Introduction
Cardiac disease is common in pet ferrets (Mustela putorius furo) [1–5]. The clinician should expect the full spectrum of cardiac diseases in ferrets found in small companion mammal practice. Dilated cardiomyopathy (DCM), arrhythmias, and acquired valvular disease (AVD) are the most common heart diseases this author sees in practice. Congenital defects in heart development are seldom reported, but atrial septal defect, ventricular septal defect, and Tetralogy of Fallot occur [6–8]. Inflammatory myocardial diseases including toxoplasmosis, Aleutian disease virus, fungal and bacterial sepsis are rarely diagnosed in practice. Neoplasia of the heart is rarely reported, with lymphosarcoma being most common. Pericardial fluid from chyle or neoplasia (lymphosarcoma) occurs, with few reports in the literature. Nonbacterial thrombotic endocarditis is associated with myxomatous aortic valve degeneration [9]. Ferrets are definitive hosts for Dirofilaria immitis, and heartworm disease should be on the differential list in endemic areas.
Clinical presentation of cardiac disease in the ferret ranges from an asymptomatic, incidental finding to fulminant heart failure [1–4]. Ferrets with clinical heart disease are generally weak, exercise intolerant, dyspneic, and often have pale or cyanotic mucous membranes with prolonged capillary refill time. Ferrets occasionally cough from pulmonary edema, heartworm infections, or compression of the main stem bronchi from an enlarged heart. Rear leg weakness can be seen and is thought to be associated with general weakness or congestive heart failure (CHF) and associated hypoperfusion. Left-sided CHF may produce crackles associated with pulmonary edema, and right-sided CHF may result in decreased lung sounds associated with pleural effusion, ascites, or organomegally. Respiratory rate may be increased, especially if thoracic effusions are present. Heart murmurs are common with DCM and valvular insufficiency but are uncommon in hypertrophic cardiomyopathy (HCM). Clinicians may have difficulty determining the origin of heart murmurs. Often, only a nonspecific left parasternal systolic murmur is heard. Holo-systolic murmurs are most often caused by valvular regurgitation. Left apical murmurs are usually of mitral valve origin, and right parasternal murmurs may be tricuspid valve insufficiency. Heart murmurs not only vary in location and duration but intensity. Anemia can cause low-intensity, variable location murmurs in ferrets due to low fluid viscosity. The normal heart rate is between 180 and 250 bpm. Sinus arrhythmia may produce a pronounced decrease in heart rate with occasional pauses ausculted; this should not be confused with pathologic bradycardia. Increased heart rate (>250 bpm) and gallop rhythms (third and fourth heart sounds ausculted) are common with DCM and HCM, respectively. Tachyarrhythmias can be seen with either form of cardiomyopathy or AVD. Arrhythmias due to premature ventricular contractions (PVCs), second-degree or third-degree heart blocks may be the only physical exam finding and should be thoroughly evaluated to determine their cause and significance. Sinus rhythm, sinus tachycardia, and bradycardia due to second-degree heart block can be seen in normal ferrets, asymptomatic ferrets with cardiac disease (Fig. 18.12A), and ferrets with clinically evident cardiac disease. Ferrets with third-degree heart block generally have clinical signs of cardiac disease [10].
Cardiomyopathy is common in middle-aged and older domestic ferrets. DCM is most common, but HCM is occasionally seen. An arrhythmogenic form of cardiomyopathy with little or no ventricular dilation or hypertrophy is probably a variant of DCM in which the conduction system is the primary site of degeneration and fibrosis [Bruce Williams, personal communication, 2008]. Histologically, cardiomyopathies have myocyte degeneration, myofiber loss, and occasional inflammatory infiltrates which eventually lead to fibrosis and myocardial dysfunction. Although DCM was an infrequent echocardiographic diagnosis in a recent retrospective study of ferrets (compared with valvular disease), it was highly associated with CHF [10]. Most cases of ferret cardiomyopathy have a good prognosis with proper diagnosis and treatment and may live a year or more from initial diagnosis. However, cases of HCM have a poor prognosis, with sudden death and a survival expectation of only months after diagnosis.
