▲ Visible light spectrum
What is to give light must endure burning.
—Viktor Frankl
What if you could watch music as it streamed out from a radio tower, like an enormous lightbulb shining in the sky? What if turning on your television made your eyes blink from the bright flash—not from the screen but from the tip of your remote control? What if your microwave heated your food with light, like one of those old toy ovens from your childhood? In fact, all these things—radio, cell phones, microwave ovens, and remote controls—are based on light. They use light that, even though we cannot see it, is nevertheless the same in every way as the light that we can see.
We are constantly awash in an astonishing spectrum of light, everflowing and everlasting. Even in the darkest room we cannot escape light, if only because our own bodies radiate it through the very act of living.
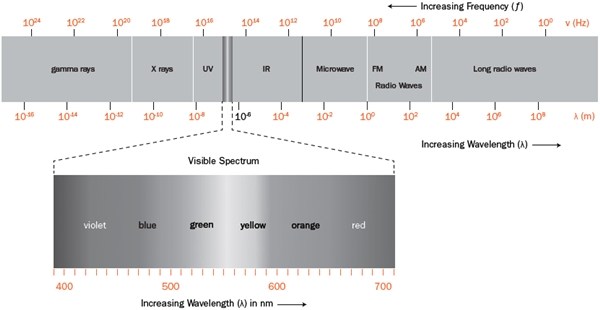
Of course, humans are mercifully sensitive to only a small portion—less than a thousandth of 1 percent—of the full spectrum of light. Our eyes see the edges of a rainbow fade gradually away to what seems like nothingness. But electronic instruments uncover for us a world far beyond the red (on one side) and violet (on the other). The universe truly is far stranger (and brighter!) than we can imagine.
Such Stuff as Light Is Made On Light—that is, the light that the physiology of our eyes is tuned to see—is part of a phenomenon called electromagnetic radiation (or EMR) that describes how electricity and magnetism radiate, or travel, from one place to another. Understanding EMR is necessarily technical, but the more you understand about light, the more amazing it is.

▲ Visible light spectrum
When an electrical current flows from one place to another, such as through a wire or the nerve system in your body, it generates a magnetic field. That’s why an electrical current flowing near a compass moves the needle. It’s why your favorite tune can be converted from an electrical pulse in a wire, to a magnet in a speaker, to the sound you hear. Control the current, and you control the magnet, which controls the speaker vibrations we hear as sound.
Conversely, when you move a magnet near a coil of wire, it creates electricity in that wire. This intimate bond between electricity and magnetism is what makes it possible for us to create motors, or, in fact, to create electricity itself in a generator.
So a changing electrical current creates a magnetic field, and a changing magnetic field creates an electrical field. And an amazing thing happens if you oscillate them by varying these fields back and forth: You create a wave effect, where the changing electric field actually generates the magnetic field, which creates the next electric field, and so on, potentially forever.
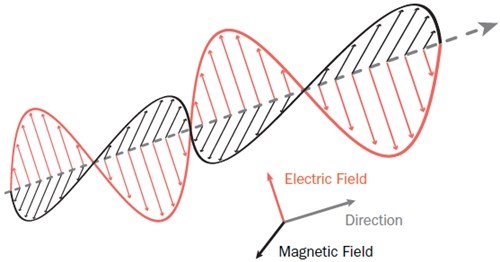
▲ Electromagnetic radiation
This self-propagating “electromagnetic” wave is what we call “light,” and it can travel through the vacuum of space without slowing, without fading. This explains why the burning plasma of a distant star, aglow with the dance of free-floating electrons, can throw its radiation waves out across trillions of kilometers to be captured by our eyes and instruments.
Electromagnetic radiation—where energy is moved through space as light enables us not only to see our own sun but also to feel its warmth and, indeed, be burned by it, even on a cloudy day. And it allows us to send and receive seemingly invisible messages with satellites in orbit or with a cell phone tower on a hill.
Making Light Technically, there are two ways to create light: incandescence and luminescence. The former comes from heating a material, such as the nuclear reactions in the sun, or from electricity being passed through a tiny wire filament inside an incandescent lightbulb until it glows white-hot at over 2,000°F.
