Answers and Explanations
Diagnostic Test
Part A
- A
Atomic mass is the averaged mass of the atoms of an element, taking into account the relative abundance of the various isotopes in a naturally occurring substance. - D
Electronegativity is a measure of the ability of an atom to attract the electrons in a bond. A common scale is the Pauling scale. - C
The atomic radius is defined as the average distance between a nucleus and the outermost electrons. It is usually measured as half of the distance between two nuclei of an element in its elemental form. - B
The atomic number is defined as the number of protons in a given element. It defines an element. - E
The ionization potential, or ionization energy, is the energy required to remove an electron from the valence shell of a gaseous atom. - D
Krypton is the first element with a filled 4p orbital, so the element must fall after it in the periodic table. Rb is the only element with enough electrons to have a filled 4p subshell. - C
Of the two ions Cl– and Ca+, Cl– has the same electron configuration as argon, a noble gas, and therefore has all paired electrons. - B
Fe has 26 electrons, configured as listed. - E
An element is a substance that cannot be further broken down by chemical means. - A
A cation is an ionic species with a positive charge. An anion is an ionic species with a negative charge. - B
Inert gases, elements in Group 0 (or VIII) of the periodic table, contain a full octet of valence electrons in their outermost shells; this makes these elements the least reactive. - E
All atoms of a given element have the same numbers of protons. - D
A calorimeter is an apparatus used to measure the heat released or absorbed by a reaction. - B
A barometer is used to measure atmospheric pressure. - C
A balance is used to measure weight. - A
Centrifuges can spin at very high rotor speeds (e.g., 60,000 rpm) to sediment out particles in suspension. - A
The Gibbs free energy is the energy of a system available to do work. The change in Gibbs free energy, ∆G, can be determined for a given reaction with the equation ∆G = ∆H – T∆S. Reactions with a negative change in Gibbs free energy are spontaneous. - C
Specific heat is the amount of heat required to raise the temperature of one unit mass of a substance one degree Celsius. - B
The heat of formation is the heat absorbed or released during the formation of a pure substance from its elements at constant pressure. - A
NaOH completely dissociates in water to form Na+ and OH– ions, the latter of which is a very strong base. This results in a solution with a very high pH (>7). - D
CH3COOH does not completely dissociate in water. There would be some undissociated CH3COOH and some CH3COO– + H+; therefore, it would be a weak acid with a pH slightly less than 7. - C
HBr completely dissociates into Br– and H+ in water; this solution would have a very low pH (<7). - B
CsCl is a salt; it completely dissociates in water into Cs+ and Cl–. This will form an ionic solution with a neutral pH. Part B
- T, F
Diamond and graphite are different carbon compounds with different properties because of different bond structures. They are allotropes, not isotopes, of one another. Diamond has a covalent crystal structure (lattice positions occupied by atoms with covalent bonds) while in graphite, the carbon molecules are in parallel sheets.
- T, F
Na donates an electron to become Na+ while Cl picks up an electron to become Cl–.
- T, T, CE
In a concentrated NaCl solution, AgCl will not dissociate because of the common ion effect that states that the solubility of one salt is reduced by the presence of another salt having a common ion.
- F, F
Hydrogen and deuterium are different isotopes of the same element. Hydrogen has one neutron while deuterium has two. Because they correspond to the same element, they have the same number of protons.
- F, T
In the periodic table, atomic radius decreases from left to right as electrons are added one at a time to the outer electron shell. Therefore, electrons within a shell cannot shield one another from the attractive pull of protons. Since the number of protons is also increasing, there is a greater positive charge pulling the electrons in close to the nucleus, reducing the atomic radius.
- T, F
The mass number of an element is equal to the total number of protons and neutrons. The atomic number is the number of protons. Therefore, an element with an atomic number of X and a mass number of N has N–X neutrons.
- T, T, CE
An example of a first-order reaction is radioactive decay. In first-order reactions, the rate is proportional to the concentration of one reactant. The half-life (τ1/2) of a reaction is the time needed for the concentration of a substance to decrease to one half its original value.
- T, F
Elements in the same group have the same valence electrons and therefore have similar chemical properties. Oxygen and sulfur are both in Group VI and have six valence electrons. A period is a horizontal row in the periodic table; oxygen and sulfur are in different periods.
- T, T
Nonelectrolytes do not dissolve in water and do not conduct electricity. Strong electrolytes dissociate completely in water. For example, NaCl will conduct electricity very well. Weak electrolytes such as formic acid (HCHO2) will dissociate slightly in water to H+ and CHO2– and will conduct electricity. Statement II, however, is not the correct explanation for statement I. In fact, it is the other way around: A solution of nonelectrolytes does not conduct electricity because no ions are formed.
