CHAPTER 4
Patient Safety and Quality Assurance
PTCE Knowledge Domain: Patient Safety and Quality Assurance
26.25% of Exam
Knowledge Areas
▪ High-alert/risk medications and look-alike/sound-alike (LASA) medications
▪ Error prevention strategies (e.g., prescription or medication order to correct patient, tall man lettering, separating inventory, leading and trailing zeros, bar code usage, limit use of error-prone abbreviations)
▪ Issues that require pharmacist intervention (e.g., drug utilization review [DUR], adverse drug event [ADE], OTC recommendation, therapeutic substitution, misuse, adherence, post-immunization follow-up, allergies, drug interactions)
▪ Event reporting procedures (e.g., medication errors, adverse effects, product integrity, MedWatch, near miss, root-cause analysis [RCA])
▪ Types of prescription errors (e.g., abnormal doses, early refill, incorrect quantity, incorrect patient, incorrect drug)
▪ Hygiene and cleaning standards (e.g., handwashing; personal protective equipment [PPE]; cleaning counting trays, countertop, and equipment)
After reading Chapter 4 you will be able to:
▪ Identify high-alert/high risk and look-alike/sound-alike (LASA) medications
▪ Understand the importance of error prevention strategies, including tall man lettering and bar codes
▪ Explain error-prone abbreviations and the importance in eliminating use
▪ Differentiate between prescription errors and the impact of each on patient safety
▪ Describe the process of medication error reporting, including MedWatch and conducting a root cause analysis
▪ Identify types of pharmacist interventions and the importance of each
▪ Understand the importance of pharmacy technician support in adherence and how to calculate adherence percentage
▪ Describe quality control in a pharmacy, including hygiene and cleaning standards
As a pharmacy technician, you will play a vital role preventing medication errors and maintaining patient safety. There are several safety strategies and measures that can be taken to help prevent medication errors. This chapter reviews these strategies, as well as pharmacist interventions and reporting for medication errors.
ISMP Safe Medication Practices
The Institute for Safe Medication Practices (ISMP) is an organization devoted to preventing medication errors. ISMP publishes real-time medication error information in newsletters and offers educational tools and guidelines for healthcare facilities and pharmacies.
High-Alert/Risk Medications
To help prevent errors, ISMP has developed a list of high-alert medications, which are drugs that may cause greater harm if used in error. These drugs do not have a higher rate of error, but can be more devastating if used in error. ISMP publishes this high-alert list to help organizations identify which medications should have special strategies for error prevention, such as limiting access, using auxiliary labels, and standardizing ordering and preparation of these medications. Each pharmacy should also use the ISMP list to develop its own internal list of high-alert/high-risk medications.
The ISMP List of High-Alert Medications in Acute Care Settings follows.
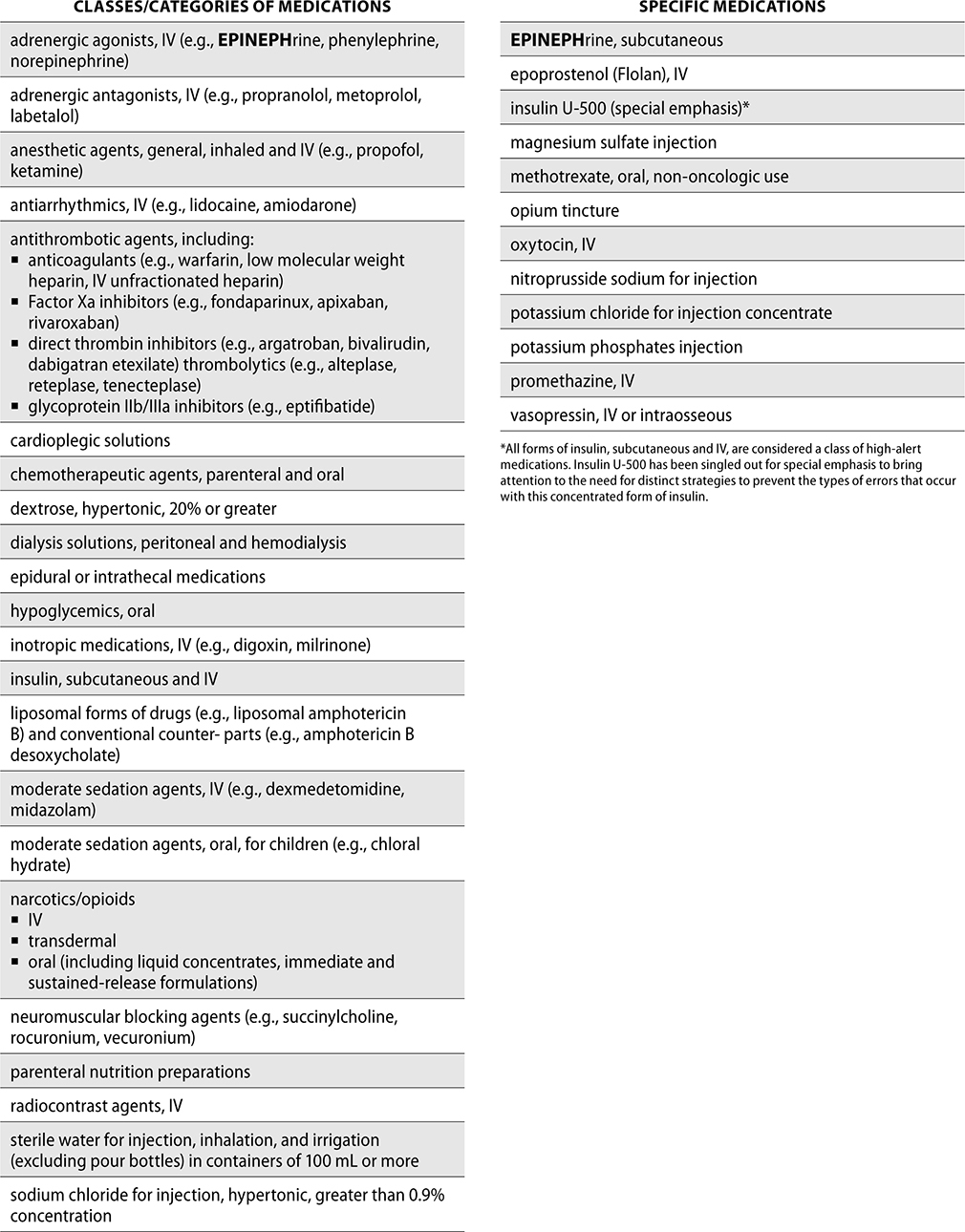
