For a given atom or ion, the pattern by which orbitals are filled and the number of electrons within each principal level and subshell are designated by an electron configuration. In electron configuration notation, the first number denotes the principal energy level, the latter designates the subshell, and the superscript gives the number of electrons in that subshell. For example, 2p4 indicates that there are four electrons in the second (p) subshell of the second principal energy level.
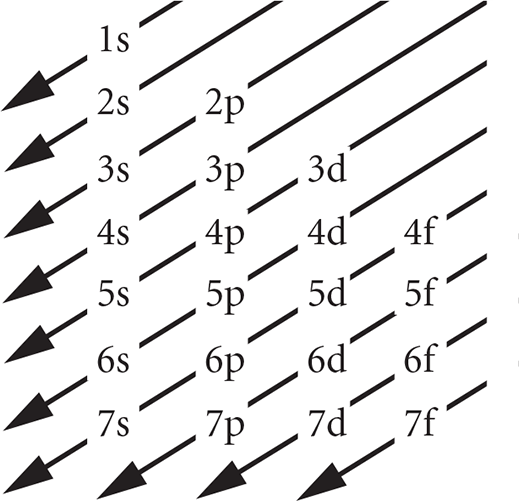
When writing the electron configuration of an atom, remember the order in which subshells are filled. Subshells are filled from lowest to highest energy, and each subshell will fill completely before electrons begin to enter the next one. The (n + l) rule is used to rank subshells by increasing energy. This rule states that the lower the values of the first and second quantum numbers, the lower the energy of the subshell. If two subshells possess the same (n + l) value, the subshell with the lower n value has a lower energy and will fill first. The order in which the subshells fill is shown in the following chart, which is arranged so that it is easily remembered: One simply lists the subshells in order, starting each shell with a new line. The order of filling them is found by crossing them with diagonal arrows.

To determine which subshells are occupied, you must know the number of electrons in the atom. In the case of uncharged atoms, the number of electrons is equal to the atomic number. If the atom is charged, the number of electrons is equal to the atomic number plus the extra electrons if the atom is negative, or the atomic number minus the missing electrons if the atom is positive.
In subshells that contain more than one orbital, such as the 2p subshell with its 3 orbitals, the orbitals will fill according to Hund’s rule. Hund’s rule states that within a given subshell, orbitals are half-filled so that they each have one electron, all with parallel spins, before any orbital is fully occupied with two electrons of opposite spins. In other words, electrons would tend to avoid pairing as much as possible.
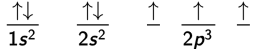
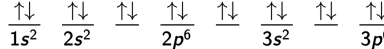
The presence of paired or unpaired electrons affects the chemical and magnetic properties of an atom or molecule. If the material has unpaired electrons, a magnetic field will align the spins of these electrons and weakly attract the atom. These materials are said to be paramagnetic. Materials that have no unpaired electrons and are slightly repelled by a magnetic field are said to be diamagnetic.