CHAPTER 2
What Is Metabolism Anyway?
How does music get inside the radio?”
It wasn’t a question I was expecting. Brian Wood and I, along with his wife, Carla, and our field assistant Herieth, had just finished setting up our tents under low acacia trees near a Hadza camp in the rambling, arid flat that separates Lake Eyasi from the rocky Tli’ika Hills. Brian and I were relaxing on the dusty ground in camping chairs, chatting about work in the gray, late afternoon light. Two Hadza men, Bagayo and Giga, were sitting on the ground nearby, having what sounded like a heated discussion in Hadza. They had a small battery-powered radio, a prized possession in Hadzaland, where the entertainment options are limited. At some point they decided to bring us into the conversation, switching to Swahili to ask their question. But Brian and I must have both looked bewildered, because Bagayo asked again.
“How does music get inside the radio?”
Shit, we should know this . . .
Exposure to new ideas and knowledge is one of the best things about travel, and with the Hadza it’s always a two-way street. Their deep understanding of the natural world is mind-blowing. A typical Hadza kid can tell you about the physical characteristics and behavioral tendencies of dozens of animal species, and tell you about the uses—food, fire, housing, tools—of every shrub, grass, and tree on the landscape. Watching a Hadza man track a wounded impala for miles without any obvious signs, or a Hadza woman determine the size and ripeness of a wild tuber three feet below the surface by tapping the ground with a rock, feels like nothing short of magic.
For our part, we share what we know about the outside world. We share our books and gadgets, and occasionally hold movie nights, playing nature documentaries or action movies on our laptops (the Jurassic Park films are perennial favorites). The innate curiosity that we’re all born with, the lifeblood of any scientist, seems to be well-nurtured in Hadza culture. They want to know.
Conversations usually start off innocently enough, but can develop into far-reaching discourses on geography, cosmology, or biology. “How long would it take to walk to your house?” is a simple enough question, but a real answer requires a discussion about the Earth being both round and unimaginably big, with huge continents separated by massive oceans (they were familiar with those concepts but remained noncommittal). “Are walruses real?” [And if so, what the hell are they?] is another fair question, particularly if you’ve just watched a documentary on Arctic wildlife and are unfamiliar with ice, oceans, or marine mammals. I tried to explain that walruses were, in fact, real (though admittedly absurd) creatures, like hippopotamuses with tusks of an elephant and feet like a fish. I’m not sure anyone believed me.
There’s a great quote of uncertain provenance, often attributed to Einstein, that “if you can’t explain something simply, you don’t really understand it.” Discussions with the Hadza brought this to life. Between the limits of my Swahili and their lack of formal schooling, it was always a fun challenge explaining how different research equipment worked, how the dinosaurs in Jurassic Park were created by computer, or what a blood pressure cuff measures. It often exposed gaps in my own understanding of which I hadn’t been aware. They’d been hidden, papered over in my mind with empty jargon that sounded smart but didn’t hold any real meaning for me.
Come to think of it, how did music get inside the radio?
Tentatively, I started in. In Arusha, the nearest big town (which all Hadza knew about, even though few had ever ventured that far), there was a building. Inside, a person played music from a tape or a record. (So far, so good. They’d seen tape players.) Now, the building had a machine that listened to the music and sent it through the air, from an antenna—a big metal pole. The radio, with its own antenna, captured the music out of the air and played it through the speaker.
“OK. But what is sent through the air from the building in Arusha, all the way out here?”
“Uh, radio waves,” I answered, knowing immediately that I was in trouble.
“OK . . . What are radio waves?”
Good question. “Well, they travel through the air invisibly, and you can’t hear them, but they carry the music . . .” I trailed off. I had no idea how to describe radio waves, because I didn’t really understand them myself. In my mind they weren’t much more than the little arcs emanating from an antenna in some cartoon. I knew they were a type of “electromagnetic energy,” but that was just another piece of empty jargon. It was like light, right? But how would I explain invisible light emanating from a metal pole, carrying music? Was that even an accurate way to describe it?
“Ah!” says Bagayo, picking up his hunting bow. “It’s like this,” and he plucks the string of the bow. The sound travels invisibly through the air, from the bowstring to our ears. Great analogy! Yes, that’s exactly the sort of thing we’re talking about here! I knew sound waves and radio waves were different things, but I also knew I couldn’t do better than Bagayo at explaining them.
Giga and Bagayo are satisfied. Brian and I are off the hook.
The next time we’re in town to resupply, I google “radio waves.”
Demystifying Metabolism
If we’re going to discuss the cutting-edge science in human metabolism we’ll need to have a real understanding of what metabolism is and how it works—certainly a better understanding than the typical biologist has of radio waves. No placeholders, not too much jargon, and zero bullshit. Let’s start at the beginning.
Metabolism is a broad term that covers all of the work your cells do. The vast majority of this work involves pumping molecules in or out of cell membranes (their walls) and converting one kind of molecule into another. Your body is a walking, sloshing bucket of thousands of molecules interacting—enzymes, hormones, neurotransmitters, DNA, and more—and hardly any of it comes in its usable form directly from your diet. Instead, cells are constantly bringing nutrients and other useful molecules circulating in the bloodstream in through their walls for use as fuel or building blocks, converting those molecules to something else, and then pushing the stuff they’ve built out of their walls to be used elsewhere in the body. Cells in the ovaries pull cholesterol molecules inside, build estrogen out of them, and then push the estrogen—a hormone with effects all over the body—out into the bloodstream. Nerves and neurons are constantly pumping ions (positively or negatively charged molecules) in and out to maintain a negative internal charge. Pancreas cells, guided by DNA, assemble insulin and a long list of digestive enzymes from amino acids. The list goes on and on. The amount of metabolic work happening right now in your body is staggering.
All of this work requires energy. In fact, work is energy. We measure work and energy using the same units and can talk about them interchangeably. Throw a baseball, and its kinetic energy as it leaves your hand is, by definition, exactly equal to the amount of work you did to accelerate it. Heat is another common form of energy. Microwave a cup of milk to warm it for your kid, and the increase in temperature tells you how much electromagnetic energy was captured by the milk. The energy released from burning gasoline is equal to the work done to move the car along the road plus the heat generated by the engine. Energy consumed is always equal to the combination of work done and heat gained, whether we’re talking about your body, your car, or your smartphone. We all play by the same laws of physics.
Energy can also be stored in things that have the potential to do work or create heat, like the gasoline in a fuel tank. A stretched rubber band or the spring of a mousetrap set to go off has strain energy. A bowling ball set precariously on a high shelf, one that could crash to the floor, has potential energy. The bonds that hold molecules together can store chemical energy, which gets released when the molecules break apart. When the molecules in a pound of nitroglycerin (chemical formula: 4C3H5N3O9) are broken into nitrogen (N2), water (H2O), carbon monoxide (CO), and oxygen (O2) during detonation, it violently releases enough energy (730 kilocalories) to launch a 165-pound man two and a half miles straight up into the sky (which would be work) or vaporize him (which would be heat), or some combination of the two. This brings us to our last point about energy: it can be converted among its many forms—kinetic energy, heat, work, chemical energy, and so on—but it can never be lost.