Luminescence is light without heat. For example, a watch dial painted with a phosphorescent substance will absorb energy in a lit room and then gently continue to glow in the dark. A firefly generates bioluminescence with a chemical reaction inside its abdomen. The phosphor in some laundry detergents is called a “brightener”—making white clothes “whiter”—because it literally glows with cool, visible light when energy from the sun hits it.
You can see this effect most clearly in a disco or an amusement park fun house that has black lights—special lightbulbs that emit ultraviolet radiation and make the phosphorescent materials in the room glow. Laundered white shirts and socks, natural phosphors in your teeth, and fluorescent paint all pop out brightly because they respond to the energy by giving off light we can see.
If you paint the inside of one of these lights with a phosphorescent coating, the surface itself glows white instead of your T-shirt. The result is the most common form of luminescence we see around us: a plain fluorescent lightbulb.
Both incandescence and luminescence derive from the same underlying mechanism: Electrons in an atom absorb energy of some sort and then emit it in the form of a bit of electromagnetic radiation. In incandescence, atoms are heated until their electrons get so excited that they break free and travel from one atom to another before settling down, releasing their energy as light. In luminescence, electrons are energized but remain within their atoms, quickly emitting the absorbed energy and dropping back to their original level. In both cases, the process continues until you stop adding energy, flicking off the switch.
If you chew a Wint-O-Green Life Saver in a dark room, you can experience triboluminescence—generating light from crushing, rubbing, ripping, or scratching. All hard candy emits light when broken, but because wintergreen oil (methyl salicylate) is fluorescent, it converts the generated ultraviolet light into blue light that is easy to see. Quickly pull open a Band-Aid wrapper, or a piece of cloth friction tape from a roll, and you’ll see a thin line of light appear. Pulling off a strip of Scotch tape generates X-rays so strong that they can leave an image on dental photographic paper.
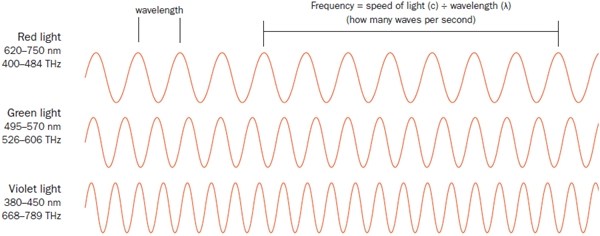
How Fast, How Red Of course, there are many kinds of light: blue, infrared, ultraviolet . . . and the difference? It’s the result of wavelength. Like ripples on a pond, or waves in an ocean, light waves radiate with energy, created by the constant rise and fall of electric and magnetic fields. If the waves gently undulate back and forth, we say they have a long wavelength and a low frequency. That is, it takes more time for each long wave to crest, so the frequency of those waves is slower.
On the other hand, a fast wave—one that goes from electric to magnetic, crest to trough, quickly—has a very short wavelength and a fast frequency.
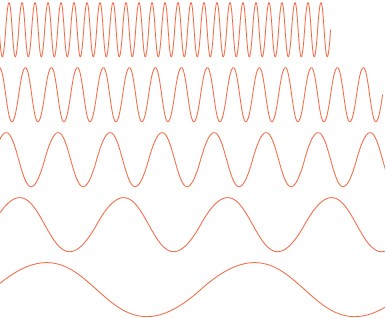
▲ Low frequency, long wavelength, less power
Frequency and wavelength are always inextricably tied because of a crucial universal constant: the speed of light. This law tells us that there is one speed at which all light—indeed all electromagnetic radiation—travels in a vacuum. It’s important to add the vacuum caveat because light does travel slightly slower in a gas, liquid, or clear solid. Place the tip of a stick in a clear brook and you’ll see it “bend” underwater because the speed of light is literally slower beneath the surface than it is in air.
“Come forth into the light of things,
Let Nature be your teacher.”
—William Wordsworth, “An Evening Scene on the Same Subject”
Pass light through a diamond and it slows to about 40 percent its normal speed. In fact, through some exceedingly clever tricks involving shooting lasers into extremely cold clouds of rubidium and helium gas, scientists have even been able to slow a beam of light until it is virtually standing still, apparently extinguished, but actually just on pause.
