufacturer to initiate a recall. A recall is the process of removing a product from supply or correcting a defect before use. The FDA regulates recalls, and this applies to more than medications. The following are FDA-regulated products that could be recalled:
▪ Medications used for humans
▪ Medications used for animals
▪ Medical devices
▪ Radiation-emitting products
▪ Vaccines
▪ Blood and blood products
▪ Transplant tissue
▪ Animal feed
▪ Cosmetics
▪ Most of the foods eaten in the United States
Most recalls are voluntary, meaning the manufacturer discovers a problem and recalls the product on its own. The FDA oversees the recall process and ensures a company is addressing the strategy and recall efforts appropriately.
Recalls are categorized into three classes. These are defined as follows with examples of each.

As a pharmacy technician, you may help with the recall process by identifying any medications that have been recalled and removing them from pharmacy stock. These medications are quarantined, and the pharmacy will receive instructions from the manufacturer on the recall process. Some manufacturers will require the medications be sent back, while some give additional education to help correct the recall.
Hazardous Drugs
Proper handling and disposal of hazardous drugs and waste is important in both employee and patient safety. Through the Occupational Safety and Health Act of 1970, the Occupational Safety and Health Administration (OSHA) was created. OSHA helps ensure safe working conditions by establishing standards and providing education and training.
The Occupational Safety and Health Act of 1970 also established the National Institute for Occupational Safety and Health (NIOSH). NIOSH is a research agency and is part of the Centers for Disease Control and Prevention (CDC). NIOSH establishes a list of hazardous drugs and defines what makes a drug hazardous. This list is used in most pharmacies as a reference to determine what inventory should be handled as a hazardous drug. Although many of the medications on this list are antineoplastic medications (chemotherapy), there are many other medications that meet the criteria for hazardous drug.
NIOSH uses six characteristics to define a hazardous drug. If a drug has one or more of these traits in either humans or animals, it is considered hazardous.
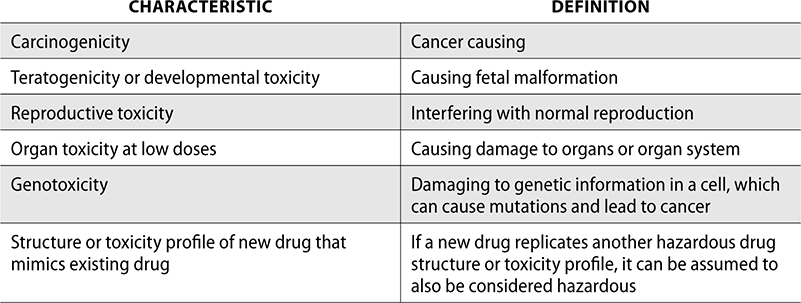
The risk of hazardous drug exposure for employees depends on the following factors:
▪ Entrance of drug into the body (inhaled, ingested, transdermal, etc.)
▪ Manipulation needed (e.g., IV needs compounding or tablet needs crushing)
▪ Personal protective equipment (PPE) worn when handling
▪ Engineering controls in place (clean room and IV hood)
In the NIOSH Hazardous Drug list, drugs are separated into three tables. Table 1 drugs must always be handled with PPE regardless of formulation. These drugs are mostly antineoplastic medications. Table 2 drugs are nonantineoplastic drugs, and if they are unopened or intact (such as tablets or capsules), they do not pose the same hazardous risk. If Table 2 medications are crushed, split, or otherwise manipulated from the final dosage form, safe handling is recommended. Table 3 drugs are nonantineoplastic also, but they are considered reproductive hazards and are also less hazardous if they are not manipulated from the final dosage form. An example of each table is listed as follows.