Look-Alike/Sound-Alike (LASA) Medications
The ISMP also compiles a list of confused drug names, which includes look-alike and sound-alike (LASA) name pairs of medications. Pharmacies and healthcare facilities can use this list to develop their own list of LASA drugs and strategies to prevent mix-ups. This could include using both the brand and generic names on prescriptions or labels, including the medication purpose on prescriptions, and configuring ordering solutions to prevent LASA names from appearing next to each other.
The FDA and the ISMP have a list of confused drug names. The following is the FDA and ISMP list of confused drug names:
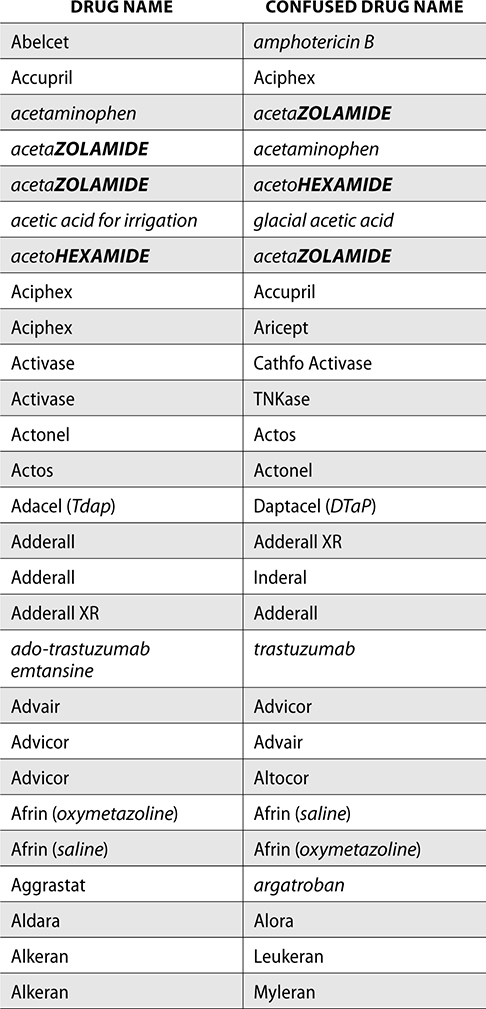
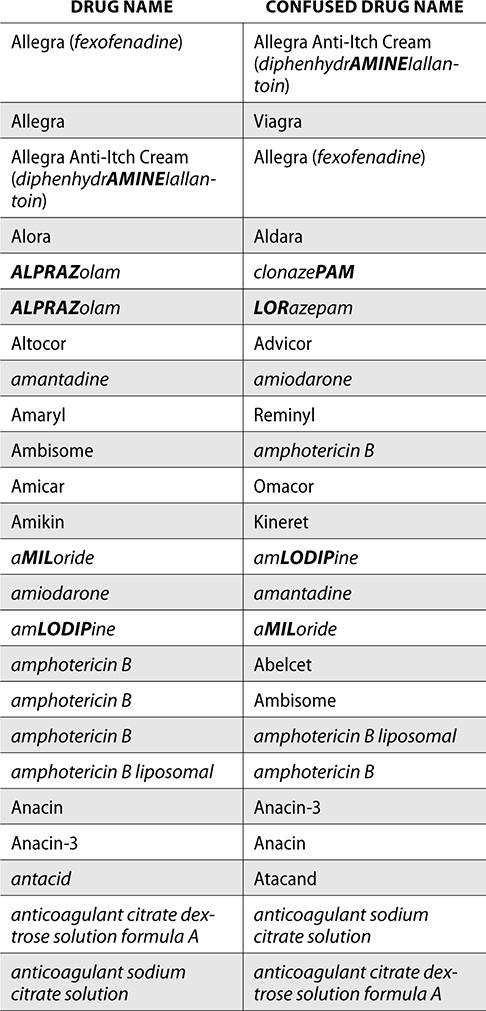
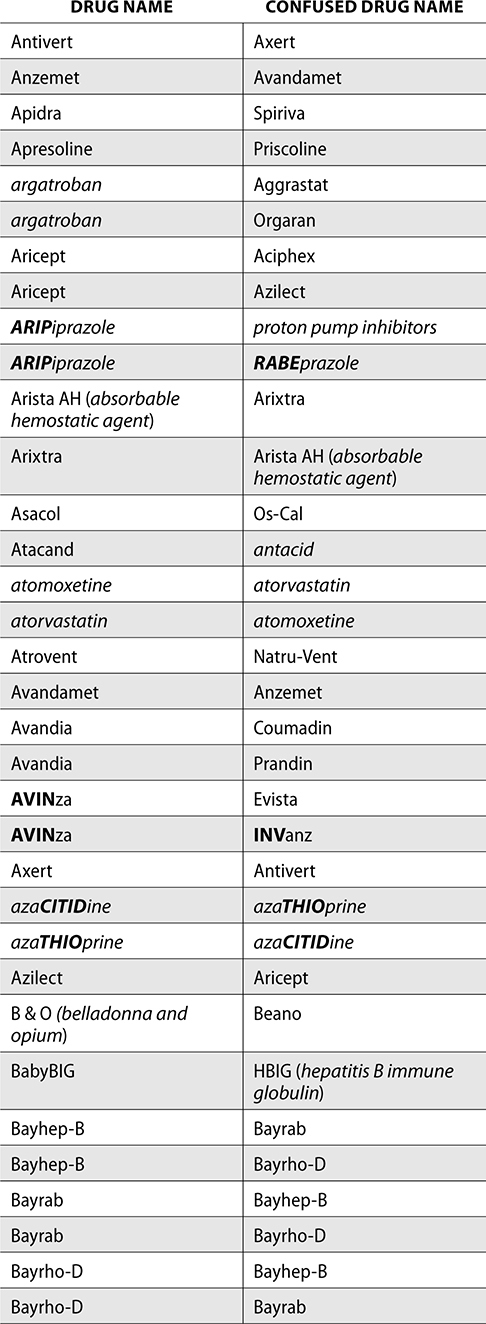
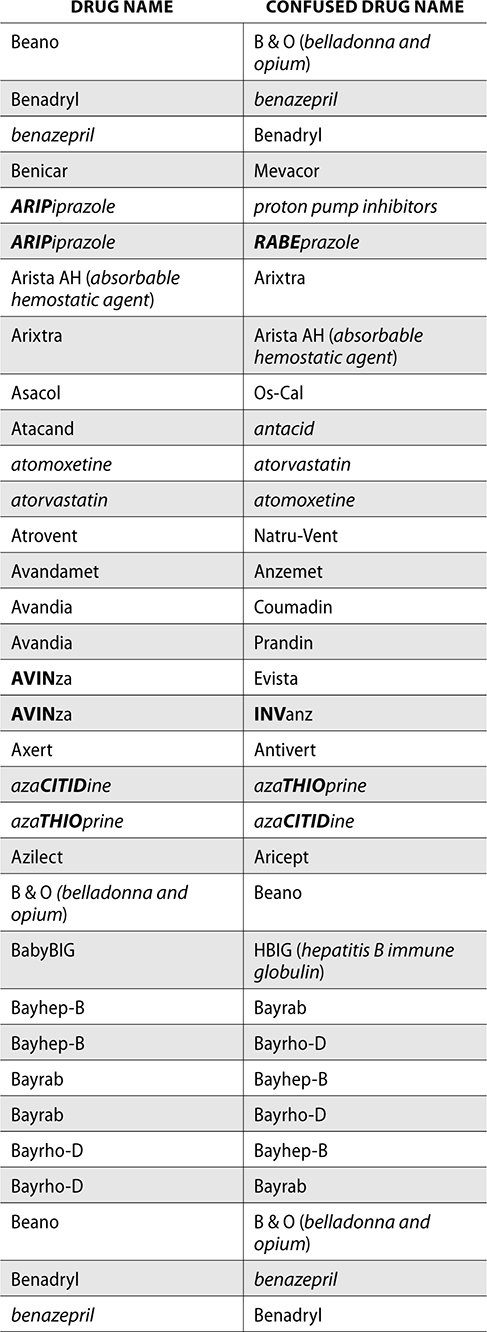
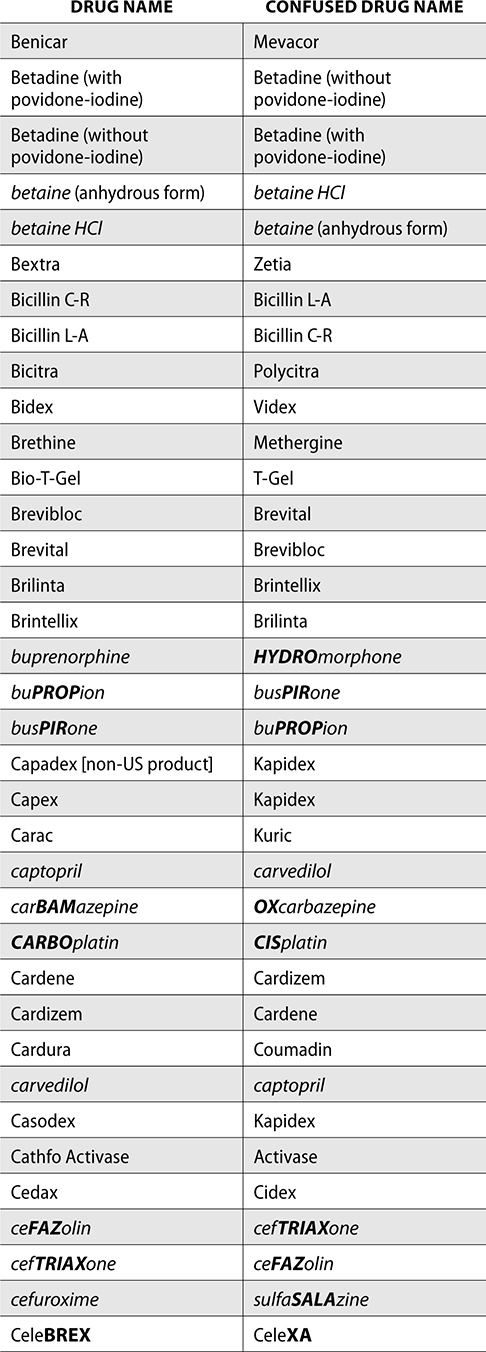
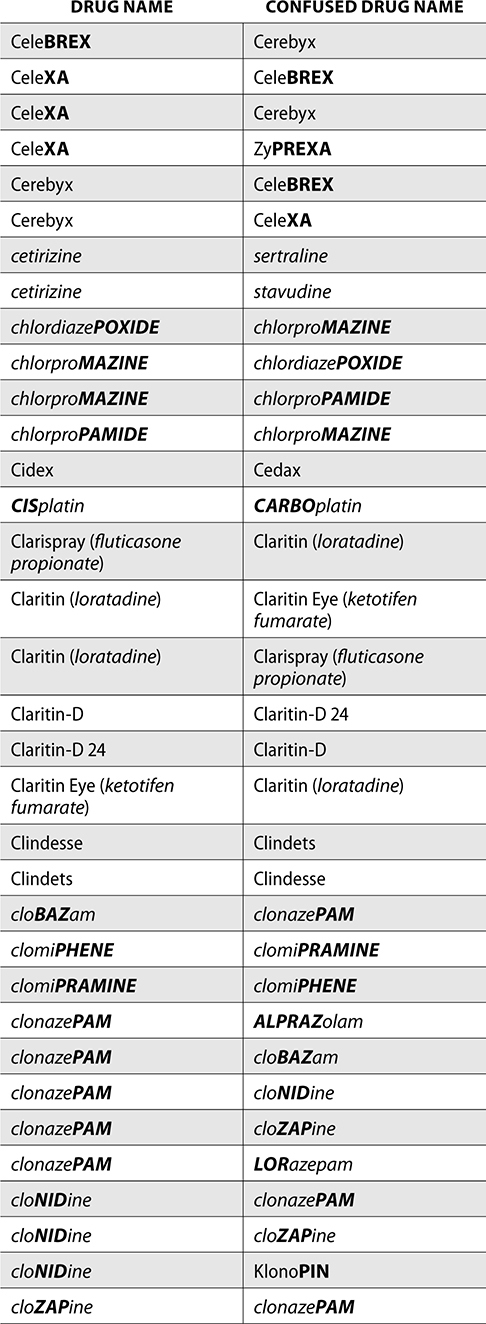
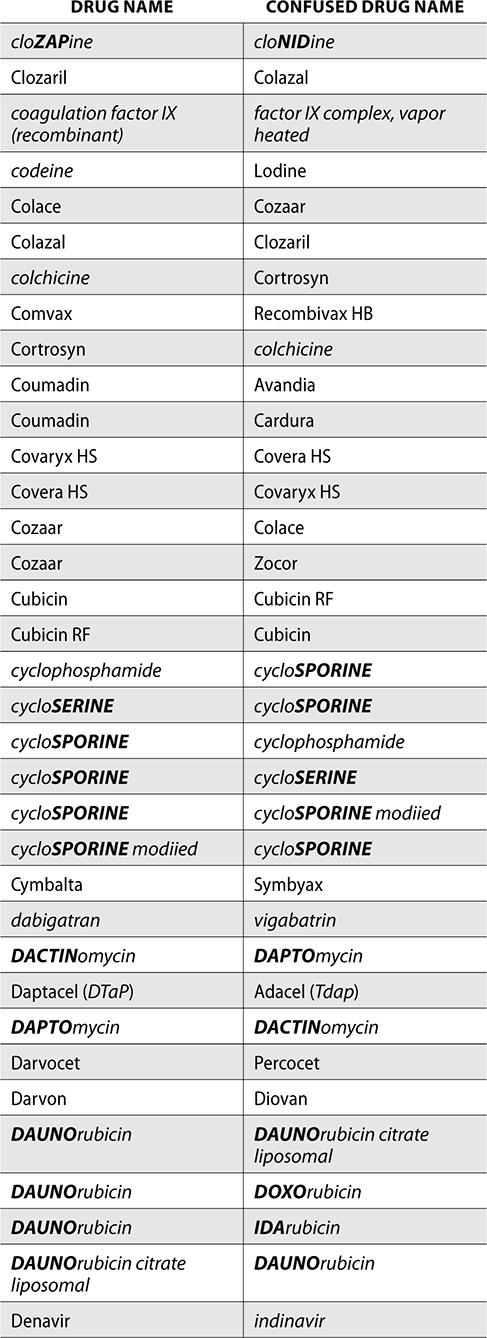
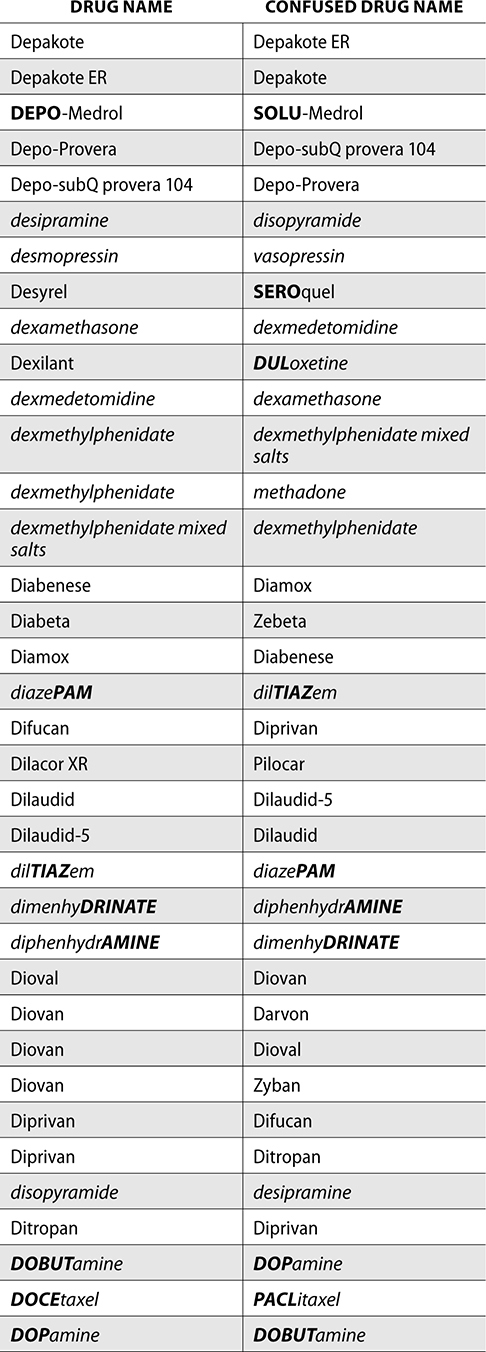
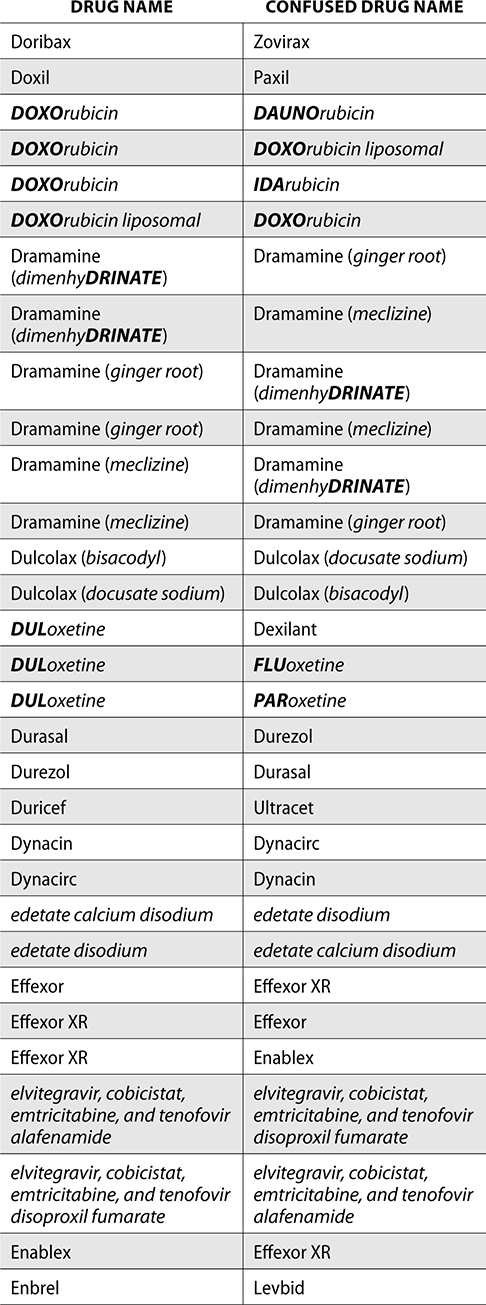
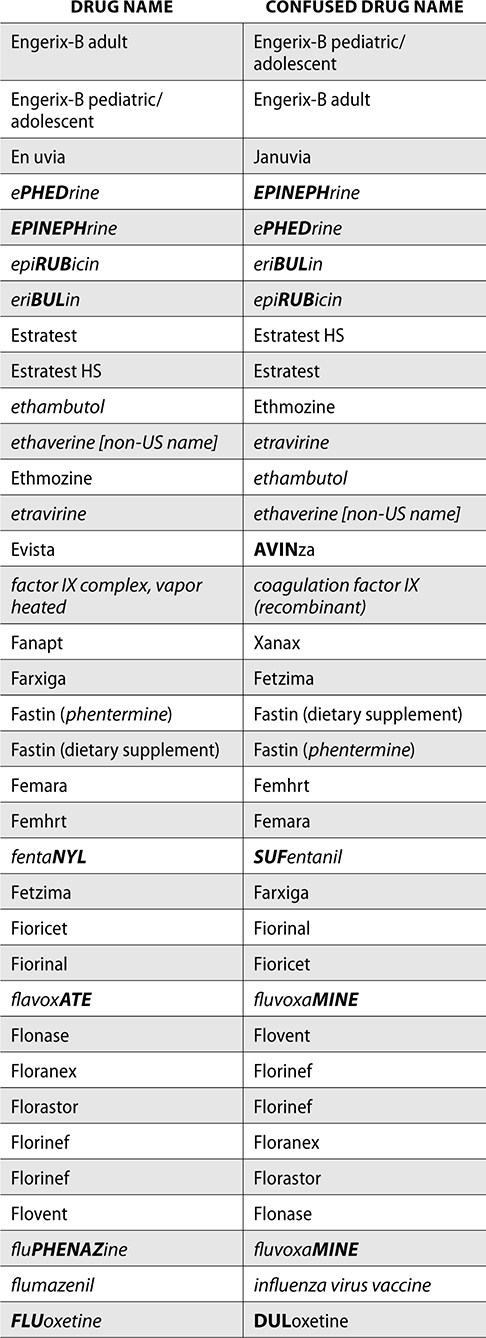
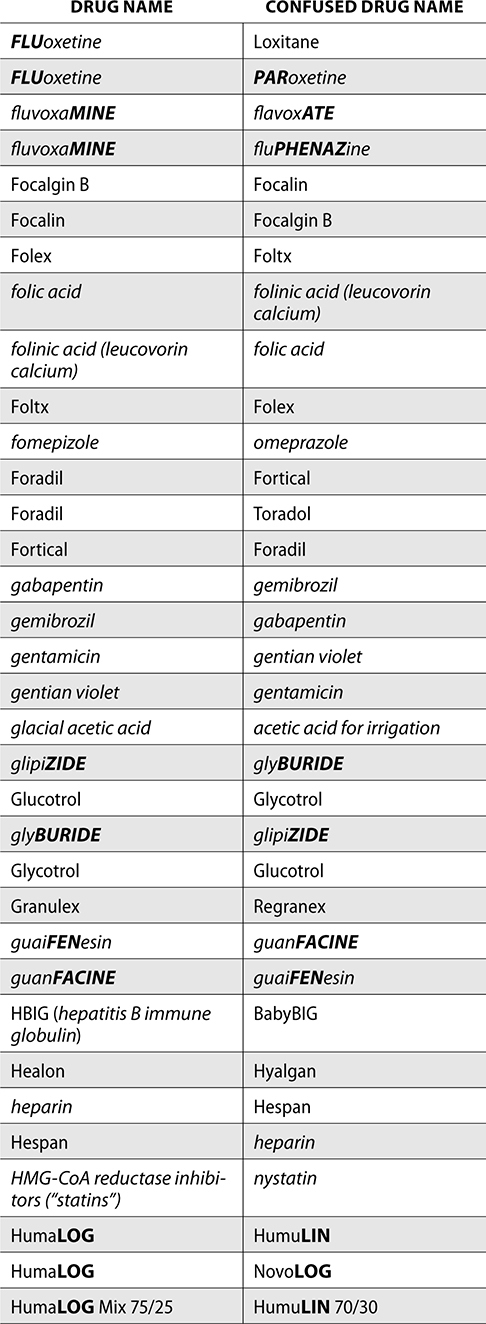
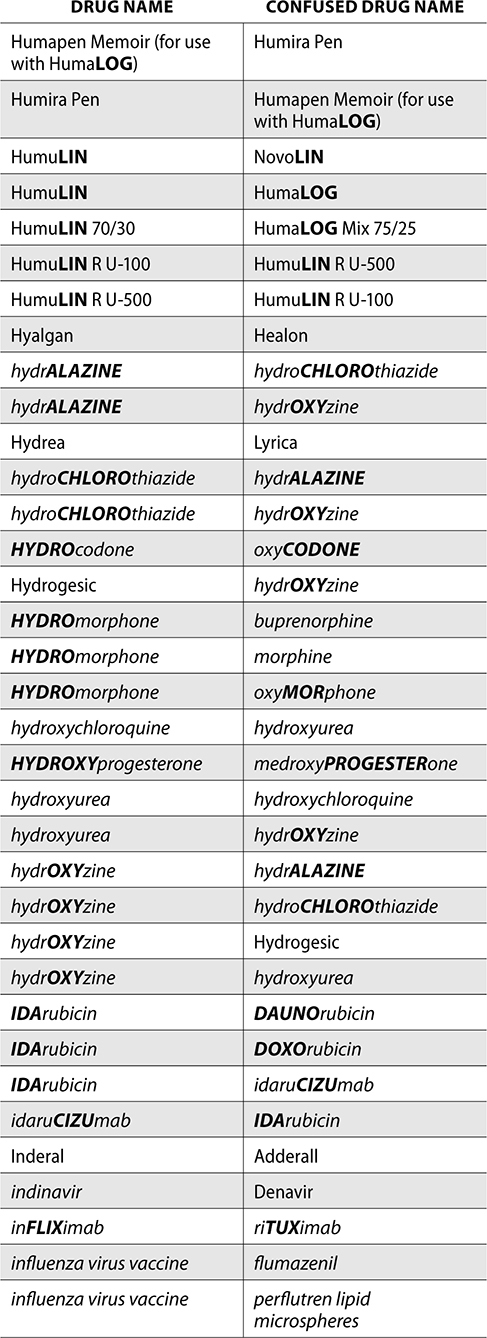
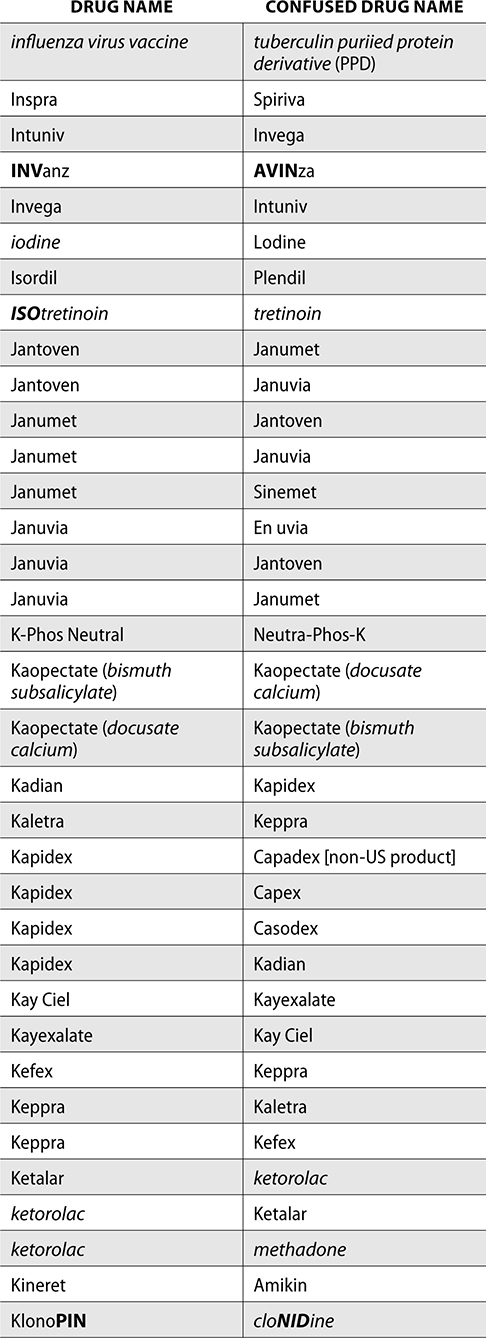
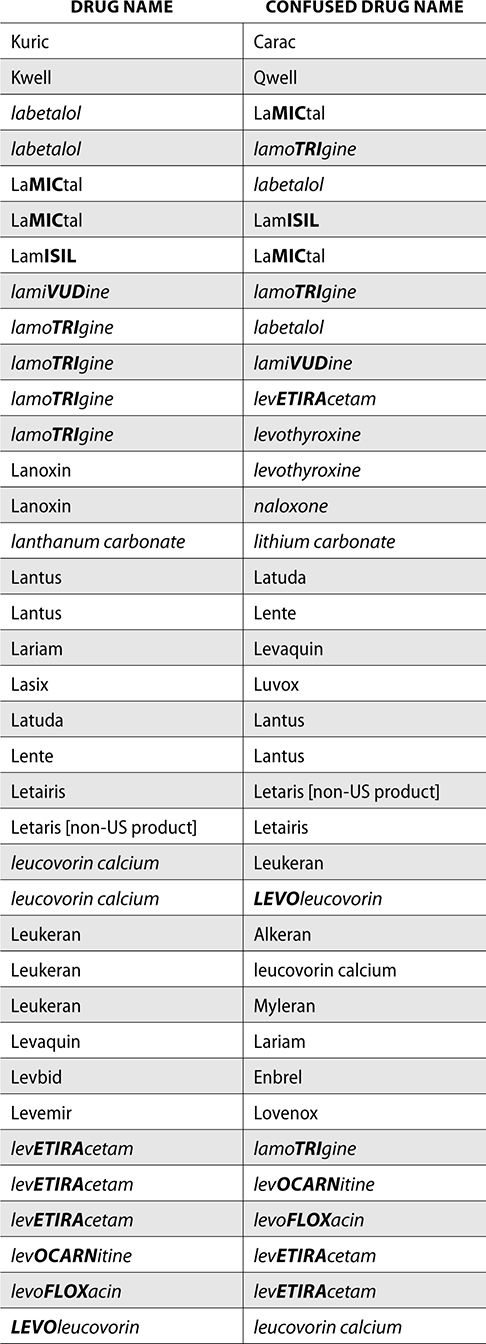
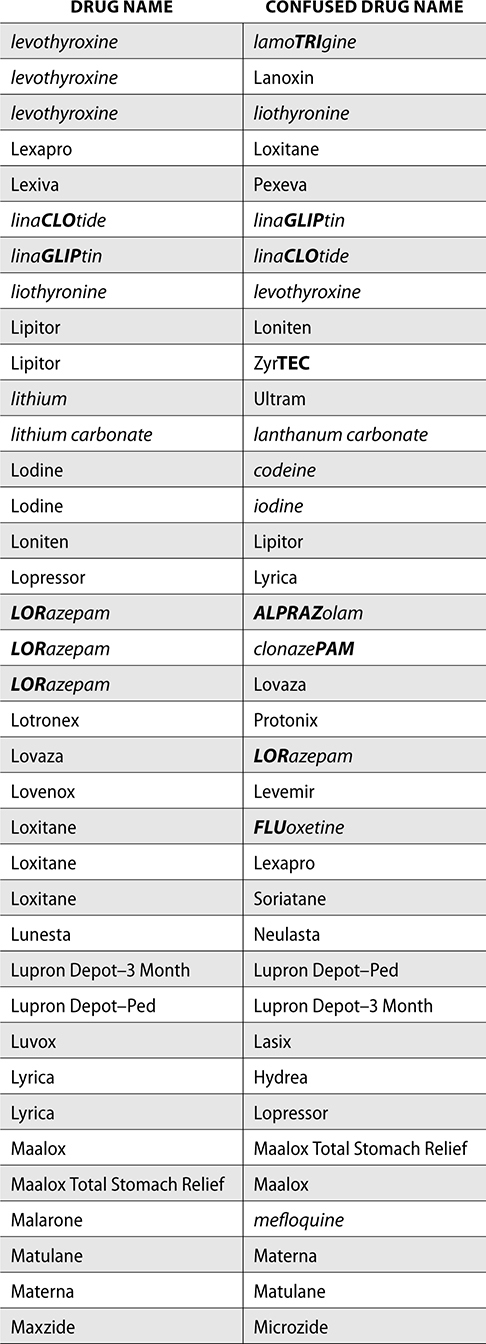
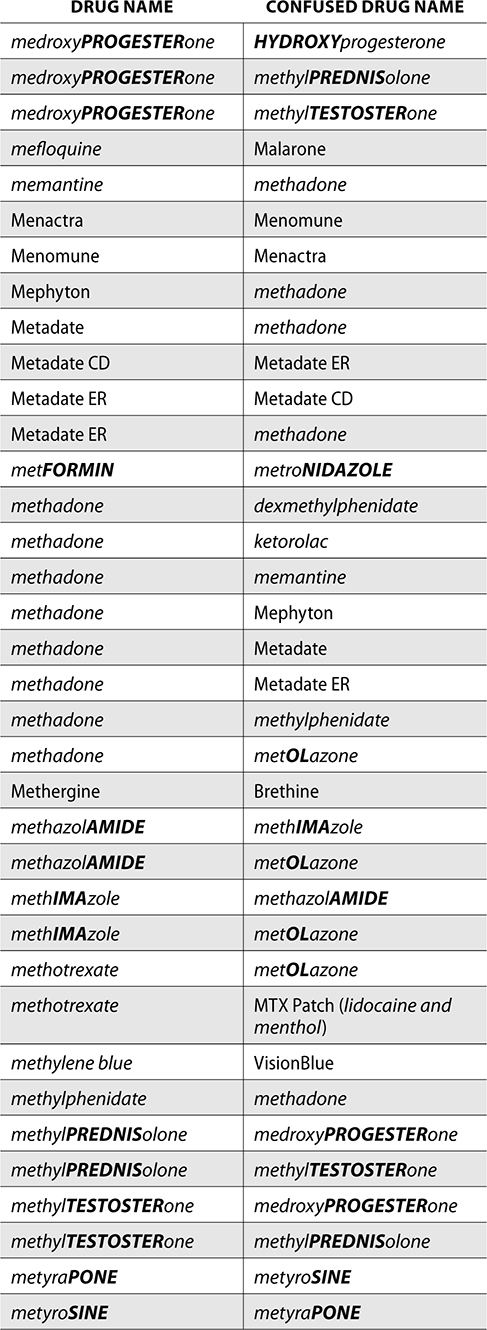
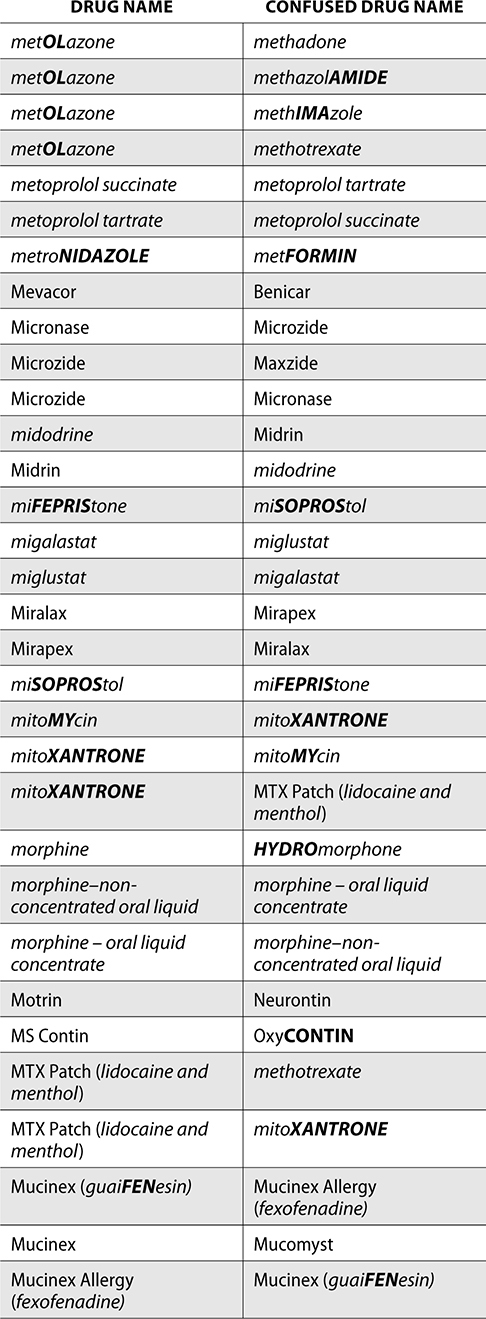
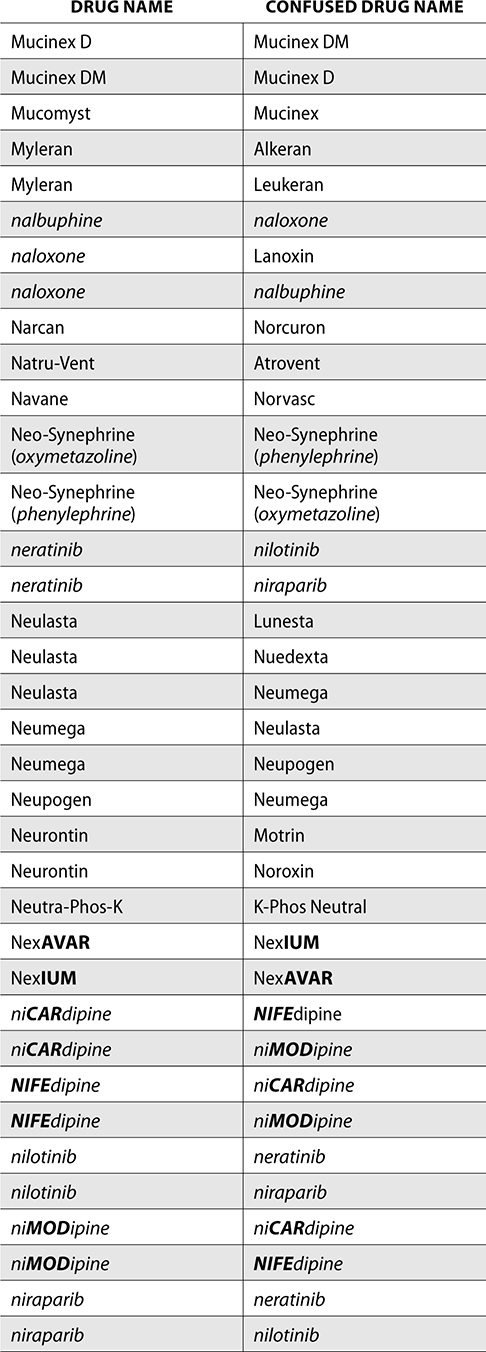
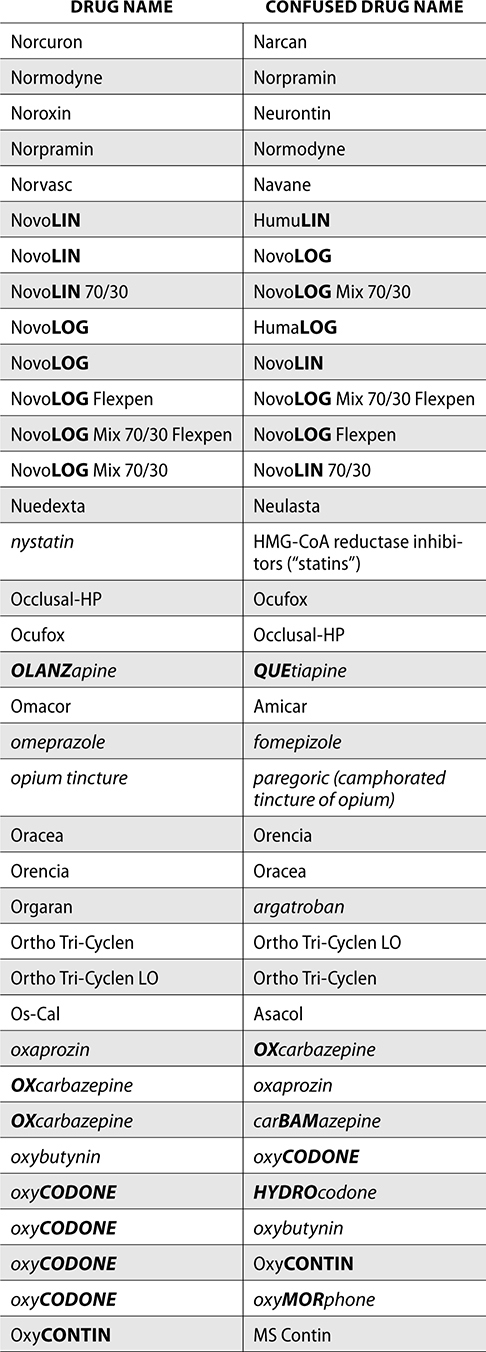
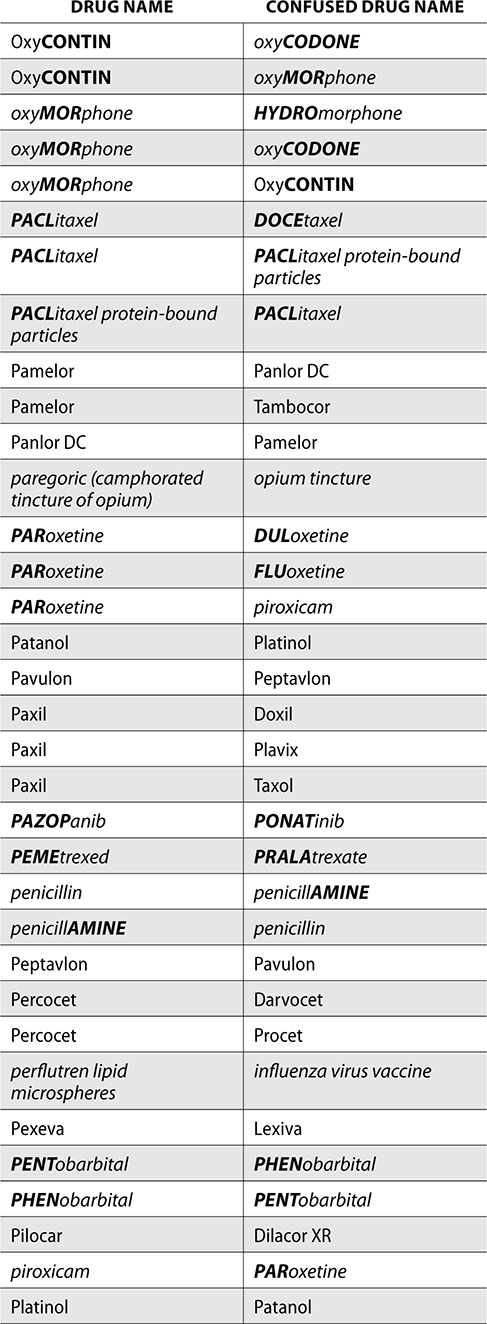
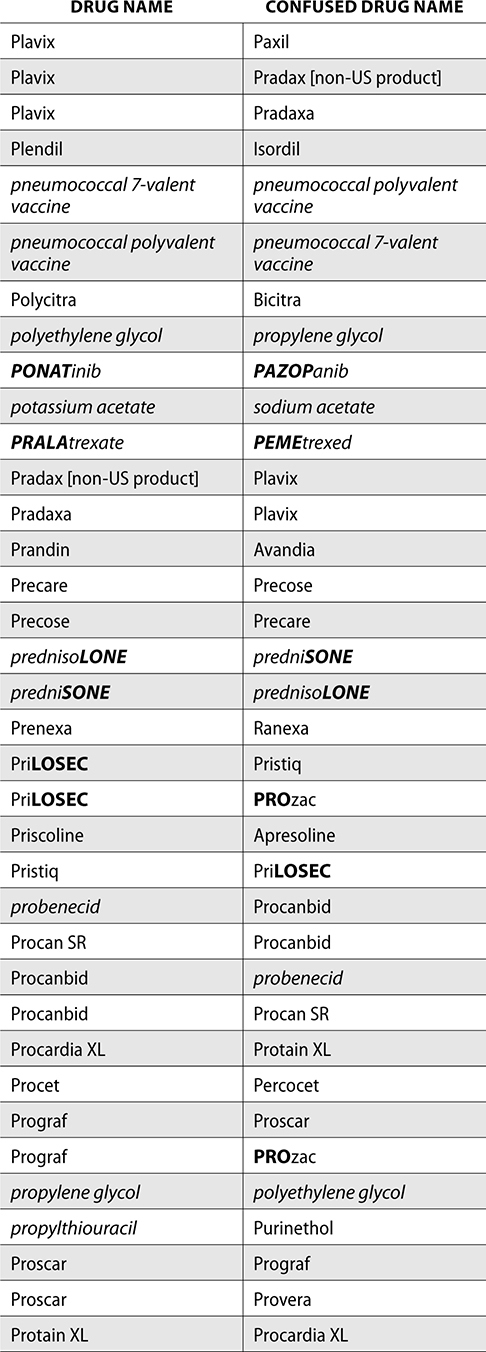
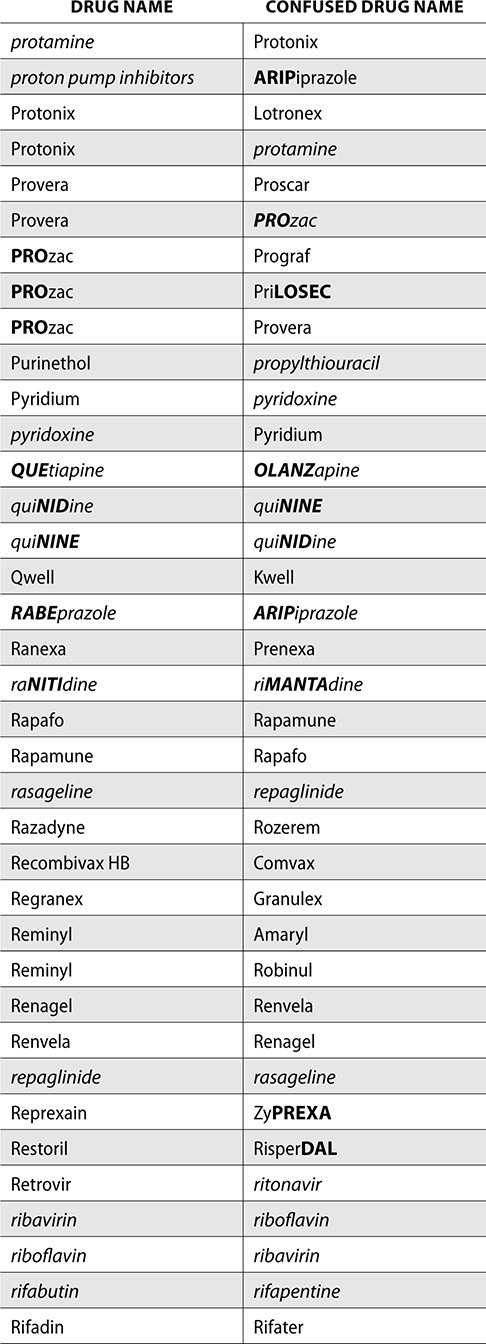
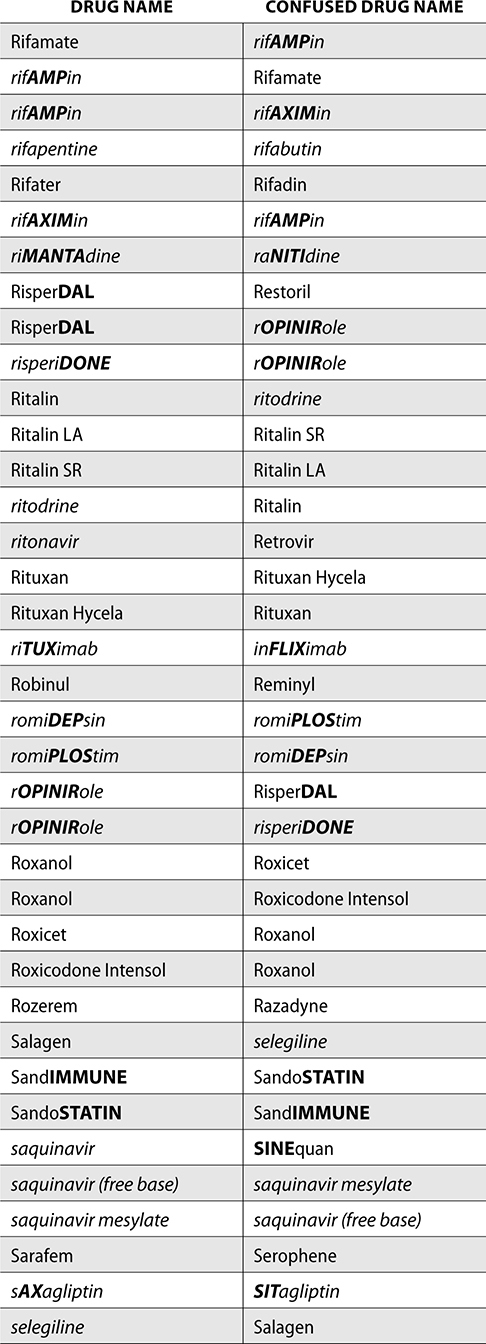
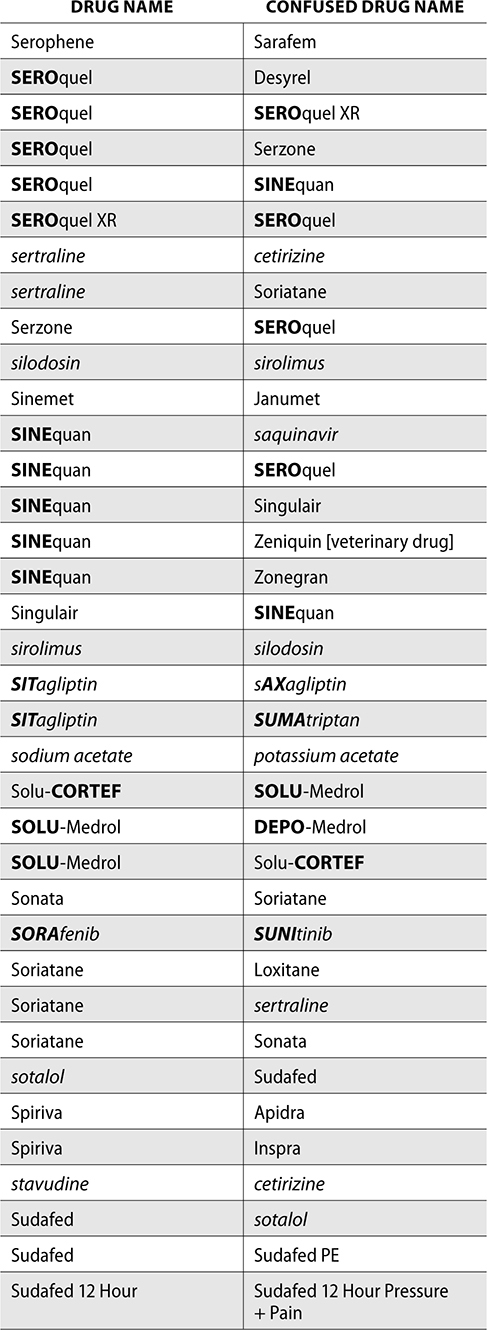
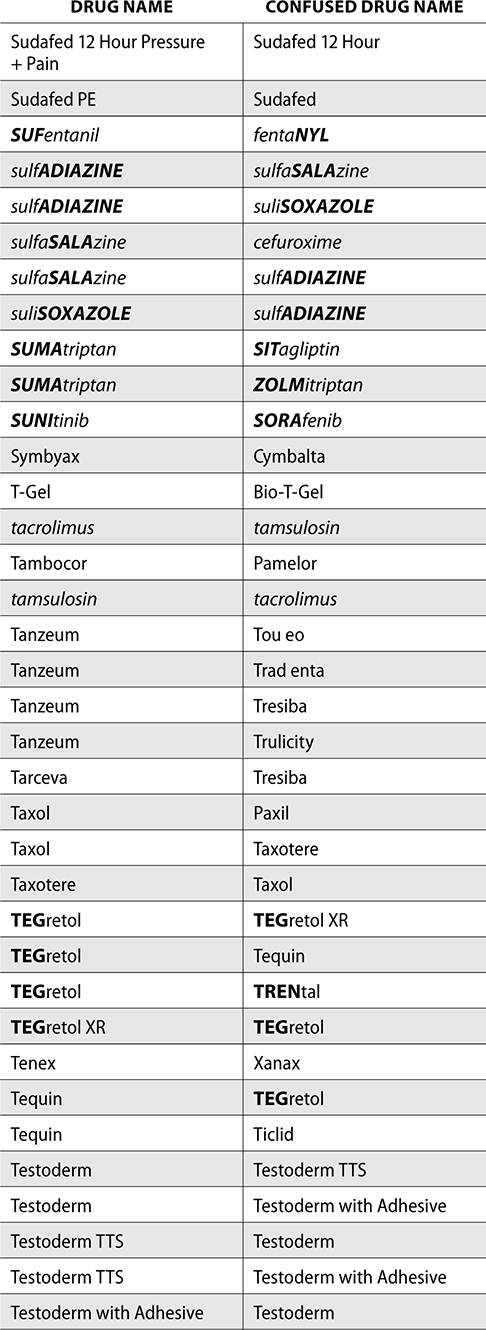
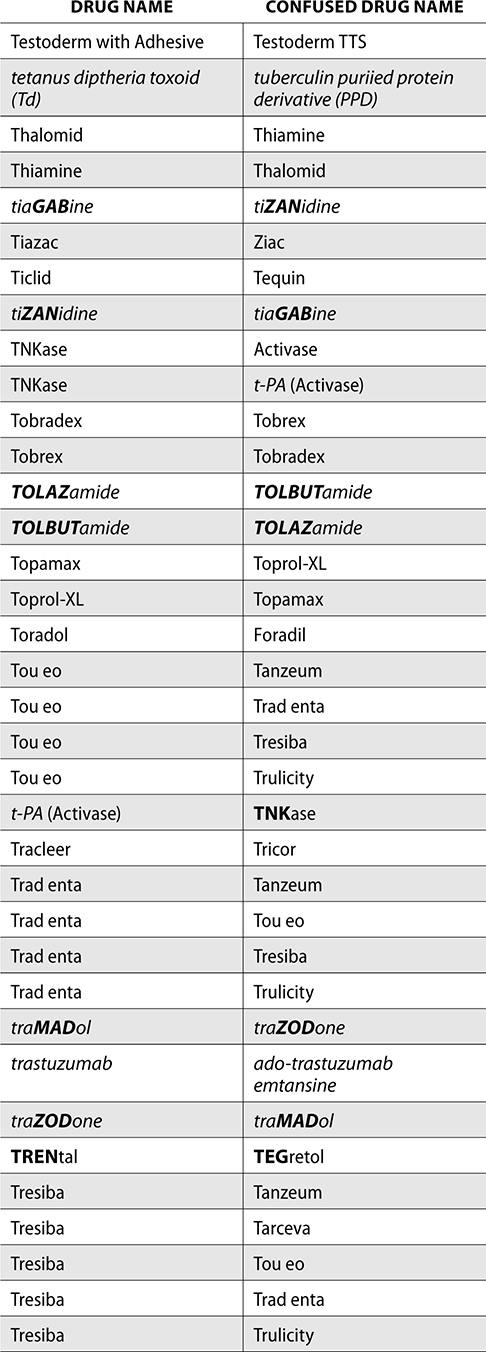
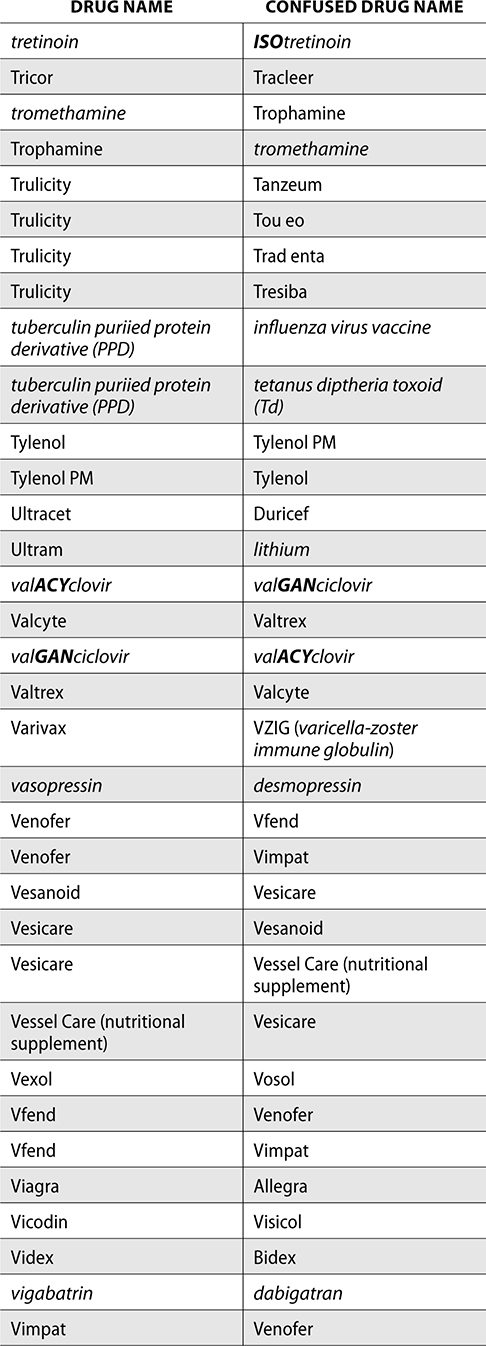
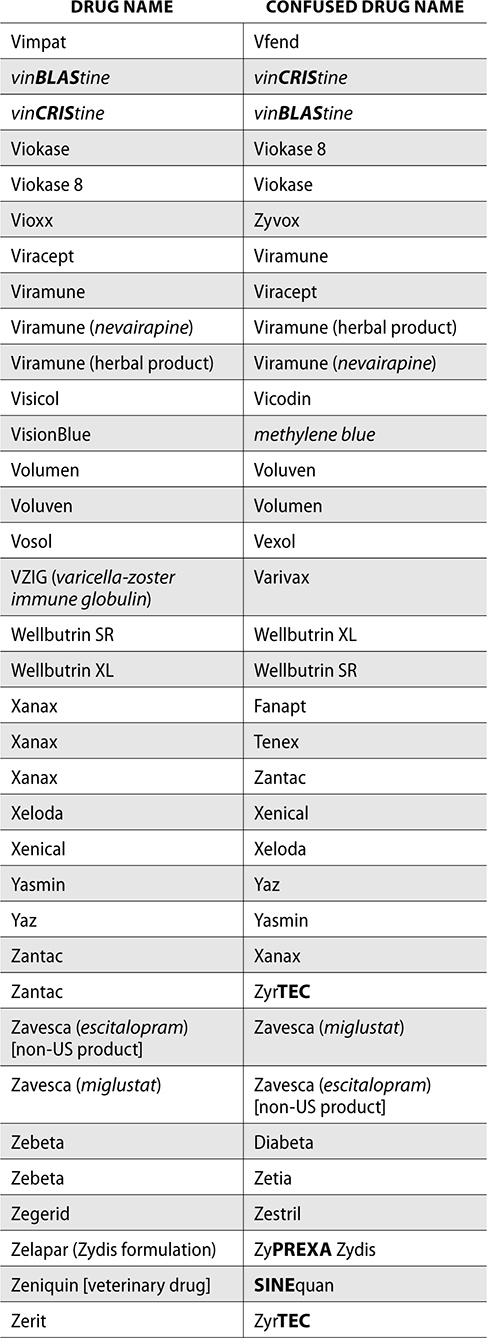
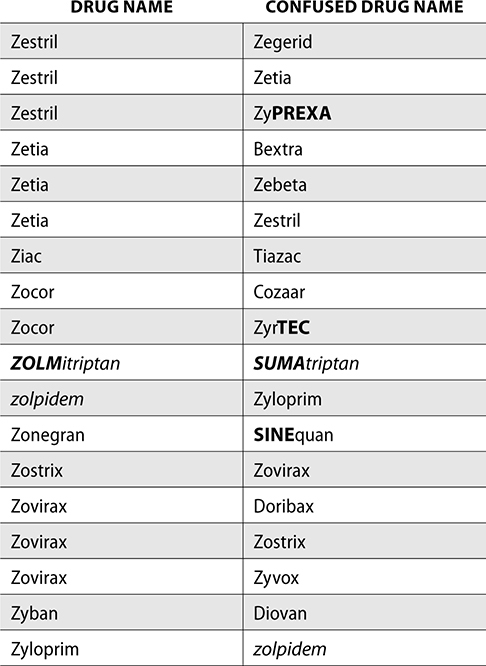
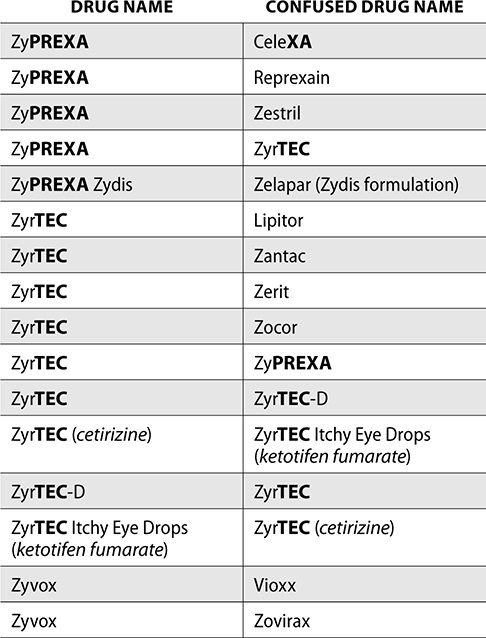
Error Prevention Strategies
A medication error is an event that leads to patient harm or inappropriate medication use that was preventable. Medication errors can be caused by many factors, though some common causes can be communication failures, failure in procedure, and human error.
There are several strategies to reduce or prevent medication errors.
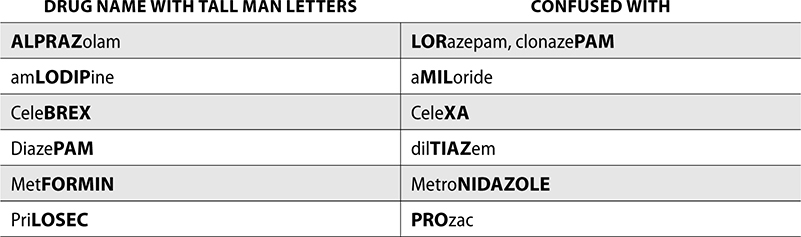
Tall Man Lettering
Medications on the confused drug list contain uppercase and bolded letters that are used to draw attention to the differences in each drug name. This is known as tall man lettering, and it helps distinguish between two drugs that look or sound similar. Pharmacies can also use tall man lettering in computerized ordering systems to allow prescribers to differentiate between LASA drugs safely.
The following examples are from the ISMP list for confused drug names with tall man lettering.
Leading and Trailing Zeros
Incorrect or misread doses can be a cause of medication errors. Leading zeros help minimize errors and should always be used. The leading zero comes before the decimal point, such as 0.7. If a leading zero is not used, .7 can be misread as 7 and a tenfold error could occur. Trailing zeros are after decimal points, such as 7.0. Trailing zeros should never be used, as they can cause confusion if the decimal point is missed. This could also lead to a tenfold error. 7.0 can be misread as 70.
