Review Questions
-
All of the following are true statements concerning reaction orders EXCEPT:
- The rate of a zero-order reaction is constant.
- After three half-lives, a radioactive sample will have one-ninth of its original concentration.
- The units for the rate constant for first-order reactions are s−1.
- If doubling the concentration of a reactant doubles the rate of the reaction, then the reaction is first order in that reactant.
-
The half-life of radioactive sodium is 15.0 hours. How many hours would it take for a 64 g sample to decay to one-eighth of its original concentration?
- 3
- 15
- 30
- 45
-
Consider the following hypothetical reaction and experimental data:
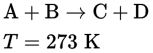
[A]0 (mol/L) [B]0 (mol/L) Rate Exp 1 0.10 1 0.035 Exp 2 0.10 4 0.070 Exp 3 0.20 1 0.140 Exp 4 0.10 16 0.140 - What is the order with respect to A?
- What is the order with respect to B?
- What is the rate equation?
- What is the overall order of the reaction?
- Calculate the rate constant.
-
Consider the following chemical reaction and experimental data:
A (aq) → B (aq) + C(g)Trial 1 Trial 2 [A] (mol/L) Rate [A] (mol/L) Rate 0.10 0.6 0.10 0.9 0.20 0.6 0.20 0.9 0.30 0.6 0.30 0.9 0.40 0.6 0.40 0.9 - What is the rate expression for trial 1?
- What is the rate constant for trial 1?
- What is the most likely reason for the increased rate in trial 2?
-
Consider the following reaction and experimental data:
SO3 + H2O → H2SO4[SO3] (mol/L) [H2O] (mol/L) Rate Trial 1 0.1 0.01 0.013 Trial 2 0.2 0.01 0.052 Trial 3 X 0.02 0.234 Trial 4 0.1 0.03 0.039 - What is the value of X?
- What is the order of the reaction?
- What is the rate constant?
- What would be the rate if [SO3] in trial 4 were raised to 0.2?
-
In the following diagram, which labeled arrow represents the activation energy for the reverse reaction?

- A
- B
- C
- D
-
The activation energy for a reaction in the forward direction is 78 kJ. The activation energy for the same reaction in reverse is 300 kJ. If the energy of the products is 25 kJ, then:
- What is the energy of the reactants?
- Is the forward reaction endothermic or exothermic?
- Is the reverse reaction endothermic or exothermic?
- What is the enthalpy change for the forward reaction?
-
According to chemical kinetic theory, a reaction can occur
- if the reactants collide with the proper orientation
- if the reactants possess sufficient energy of collision
- if the reactants are able to form a correct transition state
- All of the above
-
The number of undecayed nuclei in a sample of bromine-87 decreased by a factor of 4 over a period of 112 s. What is the rate constant for the decay of bromine-87?
- 56 s
- 6.93 × 10−1 s−1
- 1.24 × 10−2 s−1
- 6.19 × 10−3 s−1
-
Which of the following is most likely to increase the rate of a reaction?
- Decreasing the temperature
- Increasing the volume of the reaction vessel
- Reducing the activation energy
- Decreasing the concentration of the reactant in the reaction vessel
-
All of the following are true statements concerning catalysts EXCEPT
- A catalyst will speed the rate-determining step.
- A catalyst will be used up in a reaction.
- A catalyst may induce steric strain in a molecule to make it react more readily.
- A catalyst will lower the activation energy of a reaction.
-
The equilibrium constant, Keq, of a certain single-reactant reaction is 0.16. Suppose an appropriate catalyst is added.
- What will be the equilibrium constant?
- Will the activation energy increase or decrease?
-
At equilibrium
- the forward reaction will continue
- a change in reaction conditions may shift the equilibrium
- the reverse reaction will not continue
- Both A and B