Table 1. Group 1: Antineoplastic drugs, including those with the manufacturer’s safe-handling guidance (MSHG)
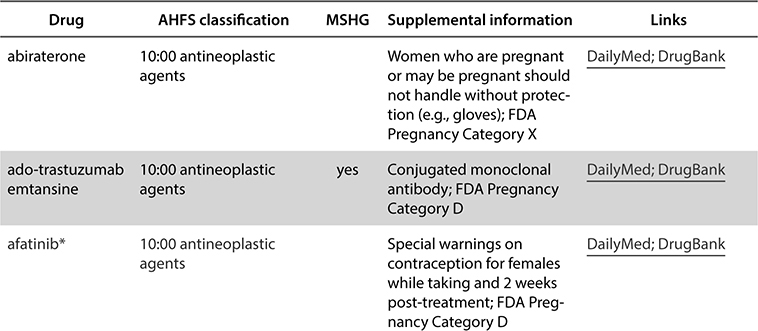
Table 2. Group 2: Non-antineoplastic drugs that meet one or more of the NIOSH criteria for a hazardous drug, including those with the manufacturer’s safe-handling guidance (MSHG)
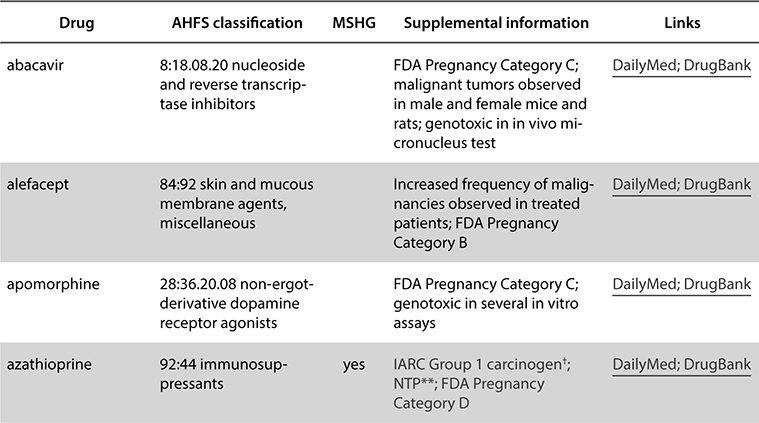
Table 3. Group 3: Non-antineoplastic drugs that primarily have adverse reproductive effects
Federal Requirements for Handling Pharmaceutical Substances
In addition to NIOSH and OSHA, the United States Pharmacopeia (USP) is an organization that develops standards for medications and other substances. These standards are not enforceable by USP, but are adopted by state boards of pharmacies and accrediting bodies for compliance. USP published Chapter 800 (USP<800>) Hazardous Drugs—Handling in Healthcare Settings. This chapter describes standards and best practices for handling hazardous drugs in order to protect healthcare workers and patients.
An overview of USP<800> chapters and facility requirements as follows:
• Maintain a facility-specific list of hazardous drugs reviewed annually based on the NIOSH list
• Complete an assessment of risk for medications on the hazardous drug list that defines risk of exposure and requirements for handling and manipulation
• Designate a qualified and trained person responsible for developing and implementing procedures and overseeing compliance
• Designate area for receiving, unpacking, and storing hazardous drugs
• Engineering requirements for sterile and nonsterile compounding, which include ventilation, appropriate air exchanges, and negative pressure
• Use of closed system drug-transfer devices (CSTDs) when compounding a hazardous drug
• Appropriate use of PPE for exposure reduction, including gowns, head, hair, shoe covers, and two pairs of chemotherapy-rated gloves
• Competencies and training for each personnel who handle hazardous drugs
• Procedures for packaging, transporting, and disposing of hazardous drugs
• Development of a written procedure for decontaminating, deactivating, and cleaning areas used in hazardous drug compounding and storage
• Development of a facility hazard communication plan that describes the use of safety data sheets (SDS) and hazardous drug communication
The development of a hazard communication plan is a requirement of UPS<800> and also OSHA if a facility has any hazardous chemical. This program is designed to ensure information regarding the hazards in a facility is communicated to employees. OSHA requires this program to include information regarding the labeling, handling, and disposal requirements of hazardous chemicals (including drugs). A hazardous communication program must also include steps to take if a spill occurs and an updated list of safety data sheets (SDS).
