Answers and Explanations
Practice Test 2
Part A
- D
O− has 9 electrons, while S+ has 15 electrons. - A
Ca+ has 19 electrons, as does K. - C
Cl− has 18 electrons, while F has 9 electrons. - B
H+ has no electrons, while He has 2 electrons. - E
Na+ has 10 electrons, while O– has 9 electrons. - A
Solute is defined as something that is dissolved in a solvent to make a solution. It is the component that is present in a lesser amount than solvent. - D
In aqueous solutions, the solvent is H2O. - B
Solvent is the component of a solution present in the greatest amount. It is the substance in which the solute is dissolved. - E
Solvation is the interaction between the solute and the solvent molecules; it is often the development of a cagelike network of a solution’s solvent molecules about a molecule or ion of the solute. - D
Deposition is the conversion of a gas directly into a solid without passing through the liquid state. - B
Condensation is the conversion of a gas into a liquid state, an instance of which you can see on a pot lid during the boiling of water. - A
Sublimation is the conversion of a solid into a gas without going through a liquid phase. - B
A neutron is a subatomic particle with a charge of zero and a mass of 1 amu. Neutrons can be found in all nuclei, except for one of the hydrogen isotopes. - A
A proton is a subatomic particle with a charge of +1 and mass of 1 amu. It is found in all atomic nuclei. - C
An electron is a subatomic particle with a charge of −1 and negligible weight. It is found around the nucleus. - C
See the explanation to question 15. - B
Metalloids’ properties lie in between those of metals and nonmetals and are found between them on the periodic table. They are semiconductors in that they do conduct electricity, but not very well. They lie in a diagonal from B to At. - D
Nonmetals have no metallic character. Three examples are N2, O2, and carbon. They are typically poor conductors of heat and electricity. - A
Class IA metals are found on the first column of the periodic table. Metals tend to have shine and luster, which makes them easily recognizable. They conduct heat and electricity very well. - E
Helium is an inert gas that is not very soluble in H2O. - D
H2SO4 is a very strong acid (sulfuric acid) that when bubbled through H2O will make a very acidic solution. - C
N2 is not very soluble, but will burn with a blue flame. - B
CO2 will extinguish a glow and will slightly associate with H2O to form carbonic acid (H2CO3 → H+ + HCO3− ). Part B
- T, T, CE
In the periodic table, groups represent elements that have the same electronic configuration in their outermost shell and share similar chemical properties. These valence electrons are involved in chemical bonding and determine the chemical reactivity of an element.
- T, T
NH3 is a Lewis base because it can donate an electron pair; it is also a Brønsted-Lowry base because it can accept an H+ from a solution, but this is not what makes it a Lewis base.
- F, F
An element with the atomic number of 16 will have 16 protons and electrons in a neutral atom. The anion with −2 charge is formed upon the addition of 2 more electrons.
- F, T
Chlorine is a better oxidizing agent because it has a greater electronegativity, which is the ability to attract electrons. Remember that as you go up and from left to right on the periodic table, electronegativity increases. Bromine has a larger atomic radius than chlorine. Also remember that as you go from right to left and down on the periodic table, atomic radius increases.
- F, F
Exothermic reactions have a negative ∆H and give off heat to their surroundings as the reaction progresses.
- T, T, CE
An element such as Fe is a substance in which all atoms have the same atomic number. It cannot be broken down by chemical reactions into anything that is more stable or simpler.
- T, T, CE
CCl4 is a nonpolar covalent bond. CCl4 has four polar C–Cl bonds so that the shape is tetrahedral. The four bond dipoles point to the vertices of the tetrahedron and cancel each other out, resulting in a nonpolar molecule.
- F, T
A basic solution has more OH− ions than H+ ions, but pH is defined as −log[H+]. pOH is −log[OH−].
- F, F
Atomic radii increase down a group because the valence electrons are farther from the nucleus and are able to resist the positive charge of the protons, which results in a larger atom.
- F, T
V1/T1 = V2/T2. In an ideal gas, temperature and volume are proportional, so when a gas is cooled, the volume decreases.
- T, T, CE
Carbonic acid will not dissociate completely in H2O.
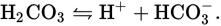
Therefore, in a 0.2 M solution, there will be fewer ions than in a 0.2 M solution of HBr (which dissociates completely). Electrical conductivity depends on the number of ions in solution, so HBr would be the better electrical conductor.
- T, F
In the equation
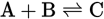 , when you increase pressure, the system will try to relieve the
stress and skew the equilibrium to the side of the equation with fewer
moles of gas formed, and more C will be produced.
, when you increase pressure, the system will try to relieve the
stress and skew the equilibrium to the side of the equation with fewer
moles of gas formed, and more C will be produced. - F, T
Water does not make a good buffer. A good buffer is defined as a pair of solutes (salts) that can keep the pH of a solution almost constant if either acid or base is added. Water is unable to do this.