But in the vacuum of space, light travels at about 300,000 kilometers per second (just over 186,200 miles per second). Nothing travels faster.
So if light is radiating out at, well, the speed of light, then the number of waves that pass by a given point in space each second (frequency) is always tied to how long those waves are. Longer wave, fewer of them can go by each second; short little wave, you can cram a bunch of them in each second.
Note that because the speed of light is so (unbelievably, shockingly) fast, when you look at wavelength and frequency, you have to deal with some really huge (and small) numbers.
Red light—that is, light that our human eyes perceive as red—has a frequency of about 420 THz (teraherz). That means those electric fields and magnetic fields are flipping back and forth about 420 trillion times each second. If your car wheels revolved that quickly, you could drive from one end of our solar system to the other in the time it takes to blink an eye.
“Colours seen by candle-light
Will not look the same by day.”
—Elizabeth Barrett Browning, “The Lady’s ‘Yes’ ”
At that rate, each of those red-colored light waves is about 700 nm (700 billionths of a meter) long, about a tenth the size of a red blood cell, even smaller than a typical microscopic bacterium.
As you shorten the wavelength—that is, increase the frequency, so you get more waves per second—red becomes yellow, then green, then blue, and then, at about twice the frequency of red, purple. Speed up the frequency even more and the light changes, moving beyond what we can see into the ultraviolet (UV) range, then X-rays, then gamma rays. More about those bad boys in a minute.
On the other side of the spectrum, if you slow the frequency (stretching out the wavelength), your red light becomes invisible infrared (“below red”), then microwaves, and then radio waves. The electromagnetic waves of your favorite FM radio station are radiating only at somewhere between 88 and 107 MHz (megahertz, or million cycles per second). Do the math and you’ll find that each of those musical waves is about 3 meters (9.8 ft) long. That’s longer than you might expect, but remember that each of those light waves speeds by unbelievably quickly—if radio signals could bend, they could travel around the globe 7 times each second.
“It is sometimes said that scientists are unromantic, that their passion to figure out robs the world of beauty and mystery. But . . . it does no harm to the romance of the sunset to know a little bit about it.”
—Carl Sagan
We know of no longest wavelength in the universe. On Earth, telluric currents—extraordinarily subtle shifts in electromagnetic waves within Earth’s crust or oceans, often due to weather or even the silent interaction between the solar wind and Earth’s magnetosphere—may be as slow as a few hundred cycles per second. Waves at that rate are called extremely low frequency (ELF). Even longer, in the field of ultra low frequency, are the intricate but slow-flipping waves of our own brains. Each message that fires through the brain’s electrochemical connections has an electromagnetic result—we are each walking transmitters, though fortunately our signals are weak.
As the frequency drops even lower, to a single cycle each second (1 Hz), such as can be found deep in the movements of Earth itself, the wavelength becomes stretched out across 300 million meters—about 80 percent of the way from Earth to the moon.
It’s All Energy As light radiates, it carries energy. You might even say that light is energy in motion. And the amount of energy transmitted through light is based entirely on the light’s frequency or wavelength. The higher the frequency, the more energy. In other words, visible light contains more energy than infrared or radio waves. However, more energy doesn’t necessarily result in effects you might expect.
“To gaze is to think.”
—Salvador Dalí
While the light we can see will illuminate us, it doesn’t warm us much. Most visible light bounces off our skin or is absorbed in ways that don’t translate into heat. On the other hand, we are warmed by infrared light; it’s invisible and transmits less energy, but it penetrates deeper, and is quickly absorbed by many materials, causing them to warm up.
Lower the frequency even more and you get microwaves, which can melt butter in seconds! Turn on a microwave oven and you send invisible electromagnetic radiation through the air to your food. This light energy has a frequency of 2.45 GHz (gigahertz, or billions of cycles per second)—lower than infrared but still far higher than radio waves. The choice of frequency results in some interesting effects: Microwaves at these frequencies are absorbed by particular kinds of molecules, such as those in water, fats, and sugars, causing them to vibrate, bang into each other, and heat up. The waves just pass by other molecules, such as the dry exterior of a kernel of popcorn or a potato, leading people to exclaim, erroneously, that microwaves cook from the inside out.