The cause of cardiomyopathy is unknown or idiopathic in ferrets. In humans, cardiomyopathy may occur as a result of preexisting endocrine diseases, viral disease, toxicities, and nutritional deficiency. In cats, DCM may be prevented and treated with the addition of taurine to the diet, and in certain dog breeds, carnitine improves DCM. Similar dietary relationship may exist in ferrets, but this has not been proven. In humans, 40% of DCM and most HCM are thought to be genetic in origin [11]. Certain breeds of dogs have a genetic link to DCM, and the Maine Coon cat has a genetic basis for HCM. There is accumulating evidence in cats that most HCM is genetic in nature, with DNA coding aberrations resulting in shortened telomeres and apoptosis of cardiomyocytes leading to myocardial dysfunction despite minimal inflammation [Bruce Williams, personal communication, 2008]. The inflammation seen may be the result of, and not the cause of, myofiber loss. Idiopathic cardiomyopathy may have a similar genetic association in the ferret. Other known causes of cardiomyopathy in the ferret are limited, but in one ferret, cardiomyopathy was associated with a cryptococcal infection [12].
DCM is characterized by increased diastolic dimension of the left and right ventricles due to myocardial dysfunction [13]. The normal afterload of systemic blood pressure does not allow normal end systolic emptying of the diseased ventricle so the ventricle remains dilated during and after systole. Indices of function such as fractional shortening (FS) are decreased, and indices of heart size such as end diastolic volume and end systolic volume are increased. Ventricular wall thickness is decreased despite an increase in cardiac mass. The atria often dilate due to increased left ventricular filling pressure (LVFP) and decreased diastolic blood flow through the mitral valve; myocardial remodeling occurs subsequent to the increased pressure and chamber dilation. Increased atrial chamber workload promotes increased atrial chamber dilation and fatigue, which is an initiating factor in CHF. Left atrial chamber dilation and CHF are common consequences of DCM and valvular disease in ferrets. Lowering LVFP is essential for controlling CHF in humans, cats, and dogs. An S3 gallop is due to the rapid filling of a dilated left ventricle (DCM or chronic volume overload in mitral valve disease). S3 is very specific for an elevated LVFP and is highly predictive of CHF in humans [14]. Systolic heart murmurs and S3 gallop rhythm often improve with therapy in ferrets, suggesting that LVFP is lowered and that CHF is averted. In some cases. CHF is manifested as right-sided CHF with abdominal and/or pleural effusion, or as left-sided CHF with pulmonary edema. It is unclear which ferrets will develop each form of CHF, but both conditions are seen in clinical cases.
Hypertrophic cardiomyopathy in the ferret is hyperplasia of individual muscle fibers of the heart, primarily the left ventricle. There is gross thickening of the septum, left ventricle free wall (LVFW), and decreased left ventricular dimensions. The heart size can be normal on radiographic projections with HCM, but chamber sizes of the heart decrease, reducing ventricular filling and cardiac output. These small chambers are apparent on ultrasound (US) examination or at autopsy when the heart muscle is grossly sectioned (Fig. 18.1). US is used to diagnose this form of cardiomyopathy and formulate a treatment plan to increase ventricular filling and cardiac output.
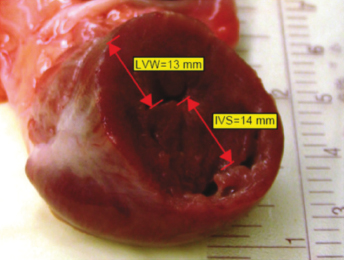
Because of the rarity of clinical cases of HCM and the presumed low prevalence in ferrets, little is known about effective therapy for this condition either prior to, or following the onset of CHF.
Treatment is aimed at improving diastolic filling of the ventricles and eliminating CHF if present. Beta blockers slow the heart rate, and calcium channel blockers reduce afterload and vasodilate coronary arteries, increasing myocardial perfusion, and reducing heart rate and blood pressure. This combination of drug effects reduces myocardial oxygen consumption and increases cardiac output. Cardiac output and coronary flow (myocardial oxygen supply) must meet oxygen demand or anaerobic conditions prevail. An anaerobic myocardium results in reduced contractility and dysrhythmias commonly seen in HCM. Diuretics and angiotensin-converting enzyme (ACE) inhibitors are used for treating CHF. Aspirin or heparin therapy is not needed because arterial thromboemboli as seen in cats with HCM is rarely seen in ferrets.