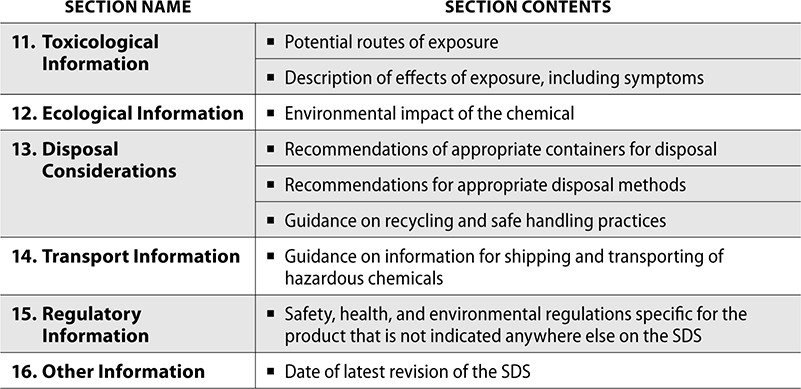
SDS contain handling requirements of hazardous drugs and chemicals. These documents are produced by the manufacturer of a chemical or drug if it is considered hazardous. OSHA requires specific information to be included in a 16-section format for standardization. The first 8 sections contain information regarding the chemical, including safe handling practices, emergency control measures, and composition. The remaining sections contain other information such as physical or chemical properties and date of last revision.
The following is a description of all 16 sections in an SDS and the contents in each:

OSHA also requires PPE be available for all employees. As a pharmacy technician, PPE is worn to protect from hazardous drug exposure when compounding, receiving or unpacking, storing, and delivering hazardous drugs. PPE includes gloves, gowns, shoe covers or booties, beard and hair covers, goggles or eye shields, and a face mask. Not all PPE is typically required for receiving, storing, or delivering hazardous drugs, but employees must follow facility procedures for hazardous drug handling.
When compounding a hazardous drug, full PPE must be worn, including two pairs of chemotherapy-rated gloves. Closed system drug-transfer devices (CSTD) should be used to compound chemotherapy and other hazardous drugs. CSTDs minimize the risk of hazardous drug exposure to the employees compounding and nurses when administering to a patient. They also protect the patient from accidental spilling or leakage.
SDS also help provide information regarding the response to a hazardous drug spill. Pharmacies must have spill kits available near the compounding area, as well as where patients may be receiving hazardous drugs. USP<800> defines the minimum components required for a spill kit, which includes:
▪ Supplies sufficient to absorb a spill of about 1000mL
▪ 2 pairs of chemo-rated gloves
▪ Hazardous-drug-resistant gown
▪ Shoe covers
▪ Goggles or face shield
▪ Disposable respirator
▪ Spill pads and towels
▪ Hazardous waste disposable bag
▪ Scoop and container for collecting any glass fragments
A spill kit must include a NIOSH-certified respirator. OSHA requires proper fit-testing to be completed for any staff using a respirator. If an employee comes into contact with a hazardous drug through touch or inhalation, the SDS can be references for treatment.
Federal Requirements for Disposal of Nonhazardous and Hazardous Pharmaceutical Substances
Disposing of pharmaceutical waste is federally regulated by the Environmental Protection Agency (EPA), though some states have specific requirements for waste management. The Resource and Conservation Recovery Act (RCRA) gives the EPA the authority to control hazardous waste. The EPA develops regulations to ensure safe cleanup and management of waste, as well as programs that help reduce or reuse waste. The RCRA defines pharmaceutical waste as P or U-listed. These pharmaceuticals must be disposed of under hazardous waste requirements.