Calories and joules are the two standard units used to measure energy, whether it’s the chemical energy stored in food, the heat from a fire, or the work done by a machine. Calories are most common in the United States when discussing food, but we’ve managed to muck up the standard usage. One calorie is defined as the energy needed to raise the temperature of one milliliter of water (one-fifth of a teaspoon) by one degree Celsius (1.8 degrees Fahrenheit). It’s a tiny amount of energy—too small to be a useful unit of measure when we talk about food (like road signs giving driving distances in inches). Instead, when we talk about “calories” in food, we’re actually talking about kilocalories, or 1,000 calories. A cup of dry Cheerios has 100 calories according to the nutrition label on the box, but they actually mean 100 kilocalories, or 100,000 calories.
So why don’t we just say “kilocalories” or “kcal” instead of abusing the term “calorie”? Bizarrely, in the late 1800s, when scientists were deciding to adopt “calories” as the preferred unit of measure for food energy, the influential and pioneering American nutritionist Wilbur Atwater decided to stick with an early, arcane convention and simply capitalize “Calories” when referring to kilocalories. That’s about as sensible as capitalizing “Yards” to refer to miles. We’ve been stuck with the confusing use of calories (or Calories) on our food labels ever since. Of course, this is just one more entry in the long, embarrassing history of measurement in the United States. A country that insists on using teaspoons, inches, and Fahrenheit obviously has deep psychological issues about discussing their units. (By the way, if you’re traveling in the civilized world and want to convert joules on their food labels to calories, divide joules by four.)
Since work and energy are two sides of the same coin, we can think about all the work that our cells do and all the energy they consume as two ways of measuring the same thing. We can use “metabolism” and “energy expenditure” interchangeably. That’s why evolutionary biologists like me, as well as doctors and people in public health, are so fixated on energy expenditure, which is how we measure metabolism: it is the fundamental measure of the body’s activity. The speed with which a cell does its work determines metabolic rate, the energy used per minute. Add up the work of all the cells in your body and you’ve got your body’s metabolic rate, the energy you expend each minute. Your metabolic rate is the full force of your cellular orchestra, 37 trillion microscopic musicians, blending together in an intricate symphony.
The sophisticated metabolic system that sustains us, and that we all take for granted, is a marvel of evolution. It took nearly a billion years—untold trillions of generations, quadrillions of false starts and dead ends—for the basic framework of today’s simplest single-cell metabolic systems to evolve on this planet, an eternity of trial and (mostly) error. It took another two billion years for the simplest multicelled organisms, with their integrated metabolic systems and divisions of labor, to evolve. Along the way, life had to confront some major challenges in basic chemistry. Oils had to mix with water. Oxygen, a chemical that burns and kills, had to be harnessed for life. Fats and sugars, holding more energy per gram than nitroglycerin, had to be burned carefully for fuel without blowing organisms up or boiling them alive.
That’s not even the strangest part. All the work our bodies do is powered by microscopic alien life-forms called mitochondria, living within your cells. Mitochondria have their own DNA and their own two-billion-year evolutionary history, including saving all life on Earth from certain doom. And much of the work done to digest your food into usable bits is done by a vast ecosystem that lives in your gut. This microbiome is made of trillions of bacteria that make their home all along your digestive tract, the long and serpentine passageway that connects your mouth to your butt.
We are all walking chimeras, part human and part other, performing the ordinary miracle of turning dead food into living people every day without a moment’s thought. It’s a story you’ve probably heard before, but likely with the magic boiled out of it and served cold from a textbook. It’s well worth another listen. If nothing else, it’s the essential foundation you’ll need to understand how diet affects your health and how your body burns energy—how life actually works.
Soylent Green Really Is People (or It Could Be)
Going at least as far back as the ancient Greeks and as recently as the 1600s, people—including very smart people like Aristotle—thought that flies, mice, and other organisms could grow spontaneously from inanimate objects like dirt and rotten meat. It made sense: one day there was a pile of old rags and some hay in the corner of the barn, the next day there were mice. Maggots seemed to explode out of old carcasses without anyone or anything putting them in there. Without a good grasp of the microscopic world or rigorous experimentation, people found it a hard idea to disprove. It didn’t fully die until Louis Pasteur’s breakthrough experiment in 1859, boiling broth and showing that nothing would grow in it if you kept out dust and bugs (we’ve been pasteurizing our food ever since). Today, the idea of “spontaneous generation” is taught to schoolkids as a classic example of how benighted people used to be and how far science has come.
It’s absurd, of course, to suggest that flies could appear spontaneously from a dead corpse. But as we’ve come to understand over the past century of scientific research on metabolism, the truth is even stranger. Animals, plants, and all other living things are essentially “spontaneous generation machines,” assembling their bodies and those of their offspring from food, water, and air. What is a fly, after all, except a little machine that builds baby flies out of rotten meat?
In the classic and campy Soylent Green, a 1973 sci-fi movie set in a dystopian future New York City, Charlton Heston’s character is appalled to discover that the green mush that everyone eats is actually made from humans. He’s carried off in the final dramatic scene, shouting to anyone who will listen, “Soylent Green is people!” Fast-forward to 2018, and in an example of life capitalizing on art, you can buy Soylent brand food mixes, gloopy tubes of nutrients that are meant to replace normal food for people on the go or without lunch friends. I have no idea how they taste, but there is a Soylent Green variety. Now, I’m pretty sure that the Soylent Green you buy online these days isn’t people. But here’s the thing: it could be. All you have to do is eat it.
Every molecule in your body, every pound of bone and muscle, every ounce of brain and kidney, every fingernail and eyelash, all six quarts of blood squirting around your vessels, all of it is made of reassembled bits of food you’ve eaten. The energy that keeps you moving and keeps you alive comes from your diet as well. You are what you eat isn’t just a well-worn cliché, it’s how life actually works. One shudders to think about the sizable proportion of Americans who are literally walking, talking, reformulated Big Macs. My kids are built and powered almost entirely from chicken nuggets, pasta, yogurt, and carrots. I myself am fueled largely by pretzels and beer. How does it all work?
Follow the Pizza
Let’s start with lunch. You’re sitting down to a hot, glistening slice of pepperoni pizza (vegans may substitute meat and cheese alternatives into this thought experiment). You take a bite and begin to chew, a luxurious mélange of bread, sauce, meat, and cheese dancing across your taste buds, the crust fighting against your teeth, the smell wafting up the back of your palate and filling your nose. It is transcendent.