Selecting Correct Patient
When entering prescriptions, verifying orders, or administering medications, selecting the correct patient is crucial for medication safety. By following the five rights of medication safety, pharmacy technicians, pharmacists, nurses, and other healthcare providers can verify all appropriate information for each patient. The five rights are:
1. Right patient
2. Right drug
3. Right dose
4. Right route
5. Right time
Using the five rights can help prevent medication errors, but sometimes even valid attempts at verifying the five rights can fall short. This can be due to trailing zeros, misreading orders that are handwritten, and communication or procedural failure. Five rights remain a basic foundation to medication safety, but accomplishing this may require additional support from other error prevention strategies.
Bar Coding
Bar codes can also be used as an added level of medication error prevention when administering medications. Bar code medication administration (BCMA) is completed by a nurse or other healthcare professional prior to giving a patient medication. The patient’s wristband is scanned, confirming the correct patient is receiving the medication. The patient’s medications can then be scanned and documented in the medication administration record (MAR). The bar code of the manufacturer label or pharmacy label is scanned to confirm correct product selection. If the wrong medication is scanned, an alert will notify the nurse that there is a problem. BCMA should not take the place of the five rights, but help support medication safety.
Additional use of bar codes occurs in the pharmacy and nursing units. During the filling of prescriptions in a pharmacy, the bar code of the correct national drug code (NDC) is scanned to confirm product selection. The pharmacist may also scan the bar code when verifying the prescription prior to dispensing. Scanning the bar code may also deduct that stock from inventory, if a pharmacy uses perpetual inventory. This helps maintain drug replenishment and inventory levels.
In a hospital pharmacy, medications are scanned prior to loading in automated dispensing cabinets to confirm the correct product was selected. The medication is scanned when loading stock, and after the nurse dispenses, the medication is scanned again prior to patient administration. This offers additional safety measures for error prevention.
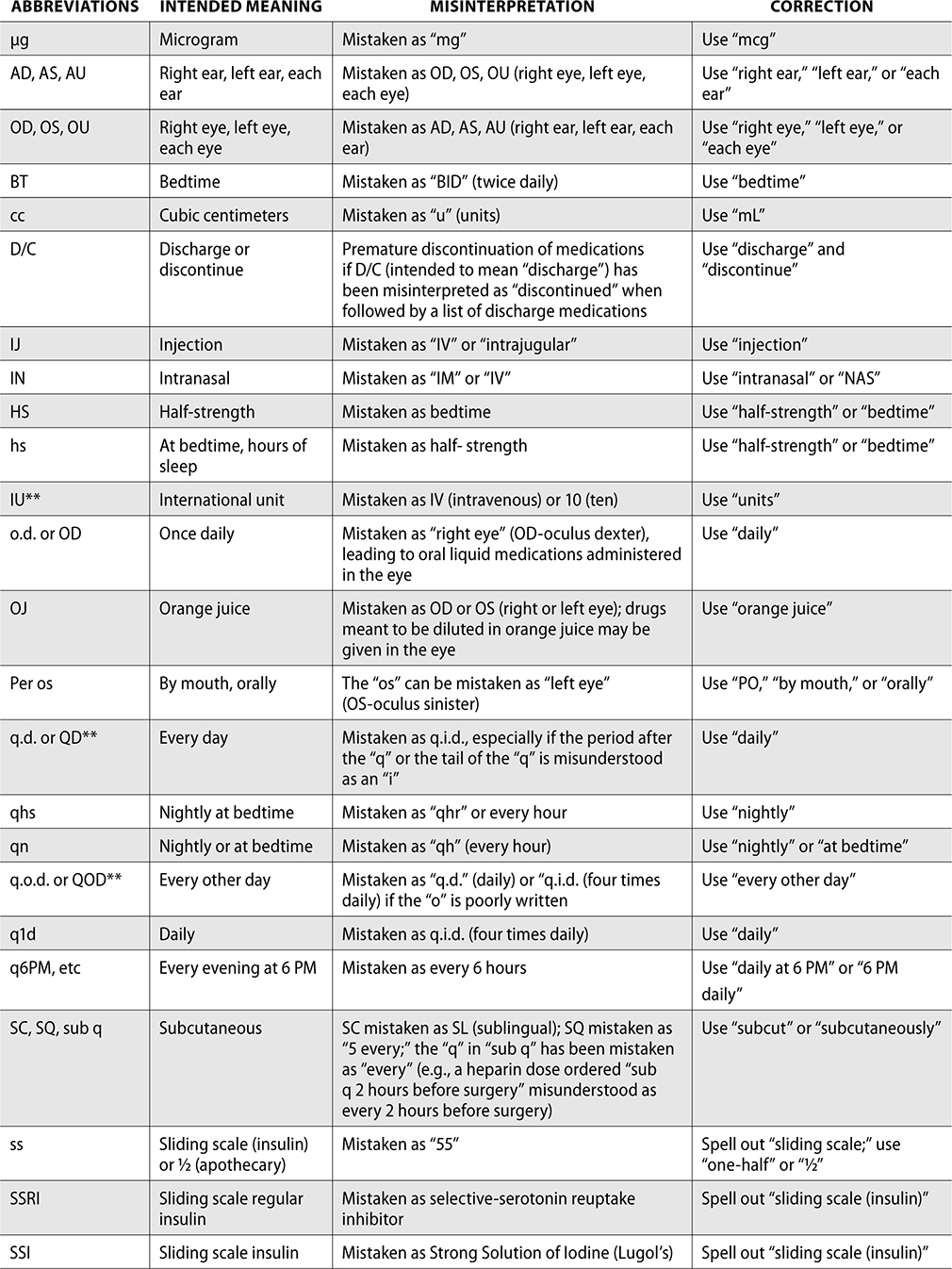
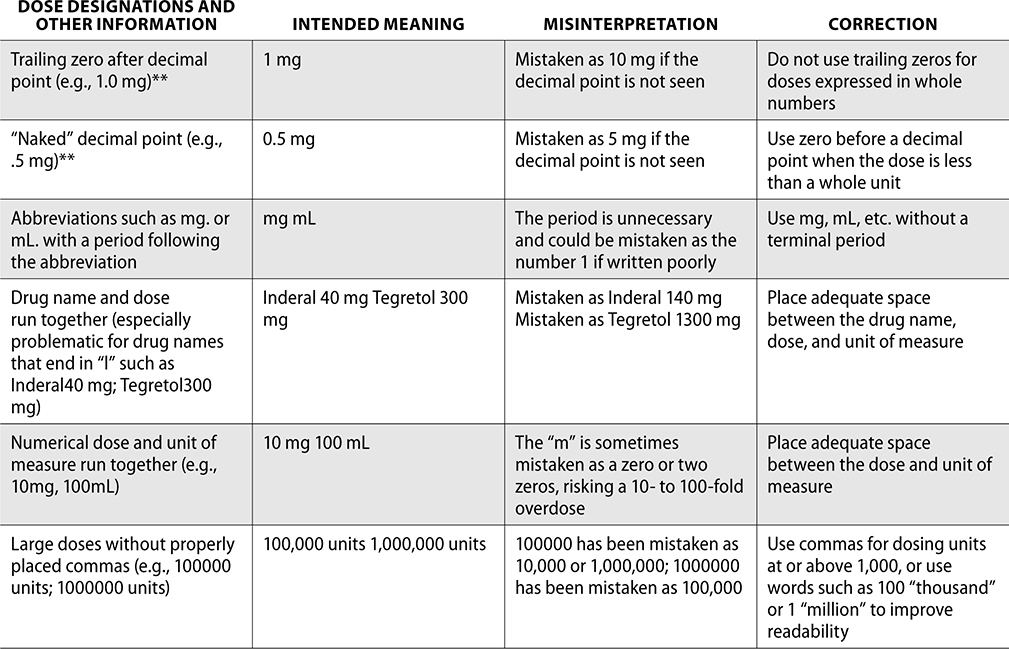
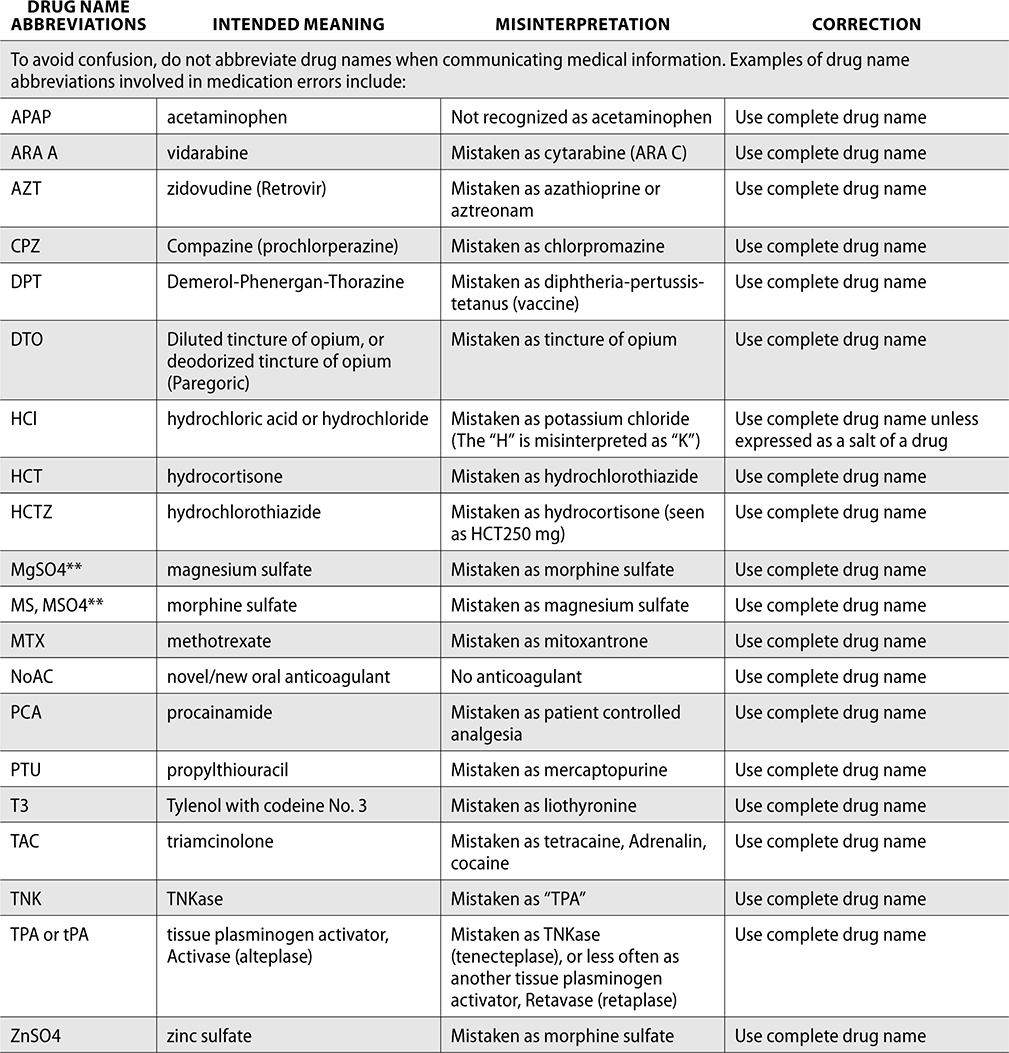
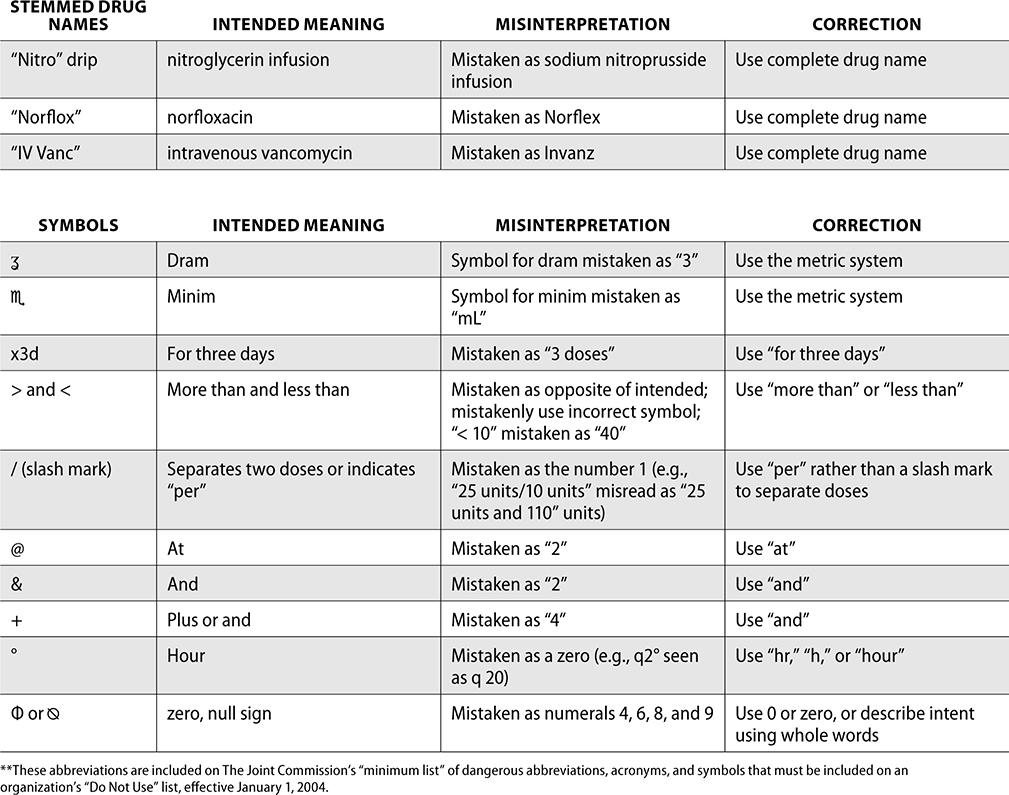
Error-Prone Abbreviations
Through the collection of data from reported medication events, the ISMP has identified error-prone abbreviations, symbols, and doses. These should never be used when communicating and always avoided whenever possible.