If you worry about those microwaves escaping the oven, fret not: Waves at this frequency are about 122 millimeters (4.8 in) long—far larger than those of visible light. So the visible light waves can escape through those tiny holes in the glass door, letting us see our food being cooked, while the long microwaves remain inside, bouncing around the chamber.
When you hear that microwaves are electromagnetic radiation, you might get nervous about that word: radiation. After all, it’s touching stuff you’re going to put in your mouth. Fortunately, EMR at the energy of visible light and lower is safe because it’s non-ionizing. Ionizing radiation in high-frequency, tiny-wavelength light (from ultraviolet light to X-rays and gamma rays) packs such an energy wallop that it can knock electrons right out of their atoms, making for an unstable chemical situation. These reactive atoms are called free radicals and are infamous for their destructive effects.
“A 60 W tungsten bulb, a normal household bulb, consumes more than six times the electrical power of a 9 W compact fluorescent lamp but they are both perceived as producing approximately equal amounts of light . . . This is because a lot of the power used by a tungsten bulb is given out in the infrared part of the spectrum where the eye has no response. The light given out by the fluorescent lamp corresponds more closely to the peak sensitivity of the eye.”
—UK National Physical Laboratory
It’s the infrared that makes you sweat on a hot day, but it’s the ultraviolet that can give you a sunburn or worse. In the upper atmosphere, seemingly innocuous chlorofluorocarbons, escapees from old refrigerators and air conditioners, are bombarded with ionizing ultraviolet light from the sun. As electrons are blasted off, the newly radicalized molecules do their best to destroy our planet’s ozone layer.
It’s the ozone layer, of course, that has traditionally helped block much of that same dangerous light from getting down to us. But inevitably some high-powered ultraviolet light pushes through anyway, striking our skin hard enough to break down the fundamental building blocks that keep us alive. When atoms in our DNA are radicalized, the result can be mutant or cancerous cells, so damaged that our internal defense mechanisms can no longer repair them.
You can run inside, but you can’t hide. Glass reflects, absorbs, or scatters about 37 percent of low-powered ultraviolet light (called UVA)—that’s about as good as putting on sunscreen, but you can still get a sunburn inside your car on a long drive. Small doses of the more powerful (higher-frequency) UVB rays are good for us—they get our body to produce essential vitamin D, but too much exposure is a major cause of melanoma.
Scientists measure the energy in light by electron volts (eV). For example, the light we can see contains only 1 or 2 eV. At 3 or 4 eV, light becomes ionizing. When the light waves speed up to about 30 PHz (petahertz, or 3 × 1016 cycles per second), they carry charges in the hundreds of electron volts. This kind of light has such peculiar properties that the researchers who first discovered them labeled them “X” rays. So powerful, with wavelengths so small, X-rays can slip between molecules of soft material like our skin and organs, stopping only when they encounter dense material such as metal or bone.
Sure, X-rays leave a path of destruction in their wake, but with short, infrequent doses the risk is low and your body generally repairs minor damage. Most of the X-rays we encounter in our lives are natural: exposure to tiny bits of radioactive rocks in the earth, radiation from the sun, and so on. Again, score one for our upper atmosphere, protecting us from the worst of it.
But as the energy in light increases, so does the danger. When you boost the energy into the tens of thousands of electron volts, the light wavelengths are reduced to picometers—trillionths of a meter, even smaller than atoms. This is the realm of gamma rays.
“Light is not so much something that reveals, as it is itself the revelation.”
—James Turrell, artist
Gamma rays are just another form of light, but with electromagnetic frequencies measured in exahertz, over a thousand times greater than some X-rays and a million times greater than visible light. Doctors can shine gamma rays emitted from radioactive materials on our body to create incredibly detailed pictures of what’s going on inside tissue or bone, or concentrate these rays on an area of cancerous cells to destroy them. Customs officials can bombard shipping containers with gamma rays to “look” through 18 cm (7 in.) of steel and find stowaways or contraband inside.
“Every man takes the limits of his own field of vision for the limits of the world.”