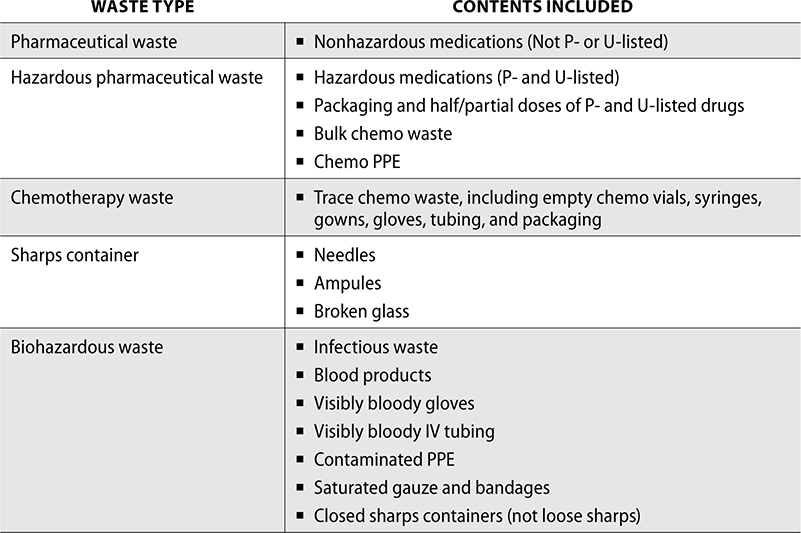
To dispose of nonhazardous pharmaceutical waste appropriately, several steps must be followed. It must first be segregated from biohazardous waste, which includes waste that may contain blood or other infectious substances. Sharps, such as used needles, are also included as biohazardous waste, though they must be disposed of in a hard sharps container. Pharmaceutical waste must also not include controlled substances, as these must be wasted in accordance with the DEA. The remaining waste should be disposed of by a waste removal company.
To dispose of hazardous drug waste, it must first be defined hazardous by the EPA. This waste must be disposed of separately from nonhazardous drug waste. Chemotherapy waste is divided into bulk and trace waste. Bulk chemotherapy waste includes vials of chemotherapy drugs that are not empty and items used to clean spills or PPE. Trace chemotherapy includes empty chemotherapy vials and IV bags, including tubing from patient administration. Trace chemotherapy waste must be disposed of in a yellow chemotherapy container, while bulk chemotherapy waste is disposed of in the hazardous drug container a facility uses for P- and U-listed waste.
The following is a summary of waste disposal:
Review Questions
The following questions help you review the chapter. Test your knowledge by working through the next 50 questions to test yourself and identify any areas you may need to review.
1. Which organization has oversight of controlled substance laws in the United States?
A. FDA
B. DEA
C. CSA
D. USP
2. Schedule I substances have which of the following?
A. accepted medical use and low potential for abuse
B. accepted medical use and high potential for abuse
C. no accepted medical use and low potential for abuse
D. no accepted medical use and high potential for abuse
3. Which of the following is a Schedule II substance?
A. fentanyl
B. heroin
C. ketamine
D. alprazolam
4. Which of the following is a Schedule IV substance?
A. diazepam
B. pregabalin
C. meperidine
D. oxycodone
5. Verbal orders for which schedule of controlled substances are discouraged but can be accepted in an emergency?
A. Schedule II
B. Schedule III
C. Schedule IV
D. Schedule V
6. How many times can oxycodone be refilled?
A. 6 times in 5 months
B. 1 time within 30 days
C. as many times as written by prescriber
D. no refills allowed
7. To register with the DEA, which form must be completed?
A. 222
B. 224
C. 106
D. 41
8. A DEA Form 222 or CSOS must be used to order
A. Schedule II
B. Schedule III
C. Schedule IV
D. Schedule V
9. Which symbol would be found on a bottle of Percocet?
A. CI
B. CII
C. CIII
D. CIV
10. If a controlled substance is destroyed, which form must be submitted to the DEA?
A. 222
B. 224
C. 106
D. 41
11. DEA Form 106 is completed when
A. a pharmacy closes
B. ordering Schedule III and IV substances
C. loss or theft occurs
D. reverse distribution occurs
12. FDA-required programs developed by drug manufacturers that help patients avoid potential safety issues of specific medications are known as