- T, T
The kinetic molecular theory of gases has five assumptions to it:
- Gases are made up of particles whose volumes are negligible compared with the container volume.
- Gas atoms or molecules exhibit no intermolecular attractions or repulsions.
- Gas particles are in continuous, random motion, undergoing collisions with other particles and the container walls.
- Collisions between any two gas particles are elastic, meaning that there is no overall gain or loss of energy.
- The average kinetic energy of gas particles is proportional to the absolute temperature of the gas, and is the same for all gases at a given temperature. That collisions between the particles and the container walls are elastic is not necessarily a consequence of the other assumptions.
- F, T
Al2(Cr2O7)3
The oxidation number of Al is 3+ while Cr2O7 must be −2.
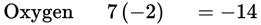
Therefore, 2Cr must be +12 to result in a net charge of −2.
2Cr = +12; Cr = +6 - T, T, CE
An increase in entropy describes an increase in randomness; gas > liquid > solid. When a solid is dissolved in water, it dissociates and entropy increases as it becomes less ordered.
Part C
- C
A Lewis base is a substance that can donate a pair of electrons. Among the answer choices, only PH3 has a pair of nonbonding electrons, making choice C the correct response. The easiest way to determine if a substance is a Lewis base is to draw its Lewis dot structure and see if a lone pair of electrons exists. - E
The difference between SO2 and SO3 is 2,300 cal. This quantity must be a negative, since this is an exothermic reaction. - D
While this is a relatively simple calculation, the question tests your ability to read carefully. The molecular weight of sulfur hexafluoride is 146 grams/mole. If you divide the mass given, 36.5 grams, by the molecular weight, you find that you have 0.25 mole of sulfur hexafluoride molecules. A mole is Avogadro’s number of particles; in other words, there are 6.022 × 1023 particles per mole. If you multiply the 0.25 mole of sulfur hexafluoride by Avogadro’s number, you’ll find the number of molecules of sulfur hexafluoride, 1.511 × 1023. 1.51 × 1023 is the number of MOLECULES of sulfur hexafluoride in the sample, not the number of atoms. There are seven atoms in each molecule, so you must multiply the number of molecules by the number of atoms per molecule, seven. The trick is in making sure all your units cancel. The answer is 1.06 × 1024 atoms. - A
Hydrogen peroxide has the empirical formula HO. - E
No reaction takes place. In order for a reaction to take place to produce H2O, energy must be added to the system (typically in the form of heat). - A
Choice A, which says that an electron may assume an infinite number of different velocities, is true in classical mechanics but not in Bohr’s model. Bohr used quantum theory in developing his atomic model and placed specific conditions on the possible values of the electron velocity. Since we’re looking for the incorrect statement, choice A is the correct answer. - A
100 g O2 divided by 32 g/mol = 3.125 moles of oxygen. You’re given 296 kJ/mol in the equation (a negative value means heat is released), so multiply 296 kJ/mole × 3.125 mole to get 925 kJ. 925 kJ is equal to 925,000 joules of heat. - E
At 10 cents/coulomb, the question is really asking you which reaction involves the transfer of the most electrons.
Cu from Cu2+ 2 electrons Na from Na+ 1 electron Cl from Cl− 1 electron H from H+ 1 electron Fe from Fe3+ 3 electrons Therefore, the production of Fe from Fe3+ involves the transfer of the most electrons and would cost the most.
- D
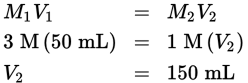
Therefore 100 mL needs to be added.
- C
This question is an application of Boyle’s law. This states that at constant temperature, the pressure and volume of a gas are inversely proportional to each other. Therefore, since the pressure of the gas in the question is increased, the volume must decrease. - D
The Lewis structure of NH3 is:
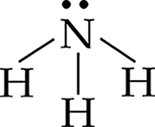
The central atom has three bonding electron pairs and one nonbonding pair for a total of four pairs. The four electron pairs will be farthest apart when they occupy the four corners of a tetrahedron. Since one of the four pairs is a lone pair, the observed geometry is trigonal pyramidal.
- B
Organic chemistry focuses only on carbon-containing compounds such as carbohydrates, alcohols, and ethers, and on their reactions. - D
Electronegativity increases in going from the lower left to the upper right of the periodic table, excepting the noble gases. So all you need to do here is locate the answer choices in the periodic table and find the one that is the closest to the upper right-hand corner and is not a noble gas. When you do this, you’ll see that choice D is the correct answer. - B
The atoms with the greatest affinity for electrons are found in the upper right corner of the periodic table (excluding the noble gases). Fluorine is an exception to the trend because of electron-electron repulsions in small atoms. - C
There are 6.023 × 1023 atoms in a mole of a compound, so 1.204 × 1024 atoms would be approximately 2 moles of Br. 2 moles of Br × 80 g/mole = 160 g. - E
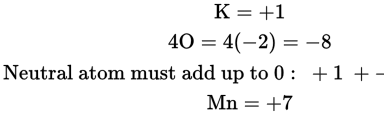
- E
γ decay is a high-energy emission that has no mass or charge. Therefore 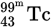 after γ decay would still be
after γ decay would still be  .