The alchemy has begun. Chewing and mixing the food with saliva is the first step in digesting your meal and its main constituent parts, the macronutrients. There are three macronutrient categories: carbohydrates, fats, and proteins. Carbohydrates are starches, sugars, and fiber. They come mostly from the plant-based portions of your food—the crust and tomato sauce in the pizza you’re eating. Fats (which include oils) come from both plant and animal sources, like the cheese and pepperoni in your slice. Proteins come mostly from animal tissue and the leaves, stems, and seeds of plants (including beans, nuts, and grains). The pepperoni and cheese are full of protein, as are the basil leaves scattered atop your slice. There’s protein in the crust as well, including the much maligned gluten that makes it chewy.
There’s also water trapped in the slice, as well as trace amounts of other stuff like minerals, vitamins, and other elements that your body needs. But the macronutrients—carbohydrates, fats, and proteins—are the main attraction. They are what build and power your body. They are the raw materials of metabolism.
The flow chart in Figure 2.1 shows where the carbohydrates, fats, and proteins from your food go in your body and what they do. Think of it as a subway map for macronutrients—a challenge to read at first, but easy enough once you follow each line from origin to destination. Each macronutrient has its own line, and each line makes three stops: Digest, Build, and Burn. Like any good transit system, there are side branches that can take you from one line to another. Off we go!
Carbohydrates
If you eat a typical American diet, carbohydrates account for about half of the calories you consume each day. In fact, despite the recent popularity of low-carb diets, humans across cultures and around the globe, including hunter-gatherers like the Hadza, typically get more calories from carbohydrates than from fats or proteins (Chapter 6). We’re primates, after all, and primates eat plants—especially ripe, sweet fruits. Carbohydrates are our main source of fuel, and we have a 65-million-year history of relying on them.
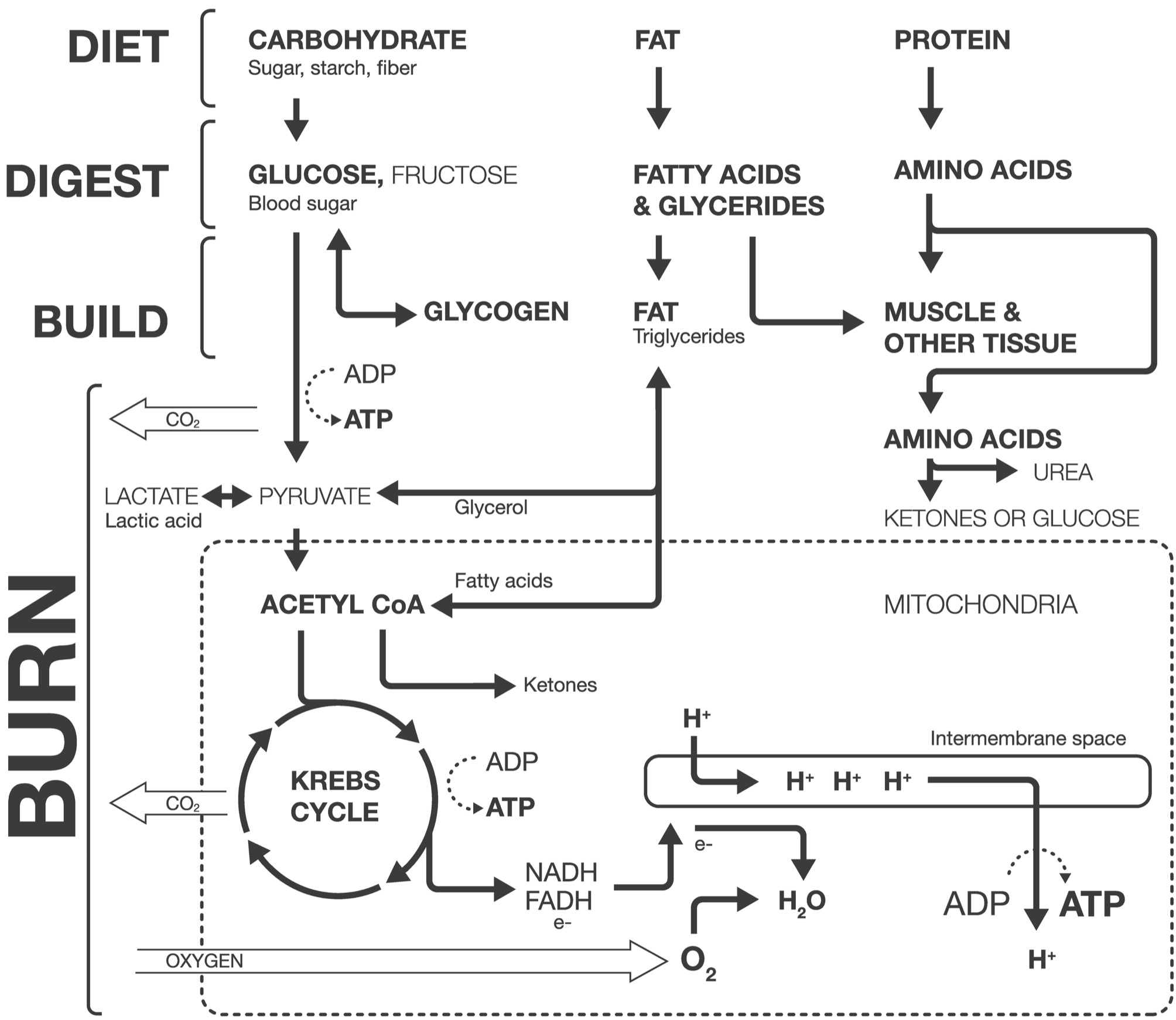
Figure 2.1. Subway Map for Macronutrients. Each macronutrient (carbohydrate, fat, protein) has its own pathway in the body, and each makes three major stops: Digest, Build, and Burn. Single-headed arrows indicate one-way paths. Paths with double-headed arrows run both directions. Some pathways are omitted for clarity. Fiber digestion by the microbiome produces fatty acids that would join the Fat pathway. Sugars are used to build some structures in the body, such as DNA. The many pathways by which amino acids can be converted to glucose or ketones aren’t shown. Galactose, the least common product of carbohydrate digestion, is also omitted. e-: electrons. H+: hydrogen ions.
Carbohydrates come in three basic forms: sugars, starches, and fiber. Sugars and starches are digested and either used to build glycogen stores or burned for energy (see above). They can also be converted into fat, as we’ll see below. Fiber is a different beast, with an important role in the gut regulating the digestion and absorption of sugars and starches, and feeding the trillions of bacteria and other critters in our intestinal microbiome. In fact, the microbiome plays an essential role in digesting fiber, and without it we’re in trouble. But first, let’s follow the starches and sugars.
Sugars are just small carbohydrates—little chains of carbon, hydrogen, and oxygen atoms. The smallest are just one sugar molecule big (hence the mono in their technical name, monosaccharides; saccharide just means sugar). The monosaccharides are glucose, fructose, and galactose. The other sugars—sucrose, lactose, and maltose—consist of two monosaccharides stuck together and are called disaccharides (“two sugars”). Sucrose (table sugar) is just a glucose and fructose bound together. Lactose (milk sugar) is glucose and galactose. Maltose is two glucoses.