Restrictive cardiomyopathy is rare with two cases reported in the literature [10]. Practicing cardiologists report a left atrial enlargement with a normal appearing left ventricle.
Acquired valvular disease (AVD) or endocardiosis is a common heart disease seen in ferrets [10]. Valvular endocardiosis is a degenerative change of unknown cause affecting the subendocardial valve leaflets and chordae tendineae in the middle aged to elderly ferret. Endocardiosis leads to progressive valvular incompetency with regurgitation of blood across the closed valve and eventually CHF. The aortic and mitral valves are most commonly affected, with tricuspid and pulmonic valve regurgitation seen less frequently [10]. Gross lesions of advanced endocardiosis include opaque nodular thickening and shortening at the free edge and base of the valve leaflets. Mitral and aortic regurgitation leads to secondary dilatation of the left atrium and left ventricle, with the left atrium often being larger than the left ventricle. Aortic regurgitation may be a common incidental finding, and isolated aortic regurgitation appears to rarely cause CHF. However, when aortic regurgitation is concurrent with mitral regurgitation, or other abnormalities such as third-degree heart block, CHF may occur [10]. As cardiac failure progresses, left-sided CHF produces pulmonary edema and pleural effusion. The right ventricle and atrium can be dilated from tricuspid regurgitation and can progress to right-sided CHF, hepatosplenomegaly, and ascites.
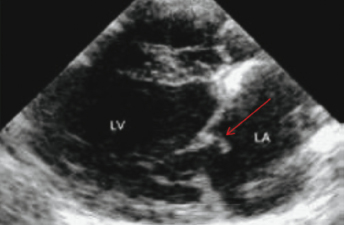
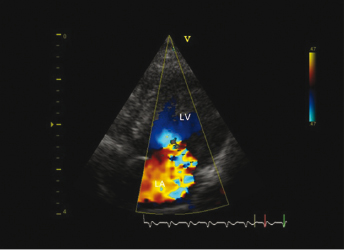
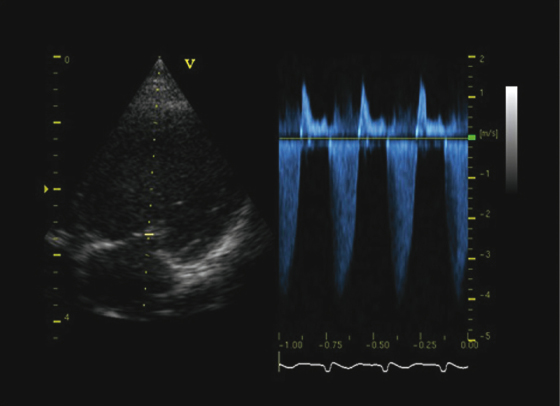
The primary differential diagnosis of chronic valvular heart disease in ferrets is DCM. US evaluation is essential to distinguish between the two disease entities and is based on the demonstration of affected valves and determination of ventricular contractility. Echocardiographic findings consistent with chronic valvular disease include cardiomegaly, thickened valves, and increased or normal FS with normal ventricular contractility (Fig. 18.2 and Fig. 18.3). An increased FS distinguishes this condition from DCM in which FS is often decreased. Valvular regurgitation is detected with color flow Doppler and pulse wave Doppler (Fig. 18.4 and Fig. 18.5). The regurgitant jet is generally large and often eccentric in ferrets with valvular disease and is less impressive and central with DCM, where it is due to a mild distraction of the mitral valve cusps and mitral annular dilation, rather than to primary valvular pathology. Small regurgitant jets of blood through the mitral, aortic, pulmonic, and tricuspid valves occur in clinically healthy ferrets and should not be mistaken as cardiac pathology during examination by US. Often, no murmur is heard with these small regurgitant jets.