- T, T
The kinetic molecular theory predicts that heavier gases will diffuse more slowly than lighter ones. This was proven in 1832 by Thomas Graham. Because of this theory, SO3 (MW 80) would diffuse more slowly than CO2 (MW 44). SO3 has a trigonal planar structure, with a bond angle of 120°. CO2 is linear, and thus has a bond angle of 180°. This, however, has nothing to do with the rate of diffusion.
- F, F
Hydrogen bonding is strongest between hydrogen and highly electronegative atoms such as F, Cl, N, O. There is no H bonding between hydrogen and noble gases.
- T, F
An electron in the 3s subshell may occasionally enter an excited state and jump into the 3p subshell. The Heinsenberg uncertainty principle states that it is impossible to simultaneously determine with perfect accuracy both the momentum and position of a particle.
- F, F
∆G, the Gibbs free energy, is the energy of a system available to do work. The change in Gibbs free energy can be determined by the equation ∆G = ∆H – T∆S. A negative ∆G indicates a spontaneous reaction, while a positive ∆G indicates a nonspontaneous reaction. Entropy never decreases in an isolated system.
- T, F
P1V1 = P2V2. An increase in pressure leads to a decrease in volume because they are inversely proportional.
- T, F
An amphoteric compound can act as either an acid or a base because it can react with either H+ or OH–, depending on the nature of the reactants.
- T, T, CE
An indicator is a chemical substance used in low concentration during a titration reaction. It will change color over a certain pH range. The color change, which occurs as the indicator undergoes a dissociation reaction, is used to identify the end point of a titration reaction.
Part C
- B
To answer this question, you need to write the Lewis dot diagrams and then use any one of several formulas to find the formal charge. One such formula is formal charge = valence electrons – [number of bonds + number of nonbonding electrons]. The Lewis dot structure of HNO3 shows that nitrogen is double bonded to one oxygen, single bonded to another oxygen, and single bonded to another oxygen that is single bonded to a hydrogen—there are no lone electron pairs on the nitrogen. Using the formula, formal charge is equal to the valence electrons of nitrogen (5) minus the sum of the number of bonds and the number of nonbonding electrons, which in this case is 4. So, the formal charge on the nitrogen is 5 minus 4, or 1. - D
In a solution, HCl contributes 1 mol of H+ ions per mol of HCl. NaOH also contributes 1 mol of OH– ions per mol of NaOH. Therefore, to neutralize the 80 mL of a 0.5M NaOH solution, you must have the same number of moles of HCl. The number of moles of NaOH you have is:
(0.80 L)(0.5 mol/L) = 0.04 mol of NaOHand therefore 0.04 mol of OH– ions.
To calculate the volume of a 1M solution of HCl, you will need to get 0.04 mol, perform the following calculation:

- D
An electrolyte is a substance that ionizes to yield an electrically conducting solution. A strong electrolyte is one that ionizes completely or nearly completely, and a weak electrolyte doesn’t ionize very much at all. Examples of strong electrolytes are NaCl, KCl, HCl, HBr, and HI. Examples of weak electrolytes are water, HF, acetic acid, benzoic acid, and ammonia. - E
If an element loses an electron, it will have more protons than electrons and with this stronger positive charge can pull the electrons in closer. Therefore, its ionic radius would be less than its atomic radius. Thus, Na, K, Mg, and Ca will all lose electrons and become smaller. Cl, on the other hand, will gain an electron; its positive nucleus cannot hold on to that extra electron as tightly, and its ionic radius is larger than its atomic radius. - A
A crystal is a solid whose atom, ions, or molecules are arranged in a regular 3D lattice structure. The basic repeating structure is known as the unit cell. - C
The oxidation state of nitrogen in N2 is 0. In N2O it is +1, in NH3 it is –3, in NO2 it is +4, and in NO3 it is +6. Oxygen is typically –2 and H is usually +1. Therefore, NH3 is the correct answer. - C
In order to answer this question correctly, you need to know the first law of thermodynamics: The change in internal energy, ∆E, equals the amount of heat transferred, q, minus the amount of work transferred, w. If heat is added to the system, q is positive; if it is transferred to the surrounding, it is negative. If work is done by the system, w is positive; if work is done on the system, w is negative. In this example, 500 joules of heat is added to the system, so q is equal to 500 joules. The system does 75 joules of work on the surrounding, so w is equal to 75 joules. Plugging these into our equation, the change in internal is equal to 500 minus 75, or 425 joules. - A
NaOH and H2SO4 (a strong base and a strong acid) both dissociate completely in H2O into their ionic components Na+, OH–, 2H+, and SO42–, which then form a salt and water. The driving force of this double displacement reaction is the formation of water by the OH– and H+ ions. - D