The ISMP has published a list of error-prone abbreviations, doses, and symbols, and The Joint Commission uses a similar list as a standard for safety within a hospital. Hospitals accredited by The Joint Commission are expected to use the dangerous abbreviations list from The Joint Commission as a “minimum list” of dangerous abbreviations, and follow this policy in practice as well as in the electronic medical record (EMR). When The Joint Commission surveys organizations, it reviews this list and ensures safety practices are being followed to prevent medication errors.
The ISMP List of Error-Prone Abbreviations, Symbols, and Dose Designations follows. Indicated with ** within this chart are those abbreviations included on The Joint Commission’s minimum list that must be used on an organization’s “Do Not Use” list.

Separating Inventory
Another way to help prevent medication errors is by segregating inventory that may be more prone to errors or have a more adverse event if an error occurred. ISMP has published guidance on standardization of drugs, storage, and distribution as a way to help reduce the risk of a medication event.
Some of the identified strategies from ISMP include:
▪ Use signing programs that draw attention to LASA drugs and high-risk/alert medications, such as stickers or separate inventory
▪ Avoid stocking LASA drugs in “fast mover” sections
▪ Do not store nondrug supplies, such as isopropyl alcohol, near diluents needed for reconstitution
▪ Immediately remove outdated, recalled, or discontinued drug products from inventory
▪ Store refrigerated medications in separate bins, such as insulins or other similar items
▪ Maintain a pickup/will-call area in a retail pharmacy free from clutter that has enough space to prevent spillage into another bin or basket
▪ Use dividers on crowded shelves to separate inventory
▪ All stock, including vials returned to stock, must be labeled with drug name, strength, expiration date, NDC number, and bar code if possible
Additionally, there are some specific medications that must be identified or separated from inventory:
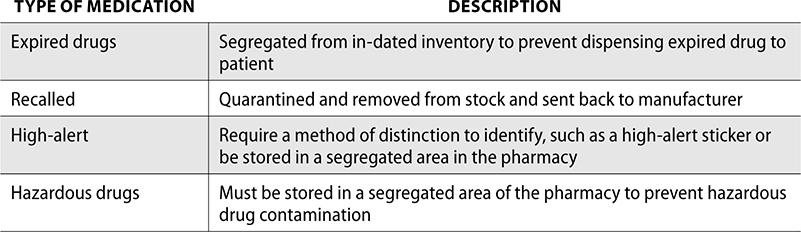
Medication Error Reporting
A medication error is a preventable event that may lead to patient harm. Medication errors can occur at prescribing, filling and dispensing, administering, and even patient monitoring. If an error occurred and does not harm the patient, it is still deemed an error. A near miss, on the other hand, is a potential medication error that was caught before it reached the patient.
Adverse drug events (ADE) occur when a medication causes harm to a patient. ADEs consist of medication errors, adverse drug reactions, allergic reactions, or overdose. An adverse drug reaction (ADR) is an unwanted and undesirable effect of a medication that occurs during the standard clinical use or dose. An allergic reaction occurs when there is an abnormal immune response to a normal dose. An overdose occurs when an excessive or dangerous dose of drug is taken.
For all ADEs, documentation and reporting are the key to preventing future patient injuries. A near miss is just as crucial as an actual event to report, as this can help identify protocol failures, dangerous trends, or workflow issues prior to patient harm. Though the FDA requires extensive research and clinical trials for new drug approval, there are often adverse events that occur post-approval when the drug is on the market. Reporting these events is important for patient safety.