Metabolic disturbances, often secondary to neoplasia, can manifest as cardiovascular (CV) disease. Hypoglycemia due to insulinoma, liver disease, or fasting can cause bradycardia in ferrets. Functional pheochromocytomas can cause sinus tachycardia, hypertension, and weakness. Antemortem diagnosis of pheochromocytoma may be difficult to differentiate from primary CV disease. Determination of blood pressure, US exam of the chest and abdomen, and determination of urine or plasma catecholamine levels can help differentiate these diseases (Table 18.1). Hypoadrenocorticism (Addison's disease) occasionally occurs in ferrets after bilateral adrenalectomy and can cause hypotension and hyperkalemia-induced cardiac arrhythmias. Hypothermia from anesthesia or cold exposure, hyperkalemia from urinary outflow obstruction, toxins, excess vagal tone, infectious endocarditis, and myocarditis can cause severe bradycardia and heart block. Treatment should be aimed at addressing the underlying cause, when possible.
Table 18.1. Normal Plasma and Urine Catecholamine Concentrations in Ferrets
Source: Wagner RA, unpublished data.
| Plasma (pg/m) n = 5 | Urine (mg/g creatinine) n = 4 | |
|---|---|---|
| Norepinephrine | 159 | 53.3 |
| Epinephrine | 64 | 28.2 |
| Dopamine | 62.8 | 5.5 |
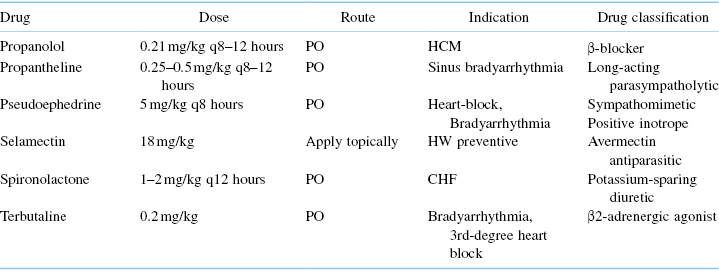
Bradyarrhythmias may be asymptomatic or have a history of lethargy, weakness, exercise intolerance, and syncope [15]. A low HR (<120 bpm) and an arrhythmia will often be identified in symptomatic ferrets; occasionally, a murmur is ausculted. With high grade second- and third-degree AV block, faint atrial beat sounds (S4) may be heard between the ventricular beats. CHF and hypoperfusion develop once heart rates consistently drop below 80 bpm. In ferrets with high degree second- and third-degree AV block, certain oral medications may raise the HR enough to improve clinical signs. Anticholinergics such as propanetheline, beta adrenergics such as terbutaline or isoproterenol, and phosphodiesterase inhibitors such as aminophylline and theophylline can be tried (Table 18.5). Most of these drugs are minimally effective at increasing the HR enough to improve clinical signs. Oral beta adrenergic agonists are incompletely or transiently effective because of first pass effect. Subcutaneous beta adrenergic agonists can effectively increase heart rate and reverse CHF, but their short half-life necessitates frequent administration. Pacemakers, transvenous, and epicardial implantation have been shown to effectively treat clinical bradycardia [15,16].
Heartworm disease in ferrets [2,5,17,18] is uncommon or possibly underdiagnosed, but more cases are being reported in D. immitis endemic areas, especially in ferrets kept outdoors. Ferrets in heartworm endemic areas should be maintained on monthly heartworm prevention. No drugs are FDA-approved for the treatment or prevention of heartworm disease in ferrets. However, drugs approved for dog or cat prophylaxis can safely and effectively be prescribed in an extra-label manner (Table 18.5).
Due to the small size of the ferret heart, as few as two heartworms may result in fatal cardiac insufficiency. If heartworms are present in the right ventricle or pulmonary artery at necropsy in any ferret, they are the likely cause of death.
Clinical signs of heartworm disease in the ferret are similar to those in the dog, but progress more rapidly, making early diagnosis extremely important. Caval syndrome has been reported in ferrets and is life threatening [19]. Clinical signs may include dyspnea, tachypnea, anorexia, pulmonary crackles, holosystolic heart murmur, ascites, coughing, pleural effusion, and sometimes sudden death. Clinical heartworm disease in ferrets presents similarly to other cardiac disease, is nonspecific, and must be considered in endemic areas. The efficacy of ELISA based on antigen detection is significantly limited by the fact that in most cases, a positive reaction requires the presence of at least two sexually mature female worms. US is often the best diagnostic test for heartworm infection in the ferret [19] (Fig. 18.6). Aberrant larval migration and consequent central nervous system signs have been documented in ferret D. immitis infection [20]. With a poor prognosis with adulticide treatment alone, transvenous heartworm extraction is a viable option [15,19,21].