To answer this question, you simply need to know the definition of ground state and excited state. The ground state of an atom is the state in which all the electrons in the atom are in their lowest energy state. If any electron has absorbed energy and been promoted to a higher energy orbital in the atom without actually leaving the atom, the atom is said to be in an excited state. An atom can have any number of excited states depending on how many electrons have been promoted and what orbitals they end up in. Usually, excited states are unstable and the atom will release energy and return to the ground state. So which of these choices explains the difference between these two definitions? Choice D: When electrons gain energy and change which atomic orbital they’re in, they change their quantum numbers, which in turn changes the electronic configuration around the nucleus. - D
This question deals with solubility constant and the common ion effect. The first step to solving this problem is to express the solubility product constant, which is the ion product of the saturated solution, as the product of the concentration of Mg2+ ion and the concentration of the hydroxide ion squared. Second, you must determine the minimum concentration of hydroxide necessary to precipitate the Mg(OH)2. From the equation given, you can solve for the concentration of hydroxide, which is the square root of the Ksp divided by the concentration of Mg2+. Substituting in the values provided in the question, you should find that the concentration is equal to 10–3 mol/L. That means that the pOH of the solution will be 3; therefore, the pH must be 11. - C
The random motion of a gas holds the most translational kinetic energy. - D
This question asks you which of the choices will increase the rate at which ice melts in a closed container. If you lower the temperature, you are tipping the equilibrium toward the ice, so B is wrong. To deal with pressure changes, we must apply Le Châtelier’s principle, which says that a system in equilibrium that is subject to stress will shift its equilibrium so as to relieve the stress. If the pressure is lowered, as in choice C, the system will counteract the change in pressure by shifting its equilibrium toward the phase that is less dense. In the case of water, that is the ice. Remember that water has a strange property in that the solid form, ice, at zero degrees Celsius is less dense than the liquid phase, water, at that temperature. Since the reduction of pressure drives the systems to produce ice, choice C is incorrect. However, choice D, an increase in pressure, will have the opposite effect and the water will be produced preferentially, meaning that the ice is melting faster. Choices A and E both favor ice formation. - D
The most polar bond is the one that has the greatest difference in the electronegativities of the two elements. Of the choices, H–F is the most polar since hydrogen and fluorine are the farthest apart in the periodic table. - E
This question asks you to determine the rate law of the reaction for the formation of ammonia, NH3. A rate law is an equation that gives the relationship between the rate of the reaction and the concentration of the reactants, each raised to an appropriate power that depends on the exact reaction. For example, the rate of this reaction is equal to k [N2]x[H2]y, where k is the rate constant and x and y are real numbers. k, x, and y can only be determined experimentally by systematically varying the initial concentration of one reactant. Since there is no experimental data, there is no way for us to know what these values are. - E
10 N H2SO4 is equal to 5 M H2SO4 as each molecule has 2H+ ions associated with it. Use the equation M1V1 = M2V2. (100)(5) = M2(800). M2 = 5/8. - C
This question asks you which molecule contains both ionic and covalent bonds. Choice C is ammonium sulfate. The bonds between nitrogen and hydrogen in the ammonium ion and between sulfur and oxygen in the sulfate ion are all covalent. However, the bond between the ammonium ion and the sulfate ion is an ionic bond. - D
Charles’s law states that V1/T1 = V2/T2, and you must convert Celsius to Kelvin. Rearrange your equation to V1/V2 = T1/T2. T1 = 303 and T2 = 293, and the ratio is 303/293. - B
This question tests your knowledge of Le Châtelier’s principle. What will happen to this reaction if more chlorine is added? Adding more chlorine puts a stress on the system, and the system alleviates that stress by using up that added chlorine: The equilibrium shifts to the left. - A
This is a redox reaction. We can tell this because the oxidation states of the species change during the reaction. The oxidation state of bromine in the product, molecular bromine, is 0. The oxidation state of bromine in the bromide reactant is –1, and the oxidation state of the bromine in the bromate is +5. The fact that the bromine of one reactant reduces the bromine of the other should not bother you, since it is immaterial. The important thing in redox reactions is the number of electrons. Since bromide is acting as a reducing agent, going from the –1 oxidation state to the 0 oxidation state, it transfers one electron per bromide. Bromate, however, is going from the +5 oxidation state to the 0 oxidation state, requiring 5 electrons. Bromate gets these electrons from bromide, requiring 5 of them. The ratio of bromate to bromide is therefore 1 to 5. - C