—Arthur Schopenhauer
Like X-rays, gamma rays naturally occur all around us in tiny amounts. When government officials attempt to locate nuclear material with gamma ray detectors, they’re often stymied by a wide variety of food. Bananas and Brazil nuts, for example, tend to have higher than average quantities of naturally occurring radioactive material, causing false positives for investigators. It gives new meaning to getting high energy from eating fruits and nuts.
Of course, while these ubiquitous gamma rays are powerful, they’re paltry compared to the far end of the electromagnetic spectrum, where gamma rays carrying 20 million electron volts flash from the tops of thunderclouds during lightning storms here on Earth. Traveling outside our terrestrial bubble, the most energetic phenomena in the universe—black holes and supernova—blast out gamma rays that radiate at 1027 Hz and more than 5 trillion electron volts.
If these numbers seem extraordinary, it’s worth putting them in perspective. It takes 1 joule3 of energy to lift an apple off a table, and 1 joule is about 6 exa-electron volts—6 billion billion (6 × 1018) eV. In other words, light is extremely powerful . . . to extremely small things. Light can shatter the infinitesimal world of subatomic particles, but it exerts hardly any pressure on our everyday “macro” world.
The sun is like a 4 × 1026 watt lightbulb. That’s brighter than the average star, but there are far brighter ones out there. Epsilon Orionis (the middle star of Orion’s belt) is 1,300 light-years away and 400,000 times as bright as the sun. There’s a star in the Large Magellanic Cloud called R136a1 that is as bright as almost 9 million suns. Of course exploding stars, called supernovae, are even brighter; the brightest on record peaked at about 100 billion suns.
That said, with enough light you can achieve the seemingly impossible. Proof came in 2010 with the launch of the Japanese spacecraft IKAROS (Interplanetary Kite-craft Accelerated by Radiation Of the Sun). Outfitted with a microthin solar sail 14 m (45 ft) wide filled with pressure from gentle waves of light, this lightweight unmanned vessel slowly, methodically gained momentum outside the drag of Earth’s gravity, like Aesop’s tortoise. Once considered the stuff of science fiction, IKAROS has already sailed past Venus on the force of light, and solar sailing is thought by many to be the future of interplanetary travel.
“In the beginning there was nothing. God said, ‘Let there be light!’ And there was light. There was still nothing, but you could see it a whole lot better.”
—Ellen DeGeneres
A Discrete, Not a Continuous Spectrum So far we’ve been discussing light as though it were simply “wave energy,” but the bigger picture is far more weird.
If you reduce the energy of light, you would expect a smooth continuum, smaller and smaller, like turning down the volume on a stereo until you finally hit zero. But light doesn’t work like that. It turns out that light can exist only at particular energy levels—as though the volume knob had notches in it at 3, 2, 1, and 0, nothing between.
The only reasonable explanation for this is that light, at its core, is made of particles. A single particle of light, called a photon, is like a little packet with a discrete amount of energy.
The intensity of light involves the number of photons, but the energy of the photons is something completely different. Imagine a photon as a Ping-Pong ball. If the ball is moving slowly when it hits you, you barely feel it. Now let’s increase the intensity by throwing 100 slow-moving Ping-Pong balls at you. More pressure, but because each ball is low powered, it isn’t much more than annoying. Now let’s shoot just one of these little balls out of a cannon at you. Ouch.
Photons are all moving at the same speed (the speed of light), but they contain different energies—what we’ve expressed before as frequencies or wavelengths. So a low-frequency photon doesn’t deliver much kick, but a high-frequency photon can pack a wallop.
Unfortunately, there’s a problem with this argument: It’s relatively easy to prove that light absolutely, positively behaves like a wave, not a particle. The fact that light refracts (bends) when moving from one medium to another; the fact that it diffracts (like ripples in a pond interacting with a stick poking through the surface) . . . waves do these things, not particles, which travel in straight lines. But if you fire light through two slits in a piece of cardboard, and use very careful measurements, you can determine that each photon is going through only one or the other slit, so there’s also no doubt that it behaves like a particle.