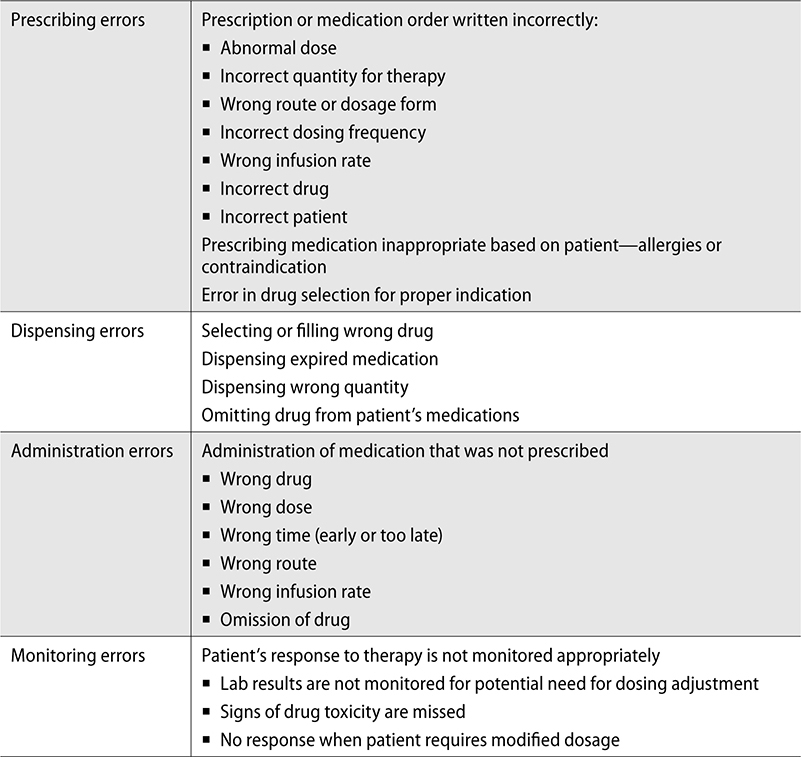
Types of Prescription Errors
Understanding how, where, and when prescription errors might occur can help mitigate potential issues before they happen. Prescription errors can be broken down into four main categories:
▪ Prescribing
▪ Dispensing
▪ Administration
▪ Monitoring
Prescribing errors occur from the written order of a provider, either a prescription or a medication order. This includes errors in prescribed dosage or drug strength, quantity (excessive or insufficient) needed for drug therapy, route or dosage form, rate of infusion, or drug ordered. A prescribing error can also include omitting necessary refills or approving early refills that may alter appropriate therapy. Incorrect patient errors can also occur during prescribing, including writing an order for a medication to a patient with a known allergy or contraindication.
Prescribing errors are often caused by communication failures, such as misinterpretation of handwritten or verbal orders. This can often be the result of using a confusing or dangerous abbreviation. Clarifying these orders is essential for patient safety. Computerized provider order entry (CPOE) can help prevent errors by removing the potential for misreading handwriting.
A dispensing error is a difference in what was prescribed and what is dispensed to a patient. Dispensing errors can be caused by anyone involved in this process—including both pharmacists and pharmacy technicians. It can result from a wrong product selection—including wrong strength or dosage form. Storing LASA drugs away from each other can help prevent these errors from occurring. Expired medications must also be removed from stock prior to expiration, as dispensing an expired medication would also be considered a dispensing error. Dispensing errors can also include the wrong quantity, such as giving only 30 tablets in a 60-tablet prescription. Omission of a drug can be considered a dispensing error. Using bar-coding to confirm the correct product, strength, and dosage form can help prevent dispensing errors.
An administration error occurs when there is a difference in what the patient is administered and what was prescribed. This includes administering the wrong drug, wrong dose, or at the wrong time. If a medication is given at the wrong time, it can exacerbate a disease. This could be the case when a patient has Parkinson’s disease and the medications are given late. The patient’s symptoms could worsen.
An administration error can also occur if a medication is given via the wrong route. For example, a patient is prescribed a subcutaneous dose of heparin. The nurse administers the dose IV push instead of subcutaneous. An omission error is also considered an administration error and occurs if a patient does not receive a medication at all. Errors in IV infusion rate can be prevented through the use of IV smart pumps. IV pumps have a built-in drug library, and some even have bidirectional capability, which allows the order from the MAR to flow to the pump and prevent overriding or incorrect infusion rates.