To figure out how many moles of Na2CO3 are in 120 mL of a 1.5 M solution, perform the following calculation: (1.5 mol/L)(0.12 L) = 0.18 molTo figure out how many grams are in 0.18 mol, you need to multiply the number of moles by the formula weight:
(106 g/mol)(0.18 mol) = 19 grams - D
Thomas Graham stated in 1832 that the rates for two gases diffusing is inversely proportional to the square root of the molar mass. Therefore, H2:O2 = [32/2]1/2. Simplify this equation and you have a ratio of diffusion rates of 4:1 for H2:O2. - D
Questions of nuclear chemistry can, as in this case, often be translated into basic arithmetic problems. Based on the law of conservation of mass, the sum of the mass numbers (superscripted) must be the same on each side of the arrow. Similarly, conservation of charge mandates that the sum of the nuclear charges (subscripted) be the same on each side of the arrow. We can thus translate the nuclear question posed into two elementary arithmetic questions:
27 + 4 = 30 + ? and 13 + 2 = 15 + ?Solving these two problems results in a mass number of 1 and a nuclear charge of 0; this set of values corresponds to the neutron
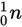 . As for the wrong choices, in choice A, the beta particle is a nuclear electron and thus has mass and charge numbers of 0 and –1, respectively. The positron, choice B, has the mass of an electron but the opposite charge; the numbers are thus 0 and +1, respectively. Alpha particles are helium nuclei; choice C corresponds to a mass number of 4 and a nuclear charge of +2. Choice E, gamma ray, is short-wavelength electromagnetic radiation, i.e., light; as such, it has no mass and no charge.
. As for the wrong choices, in choice A, the beta particle is a nuclear electron and thus has mass and charge numbers of 0 and –1, respectively. The positron, choice B, has the mass of an electron but the opposite charge; the numbers are thus 0 and +1, respectively. Alpha particles are helium nuclei; choice C corresponds to a mass number of 4 and a nuclear charge of +2. Choice E, gamma ray, is short-wavelength electromagnetic radiation, i.e., light; as such, it has no mass and no charge. - D
Cr metal in its elemental state has an oxidation number of 0. In K2Cr2O7, O has an oxidation number of –2 and K has an oxidation number of +1.

Cr must cancel out the –12, so the two Cr molecules must have a charge of +12. 12/2 = +6 for each Cr molecule.
- A
When a Ca molecule loses an electron to become Ca+, it goes from 20 to 19 electrons. K in its elemental state has 19 electrons. - C
This question requires you to know something about the periodicity of the elements. Basically, all you are asked is why all the halogens behave so similarly in reactions. You know from studying the periodic table that there must be something that repeats or the table would not be periodic. What is it about the elements in a column that are the same? The number of valence electrons. Since the identity of the valence electrons is the same in each column, or group, and columns in the s block have valence s electrons, columns in the p block have valence s and p electrons, and columns in the d block have valence s and d electrons, the only thing that can make them act similarly is the number of electrons in these shells. Furthermore, the number of electrons in the valence shell affects the reactivity and stability of the shell. The number of electrons affects the ionization energy and electron affinity, whether the atom will form cations or anions, and even the number of bonds the atom can participate in. - B
The atomic weight of an element depends on the number of protons and neutrons in its nucleus; those numbers are always unique to a particular element, regardless of the number of electrons in the species. The ionization potential depends chiefly on the radius of the parent atoms and the effective charge of the nucleus. Since K+ and Cl– have different numbers of protons by definition, they will have different effective charges, different atomic radii, and therefore different ionization potential. Choice B, however, says that the two ions have the same electronic configuration. Chlorine belongs to the third period and potassium belongs to the fourth, so in their unionized forms, chlorine’s third shell contains seven electrons and potassium’s fourth shell contains one electron. If chlorine gains one electron and potassium loses one electron, both will have eight electrons in the third shell, which becomes the valence shell. So, the potassium and chlorine ions described in the question both contain the same number of electrons and the same number of occupied orbitals and thus share the same electronic configuration. - E
Cl– has the largest ionic radius. When an atom gains an electron, positive protons in the nucleus cannot hold on to the electrons as well and therefore the electron shells are able to spread farther out. - D
The balanced equation looks like this:
8H+ + MnO4– + 5Fe2+ → Mn2+ + 5Fe3+ + 4H2O.One Mn2+ and five Fe3+ yield a total charge of +17.
