So far we have ignored the fact that the valence electrons that interact originally occupy different atomic orbitals in the atoms they come from. What does it mean for an electron in the 2p subshell of an oxygen atom to be “shared” with another atom, for example? Does it still occupy the same dumbbell-like region of space? Does it matter whether the orbital is 2px, 2py, or 2pz? Can it not be a valence electron from the 2s subshell instead? All these questions are addressed (or perhaps more correctly, as you shall see, sidestepped) in the orbital hybridization picture of bonding, which “scrambles” the atomic orbitals of the central one to form new, hybrid ones that participate in bonding. It is not so much an alternative to VSEPR theory as an extension of it that gives a fuller understanding of the nature of bonding.
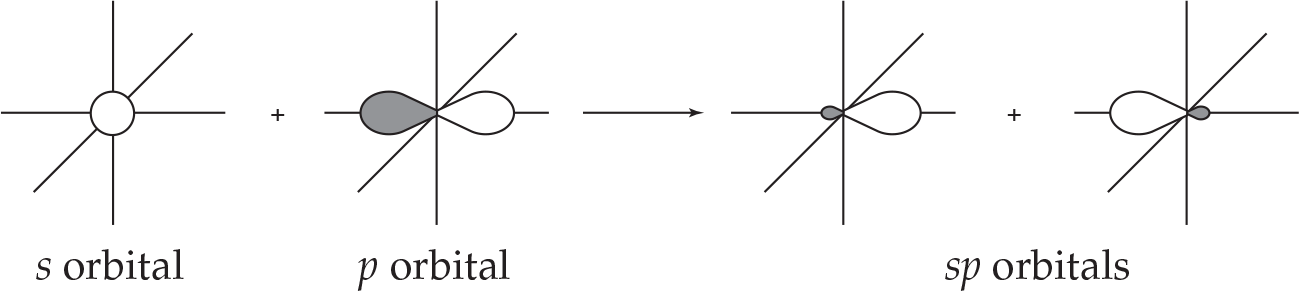
A molecule such as BeH2 has a linear geometry. The two valence electrons of Be, originally in the 2s orbital, are shared with the hydrogen atoms (which in turn share their 1s electrons with Be). In the orbital hybridization picture, each of the two electrons in Be actually occupies an orbital that is a mix of an s and a p orbital, called an sp hybrid orbital.

These two hybrid orbitals are oriented antiparallel to each other, and interact with the 1s orbitals of the hydrogen atoms on each end of the molecule. This leads to the 180° bond angle predicted in the VSEPR theory (implied by the linear geometry). The general convention adopted is to call the p orbital that participates in hybridization the pz orbital, even though such designations are by and large arbitrary. Also, note that we mix two atomic orbitals and end up with two hybrid orbitals: In general, the number of atomic orbitals that “go in” has to equal the number of hybrid orbitals that “come out.”
Three sp2 hybrid orbitals are formed by mixing an s and two p orbitals.
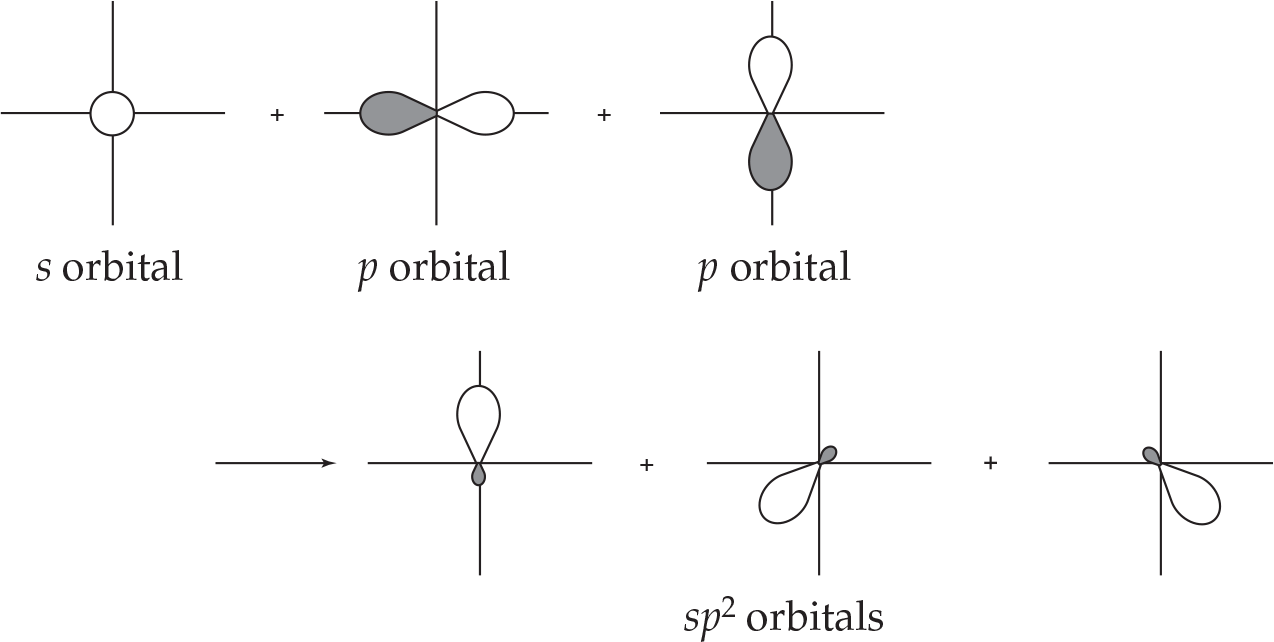
Again, three orbitals are mixed to generate three new hybrid orbitals. These are oriented 120° apart from each other and thus the spatial arrangement is in accordance with the trigonal planar geometry of molecules like BF3.
Four sp3 hybrid orbitals are formed by mixing an s and all three of the p orbitals. These hybrid orbitals point toward the four corners of a tetrahedron.