Starches are simply a bunch of sugar molecules strung together in a long chain. Because there are so many sugar molecules stuck together, starches are also called polysaccharides (“poly” meaning many) or complex carbohydrates. By far the most common sugar molecule in plant starch is glucose, and plant starch molecules can be hundreds of glucose molecules long. Starch is how plants store energy, which is why it’s in huge supply in energy storage organs of plants like potatoes and yams. Nearly all plant starch (the starch in our food) is a mix of just two polysaccharides, called amylose and amylopectin.
No matter what foods they come from, starches and sugars all get digested into one of the three monosaccharides. Starch digestion starts in your mouth, with an enzyme in your saliva called amylase, which begins the process of breaking long amylose and amylopectin molecules down into smaller and smaller pieces. Enzymes are proteins that break apart molecules or promote chemical reactions (their names usually end in -ase). Digestive enzymes like amylase chop food molecules into smaller and smaller bits. Starches have been so important in human evolution that we’ve evolved to make more amylase than any of the other apes, something we’ll discuss in Chapter 6.
After you swallow, the mushy bolus of food ends up in your stomach, where the acid kills bacteria and other nasties that have hitched a ride in your food. After that, the food is pushed out of your stomach and into the small intestine, where most of the digestive work takes place. In the small intestine, the starches and sugars are hit with enzymes produced by the intestine and pancreas to break them down further. The pancreas, an organ about five inches long and the shape of a skinny chili pepper, sits just beneath the stomach and is attached to the small intestine with a short duct. It’s most famous for its production of insulin, but the pancreas also produces most of the several dozen enzymes used in digestion (along with bicarbonate, which neutralizes the stomach acid as it enters the intestine). The assembly of these enzymes (their specific shape and makeup) and the levels of production (whether to make a lot or a little of a particular enzyme) are controlled by your genes. For example, if you’re lactose intolerant and can’t digest milk, that means your genes have shut down the assembly and production of the enzyme lactase, which is needed to break the disaccharide lactose into glucose and galactose. No other enzyme can do that job, so the lactose heads intact to the large intestine, sending the bacteria there into a feeding frenzy that produces a lot of gas and all the other lovely side effects of milk intolerance.
Starch and sugar digestion continues until all the polysaccharides and disaccharides are broken down into monosaccharides. Since much of the carbohydrate in your diet comes from starch, and starch is made entirely from glucose, about 80 percent of the starches and sugars that you eat end up as glucose. The rest is broken down to fructose (about 15 percent) or galactose (about 5 percent). Of course, if you eat a diet high in processed foods full of sugar (i.e., sucrose, which is glucose plus fructose) or high-fructose corn syrup (which is about 50 percent fructose and 50 percent glucose mixed with water), the percentage of fructose might be a bit higher for you, and the percentage of glucose a bit lower.
These sugars are absorbed through the intestinal wall and into the bloodstream. The walls of our intestines are chock-full of blood vessels, and blood flow to our guts more than doubles after a meal to carry away nutrients. The result is the familiar rise in blood sugar (almost all glucose) after a meal, particularly one high in carbs. If the food you eat is processed, low in fiber, and easily digested, the carbs are digested quickly and the sugars rush into the bloodstream, creating a huge, steep spike in blood sugar. Those foods are said to have a high glycemic index, which is the rise in blood glucose measured two hours after ingesting a particular food, relative to the rise you’d experience from eating pure glucose. Foods that are harder to digest (more complex carbohydrates, fewer sugars, more fiber) take longer to digest and absorb, resulting in a long, low rise in blood sugar—and a low glycemic index. We’ll discuss diets in Chapter 6, but there’s some evidence that low glycemic index foods might be better for you.
The unsung heroes in all this digestive work are dietary fiber and your microbiome. Fiber is a class of carbohydrate (there are many varieties of fiber) that our bodies can’t digest—at least, not on their own. These tough, stringy molecules are what give plant parts their strength and structure. Fiber from our food covers the intestinal walls like a wet knit blanket, forming a lattice-like filter that slows the absorption of sugars and other nutrients into the bloodstream. That’s why the glycemic index—the rush of sugar into the blood—is about 25 percent higher for orange juice, which doesn’t have much fiber, compared to a piece of orange, which does.
Fiber also feeds our microbiome, the steamy ecosystem of organisms that live in our guts and help us digest food. Most of the microbiome lives in the large intestine, or colon, where it plays a critical role dealing with fiber and all the other stuff we can’t digest in the small intestine. We are only beginning to appreciate the importance of the microbiome, but the scale is stunning. With trillions of bacteria, each with their own thousands of genes, the microbiome is like a four-pound superorganism living inside of you. These bacteria digest much of the fiber we eat, using enzymes our own cells can’t make and producing short-chain fatty acids that our cells absorb and use for energy. Our microbiome also digests other stuff that escapes the small intestine, aids in immune system activity, helps produce vitamins and other essential nutrients, and keeps the digestive tract running properly. The effects on our health, from obesity to autoimmune diseases, are wide-ranging, and new discoveries are happening every day. What we know for sure at this point is, if your microbiome isn’t happy, you aren’t happy.
The main reason we eat and crave carbohydrates, their reason for existence as far as our cells are concerned, is to power our bodies. Carbs are energy. Once the sugars are absorbed in your bloodstream, there are only two places for them to go—they can be burned now or stored for later (Figure 2.1). This is where the hormone insulin, produced by the pancreas, comes in. Some cells use insulin to get glucose molecules inside of them, through their membranes.
Burning carbohydrates for energy is a two-stage process that we’ll discuss in detail below. Blood sugar that isn’t burned immediately is packed away into glycogen stores in your muscles and liver. Glycogen is a complex carbohydrate similar to plant starch. It’s easy to tap into when energy is needed, but relatively heavy because it holds an equal proportion of carbon and water (hence the term “carbohydrate”). It’s like canned soup: quick to prepare, but heavy and bulky because it’s stored with water. Humans, like other animals, have evolved hard limits on the amount of glycogen our bodies can hold. Once those buckets are full, blood sugar has to go somewhere else. And the only place left to go is fat.
When your body’s energy needs are met and your glycogen stores are full, the excess sugar in your blood is converted to fat, as we’ll discuss below. Fat stores are a bit more difficult to use for fuel—there are more intermediate steps to convert them to a burnable form. But fat is a much more efficient way to store energy than glycogen, because it’s energy dense and doesn’t hold water. And as we know all too well, there’s virtually no limit to how much fat the human body is able to store.
Fats
Fats have a fairly simple itinerary—they are digested down into fatty acids and glycerides and then built back up into fat in your body, which is eventually burned for energy. The challenge, though, is that fats are hard to digest. It comes down to basic, familiar chemistry: oil and water don’t mix. Fats (including oils) are all hydrophobic molecules, which means they won’t dissolve in water. But like all life on Earth, our body’s systems are water-based. Breaking big globs of fat down into microscopic bits isn’t possible with just water—it’s like trying to clean a greasy pan without soap. The evolutionary solution? Bile.