- D
The balanced equation looks like this:

- C
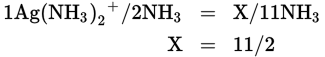
5.5 moles
- C

Total molecular weight: 108 + 28 + 6 = 142
Percent Ag: (108/142) × 100% = 76.1%
- C
Balanced equation: C3H8 + 5O2 → 3CO2 + 4H2O

Therefore, oxygen is the limiting reagent; 5 moles of O2 and one mole of C3H8 will form 3 moles of CO2.
3 moles x 44 g/mol = 132 g CO2 - A
P1V1 = P2V2 is the equation you will use.

- D
60 is the mass number (the number of protons + the number of neutrons). 27 is the atomic number (the number of protons). In a beta-decay reaction, the atomic number increases by one as a neutron turns into a proton and an electron that is ejected. - C
Ionic solutions will conduct electricity. The NaCl will dissociate to become Na+ and Cl–. The same occurs when you melt the NaCl. Solid NaCl and pure water are not ionic solutions. - B
NaCl is an ionic compound with ionic bonds because it is formed when Na donates an electron to Cl. The bond is formed by the electrostatic interaction between the positive and negative ions. - B
Glucose molecules carry no electrical charge, so there are no electrical charges in the solution to provide conduction. - C
This Roman-numeral question asks which of the given statements describe a galvanic cell (remember more than one of the answers may be correct). Galvanic cells are capable of spontaneous reactions. In all electrochemical cells, oxidation occurs at the anode and reduction occurs at the cathode. In addition, the anode in a galvanic cell is negative, meaning that it is a source of electrons. Since a species loses electrons when it is oxidized, this should make sense. There is a trick to remembering these facts. In a galvanic cell, oxidation occurs at the anode, which is negative. Alphabetically, anode comes before cathode, oxidation before reduction, and negative before positive. However, this little trick only works for galvanic cells. - E
34 g of NH3 at 17 g/mol equals 2 mol combusted. Two moles are combusted at 81 kcal/mol. This equation would be (2 mol)(81 kcal/mol) = 162 kcal. - D
The s subshells contain only 2 electrons. Their principal, azimuthal, and magnetic quantum numbers are identical, but due to Pauli’s exclusion principle their spin quantum numbers must be +1/2 and –1/2. Therefore, they have opposite spins. - C
A buffer is a solution made from a mixture of a weak acid and a salt containing its anion. Buffers resist pH change due to the addition of acid or base. Thus, to make a buffer solution with a weak monoprotic acid, the addition of the corresponding salt is required. This salt must contain the anion, or conjugate base, of the acid. - B
If the temperature of an exothermic reaction is increased, the reaction shifts to the left. If the temperature of an endothermic reaction is increased, it shifts to the right. The reaction in question has a negative enthalpy of reaction, meaning that it is an exothermic reaction. If the temperature of this reaction is increased, the reaction will shift to the left. - E
Boyle’s law states that at constant temperatures the volume of a gas is inversely proportional to its pressure. Statement II is therefore correct. Statement III is also correct as density, defined as mass divided by volume, will increase proportionally to pressure since the volume is decreasing. - A
Molality equals the number of moles of solute/kg of solvent. 200 g/[24 g/mol Mg + 71 g/mol for 2 Cl] is the number of moles of solute. This is also the value of molality since it is added to 1 kg of solvent. - C
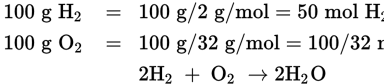
Therefore, oxygen is the limiting reagent since it is the compound in the least amount. According to the balanced equation, for every mole of oxygen you will get two moles of water. Therefore, to discover how many moles of water would be produced, multiply the number of moles of oxygen you begin with by two, to account for the fact that two moles of water are produced for every mole of oxygen. Thus, the correct equation would be (2 mol H2O2/1 mol of O2)(100/32 mol of O2).
- D
Perhaps the easiest way to solve such a problem is to imagine a particular sample mass of the compound. For the sake of convenience, choose 100 grams, though any mass will let you arrive at the correct answer. Because the oxide of arsenic contains only arsenic and oxygen, a 100-gram sample would contain 65.2 grams of arsenic and the remainder, 34.8 grams, must be oxygen. To find the ratio between these two elements in the compound, divide the mass of arsenic by the atomic weight of arsenic, 74.7 grams/mol, and the mass of oxygen by the atomic weight of oxygen, 16 grams/mol. This will give the mole ratio between arsenic and oxygen in the compound. To convert to a more easily useful ratio, divide both by the lowest number of the two, in this case arsenic. This gives us a ratio of 1 mole of arsenic to 2.5 moles oxygen, which is better stated by doubling both numbers and getting 2 moles of arsenic to 5 moles of oxygen. This would correspond to the formula in choice D, As2O5.