. - C
The number of moles of solute in the solution will be the same after dilution as before, and the number of moles in each case is equal to the molar concentration multiplied by the volume of solution. This means that the initial concentration times the initial volume will be equal to the final concentration times the final volume (M1V1 = M2V2). So, the final volume will equal M1(V1)/M2. Plugging into this equation, we find that V2 = 200 mL (50 mL + 150 ml). - C
All members of Group IA have similar reactivities because they have a similar valence shell configuration (one loosely bound electron). They lose it easily to form univalent cations and react readily with nonmetals, especially halogens. - E
One mole of a gas at STP will have a volume of 22.4 L. If the pressure is doubled, the volume will halve since they are inversely proportional. If the temperature is tripled, the volume will triple since they are proportional. 3/2(22.4 L) = 33.6 L. - A

- D
If the reverse reaction is exothermic, the equation can be written as such:

If you remove heat, the equilibrium will shift to the left in an attempt to produce more heat.
- A
An increase or decrease of entropy is easy to predict when the chemical reactions involve gases. If there are more moles of gas on the products side than on the reactants side, there is an increase in entropy. If there are fewer moles of gas on the products side than on the reactants side, there is a decrease in entropy. For choice A, there are two moles of gas on the reactants side and one mole of gas on the products side—there is, therefore, a decrease in entropy for this reaction. - D
Mendeleev discovered the properties of the elements and how they had regular intervals, and formulated the first periodic table. Faraday found that chemical changes could occur when an electrical current was sent through certain chemical solutions. Millikan did the famous oil drop experiment that determined the charge on an electron. Rutherford discovered the nucleus by striking thin metal foils with alpha particles. - A
Electrochemical reactions that are nonspontaneous (those having a positive ∆G) can be driven to completion by passing an electric current through the solution. This process is known as electrolysis and the cell is called an electrolytic cell. In an electrolytic cell, the anode is positively charged and the cathode is negatively charged—the opposite of a galvanic cell. Just as with a galvanic cell, oxidation occurs at the anode and reduction occurs at the cathode. - C
Ionization energy is defined as the amount of energy required to remove an electron from a given species. Ionization energy is usually expressed in energy per particle (as it is here) or in energy per mole, and energy, in turn, is usually expressed in electron volts (eV), joules, or kilojoules. The first ionization energy of an element is the energy required to remove an electron from a neutral atom of that element, while the second ionization energy is the energy required to remove a second electron, i.e., to remove an electron from the +1 cation. A particularly useful piece of logic/knowledge here is that it will be more difficult to remove an electron from a positively charged species than it will be to remove an electron from a neutral version of the same species. In other words, the second ionization energy of an element is always greater than its first ionization energy. Based on this fact alone, choices A, D, and E can be eliminated. To choose between the remaining two choices, we must reason that a small value for ionization energy corresponds to relative ease of removal of the electron. A Group IA metal atom loses its first electron with relative ease, but after that it will possess an electronic configuration similar to that of a noble gas. It will therefore be difficult to remove another electron, implying a high second ionization energy. Looking at the numbers in the table, we see that both remaining choices have relatively small values for first ionization energy, but that X has a much higher second ionization energy. It follows that element V is most likely a Group IIA element (e.g., Mg, Ca) while element X is most likely a member of Group IA (e.g., Na, K). Choice C, then, is the best choice. - A

Pressures are additive as described by Dalton’s law of partial pressures:
40 torr + 140 torr = 180 torr - B
This question asks you to determine why water has a higher boiling point than hydrogen fluoride. Choice B says that water has a higher boiling point because it can form more hydrogen bonds. Hydrogen bonding does affect boiling points by increasing the attraction between the molecules of a compound. Water is capable of forming as many as four hydrogen bonds per molecule, while hydrogen fluoride can only form two. This intermolecular attraction leads to a complexation of water molecules and contributes to the high boiling point of water. - A
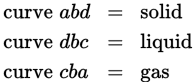
At pressures less than (b), solids are able to convert directly to gas (sublimation) and gases are able to convert directly to solid (deposition).
- D
Line bd describes the conversion between liquid and solid and therefore between melting and freezing. - E
Line bc describes the conversion between liquid and gas and therefore between evaporation (boiling) and condensation. - A
In a total volume of 12 L, you must calculate the number of moles you have.