Bile was long thought to play a role in our moods and temperament as one of the four humors, a fun example of clever people believing dumb things. Very smart people, from Hippocrates all the way through doctors and physiologists of the 1700s, thought that too much yellow bile made people aggressive. Doctors would bleed people with leeches if they were suspected of being out of humoral balance, which is one reason that doctors probably killed more people than they saved until the advent of modern medicine a century or so ago. Today we know that bile is the stuff that makes fat digestion work.
Bile is a green juice produced by your liver and stored in your gall bladder, which is a small, thumb-sized pouch that sits between the liver and small intestine, connected to both with short ducts. When fats enter the small intestine from the stomach, the gall bladder squirts a bit of bile into the mush of food. Bile acids (also called bile salts) act like detergents, breaking up the globs of fat and oil into tiny emulsion droplets. Once the fat is emulsified, enzymes called “lipases,” produced by the pancreas, are added to the mix and break these emulsion droplets down to an even smaller size, to microscopic droplets called micelles, just a hundredth the diameter of human hair. These micelles form, break apart, and form again like the bubbles in a fizzy drink. Each time they break apart, they release the individual fatty acids and glycerides (which are fatty acids attached to a glycerol molecule) they were holding, the basic building blocks of fats and oils.
Fatty acids and glycerides are absorbed into the intestinal wall and re-formed into triglycerides (three fatty acids attached like streamers to a glycerol molecule), the standard form of fats in the body. Here the body confronts the next challenge of digesting fats: because they don’t mix well with water, they tend to clump together in water-based solutions like blood. Lumpy blood would kill you, clogging up the small vessels in your brain, lungs, and other organs. The evolved solution is to pack triglycerides into spherical containers called chylomicrons. This keeps the fats from clumping together, but results in a package too big to be absorbed through capillary walls and into the bloodstream, where they need to go for distribution throughout the body.
Instead, the fat molecules, packed in chylomicrons, are dumped into the lymphatic vessels. Part surveillance system, part garbage collection, the lymphatic vessels have their own network throughout your body, picking up debris, bacteria, and other detritus and bringing it to the lymph nodes, spleen, and other immune system organs to be dealt with. It’s well suited to pick up big particles like chylomicrons stuffed with fat. The lymphatic vessels also collect all the plasma that leaks out of your blood vessels (about three quarts a day) and returns it to your circulatory system, so it offers a port of entry into the bloodstream. Specialized lymph vessels called lacteals, embedded in the intestinal wall, pull chylomicrons into the lymph system and then dump them directly into the circulatory system, just upstream of your heart.
White, fat-filled chylomicrons are so big, and so plentiful after a fatty meal, that they can give the blood a creamy hue. Eventually, though, they are ripped apart and their contents pulled into waiting cells for storage or use. Lipoprotein lipase enzymes in the blood vessel walls first break the triglycerides into fatty acids and glycerol, which are pulled into waiting cells by aptly named fatty acid transporter molecules before being reassembled into triglycerides. Most fat is stored in fat cells (adipocytes) and muscles, forming a reserve fuel tank. These stored triglycerides are the fat that we feel in our belly and thighs, or see marbled into a nice cut of steak. Problems arise when our bodies start storing substantial amounts of fat in our liver and other organs, which can lead to liver failure and a range of other health issues. The causes of fatty liver aren’t always clear, but obesity is a major risk factor.
A small proportion of the fats we eat are used to build structures like cell membranes, the myelin sheaths that coat our nerves, and parts of our brain. Some of the fatty acids needed to build these tissues can’t be made by reformulating others, and so are considered essential fatty acids—you need to get them from the food you eat. That’s why food producers often tout the omega-3 fatty acid (an essential fatty acid) content in their fish, milk, or eggs.
Like carbohydrates, the ultimate destination for fat—the reason you crave it and the reason your body goes to all the trouble to digest and store it—is to burn it as fuel. All animals are evolved to store energy as fat because it holds an incredible amount of energy in a small package, 255 kilocalories per ounce. That’s on par with jet fuel, more than five times the energy density of nitroglycerin, and nearly a hundred times better than a typical alkaline battery. Happily, the process of breaking down fats for energy is a slower process than exploding dynamite. Some fats are burned immediately after digestion, fresh from your gut. But most of the time, between meals, your body draws on stored fats for fuel. The triglycerides that make up your stored fat are broken down into fatty acids and glycerol and used to make energy (Figure 2.1), something we’ll see in more detail below.
Proteins
Proteins have an interesting itinerary. Unlike fats and carbohydrates, proteins aren’t a primary source of energy (unless you’re a carnivore). The main role of protein is to build and rebuild your muscles and other tissues as they break down each day. Your body does burn protein for energy, but it’s a small contributor to your daily energy budget.
Protein digestion begins in the stomach with an enzyme called pepsin, which starts breaking proteins apart. The cells within your stomach wall make an enzyme precursor called pepsinogen, which is converted by the stomach acid into the enzyme pepsin, which then gets all Edward Scissorhands on any proteins it comes in contact with, chopping them up. This process continues in the small intestine as food leaves the stomach, with enzymes secreted by the pancreas.
All proteins get digested down to their basic building blocks: amino acids. Amino acids are a class of molecules shaped a bit like a kite—a head attached to a tail. They all have the same head: a nitrogen-containing amine group connected to a carboxyl acid. Amino acids are distinguished by their tails, which are always some configuration of carbon, hydrogen, and oxygen atoms. There are hundreds of amino acids on Earth, but only twenty-one are used to build proteins in living plants and animals. Nine of these are considered essential for humans, meaning our bodies can’t make them on their own; we need to get them from our diet (don’t worry—if you haven’t died yet, you’re getting them). The others your body can make by itself if needed, usually by breaking down and reformulating other amino acids. But we’re getting ahead of ourselves.
The next stop for amino acids is to build the tissues and other stuff that make up the human machine (Figure 2.1). Once the proteins from our pizza slice are digested into amino acids, they are absorbed through the walls of the small intestine and into the bloodstream. From the blood, the amino acids are pulled into cells to construct proteins, which are chains of amino acids strung together. The construction of proteins from amino acids is one of the primary jobs of DNA. A gene is just a stretch of DNA that lines up a particular sequence of amino acids to make a protein (some genes are regulatory, meaning they don’t assemble proteins themselves but instead activate or suppress protein-assembling genes). Variants in DNA sequence (the string of As, Ts, Cs, and Gs) can result in different amino acid lineups and thus slightly different proteins, contributing to biological differences among individuals. Amino acids are also used to make a variety of other molecules like epinephrine, the fight-or-flight hormone; and serotonin, one of the neurotransmitters our brain cells use to communicate.