You could dismiss this paradox, called the wave-particle duality, as just another wacky this-and-that fact of nature, like the old Saturday Night Live joke “It’s a floor wax and a dessert topping.” But actually, this is one of the greatest and most troubling mysteries in science today. It calls into question everything we think we know about the universe. We like to think that “stuff” is here or it’s not, that it’s matter or it’s energy, but in fact everything is likely both: here and not, matter and energy. And if that doesn’t confuse you, you don’t understand it.
Fortunately, while the scientists and philosophers are arguing over the nature of reality, we needn’t fully understand light in order to see it, measure it, and even use it to our advantage.
▲ Sending one photon toward a board with two slits and observing to see which slit it passes through, you can prove that it acts like a particle, traveling through one slit or the other.
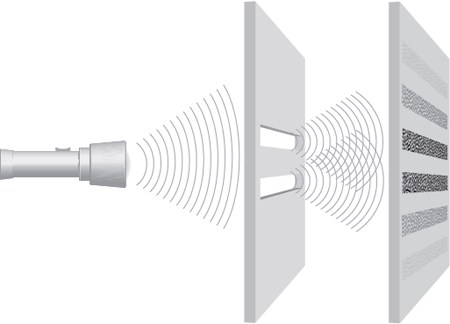
▲ If you don’t watch, or if you send many through at the same time, the light acts like a wave, diffracting and creating interference patterns.
What We See The majority of what we humans understand is due to light, whether the reflections of the physical world around us or the glimmer of far-off stars. We gather information from what we see with our eyes and—perhaps even more important—our photosensitive instruments that can detect the invisible light around us.
“Yet mystery and reality emerge from the same source. This source is called darkness. Darkness born from darkness. The beginning of all understanding.”
—Lao-tzu, Tao Te Ching
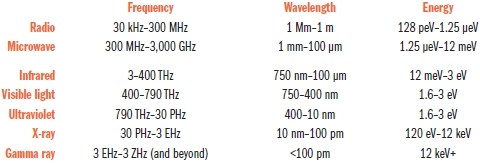
Of course, color doesn’t actually exist in the universe. We see color only because our eyes can register light at certain wavelengths and our brain attempts to make sense of those wavelengths by assigning them a visual meaning.
As we’ve seen, our ability to sense electromagnetic radiation is limited to a tiny range of wavelengths starting at about 380 nm (which we see as violet) and extending to about 750 nm (red). In musical notation, an octave is a doubling of a sound wave’s frequency, and if you do the math, our spectrum of visible light equates to only about a single octave. Compare that to our 10-octave range of hearing, or the 45 octaves between AM radio waves and gamma rays.
The only animal that can see both infrared and ultraviolet light is the goldfish.
Nevertheless, we can sense that little segment called visible light because of four particular types of nerve cells that we’ve developed in the tissue along the rear wall of our eyes: three types of cone cells and one rod cell—each named for its general physical appearance under a microscope. All are sensitive to light—that is, they can absorb electromagnetic energy and transmit it as a signal to the brain—but each is tuned to different wavelengths.
The three kinds of cones are most sensitive to light waves in the red, green, and blue frequencies, though each cone can also pick up a wide range of light. So, for example, the “green” cone can pick up some blue, yellow, and red, but it’s most sensitive to the wavelengths we see as lime green. When light enters the eye, the cones quickly react, sending signals to the brain, which combines them—first finding edges (areas of widely different color contrast), then filling in the rest with color details until we determine what we’re looking at.
Rods are far more sensitive, but they work best in very low light. Able to respond to even a single photon entering the eye, rods excel at night vision and for sensing very quick, small motions. They also tend to “wake up” more slowly than cones. For example, when you walk into a dark room, like a cinema, it can take several minutes for your eyes to adjust: Your cones aren’t receiving enough light to function well, and your rods need time to get activated. After five minutes, your rods are working great, but you can barely make out any colors—just areas of light and dark.
“The principal person in a picture is light.”