Radiography is useful in diagnosing CV disease in ferrets. It is the standard for determining cardiac size and CHF (pleural effusion, pulmonary venous engorgement, and pulmonary edema). The appearance of the normal ferret radiograph is similar to that of other carnivores. The ferret's thoracic cavity and trachea are relatively long. The normal ferret's trachea is parallel or bent slightly ventral starting around the 4th or 5th thoracic vertebra. The heart is between the 6th and 8th ribs. Occasionally on the lateral radiographic projection, the heart appears “elevated” (“floating heart”) above the sternum, mimicking pneumothorax (Fig. 18.7). However, this may be a normal finding on a ferret radiograph.
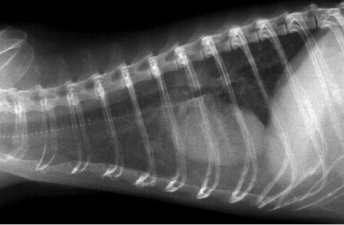
Radiographic signs of heart disease include a large globoid heart shadow, an elevated trachea, pleural effusion, and increased contact between the heart shadow and the diaphragm or sternum (Fig. 18.8). Other radiographic signs include pulmonary edema, pulmonary venous congestion, ascites, hepatomegaly, and splenomegaly. An enlarged heart shadow may indicate increased heart size, a mediastinal or cardiac mass, or pericardial effusion; echocardiography may be necessary to differentiate these lesions. Thoracic radiographs facilitate monitoring heart disease; the size of the heart and other thoracic changes become more pronounced as disease progresses.
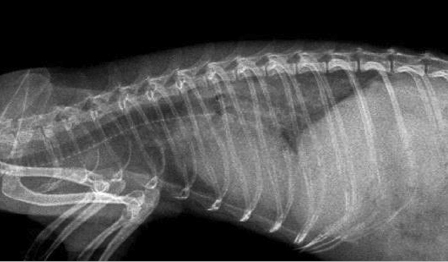
The modified vertebral heart score (VHS) method measures the heart size in vertebral units (Fig. 18.9 and Fig. 18.10) [22]. The right lateral view is preferred to detect and measure cardiac enlargement. The long axis of the heart is measured from the bottom of the main stem bronchus to the apex of the heart. The short axis is measured perpendicular to the long axis at the level where the caudal vena cava meets the heart on the right lateral view. The length and width of the heart are registered as the number of vertebrae and estimated to the nearest 1/4 of a vertebra. Calipers are placed at the onset of T5 and extend caudally parallel to the vertebral bodies to a length equal to the caliper measurements for the long axis. Place the calipers at the onset of T5 and extend it caudally, parallel to the vertebral bodies, to a length equal to the caliper measurements for the short axis. Add the number of vertebral bodies for the long and short axis to generate a VHS. Normal ferret VHS for the lateral view is 3.75 to 4.07 vertebrae. Clinicians should understand that VHS is not designed to diagnose cardiac disease, but to identify cardiomegaly (mainly secondary to volume overload). It is aimed more at reducing the rate of false positive diagnoses, but at the cost of more false negative diagnoses. Thus, the VHS does not improve the diagnosis of cardiac disease in dogs [23], and this may apply similarly to ferrets.

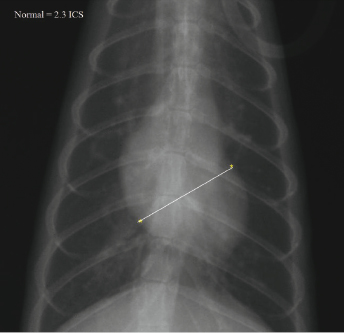
The heart shadow size can also be compared to the number of rib spaces, with ∼2.3 rib spaces being used as a gauge for normal (Fig. 18.8 and Fig. 18.9). In right lateral position, the short axis of the cardiac silhouette is measured perpendicular to the long axis at the level of caudal vena cava. The mean intercostal space (ICS) for ferrets is 2.28. The right lateral short axis ICS measurement is considered the best indicator of cardiomegaly and is predictive of cardiac disease [24].