Using BCMA can help prevent administration errors. By scanning the drug prior to administering, a nurse can identify incorrect doses, routes, dosage form, or if the drug itself is incorrect. BCMA will also identify if the time is wrong—such as a too-early dose. Patient wrist bands are scanned to help prevent wrong patient errors as well. The five rights should also be used to prevent administration errors.
A monitoring error is when a drug treatment plan for a patient is not evaluated for appropriate prescribing. It can occur when a patient’s response to therapy is not monitored appropriately, such as through lab results or signs of drug toxicity. Monitoring errors include a lack of response if a patient requires a modification to the prescribed dosing.
Preventing monitoring errors starts with proper training of providers on the potential negative effects of medications, which may indicate toxicity. Providers must also be trained in understanding monitoring methods, such as through vital signs and evaluating lab results—this includes pharmacists. Pharmacists often review lab results prior to initiating drug therapy, such as chemotherapy.
Event Reporting Procedures
Most pharmacies and healthcare facilities have an internal mechanism to report medication errors. This may be online or on paper, but regardless of the process, reporting errors is essential to patient safety. Online reporting programs offer the capability of trending data, which helps plan investigations or create task forces to reduce the potential for errors.
The FDA uses a voluntary reporting system known as MedWatch for adverse and safety events. Healthcare professionals, consumers, or patients can all report through this online program. MedWatch reporting can include drugs, biologics, medical devices, dietary supplements, or cosmetics. This can also include suspected counterfeit medications.
The FDA uses the information from MedWatch to initiate recalls, investigate manufacturers, or make recommendations on medications. Reporting is therefore crucial for patient safety. While there is no legal requirement to report adverse events, MedWatch should be used in each the following situations:
1. Unexpected side effect or adverse event
2. Product quality issue
3. Potential medication errors that can be avoided, such as labeling or packaging
4. Therapeutic failure
When completing a MedWatch form online, the following information is required:
▪ Name of drug (and if medical device, model and serial number)
▪ Description of the adverse event
▪ Any concomitant medication use or disease history
▪ Date event occurred and when medication was started or stopped
▪ Dosage and directions for use
▪ The outcome of the event—for example, did the adverse event stop after the medication was discontinued?
ISMP also has a reporting database known as the Medication Error Reporting Program, or MERP. The goal of the ISMP and compiling data from MERP is to identify potential causes of errors and create information for pharmacies and healthcare organizations to follow. These safety practices can then be used by accrediting bodies, such as The Joint Commission, to review for patient safety.