These same tissues and molecules break down over time. They are eventually converted back into amino acids, and travel through the bloodstream to the liver. There, things get a little tricky. The amine group in the amino acid has a very similar structure, NH2, to ammonia, NH3 (notice the similarity in the names as well, amine and ammonia). In the same way that drinking ammonia-based household cleaner would surely kill you, accumulating ammonia from breaking down amino acids would be fatal. Happily, we have an evolved mechanism to convert that ammonia to urea, which then travels via the bloodstream to the kidneys to be excreted in the urine. It’s the urea in our pee that lends it that zesty eye-watering smell, which makes sense because it’s made from ammonia.
We pee out the equivalent of fifty grams (about two ounces) of protein each day. Exercise adds to that total by increasing muscle breakdown. We have to eat enough protein to replace what we lose each day, lest we find ourselves in protein deficit. If we eat more protein than we need, the extra amino acids are converted to urea and cleared out by the urine. This can make for some very expensive pee if you overdo it on the protein supplements.
The last stop on the protein train line is to burn the amino acids for fuel (Figure 2.1). After the nitrogen-containing head is chopped off, converted to urea, and sent on its way, the tails are used to make glucose (a process called gluconeogenesis, which literally means “making new sugar”) or ketones, both of which can be used for energy, as we’ll see below. Proteins are typically a minor part of the daily energy budget, providing around 15 percent of our calories each day. But they are a vitally important emergency energy supply if we’re starving, a bit like setting the furniture on fire to heat your house. The skeletal figures of concentration camp victims are a terrifying example of this process taken to the extreme, their bodies consuming themselves in a desperate effort to stay alive.
Burn, Baby, Burn
All roads on our metabolic train map lead, eventually, to one place: fuel. Carbs, fats, and proteins all hold stored chemical energy in the bonds that hold their molecules together. Breaking those bonds apart releases that energy, the energy we use to power our bodies.
In all biological systems, including our bodies, energy has one fundamental, common form: adenosine triphosphate, ATP. ATP molecules are like microscopic rechargeable batteries, which are “charged” by adding a phosphate molecule onto a molecule of adenosine diphosphate, ADP (note the “tri” versus the “di” in their names, indicating three phosphates on ATP versus two on ADP). A gram of ATP holds about fifteen calories of energy (that’s calories, not kilocalories), and the human body only holds about fifty grams of ATP at any given time. That means each molecule cycles from ADP to ATP and back over three thousand times per day to power our body. Burning carbs, fat, and protein, then, is the process of transferring the chemical energy in the sugar, fat, and amino acid molecules to the chemical bond that holds the third phosphate onto molecules of ATP. When we use food to make energy, what we’re making is ATP.
Let’s start with one molecule of glucose, the predominant form of sugar that our bodies use for energy (the story is essentially the same for fructose and galactose). This glucose molecule might come directly from carbs we just ate, or might come from stored glycogen that’s been reconverted to glucose. As we began to discuss at the end of the section on carbohydrates, burning sugars for energy is a two-stage process. First, glucose (C6H12O6) is converted to a molecule called pyruvate (C3H4O3) in a ten-step process that is powered by two ATP molecules but produces four ATP molecules, resulting in a net gain of two ATP. It’s a relatively fast process, and it’s what we use to power short bursts of activity like a 100-meter sprint or a powerlift at the gym.
This first stage of metabolism is called anaerobic because it doesn’t require oxygen, which you can appreciate when you watch the Olympics on TV: elite sprinters hardly seem to breathe at all, and powerlifters hold their breath. If there’s not enough oxygen present, either because we’re not breathing effectively or (more likely) because our muscles are working too hard, too fast for oxygen supply to keep pace with all of the pyruvate being produced, the pyruvate gets converted to lactate. Lactate can be reconverted to pyruvate to be used for fuel, but if it builds up, it can also become the dreaded lactic acid, which makes our muscles burn when we’re working hard and pushing our limits.
The second stage, aerobic metabolism, is where we need oxygen. If there’s sufficient oxygen in the cell, the pyruvate produced at the end of the first stage is pulled into a chamber within the cell called the mitochondria. There are dozens of mitochondria in a typical cell, and they are known as the “powerhouse of the cell” because the bulk of ATP production happens within them. This is where the magic happens, the chemical choreography that keeps us alive.
In the mitochondria, pyruvate is converted to acetyl coenzyme A, or acetyl CoA, which would vie with ATP for the title of the most important chemical you’ve probably never heard of or have completely forgotten. Acetyl CoA is like a train car full of passengers—carbon, hydrogen, and oxygen atoms—without an engine to pull it. Along comes oxaloacetate, which is hitched to acetyl CoA and begins to pull it along a circular track called the Krebs cycle. The train will make eight stops, and at each, some carbon, hydrogen, and oxygen passengers get on or off. The coming and going of those atoms generates two ATP. By the final stop, only the oxaloacetate engine is left. It’s hitched up to another acetyl CoA and the cycle repeats.
Importantly, some of the passengers are robbed as they get on and off the Krebs cycle train, their electrons stolen away by the molecules NADH and FADH. These NADH and FADH molecules scurry away to the back alleys of the mitochondria and unload their purloined electrons into a special receptor complex in the membrane—a door in the wall. Mitochondria are double-walled structures, like a thermos; there’s a small space between the inner and outer membranes called the intermembrane space. When the stolen electrons are deposited into the inner membrane complex, positively charged hydrogen ions (which are in plentiful supply) chase the negatively charged electrons and end up trapped in the intermembrane space. The hydrogen ions are like fish caught by a weir: they flow through the inner membrane, pulled by the electrons, only to find themselves trapped and crowded in the intermembrane space.
With all the positively charged hydrogen ions packed together, there is an electrochemical force pushing them out to balance the charge on either side of the inner membrane. But there’s only one way for the hydrogen ions to escape the inner membrane space: a special portal in the inner membrane that’s built like a turnstile. The hydrogen ions stream through the turnstile, driven by the electrical charge. As the turnstile spins, it forces together ADP and phosphate molecules, making ATP. This is the real moneymaker, producing thirty-two ATP. The complex choreography of electrons and hydrogen ions dancing along the inner membrane, called oxidative phosphorylation, is the primary energy generator that powers your body.
And what becomes of the glucose molecule itself, the carbon, oxygen, and hydrogen atoms that we started with at the beginning? Remember that it’s the energy in the bonds holding these atoms together that we use to charge our ATP, not the atoms themselves. Instead, the carbon and oxygen atoms, which make up 93 percent of the mass of a glucose molecule, are converted to carbon dioxide (CO2) in the conversion of glucose to pyruvate and in the Krebs cycle. The hydrogens bind with oxygen at the end of oxidative phosphorylation, forming water, H2O. We eat carbohydrates only to breathe them out, filling the air around us with the skeletons of potatoes past; a remaining fraction ends up as drops of water in the ocean of our body.