A. REMS
B. CSA
C. USP
D. NIOSH
13. Which of the following must be dispensed with a med guide?
A. albuterol
B. citalopram
C. amoxicillin
D. rosuvastatin
14. Which medication requires certification with iPLEDGE?
A. clozapine
B. thalidomide
C. isotretinoin
D. suboxone
15. Which medication requires REMS due to the potential for misuse causing accidental overdose and death?
A. clozapine
B. thalidomide
C. isotretinoin
D. suboxone
16. A recall that could cause serious health problems or death is which class?
A. Class I
B. Class II
C. Class III
D. Class IV
17. A medication that is teratogenic causes
A. cancer
B. damage to organs
C. damage to genetic information
D. fetal malformation
18. Which federal organization helps ensure safe working conditions?
A. USP
B. FDA
C. OSHA
D. DEA
19. Which agency defines hazardous drug and establishes a list of hazardous drugs?
A. FDA
B. OSHA
C. USP
D. NIOSH
20. USP<800> is a chapter published that defines standards for
A. sterile compounding
B. nonsterile compounding
C. hazardous drug handling
D. radiopharmaceuticals
21. Where should a broken ampule be disposed?
A. hazardous waste container
B. sharps container
C. biohazard container
D. chemotherapy waste container
22. Trace chemo waste, such as empty chemo vials and IV tubing, should be disposed of in the
A. nonhazardous waste container
B. hazardous waste container
C. chemotherapy waste container
D. biohazard waste container
23. Which of the following should be used when compounding chemotherapy and other hazardous drugs and when administering to patients?
A. spill kit
B. standard needle and syringe
C. three pairs of chemo-rated gloves
D. CSTD
24. A hazard communication program must include
A. an updated list of SDS and steps to take if a spill occurs
B. all requirements from USP<797>
C. FDA recall information
D. DEA inventory
25. Designating a qualified and trained person responsible for developing and implementing procedures and overseeing compliance of hazardous drugs is a requirement for
A. USP<795>
B. USP<797>
C. USP<800>
D. USP<847>
26. Which of the following was created to combat the growing drug problem in America?
A. FDA
B. CSA
C. USP
D. NIOSH
27. Methylphenidate is which schedule of controlled substance?
A. Schedule I
B. Schedule II
C. Schedule III
D. Schedule IV
28. Which of the following is a Schedule III substance?
A. anabolic steroids
B. oxycodone
C. methadone
D. alprazolam
29. Which schedule substance has low to moderate potential for abuse?
A. Schedule I
B. Schedule II
C. Schedule III
D. Schedule IV
30. Pregabalin is which schedule of controlled substance?
A. Schedule II
B. Schedule III
C. Schedule IV
D. Schedule V
31. According to federal law, if a pharmacy accepts an emergency verbal order for a Schedule II substance, when must the prescriber provide a valid written prescription?
A. 24 hours
B. 72 hours
C. 7 days
D. 30 days
32. Which of the following is required for a controlled substance prescription to be transferred to another pharmacy?
A. number of refills remaining
B. patient Social Security number
C. date patient was seen by the prescriber
D. name of technician who answered call
33. How many times could a prescription for zolpidem be refilled?
A. no refills permitted
B. up to 6 times in 30 days
C. up to 5 times in 6 months
D. up to 10 times in 180 days
34. Controlled substance prescriptions must be stored for a minimum of
A. 30 days
B. 180 days
C. 1 year
D. 2 years
35. A DEA 222 form can be completed by
A. any pharmacy staff members
B. a pharmacy technician only
C. a pharmacist only
D. a responsible pharmacist only
36. Which of the following is a benefit of CSOS?
A. larger inventory needed
B. slower transmission time
C. no need for record retention
D. more efficient and typically faster delivery
37. Ketamine would have which symbol on a stock bottle?
A. CI
B. CII
C. CIII
D. CIV
38. Removing expired or unused controlled substances from a pharmacy and returning them to the manufacturer for credit is known as
A. take-back days
B. reverse distribution
C. credit/rebill
D. diversion
39. Which drug would require a PPI?
A. atorvastatin
B. ethinyl estradiol and norethindrone
C. ergocalciferol
D. lidocaine
40. Which of the following is a handout given with certain medications that contains FDA-approved information to help patients understand the potential for serious adverse events?
A. REMS
B. medication guide
C. Orange Book
D. reverse distribution
41. Clozapine has a REMS to help prevent
A. birth defects
B. accidental overdose
C. tendon rupture
D. severe neutropenia
42. A pharmacy has 21-count boxes of pseudoephedrine with 30mg in each tablet. According to federal law, what is the maximum number of boxes that can be purchased in one day without violating the CMEA?