When reporting a medication error in MERP, the following information is required:
▪ What went wrong or could go wrong
▪ Causes and contributing factors
▪ How the event was discovered or intercepted
▪ Actual or potential outcome of the involved patient(s)
▪ Your recommendations for error prevention
▪ Product names, dosage forms, and strength/dose
▪ Specific information regarding the model, build, and manufacturer of involved healthcare information technology and medication-related devices
▪ Any associated materials that help support the report being submitted (e.g., images of devices, display screens, products, containers, labels, de-identified prescription orders)
Root Cause Analysis
When a medication error occurs, it may require a more thorough investigation, or root cause analysis (RCA), to identify any underlying issues that could result in additional errors. An RCA is a process to find the “root cause” of a problem through a comprehensive review of all workflows and systems in place. This will then not only identify the problem, but help develop a way to prevent it from occurring again. Not all errors have one root cause, and an RCA can help identify additional causes if there are more than one.
An RCA should be conducted in a team approach. It is typically more beneficial to have the team be comprised of those who were not involved in the original error itself, but familiar with the workflows, processes, and procedures in the area it occurred.
The first step in an RCA is collecting data and reconstructing the error through a review of records and interviews of anyone involved. The RCA team then analyzes the events leading to the error, with the goal of understanding how and why the event occurred. The end result of an RCA is to prevent future adverse events from occurring.
A team conducting an RCA recognizes that human error can be a factor for all errors, but will instead focus on potential systematic issues that may have contributed to the event. Review the following medication error and think about how human error can play a role in this medication event.
A nurse has an infusion of lipids to administer to a patient on TPN. The nurse is to program the pump to infuse over 12 hours, but instead programs the pump to infuse over 12 minutes. The patient receives the entire infusion in 12 minutes.
This example can be considered human error—the nurse did not enter the infusion rate correctly. However, an RCA identifies factors that may enhance the possibility of an error occurring. These are called contributing factors and can include circumstances or conditions that may increase the probability of an adverse event. Contributing factors to the preceding error were as follows: this unit was short staffed so no other nurse was available as a double-check on the infusion rate; this nurse was working her seventh straight day because of staffing issues; this nurse had four patients over a typical max patient load for one nurse.
While the error that occurred can be considered human failure, an RCA can identify the contributing factors and the root cause of the medication event. This medication error can be analyzed to be caused by an organizational failure—staffing was not at an appropriate or safe level for a nurse to care for patients. RCA can help prevent punitive responses to human error, and instead investigate the organizational or systemic failure behind the mistake to help prevent the same error from occurring in the future.
Product Integrity
MedWatch can also be used to report issues with product integrity. Integrity issues could include early degradation of a medication (after proper storage) and physical appearance that may suggest a medication is counterfeit. A counterfeit medication may contain no active ingredient, or a drug different than what is specified. It could also contain the wrong dose and has the potential to be harmful if taken. As a pharmacy technician, it is important to be on the lookout for counterfeit drugs. A visual inspection is the first step in identifying any potential integrity issues. The drug package should be examined for tampering of the security seal, unusual fonts or print colors, and spelling errors, and the manufacturer address should be traceable. The dosage form itself can also be inspected for excessive powder or broken tablets in the bottom or discoloration of the tablet or capsule.
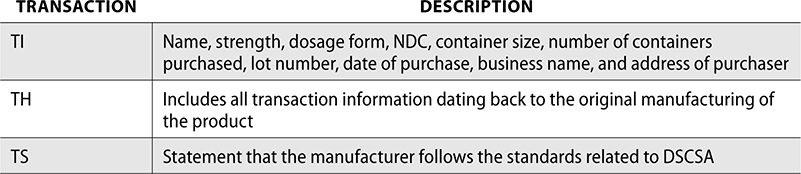
The Drug Supply Chain Security Act (DSCSA) was enacted in 2013 to help detect and remove counterfeit, stolen, or contaminated drugs from the US supply chain. Under the DSCSA, pharmacies must verify licensing of wholesalers and registration of manufacturers. All drugs must be accompanied by transaction information (TI), transaction history (TH), and a transaction statement (TS), and these must be stored for six years to provide tracking information. This information can be stored electronically.
Pharmacist Intervention
Pharmacist interventions are an important part of patient safety and daily medication review. Pharmacy technicians can help identify the need for interventions by alerting pharmacists in the event a clinical review or consult is needed. Some potential issues requiring pharmacist intervention include drug utilization review (DUR), recommendation of OTC, therapeutic substitution, misuse or adherence issues, immunization follow-up, allergies, or drug interactions.
Drug Utilization Review (DUR)
A pharmacist completes a DUR by reviewing a prescription for potential drug interactions, allergies, contraindications, and compliance issues. A DUR provides a comprehensive review of prescriptions and medication data before and after dispensing to ensure a positive outcome.
The Omnibus Budget Reconciliation Act of 1990 (OBRA90) required a DUR to be completed for all Medicaid patients, though now a DUR is conducted on most claims submitted through insurance adjudication. Medicaid DUR is an ongoing program that reviews data submitted for patterns of drug use in Medicaid programs. A DUR can be considered prospective (screening of medication before it is dispensed) or retrospective (ongoing review of claims data).
Prospective DUR identifies issues such as:
▪ Therapeutic duplication
▪ Drug–disease interaction
▪ Drug–drug interaction
▪ Inappropriate prescribing, such as incorrect dosage, frequency, or duration
▪ Recommendations for substitutions and therapeutic interchange
▪ Allergies
▪ Misuse or abuse of a medication
▪ Appropriateness of medication for patient
▪ Pregnancy alert
Medicaid periodically completes retrospective DUR reviews claims data to identify patterns such as:
▪ Adverse events
▪ Therapeutic appropriateness
▪ Use of generic products
▪ Incorrect duration
▪ Abuse or fraud
▪ Unnecessary medical care
A DUR will notify the pharmacist if an intervention is necessary. The pharmacist may choose to proceed with the prescription as is (override the DUR), contact the provider for clarification or modification, or contact the insurance for approval or clarification.
Over-the-Counter Recommendations
Self-medicating with over-the-counter (OTC) products is very common in pharmacy practice. Pharmacy technicians can assist in selection of OTC products by helping patients locate specific products and answering any nonclinical questions. For instance, if a patient asks if the box of diphenhydramine on the shelf is the generic for Benadryl, a pharmacy technician can answer that question. If the patient asks what is recommended for allergies, a pharmacy technician must have the pharmacist answer for a clinical recommendation. The pharmacist must consider additional factors such as potential contraindications, concurrent medications, or disease state.
Therapeutic Substitution
A therapeutic substitution occurs when a medication is substituted with a drug that has a different active ingredient but with the same intended clinical effect. These drugs are typically in the same drug class. The intent of therapeutic substitution is to use the lowest-cost medication for drug therapy. In hospitals, therapeutic substitution is a method of reducing inventory. For example, if a patient is admitted to the hospital and typically takes lisinopril at home, but the hospital only stocks enalapril, a therapeutic substitution would occur so the patient could continue taking an ACE inhibitor while in the hospital, but a different medication than what was originally prescribed. The hospital can then stock only one ACE inhibitor, instead of many different drugs in the same class.
In retail pharmacy, insurance companies often dictate therapeutic substitution rules by requiring a step therapy or rejecting claims for more expensive drugs that have a cheaper or generic option in the same drug class. Patients may have to try the cheaper option first before getting an authorization for the more expensive option if there is treatment failure associated with the substitution. There are certain drug classes that must be careful about substituting, such as antidepressants, anticonvulsants, or hormone therapy. Patients often respond uniquely to these medications, and an alternate brand, generic, or different drug can have a negative impact on outcomes.
Misuse
Misuse of a prescription is when a patient intentionally or unintentionally takes a medication outside of the prescribed directions. This could include taking a prescription that is not prescribed to you, or using a medication for a high or euphoric feeling. Prescription drug abuse and misuse is a continued problem in the United States. It is often driven by the misperception that abusing prescription drugs is safer than abusing illicit substances. This has led to an increase in emergency room visits due to overdose and overdose deaths.
There are three classes of drugs that are most frequently abused:
1. Opioid analgesics used for pain
2. Sedatives, such as benzodiazepines or hypnotics used for anxiety and sleep
3. Stimulants used for ADHD
Adherence
Medication adherence is defined as the extent to which a patient takes a medication as prescribed. Adherence is a change in a patient’s behavior by following prescribed regimen. Nonadherence can be caused by many issues such as cost of medication, side effects resulting in adherence issues, or cognitive deficits in understanding instructions or forgetting to take the drug. Pharmacy technicians can help identify these issues with patients and alert the pharmacist for potential adherence problems. If a patient is consistently late refilling a medication or asks for help with treatment of side effects, a pharmacist should intervene to provide clinical support. If a patient is forgetting to take their medication, recommending a pillbox reminder is a good tool to help improve adherence.
Adherence can be calculated as a percentage by dividing the total days’ supply a patient filled by the total number of days and multiplying by 100.
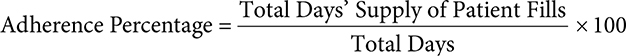
For example, if a patient filled a medication for a 90-day supply, one time in 6 months, this would be 90 days of total therapy divided by 180 days of total time.
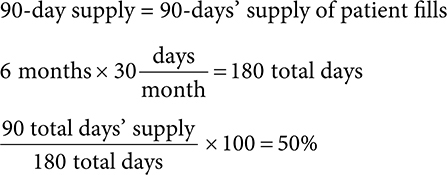
This patient would have a 50% adherence rate.
Immunization
Depending on the state in which you live, pharmacists (and even pharmacy technicians in some states!) are able to give immunizations, though which specific vaccines depend on state law. Some states allow pharmacists to administer all vaccines to patients of all ages, while other states restrict which vaccines have a protocol for pharmacists and a minimum age for administration. Regardless of the state you’re in, pharmacy technicians can assist the pharmacist for patients requesting vaccines by completing documentation for the patient, including informed consent for the vaccine risks and supplying a vaccine information statement (VIS). The VIS provides information on the vaccine, including risks of a reaction, reporting procedures for post-immunization follow-up, who should get vaccinated, and information that patients should tell their healthcare provider.
After an administration of a vaccine, pharmacists must provide post-immunization follow-up with the patient to detect possible safety concerns. Any concerns reported by patients for vaccines should be reported to the Vaccine Adverse Event Reporting System (VAERS). This system is designed to detect possible problems with US vaccines.
The objectives of VAERS include:
▪ Detecting new and unusual adverse vaccine events
▪ Monitoring increases in known adverse events
▪ Indentifying risk factors for patients for specific adverse events
▪ Assess new vaccines for safety
▪ Determine if reporting clusters exist, indicating potential geographic or lot issues
▪ Recognize administration issues
▪ Provide a monitoring system for large-scale program
Adverse Drug Events (ADEs) and Allergies
An adverse drug event occurs when medication use results in an injury to a patient. The ADE can be caused by an error or an ADR. A contributing factor to ADEs can be polypharmacy, or when a patient is taking multiple drugs, often for the same disease. Pharmacist intervention may be required if a patient’s provider should be notified for a change in the drug therapy. This can help prevent a potential ADE.
Age may also be a risk factor for ADEs. Elderly patients are susceptible to disease–drug interactions due to chronic diseases requiring dose modifications. The Beers Criteria Medication List is published by the American Geriatrics Society and is a list of medications and drug classes that may be inappropriate for geriatric use. The Beers list provides recommendations on prescribing these drug classes to prevent ADEs for geriatric patients.
ADEs can also be caused by an allergic reaction. Pharmacy technicians can help prevent allergic reactions by documenting patient allergies each time a patient fills a prescription, to verify no changes have occurred. If a patient says they have no known allergies, it is important to identify if this refers to all medications only, or no allergies including other substances. This distinguishes NKA (no known allergies) from NKDA (no known drug allergies). If a patient has no known allergies, including all substances and drugs, this is NKA. If a patient has no known allergies to medications only, this is NKDA. This pharmacist intervention is caught through DUR.
Quality Control
Quality control (QC) in the pharmacy is required for Medicare Part D and for many accrediting bodies. Typically, pharmacies accomplish this through implementing a continuous quality improvement program (CQI). This helps find and assess issues, implement change, and measure progress. Reporting of medication errors often drives the processes to be reviewed in a CQI. If a trend is identified through error reporting, it can be targeted as a measure to evaluate improvement after a CQI is implemented.
Hygiene and Cleaning Standards
Quality control is also important for cleaning and hygiene standards. To prevent contamination of sterile compounds, USP has outlined standards for sterile compounding in USP chapter <797>. Compliance with these standards helps minimize sterility issues.
USP<797> provides standards and requirements on the following:
▪ Engineering controls for compounding equipment
▪ Training and competencies
▪ Certification requirements for hoods
▪ Beyond-use-dating
▪ Layout of facility
▪ Clean room design
▪ Environmental monitoring for potential contaminants
Handwashing and Personal Protection Equipment
Personal protection equipment (PPE) is required for all compounding procedures. PPE and hand hygiene help minimize microbials from entering a sterile environment needed to compound. PPE includes hair and beard cover, face mask, gloves, gown, and shoe covers. Donning, or putting on, PPE must follow a specific process to ensure proper protection. It should be completed “dirtiest” to “cleanest,” such as putting on shoe covers before putting on gloves.
Proper hand hygiene and handwashing is an important part of sterile compounding and an overall obligation of a pharmacy technician. Good handwashing prevents the spread of illness and also helps maintain cleanliness within a pharmacy. The first step in handwashing for sterile compounding begins in the ante room, which is located adjacent to the compounding (buffer) room. Jewelry must be removed and no nail polish or artificial nails can be worn. Dirt and microbials can gather under artificial nails and nail polish can chip off and contaminate a sterile area. Hands should be washed with an antimicrobial soap up to the elbows with hot water for a minimum of 30 seconds. A nail pick should be used to clean under the fingernails and remove any debris. After cleaning, hands should be dried with a lint-free paper towel.
Below is the proper order of PPE donning, or garbing, for sterile compounding:
1. Start by removing any jewelry, makeup, artificial nails or polish before donning PPE.
2. Put on shoe covers.
3. Don hair cover—including beard cover if needed.
4. Put on a face mask or shield. A face shield or goggles are typically required only when compounding hazardous drugs.
5. Perform hand hygiene.
6. Don nonshedding gown over clean scrubs.
7. Use surgical (alcohol-based) hand scrub prior to putting on gloves. Allow to dry.
8. Don sterile gloves. Two pairs of chemotherapy gloves should be used if compounding a hazardous drug.
After completion of sterile compounding, doffing, or removing garb, should be in the reverse order of the preceding steps—starting with removal of sterile gloves and ending with removing shoe covers.
Cleaning the Pharmacy
Keeping a pharmacy clean is not just limited to areas where sterile compounding is performed. Areas in a pharmacy where nonsterile compounding must also be kept clean and the pharmacy must be kept free from clutter for patient safety. Part of this cleaning includes counting trays, which are used for counting patient prescriptions. There is often a residue that remains after the medication has been counted. Trays should be cleaned with 70% isopropyl alcohol (IPA). Cleaning counting trays regularly helps prevent cross-contamination, which is the contamination of another medication due to residual residue left on a counting tray. Drugs that leave a powder on the tray can contaminate the next prescription if the tray is not cleaned in between prescriptions. This is especially true for medications that have allergy implications. For example, if a counting tray is not cleaned following the counting of amoxicillin, and a patient has a penicillin allergy, this could result in an allergic reaction if the residue remains on the tray when counting the next prescription.
Countertops should also be cleaned regularly in all pharmacies. Hospital pharmacies, or those with a clean room, follow standards in USP<797>, which require monthly cleaning of walls, ceilings, and shelves that are in the buffer or ante room. This cleaning must be documented on a log each month.