Burning Fat, Getting Fat, and Going Keto
We use the exact same steps of aerobic respiration to burn fat. Instead of starting with a glucose molecule, we start with a triglyceride molecule. It might be fresh from the pizza we just ate, packaged in a chylomicron, or newly released from our copious body fat stores. Regardless of their source, triglycerides are broken into fatty acids and glycerol and converted to acetyl CoA (glycerol is transformed to pyruvate first; Figure 2.1). And just like glucose, the atoms of carbon, oxygen, and hydrogen that make up those fatty acids and glycerols are exhaled as CO2 or formed into water. Aside from the small proportion that’s converted to water, the fat you burn leaves your body by air, excreted by your lungs. You exhale your food.
If we’re burning a lot of fat, whether we’re on an extremely low-carb diet or starving, some of the acetyl CoA generated will be converted to molecules called ketones. Most ketone production occurs in the liver. Ketones are sort of a traveling version of acetyl CoA, and can travel in the bloodstream to other cells, be reconverted to acetyl CoA, and used to generate ATP. Like a lot of metabolic conversion, most ketone production is done in the liver, but they are used throughout the body. This is the pathway that popular ketogenic diets engage, promoting a system of eating all fats and proteins and almost no carbohydrates. With the carbohydrate train line essentially shut down, all traffic shifts to the fat and protein pathways.
Because ketones travel in the blood, they show up in your pee. The curious and bored can buy test strips over the counter at most pharmacies. The presence of ketones in urine signals that the body is in “ketogenesis,” and depending heavily on fat for energy.
Once you’re familiar with the fat and glucose pathways in Figure 2.1, it’s fairly obvious why extremely low-carb, ketogenic diets like Atkins or the trendy Paleo diet (which as we’ll see in Chapter 6 isn’t Paleo at all) can lead to massive fat loss. If you consume no carbohydrates, the only way to generate acetyl CoA is by burning fat. Sure, you can also burn proteins by converting amino acids into ketones or glucose (some amino acids even form molecules that can jump into the middle of the Krebs cycle, like a kid jumping into a double-Dutch jump rope session). But protein is typically a minor player in terms of daily calories. Fat is the main fuel on a low-carb diet, and if you eat fewer calories than you burn, the deficit will be met by burning stored fat for energy. Some of this fat will be processed into ketones prior to burning. For example, the brain is a particularly picky eater and generally uses only glucose for metabolism, but if there’s no glucose available, it will switch to burning ketones.
The dark side of converting fats to energy is that the tracks run both ways. As you see in Figure 2.1, a sugar molecule (glucose or fructose) can be converted to acetyl CoA and then jump on the fatty acid track instead of entering the Krebs cycle, and voilà! You convert the sugar into fat. It’s the same process used to convert fat into acetyl CoA, just run in reverse.
In fact, like any good, flexible transit system, our metabolic pathways are evolved to respond to traffic conditions and send molecules to their most sensible destinations.* Got more sugars than you need? Send the extra glucose and fructose to glycogen. Glycogen stores full? Send the excess sugar to acetyl CoA. If the Krebs cycle train is overcrowded because energy demands are low, start sending acetyl CoA to fat. And there’s always plenty of space available in fat. Glycogen stores fill up, and you can’t store excess protein, but there’s no limit to how much fat you can layer on.
And that’s why we should be suspicious of any diets that target one specific nutrient as a hero or a villain for weight loss. Nothing is innocent if eaten in excess. Any calories that aren’t burned, no matter if they come from starches, sugars, fats, or proteins, will wind up as extra tissue in your body. If you’re pregnant or bulking up at the gym, that extra tissue might be useful things like organs or muscle. But if you’re not, those extra calories, no matter their original dietary source, will end up as fat. That’s the foundation we need to understand to begin talking about all the real-world complexities of diet and metabolic health. We’ll talk a lot more about diets and the evidence for what works and what doesn’t in Chapters 5 and 6.
Poisoned by Plants
Is it better to live in blissful, romantic ignorance? I can certainly see the argument for it. It’s easier to face the day when you feel like Mother Nature just wants to give you a big warm hug—that the natural world and even your fellow humans are essentially good. Pain and death may be inevitable, but only because we’re clumsy, fallible, and out of tune with the guiding harmonies of the universe. If only we let go and felt the karmic flow, were generous and kind, the world would surely reciprocate. If only we could return to a state of nature, like our hunter-gatherer ancestors.
Right?
Movie night on the savanna. The entire Hadza camp is gathered in the darkness around Brian’s laptop. There’s a nature documentary on, and everyone loves it. Every time a new animal protagonist walks into the frame, chatter wells up from the crowd. Ooooohhh! Look at that wildebeest! Aw man, that’s a huge giraffe! Then it’s a nighttime scene at the edge of watering hole. Elephants have come to drink, desperate for water at the height of the dry season. But lions are lurking nearby. They pounce on a baby elephant, gnawing at the back of its neck while it runs in fear. The little elephant raises its tiny trunk and bleats its pained baby elephant cry. The crowd is rapt, myself included. Adult elephants try to run the lions off, but it’s no use. There are too many and they attack like ninjas, one after the other, drawing blood from deep wounds. Soon it’s over. A baby elephant! Oh god, the horror. Surely Nature has erred. Something this repugnant wasn’t supposed to happen.
The Hadza erupt with glee. Ha! The lions got ’em!
I’m stunned. What psychopaths root for the lions?*
Then it begins to sink in. Feeling bad for the elephants is a luxury of life in the industrialized world, experiencing nature through a television screen. To grow up in it, to live in nature every day, is to understand that it doesn’t want to snuggle. There’s no majestic drama playing itself out for the benefit of your spiritual growth. Instead, you are part of a jumbled crowd of species, some malevolent, others indifferent, none of them your friends. Hadza hate elephants because elephants are massive and ornery and occasionally kill Hadza. They view elephants with about the same affection as they view snakes, and Hadza people hate snakes.
The Hadza don’t weep for the animals they hunt and kill, any more than you cry over a cup of yogurt. They are not cynical or jaded, but they know the deal. Being part of the ecosystem means eating others, whether it’s plants or animals. The wild hunting dogs that catch your scent on the breeze and turn to follow will feel no remorse as they tear your innards out. Nothing personal, it’s just business. Understanding life in a real, functioning ecosystem requires us to abandon the romantic, Disneyesque mythologies that we’re fed growing up in our sheltered suburbias.
Understanding the world through the lens of evolution is a similarly disorienting wake-up call. What Darwin saw clearly for the first time was that species are all competing for limited resources, struggling to find food without becoming dinner. There is neither “good” nor “bad” in nature—we place those cultural assessments on an otherwise amoral and indifferent cast of characters. Even things that seem clearly done for our benefit are driven by evolutionarily selfish ulterior motives. Fruits, those gifts from the tree, heavy with sweet flesh, are simply a clever way of dispersing seeds. Dogs have evolved to prey on our emotions and make us love them because humans are a great source of dog food. And the lush green plants that fill our planet with life? They’ve been quietly poisoning us for two and half billion years.