A. 3 boxes
B. 4 boxes
C. 5 boxes
D. 6 boxes
43. Which of the following rules must a pharmacy selling pseudoephedrine follow to comply with the CMEA?
A. require two forms of ID of any purchaser
B. limit access to customers by selling behind the counter
C. daily sales not to exceed 5 grams
D. records kept for a minimum of 6 months
44. A drug that is genotoxic causes damage to
A. organs
B. reproductive growth
C. cancer
D. genetic information in a cell
45. The RCRA gives the EPA authority to control
A. narcotic dispensing
B. sterile compounding
C. recall notifications
D. hazardous drug waste
46. Used IV tubing should be disposed of in the
A. biohazard waste
B. hazardous drug waste
C. chemotherapy waste
D. sharps container
47. Lorazepam is which schedule controlled substance?
A. Schedule II
B. Schedule III
C. Schedule IV
D. Schedule V
48. Marijuana is considered a
A. Schedule I substance
B. Schedule II substance
C. Schedule III substance
D. Schedule IV substance
49. Which schedule has a low potential for abuse and is often an antitussive, anticonvulsant, or antidiarrheal medication?
A. Schedule II
B. Schedule III
C. Schedule IV
D. Schedule V
50. After an injury, a patient who is nonhospice or in a long-term-care facility receives a prescription for a Schedule II controlled substance. The pharmacy must partially fill the order due to low inventory and all other nearby pharmacies are closed. The remaining supply must be filled fully within
A. 24 hours
B. 36 hours
C. 48 hours
D. 72 hours
Answer Key
1. B
The Drug Enforcement Administration (DEA) was established in 1973, and their main mission is to enforce the controlled substances laws of the United States.
2. D
Schedule I substances have no accepted medical use and a high potential for abuse.
3. A
Fentanyl is a Schedule II substance.
4. A
Diazepam is a Schedule IV substance.
5. A
Verbal orders for Schedule II prescriptions are discouraged but may be accepted in emergency situations.
6. D
Oxycodone is a Schedule II prescription and is not permitted to be refilled or transferred to another pharmacy.
7. B
A new pharmacy must register with the DEA prior to ordering controlled substances. The pharmacy completes DEA Form 224, and this registration must be renewed every three years.
8. A
DEA Form 222 must be used for Schedule I or II ordering.
9. B
Percocet is a Schedule II substance, so the symbol used for labeling would be CII.
10. D
DEA Form 41 is submitted to the DEA if a controlled substance is destroyed.
11. C
In the event a pharmacy discovers there is a loss or theft of a controlled substance, they must notify the DEA within one business day of the discovery. In addition to notifying the DEA, a DEA Form 106 must also be completed. This can be completed online or downloaded and submitted to the local DEA office.
12. A
REMS are designed to reduce the frequency and severity of adverse events by informing patients of safety concerns or requiring specific practices for safe use.
13. B
Citalopram requires a medication guide. All SSRIs require med guides.
14. C
The REMS program for isotretinoin is known as iPLEDGE. The goal of this REMS to prevent fetal exposure and inform pharmacist, providers, and patients of the potential for teratogenic effects.
15. D
Suboxone is indicated to treat opioid addiction. Misuse of suboxone may lead to accidental overdose and death.
16. A
Class I recall is a dangerous or defective product that could cause serious health problems or death.
17. D
Teratogenicity causes fetal malformations.
18. C
OSHA helps ensure safe working conditions by establishing standards and providing education and training.
19. D
NIOSH establishes a list of hazardous drugs and defines what makes a drug hazardous. This list is used in most pharmacies as a reference to determine what inventory should be handled as a hazardous drug.
20. C
USP published Chapter 800 (USP<800>) Hazardous Drugs—Handling in Healthcare Settings. This chapter describes standards and best practices for handling hazardous drugs in order to protect healthcare workers and patients.
21. B
A sharps container is used for broken glass and needles.
22. C
Trace chemo waste, including empty chemo vials, syringes, gowns, gloves, tubing, and packaging, must be disposed of in a yellow chemo waste container.