—Edouard Manet
Even more telling is the placement of rods and cones on the retina: Your eye contains about 6 million cone cells densely packed into the center, directly behind your pupil and lens. Surrounding the cones are about 100 million rod cells, like a ring around a bull’s-eye target. This explains why, when walking outside on a dark night, you might see a star shimmer in your peripheral vision: The very dim light hitting the outside edges of that target excites the rods but is often nowhere near bright enough to see when you try to focus your less-sensitive cones on it. Conversely, when you’re in good light and you want to discern color or detail (such as these words), you need to look directly at it, focusing the image on your cones.
“The French philosopher Auguste Comte demonstrated that it would always be impossible for the human mind to discover the chemical constitution of the stars. Yet, not long after this statement was made the spectroscope was applied to the light of the stars, and we now know more about their chemical constitution, including those of the distant nebulae, than we know about the contents of our medicine chest.”
—Edward Kasner and James Newman, Mathematics and the Imagination
Granted, when we talk about humans, there are always exceptions. Some people lack one type of cone, resulting in what we call color blindness. They can still see color but must make do with only two signals instead of three. Conversely, some people—primarily women—have developed a mutant fourth cone. Where most of us are trichromats, these people are tetrachromats, able to see more colors—or, more accurately, more distinctions among colors, especially in the red to yellow tones. A tetrachromatic mother might be better suited to seeing tiny changes in a child’s complexion or perhaps even notice subtle infrared heat radiating from a fever in a way that most of the rest of us could not.
It’s unclear how many bands of color a tetrachromat sees looking at a rainbow. When Isaac Newton first used a prism to split white light into a rainbow in 1672, he named the five colors that most of us identify: red, yellow, green, blue, and violet. Later, however, in an effort to synchronize the spectrum with the seven notes of a Western musical scale, he somewhat arbitrarily inserted two additional bands of color: orange and indigo. Thus the primary-school mnemonic was born: ROY G. BIV.
Today, few people identify indigo as a hue separate from blue or violet. That doesn’t mean we no longer see that color, but most of us probably don’t call it out as notably different from the colors around it.
Curiously, we can identify colors that are not in the spectrum at all. Most notable is magenta—that hot pink color found on fuchsia flowers and in nearly every color printer in the world. You can easily create magenta on a computer screen by mixing red and blue light. Our eyes pick up the red and blue wavelengths and our brains mix them together. The result should be halfway between red and blue, but on the light spectrum that color is green! We can tell that the color we’re seeing isn’t green, so, in a that-does-not-compute moment, the brain makes up a color to see: magenta.
Messages in the Light If light is energy in motion, then it is information in motion, too. At its simplest, someone might light a bonfire on a hill fifty miles away in order to warn his tribe of danger—the information from the light is able to travel far faster than a messenger or even sound. If the tribe had particularly clever gadgetry, such as telescopes and prisms, they might even be able to learn what the folks on the hill were burning. This is due to a curious (but incredibly helpful) phenomenon: Different elements, when heated, give off specific wavelengths of light. Sodium gives off a different pattern of frequencies than carbon or hydrogen.
Armed with this knowledge, we can point our telescopes toward the sun and stars, carefully analyzing the light we capture and learning things we would not otherwise know: what the sun is made of, where black holes are hiding, how light follows the warped fabric of space-time as gravity bends reality. Our ability to tease apart light, to reveal its makeup, is called spectroscopy.
Of course, the sun and stars (and everything else in the universe) exhibit more than visible light. Radio waves and microwaves reach out across the cosmos, helping us map the solar system and the constellations. X-rays and gamma rays help us determine massive centers of otherwise invisible energy in the universe, such as pulsars and quasars. Everywhere we turn our instruments, we gather information, looking for understanding, searching for meaning.
And if we can find answers in the spectrums of natural light, then we can also encode our own messages in light that we create. The trick to doing this is called modulation: taking a known wave and adjusting it over time.
“What is essential is invisible to the eye.”
Antoine de Saint-Exupéry, The Little Prince
For example, let’s say you tune in to an AM station at 700 on your radio dial—that’s 700 kHz, or an electromagnetic wave oscillating back and forth at 700,000 cycles each second. AM stands for “amplitude modulation,” which means the signal—the music, or the sportscaster yammering away—is encoded by increasing and decreasing the volume or intensity very slightly but very quickly. As we learned earlier, a light’s intensity (its strength, or amplitude) is based on how many photons are being transmitted. Your radio senses those tiny changes and converts them into (you hope) a pleasant sound.