Life requires energy, and the first fuel system to evolve on our planet was photosynthesis. The earliest bacteria to harness the sun’s energy relied on hydrogen and sulfur rather than water to make photosynthesis work. Then around bout 2.3 billion years ago, somewhere in the shallow ponds of a young, rocky Earth, a new recipe for photosynthesis evolved, converting water (H2O) and carbon dioxide (CO2) into glucose (C6H12O6) and oxygen (O2). Sunlight provided the energy needed for this conversion—energy that got stored in the molecular bonds of glucose.
This new type of photosynthesis, called oxygenic because it produces oxygen as a waste product, was a game changer. Oxygenic photosynthetic life colonized the planet, soaking up CO2 and water and spewing out oxygen. We tend to think of oxygen as a good thing, the sustainer of life, but its true chemical nature is devastating. It steals electrons and binds to other molecules, altering them completely and often tearing them apart. Oxygen is Shiva the destroyer, obliterating everything it touches either slowly (rust) or violently (fire).
At first, the new oxygen produced by plants was absorbed by the iron in dirt and rocks, creating massive, oxidized “red beds” in the Earth’s crust. Then the oceans absorbed as much oxygen as they could hold. After that, the atmosphere began to fill, climbing from 0 percent to over 20 percent oxygen as photosynthetic plants across the globe belched out the noxious stuff unabated and uncaring. As oxygen levels soared, it began to snuff out life, an event known as the Great Oxygen Catastrophe. Earth was on the brink of becoming a dead planet.
Aliens Within: Mitochondria and the O2 Joy
In the incomprehensible fullness of evolutionary time, unlikely events become routine. Consider the chances of being struck by lightning, which are 1 in 700,000 per year for a person in the United States. If you live to be seventy, your lifetime chances are still reassuringly low, 1 in 10,000. But what if you lived for three billion years, watching the full history of life on Earth unfold? Over that timescale, you could expect to be struck by lightning over 4,200 times.
The numbers are even more difficult to grasp when we consider evolution among the teeming microscopic hordes of bacteria and other single-celled organisms. There are over a million bacteria in an ounce of “clean” drinking water, and about 330 million cubic miles of water on the planet. That puts the total number of waterborne bacteria on this planet (ignoring any on land) at around 40×1027, or 40 with 27 zeros after it. Even if they only replicate only once a day, that’s 14×1030 replications a year. What are the chances of a random mutation arising that changes a metabolic pathway, making some previously unusable chemical into a source of food? Even if the odds are one in a hundred trillion, we can expect more than 100,000 trillion such mutations every year. Over the millions of years available to evolution, such mutations are almost inevitable.
As the young Earth slowly filled with poisonous oxygen over eons, it presented an opportunity. Among the untold quadrillions of bacteria living, mutating, and reproducing over billions of years, some hit upon a seemingly impossible solution, a way to harness oxygen to make fuel: oxidative phosphorylation. Shuttling electrons in and out of an intermembrane space enabled these bacteria to reverse the process of photosynthesis, using oxygen to break apart the bonds of glucose, unleashing the stored solar energy trapped within. The waste products were CO2 and water—the ingredients for photosynthesis.
It was a landmark event in the evolution of life. Aerobic metabolism opened a fresh, untrammeled frontier, a new way for life to work. Oxygen-using bacteria swept across the planet, diversifying into new species and families. Soon they were everywhere.
Then another improbable event. In the vicious cell-eat-cell world of early, simple life, the proliferating aerobic bacteria would have been a delicious new menu item. When a cell eats another cell (whether it’s an amoeba in a backyard stream gobbling up a paramecium or an immune cell in your bloodstream killing an invading bacterium), it engulfs its prey, bringing the victim inside its membrane to dismantle and burn for fuel. But as uncountable zillions of aerobic bacteria were eaten over hundreds of millions of years, a small handful (maybe only one or two) escaped destruction. Instead, against the odds, they survived intact, living on within their new host. It was a microscopic Jonah in the belly of the whale.
And it worked brilliantly.
These chimeric cells held advantages over others in the oceans of middle earth. With a dedicated energy-producing bacterium on board, these hybrid cells outcompeted others in the battle for turning energy into offspring. Having an internal bacterial engine became the norm. Every animal on Earth today, from worms to octopi to elephants, is an inheritor of this great leap forward. Like all other animals, we carry the descendants of those planet-saving aerobic bacteria in our cells. They are our mitochondria.
The revolutionary idea that mitochondria evolved from symbiotic bacteria was championed by the visionary evolutionary biologist Lynn Margulis. Researchers as far back as the 1800s had recognized the visual similarity between mitochondria and bacteria as viewed through a microscope and floated the possibility of a bacterial origin of mitochondria, but it was Margulis who gave the idea life and heft. She wrote a landmark paper on the theory in the late 1960s. It was rejected by more than a dozen journals as too outrageous, but she persevered. During the decades that followed, it became clear that Margulis’s outrageous idea was dead-on.
Mitochondria within our cells retain their own strange loop of DNA, a telltale vestige of their bacterial past. And we dutifully feed and tend to them like treasured pets, our heart and lungs dedicated to the task of supplying our mitochondria with oxygen and carting away their CO2 waste. Without them and the magic of oxidative phosphorylation, we couldn’t sustain the energetic extravagance we take for granted. Life would have never evolved into the grand menagerie we see today.
Oxygen is the essential ingredient in oxidative phosphorylation precisely because it’s an electron thief—the characteristic that makes it so destructive. Oxygen is the final electron acceptor in what is known as the electron transport chain, the bucket brigade that passes electrons along the inner membrane of the mitochondria, pulling hydrogen ions into the intermembrane space. Without oxygen, the electron transport chain stops, the Krebs cycle backs up, and the mitochondria shut down. When electrons jump onto oxygen at the end of the electron transport chain, they attract hydrogen ions, forming water, H2O. Your mitochondria form more than a cup of water (about three hundred milliliters) each day from the oxygen you breathe in.
Off to the Races
At the fundamental level of macronutrients and mitochondria, pathways and ATP production, all animals (humans included) are essentially the same. Figure 2.1 applies equally well to cockroaches, cows, and Californians. And yet, in the nearly two billion years since aerobic metabolism and mitochondria came on the scene, a staggering diversity of life has evolved, all using the same basic metabolic framework. Metabolisms have been sped up and slowed down, tweaked and shaped to fuel the myriad ways that animals move, grow, reproduce, and repair. As we saw in the last chapter, these metabolic changes have shaped our own species in essential ways.
Now that we understand the metabolic basics that all animals share, let’s explore the ways that evolution has shaped them to be different. Let’s see all the places that oxygen-eating engines can take us, and how they function day to day in the real world. How much energy do we really burn each day, and what is it all spent on? How much energy does it take to walk a mile, battle a cold, or build a baby? Can we really “boost” our metabolism with coffee, diet, or superfoods? How does our body manage to supply the right amount of fuel to meet our daily needs? And why do our metabolic engines wear out and fail? Is death the inescapable cost of burning energy, the devil’s bargain for a chance to dance among the living?
Most important, how far do I have to run to escape the guilt of a good donut?