23. D
Closed system drug-transfer devices (CSTD) should be used to compound chemotherapy and other hazardous drugs. CSTDs minimize the risk of hazardous drug exposure to the employees compounding and nurses when administering to a patient. They also protect the patient from accidental spilling or leakage.
24. A
A hazardous communication program must also include steps to take if a spill occurs and updated list of safety data sheets (SDS).
25. C
USP<800> requires a facility to designate a qualified and trained person responsible for developing and implementing procedures and overseeing compliance.
26. B
The Comprehensive Drug Abuse Prevention and Control Act of 1970 was created to combat the growing drug problem in America. This act included the Controlled Substance Act (CSA), which consolidated several laws regulating the prescribing, distribution, and scheduling of controlled substances.
27. B
Methylphenidate is a Schedule II substance.
28. A
Anabolic steroids, such as testosterone, is a Schedule III substance.
29. C
Schedule III has a low to moderate potential for abuse—less abuse potential than Schedule I or II.
30. D
Pregabalin is a Schedule V substance.
31. C
If an emergency fill is required, the pharmacy must write out the verbal order as a valid prescription and dispense a quantity sufficient only for the emergency period (e.g., a fill for Saturday and Sunday until the prescriber’s office is open). The prescriber must then provide the pharmacy with a valid written prescription for this order within seven days.
32. A
The following requirements must be met for a prescription to be transferred to a pharmacy:
▪ Date of issue of original prescription
▪ Original number of refills authorized
▪ Date of original dispensing
▪ Number of refills remaining and location of any previous refills
▪ Pharmacy name, address, and DEA number from which the prescription was transferred
▪ Name of pharmacist transferring the prescription
33. C
Refills for Schedule III and IV prescriptions are permitted but limited to five refills within six months (or whichever occurs first).
34. D
All original controlled substance prescriptions must be filed and stored for a minimum of two years.
35. C
DEA 222 forms are used for ordering Schedule II substances and must be completed by a pharmacist.
36. D
It is also more efficient, as it does not require time spent in the mail to the wholesaler, and instead is submitted electronically for more timely and accurate validation by the supplier. This also helps pharmacies maintain a smaller inventory because they can submit an electronic order and have the inventory replenished sometimes the next day. Records of electronic CSOS orders must also be maintained for two years.
37. C
Ketamine is a Schedule III controlled substance, so it would have a CIII on the manufacturer labeling.
38. B
A reverse distributor removes the expired inventory from a pharmacy and returns the drugs to the manufacturer. The manufacturer accepts the returns and may give credit back to the pharmacy.
39. B
PPIs are developed by the manufacturer and are required to be dispensed with certain drug classes such as oral contraceptives or estrogen-containing products.
40. B
A medication guide contains FDA-approved information for patients in an easy-to-read format to help inform patients about the potential for serious adverse events.
41. D
Clozapine (Clozaril) is a medication used for the treatment of schizophrenia and has been associated with causing severe neutropenia.
42. C
5 boxes. 21 × 30 = 630mg in each box. Total allowed in a one-day sale of pseudoephedrine = 3.6g. 3.6g × 1,000 = 3,600mg. 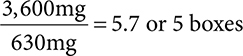 .
.
43. B
Requirements and restrictions of the CMEA include:
▪ Daily sales not to exceed 3.6 grams
▪ 30-day purchase limit of no more than 9 grams
▪ Photo ID or proof of identity of any purchaser
▪ Retrievable records of all purchases to be kept for a minimum of two years
▪ Limit access to customers by selling behind the counter
44. D
A drug that is genotoxic causes damage to genetic information in a cell, which can cause mutations and lead to cancer.
45. D
The Resource and Conservation Recovery Act (RCRA) gives the EPA the authority to control hazardous waste.
46. A
Used IV tubing and other infectious waste or blood products should be disposed of in the biohazard container.
47. C
Lorazpam (Ativan) is a Schedule IV controlled substance.
48. A
Marijuana is considered by the DEA to be a Schedule I substance.
49. D
Schedule V substances have a low potential for abuse and are often used for antitussive, anticonvulsant, or antidiarrheal purposes.
50. D
A Schedule II drug can be partially filled in an emergency only, and the patient must receive the full prescription within 72 hours.