- C
Boiling point elevation is a colligative property, one due solely to the number of particles and not the nature of the particles. Therefore, III is false and II is true. I is false because vapor pressure is lowered by addition of solute, which increases boiling point. An increase in vapor pressure would lead to boiling-point depression. - D
1K2Cr2O7 + 14HCl → 2KCl + 2CrCl3 +7H2O + 3Cl2
2 + 2 + 7 + 3 = 14
- C
The reaction is an example of β− decay, so X is an electron (  ) emitted by this type of radioactive decay.
) emitted by this type of radioactive decay. - B
See the explanation to question 58. - C
In the equation 2H2 + O2 → 2H2O, if you begin with 4 g of H2 at 2 g/mole, you have 2 mol of H2. According to the equation, a 100% yield would result in 2 moles of H2O. Two moles of H2O at 18 g/mol would weigh 36 grams. Percent yield is defined as actual/theoretical × 100%. (27/36) × 100% = 75%. - A
An increase in entropy is an increase in randomness and loss of order. Gas going to a solid is an increase in order and two molecules combining to form one molecule is also an increase in order. A solid (Na) becoming an ionic compound (NaOH) and a gas (H2) is a decrease in order and an increase in entropy. - C
This question requires you to know how to calculate the percentage by weight of an element in a compound. You need to first calculate the weight of oxygen in each compound and then divide that value by the compound’s molecular weight. The weight of oxygen in choice A is 80 grams/mole and the MW = 157 grams/mole. This is 51% oxygen. Doing the same with the other answer choices, you get 43% oxygen (choice B), 53% oxygen (choice C), and 46% oxygen (choice D). Obviously, choice C contains the most oxygen by weight and is the correct answer. - C
If you have 0.25 mole of SO2 and 0.25 mole of O2, SO2 is the limiting reagent since you need two moles per reaction compared to one mole of O2. Since 2 moles of SO2 yields 2 moles of SO3, 0.25 mole of SO2 would yield 0.25 mole of SO3. - A
A buffer solution is prepared from a weak acid and its conjugate base in near equal quantities. As long as these conditions are met, the pH should remain the same. A buffer solution with the concentrations of each of these components halved may have less ability to buffer, but the initial solution will have the same pH. - C
The molecular weight of sodium nitrite is 69 grams/mole. Since there is one nitrogen atom per formula unit, we can find the weight fraction of sodium nitrite that is nitrogen by dividing the atomic weight of nitrogen, 14 grams/mole, by the molecular weight of sodium nitrite, 69 grams/mole. If we multiply this fraction, 14/69, by 50 grams, we get 10.1 grams. - D
This question asks you to predict which combination of ∆H and T∆S values will always give a spontaneous reaction. Recall that spontaneous reactions have negative values of Gibbs free energy, ∆G, and that ∆G = ∆H − T∆S , where ∆H is the change in enthalpy, ∆S is the change in entropy, and T is the absolute temperature. This is the key equation, which you probably have memorized. From this equation, it’s clear that the best way to guarantee a negative ∆G is to have a negative ∆H value and a positive ∆S value since T, in Kelvin, is always positive. - C
The most nonmetallic compound is Si; these elements are found in the right-hand corner of the periodic table. The most metallic (it actually is a metal) is Pb; these elements are found on the left-hand side of the periodic table and through all the transition metals. Also, in Groups III through VII, nonmetallic character increases as you go up the periodic table. Therefore, the order of these elements in decreasing nonmetallic character would be Si, Ge, Sn, Pb. - C
Group IA elements are alkali metals; they have low densities, large atomic radii, low ionization energies, and low electronegativities. They are metals and have metallic bonding, and are good conductors of electricity. However, they have low melting points. - A
This question boils down to definitions. The electron affinity of an atom is defined as the change in energy that occurs when an electron is added to a gaseous neutral atom in its ground state. So electron affinities can be positive or negative, depending on whether energy is released when an atom spontaneously accepts an electron or energy is gained when an electron is forced onto an atom. So choice A is the correct answer. Electronegativity is a derived quantity, usually scaled for all atoms between 0 and 4, that characterizes the pull an atom has for the electrons in a bond. The electronegativity has nothing to do with how likely an atom is to gain an electron, just how strong its pull is on an electron in a bond. The “in a bond” part is very important. The concept of electronegativity can only be applied to atoms that are already bonded. It characterizes the polarity of the bond, not the likelihood of bond formation. So choice B is the definition of electronegativity. Choice C is the definition of the first ionization energy. This is sort of the opposite of electron affinity, since in electron affinity, electrons are gained; in ionization, electrons are lost. Choice D would give the energy of a photon released when an electron relaxed from an excited state to a lower-lying state. Since there are many quantities for this energy, depending on which excited state the electron is in, it hardly makes a good answer to this question.