Switch to 103.7 on the FM dial, and your radio begins to pick up light waves at 103.7 MHz, or 103,700,000 cycles per second. Here you experience a different kind of signal: “frequency modulation,” in which the frequency of the wave is altered up and down while maintaining its intensity. (More precisely, one wave with a varying frequency is merged with another, steady wave, and the result—an extremely complex wave—is transmitted, received, and pulled apart again.)
Awash in Light Radio broadcasts, satellite television transmissions, cell phone conversations, GPS signals, police and fire alerts, WiFi computer networks, radio-controlled toys, airport radar, garage door openers—we are constantly bombarded by light of varying wavelengths, energies, frequencies. And yet, strangely, most of this human-made radiation passes right through our bodies, even through the walls of our buildings, without affecting us or even slowing down. Why?
Light—which, remember, is made of photons, those tiny packages of energy that behave like both waves and particles—is unique in the universe in its ability to be both exceedingly tiny and extremely large. This range of size largely explains its ability to travel. Because each wave from an AM radio station is longer than a football field, the relatively small and sparse molecules that make up a wall don’t have enough presence to impact it much. But line the wall with thick metal—a material with dense connections of electrically bound atoms—and those long waves can’t get through.
You see, matter (like an atom or a molecule) can interact with a light wave in one of three ways: let it pass by, absorb it, or bend it. Which of these occurs is based on the wavelength of the light versus the type, size, density, and structure of the material. An X-ray has a tiny wavelength, so it passes through our skin like a bike zooming through a forest of widely spaced trees. Every so often it might hit a branch and cause a little damage, but in general it won’t stop until it reaches a thick bramble, like the molecules in your bones. At that point, the light will likely be either bent (forcing a quick change of course) or absorbed (like a bike crash).
Microwaves, as we saw earlier, are far longer than X-rays—small enough to be easily absorbed by some food molecules but large enough that they cannot escape from the oven into your kitchen.
Visible light, on the other hand, consists of wavelengths that are just the right size to be absorbed or reflected by most matter around us. This is, as scientists like to say, evolutionarily advantageous: If our eyes were tuned to see radio waves instead, we’d be constantly banging into “invisible” things around us. But because we see the visible light spectrum, a ripe banana tends to absorb the “blue” wavelengths and reflect “red” and “green” waves into our eyes, causing us to see a yellow object.
Rocks appear solid because they, too, reflect and absorb various frequencies of light. But if you grind rocks to sand and melt the sand to make glass, you’ve changed the molecular structure so radically that wavelengths of visible light can now pass through it largely unimpeded. Yet those same molecules that let visible light pass through are also absorbing the slightly shorter wavelengths of ultraviolet light. So the size of the wave matters, but not as much as the material composition.
Now You See It . . . Light is such an inherent part of our everyday experience—whether through the colors we see or the heat we feel—that it’s easy to forget how fundamental it is to the underlying structure of our universe, and how little we truly understand it. After all, electromagnetism is not just the study of magnets and generators and light waves. It is considered one of the four fundamental forces of nature, along with gravity and the strong and weak nuclear interactions. These are the most basic physical forces that hold our universe together and that cannot be described by any other, further reduced explanation.
Electromagnetic force pulls atoms into molecules and holds them together across enormous (to an atom) distances, forming what (from our size) appear to be solid objects. Without that subtle attraction, your chair, your floor, you, Earth would come apart as nothing but gas. At the core of this electromagnetic force is the lowly photon—called a quanta by Einstein as he laid the groundwork for quantum mechanics. Photons—known as the carrier particles of electromagnetism—are literally what make our universe possible.
Light—the oscillation of electromagnetic waves, the transmission of photons—is like the lifeblood of the cosmos, carrying packets of energy from one atom to another, or from one galaxy to another, through billions of years of space. Although it appears from our perspective that there is no medium in the vacuum between the stars (or between the atoms) through which these waves could be held and passed, that misses the point: Space itself is the medium. We are the medium. And we are the receivers.
Additional Material