Cleaning equipment in the pharmacy is typically completed in the clean room with the primary engineering control (PEC) used for sterile compounding. The ISO level in the pharmacy determines how clean the air is. If the ISO level is higher, the air is dirtier. In the ante area outside the buffer area, the air should be no higher than ISO class 8. The buffer room must have a level no higher than ISO class 7, and within the PEC, the ISO level cannot be greater than ISO class 5.
If the PEC has been shut off at any time, it should be turned on and left to run for 30 minutes prior to use. If a laminar airflow hood is being used for compounding, air is pulled into the HEPA filter and blown out horizontally toward the employee. This is why hazardous drug compounding should not be done in a laminar airflow hood. To clean, use sterile 70% IPA and start with the bar used for hanging IV bags. Next, clean the sides of the hood using a bottom to top motion. Finally, the surface should be cleaned using a side to side motion, starting in the back and working toward the front. Avoid any sprays as this could result in damage to the HEPA filter. Cleaning of the hood should be done at the beginning of every shift, every 30 minutes or before every batch, and if a spill occurs. Cleaning for all PECs must be documented on a log. The HEPA filter must be certified every six months. In addition, during this certification, surface and air samples are taken to verify proper cleaning and airflow is occuring.
For hazardous drug compounding, a vertical flow hood or biological safety cabinet is used, which provides a vertical flow of air down after HEPA filtration. This negative pressure helps prevent air exposure to an employee working. Cleaning of the vertical flow hood is done in the same manner as the horizontal flow hood. In addition to 70% IPA, a sporicidal cleaner is recommended to prevent hazardous drug contamination.
Review Questions
The following questions help you review the chapter. Test your knowledge by working through the next 50 questions to test yourself and identify any areas you may need to review.
1. The ISMP has a published list of medications that may cause greater harm if used in error. These are known as
A. LASA
B. high-alert/high-risk
C. tall man
D. confused abbreviations
2. Which drugs should not be stocked next to each other on the shelf?
A. high alert
B. generic
C. fast movers
D. LASA
3. Uppercase and bolded letters used to draw attention to differences in drug names are known as
A. tall man lettering
B. LASA
C. confused drug list
D. leading zeros
4. Which of the following should never be used as it could cause a tenfold error?
A. trailing zero
B. leading zero
C. bar coding
D. five rights
5. AD, AS, and AU are all on the ISMP List of Error-Prone Abbreviations, Symbols, and Dose Designations for being potentially confused with
A. subcutaneous, intramuscular, and intradermal
B. before lunch, with meals, and after lunch
C. right eye, left eye, each eye
D. once daily, twice daily, three times daily
6. A medication error that was caught before it reached the patient is known as a(n)
A. near miss
B. adverse drug reaction
C. high alert
D. recalled
7. A medication error that includes an incorrect strength, quantity, or dosage written would be which type of error?
A. prescribing
B. dispensing
C. administration
D. monitoring
8. A voluntary reporting system for adverse and safety events managed by the FDA is
A. MERP
B. CQI
C. RCA
D. MedWatch
9. Counting trays in a pharmacy should be cleaned with
A. soap and water
B. distilled water
C. 70% IPA
D. bleach
10. An unwanted and undesirable effect of a medication that occurs during a normal dose is a(n)
A. near miss
B. medication error
C. recall
D. adverse drug reaction
11. Which of the following ISO levels represents the cleanest air?
A. ISO class 5
B. ISO class 6
C. ISO class 7
D. ISO class 8
12. Which process helps identify the origin of a problem instead of focusing on the human error?
A. CQI
B. MERP
C. RCA
D. ISMP
13. Which of the following could be a therapeutic substitution for Vasotec?
A. lisinopril
B. olmesartan
C. propranolol
D. lansoprazole
14. The best way to prevent the spread of illness and germs is through
A. donning shoe covers
B. handwashing
C. changing scrubs hourly
D. wearing a mask
15. Implementing a plan for improvement measures in a pharmacy, including responding to medication error reporting, is known as
A. ADR
B. RCA
C. CQI
D. PPE
16. Counting trays should be cleaned regularly, especially after a powder residue, to prevent
A. cross-contamination
B. monitoring errors
C. BCMA
D. microbial infection
17. Taking multiple drugs, often for the same disease, is known as
A. Beers list
B. ADE
C. polypharmacy
D. DUR
18. A medication event caused by a vaccine should be reported to
A. MedWatch
B. MERP
C. VAERS
D. ISMP
19. Which medication is most likely to be misused?
A. levothyroxine
B. alprazolam
C. spironolactone
D. pantoprazole
20. Which of the following is used to help identify drugs that may be inappropriate for geriatric use?
A. polypharmacy
B. LASA list
C. BCMA
D. Beers list
21. Which medication could be a therapeutic substitution for Protonix?
A. Pepcid
B. Prilosec
C. Tagamet
D. Carafate
22. Which of the following questions could a pharmacy technician answer?
A. Where can I find the loratadine?
B. What should I take for my cough?
C. I’m having drowsiness with my medication, is this normal?
D. Can I take ibuprofen though I have a stomach ulcer?
23. Which of the following would flag on a prospective DUR?
A. patient change in address
B. interaction with other concurrent medication
C. declined credit card
D. expired driver’s license
24. Which of the following is a review for potential drug interactions, allergies, contraindications, or compliance issues?
A. OBRA
B. BCMA
C. OTC
D. DUR
25. The Drug Supply Chain and Security Act was passed to help prevent
A. drug shortages
B. product integrity issues
C. sterile compounding errors
D. insurance rejections
26. When a drug treatment plan for a patient is not evaluated appropriately, this would be considered a
A. prescribing error
B. dispensing error
C. administration error
D. monitoring error
27. A patient has a prescription for 10 tablets to take 1 tab q12h PRN. The patient requests a refill after 3 days. How many days early is the patient requesting the refill?
A. 1 day
B. 2 days
C. 4 days
D. 6 days
28. Which chapter of USP outlines standards for sterile compounding?
A. 795
B. 797
C. 800
D. 826
29. How often must the HEPA filter be certified?
A. 30 days
B. 6 months
C. 1 year
D. 2 years
30. When doffing PPE, what should be removed first?
A. shoe covers
B. face mask
C. gown
D. gloves
31. A patient has a 30-day supply of a medication with 4 refills. The patient fills this prescription a total of 3 times in 5 months. What is the adherence percentage?
A. 50%
B. 60%
C. 70%
D. 80%
32. A nurse accidentally misses giving a patient their morning medications. Which type of error would this be?
A. prescribing error
B. dispensing error
C. administration error
D. monitoring error
33. USP<797> provides standards on which of the following?
A. cleaning guidelines for nonsterile compounding
B. handling of hazardous drugs
C. compounding of radiopharmaceuticals
D. competencies for sterile compounding
34. Which of the following medications is considered high risk?
A. Lantus
B. Mobic
C. Zyrtec
D. Prevacid
35. A pharmacist checking a prescription notices there are only 30 tablets in a bottle for a prescription written for 60. The pharmacist has caught a potential
A. prescribing error
B. dispensing error
C. administration error
D. monitoring error
36. When a nurse scans a medication prior to administering to a patient, this is known as
A. five rights
B. tall man lettering
C. MAR
D. BCMA
37. A patient fills a 30-day supply of a medication 6 times in 9 months. What is the adherence percentage during this 9-month period?
A. 33%
B. 45%
C. 55%
D. 67%
38. When cleaning a laminar airflow hood, which should be cleaned first?
A. top of hood
B. bottom of hood
C. IV hanging bar
D. sides of hood
39. A vertical flow hood should be used when compounding
A. all IVs
B. antibiotics only
C. hazardous drugs
D. ampules only
40. Which of the following is considered a high-alert medication?
A. esomeprazole
B. warfarin
C. doxycycline
D. folic acid
41. How should three milligrams be written on a prescription?
A. 0.3mg
B. 3.0mg
C. 3 mg
D. three milligrams
42. Which of the following should be avoided and never used when communicating?
A. bar codes
B. tall man lettering
C. error-prone abbreviations
D. MAR
43. A medication is written for a patient that is contraindicated with a patient’s disease state. This would be what type of error?
A. prescribing
B. dispensing
C. administration
D. monitoring
44. ISMP has a medication error reporting database known as
A. MedWatch
B. MERP
C. RCA
D. CQI
45. Which of the following could be a therapeutic substitution for Coreg?
A. lisinopril
B. metoprolol
C. valsartan
D. hydrochlorothiazide
46. When donning for sterile compounding, which PPE should be put on first?
A. face mask
B. gown
C. gloves
D. shoe covers
47. Which medication has the highest chance of being misused?
A. Adderall
B. Lipitor
C. Aldactone
D. Bactrim
48. When a medication causes an injury to a patient, this is known as a(n)
A. ADE
B. EMR
C. VAERS
D. LASA
49. A patient has filled a 30-day supply of a prescription 6 times in the last 180 days. What is the patient’s adherence percentage?
A. 25%
B. 50%
C. 75%
D. 100%
50. HS should never be used to abbreviate “half-strength,” as this could be confused with
A. every day
B. units
C. discharge or discontinue
D. at bedtime
Answer Key
1. B
To help prevent errors, ISMP has developed a list of high-alert medications, which are drugs that may cause greater harm if used in error.
2. D
LASA (look-alike and sound-alike) drugs should not be stored next to each other to avoid a potential mix-up.
3. A
Medications on the confused drug list contain uppercase and bolded letters that are used to draw attention to the differences in each drug name. This is known as tall man lettering and helps distinguish between two drugs that look or sound similar.
4. A
Trailing zeros are after decimal points, such as 7.0. Trailing zeros should never be used, as they can cause confusion if the decimal point is missed. This could also lead to a tenfold error.
5. C
The intended meaning of AD, AS, and AU is right ear, left ear, and both ears. It is often confused with right eye, left eye, and each eye.
6. A
A near miss is a potential medication error that was caught before it reached the patient.
7. A
Prescribing errors occur from the written order of a provider, either a prescription or a medication order. This includes errors in prescribed dosage or drug strength, quantity (excessive or insufficient) needed for drug therapy, route or dosage form, rate of infusion, or drug ordered.
8. D
The FDA uses a voluntary reporting system known as MedWatch for adverse and safety events.
9. C
Counting trays should be cleaned with 70% isopropyl alcohol (IPA).
10. D
An adverse drug reaction (ADR) is an unwanted and undesirable effect of a medication that occurs during the standard clinical use or dose.
11. A
The ISO level in the pharmacy determines how clean the air is. If the ISO level is higher, the air is dirtier.
12. C
An RCA is a process to find the “root cause” of a problem through a comprehensive review of all workflows and systems in place.
13. A
Vasotec (enalapril) is an ACE inhibitor and so is lisinopril.
14. B
Good handwashing prevents the spread of illness and also helps maintain cleanliness within a pharmacy.
15. C
Pharmacies accomplish quality control through implementing a continuous quality improvement program (CQI). This helps find and assess issues, implement change, and measure progress.
16. A
Cleaning counting trays regularly helps prevent cross-contamination, which is the contamination of another medication due to residual residue left on a counting tray.
17. C
Polypharmacy is when a patient is taking multiple drugs, often for the same disease.
18. C
Any concerns reported by patients for vaccines should be reported to the Vaccine Adverse Event Reporting System (VAERS). This system is designed to detect possible problems with US vaccines.
19. B
Alprazolam is a benzodiazepine. Opioid analgesics, sedatives such as benzodiazepines, and stimulants are all drugs that are more frequently abused than other medications.
20. D
The Beers list is published by the American Geriatrics Society and is a list of medications and drug classes that may be inappropriate for geriatric use. The Beers list provides recommendations on prescribing these drug classes to prevent ADEs for geriatric patients.
21. B
Prilosec is the name brand for omeprazole. Protonix is a proton pump inhibitor and the generic is pantoprazole. Omeprazole would be the only suitable substitution for pantoprazole.
22. A
A pharmacy technician can instruct a patient where to find an OTC medication, but cannot give clinical guidance.
23. B
Prospective DUR identify issues such as drug–drug interaction, therapeutic duplication, and drug–disease interaction.
24. D
A pharmacist completes a DUR by reviewing a prescription for potential drug interactions, allergies, contraindications, and compliance issues. A DUR provides a comprehensive review of prescriptions and medication data before and after dispensing to ensure a positive outcome.
25. B
The Drug Supply Chain Security Act (DSCSA) was enacted in 2013 to help detect and remove counterfeit, stolen, or contaminated drugs from the US supply chain.
26. D
A monitoring error is when a drug treatment plan for a patient is not evaluated for appropriate prescribing. It can occur when a patient’s response to therapy is not monitored appropriately, such as through lab results or signs of drug toxicity.
27. B
2 days. The patient is prescribed to take 2 tablets daily as needed. This is a max of 2 tablets daily. Since the patient has 10 tablets, this should last the patient 5 days.
28. B
USP<797> provides standards for sterile compounding.
29. B
The HEPA filter must be certified every 6 months.
30. D
After completion of sterile compounding, doffing or removing garb should start with removal of sterile gloves and end with removing shoe covers.
31. B
The patient fills a 30-day supply 3 times in 5 months. 3 × 30 = 90. 5 months = 150 days.
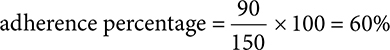
32. C
An administration error occurs when there is a difference in what the patient is administered and what was prescribed. This includes administering the wrong drug, wrong dose, or at the wrong time. An omission error is also considered an administration error and occurs if a patient does not receive a medication at all.
33. D
USP<797> includes standards related to sterile compounding such as competency and training requirements, facility layout, cleanroom design, and environmental monitoring.
34. A
Lantus is an insulin, and all insulins are considered a high-risk medication.
35. B
A dispensing error is a difference in what was prescribed and what is dispensed to a patient. Dispensing errors can also include the wrong quantity, such as giving only 30 tablets in a 60-tablet prescription.
36. D
Bar code medication administration (BCMA) is completed by a nurse or other healthcare professional prior to giving a patient medication.
37. D
67%. Start by determining the total days of patient fills. 30 × 6 = 180. Next, calculate the amount of days in 9 months. 9 × 30 = 270.
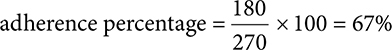
38. C
To clean, use sterile 70% IPA and start with the bar used for hanging IV bags.
39. C
For hazardous drug compounding, a vertical flow hood or biological safety cabinet is used, which provides a vertical flow of air down after HEPA filtration.
40. B
Warfarin is on the ISMP high-alert medication list.
41. C
3 mg does not have a decimal point, so it does not need a leading zero and trailing zeros should never be used.
42. C
Through the collection of data from reported medication events, the ISMP has identified error-prone abbreviations, symbols, and doses. These should never be used when communicating and always avoided whenever possible.
43. A
Incorrect patient errors can occur during prescribing, including writing an order for a medication to a patient with a known allergy or contraindication.
44. B
ISMP is also a reporting database known as the Medication Error Reporting Program (MERP).
45. B
Coreg (carvedilol) is a beta blocker and so is metoprolol.
46. D
PPE is donned dirtiest to clean, with shoe covers first and gloves last.
47. A
Adderall is a stimulant used for ADHD and has a more likely chance of being misused.
48. A
An adverse drug event (ADE) occurs when medication use results in an injury to a patient.
49. D
100%. Start by determining the total days of patient fills. 30 × 6 = 180.
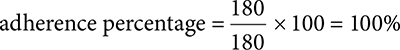
50. D
At bedtime. Every night at bedtime and half strength can both be abbreviated HS.