In cases with pleural effusion, thoracic fluid cytology should be performed to differentiate cancer, pyothorax, and chylothorax. Thoracic masses usually occur in the cranial mediastinum and are common in ferrets. Lymphosarcoma is the most common type of thoracic neoplasia in the ferret, but other cancers occur.
Ascites, while less common than pleural effusion, is seen in later stages of CHF. Pulmonary venous congestion and pulmonary edema with a mixed interstitial and alveolar pattern containing air bronchograms is commonly seen in CHF. Hepatosplenomegaly may be seen with CHF, but noncardiac disease must also be considered.
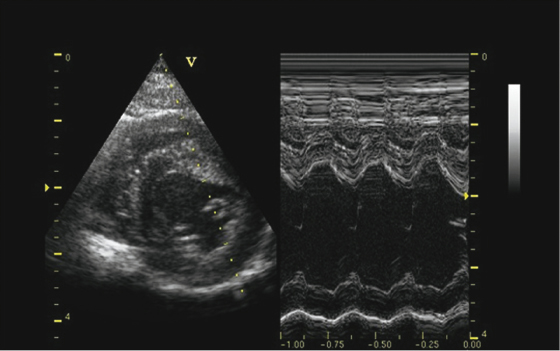
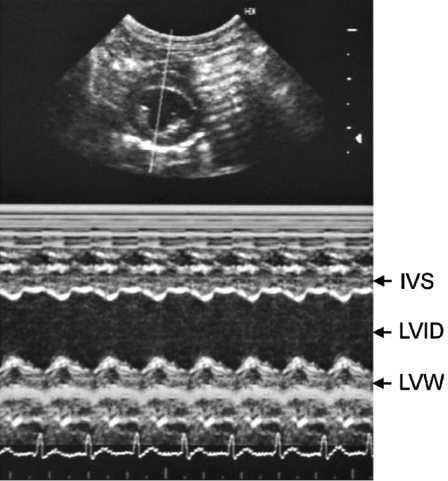
Ultrasound (US) is the method of choice for diagnosing structural and functional cardiac abnormalities. Pleural and pericardial effusions, heartworms, and mediastinal masses are readily identified with US. Echocardiography can be used to diagnose heartworms but may be of low sensitivity and requires good sonography skills. Heartworms appear as hyperechoic parallel bright lines within the right atria, ventricle, or pulmonary artery. US is essential in differentiating DCM from HCM and in tailoring treatment course. Standard M-mode measurements (Table 18.2 and Table 18.3; Fig. 18.11) include chamber dimensions, wall thickness, and indices of systolic function [25,26]. Body weight does not affect M-mode measurements [26]. In DCM (Fig. 18.12A), there is an increased left ventricular internal diameter in diastole and in systole (LVIDd and LVIDs). There is also decreased FS, and the left atrium is often dilated (left atrium appendage diameter, LAAD), the right ventricle can also be dilated, but this occurs less often. HCM is characterized by gross thickening of the septum (IVSd and IVSs) and left ventricular free wall (LVWd and LVWs) and decreased left ventricular chamber dimensions (LVIDd and LVIDs). The walls of the left ventricle may be thickened symmetrically or asymmetrically. FS may be normal, increased, or slightly decreased with HCM in ferrets (Fig. 18.12B).
Table 18.2. Echocardiographic Reference Values for Ferrets Anesthetized with Isoflurane
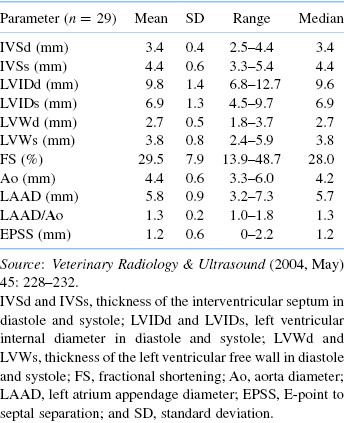
Table 18.3. Two-Dimensional, M-Mode, and Doppler Echocardiographic Values Obtained Normal Adult Ferrets Sedated with Ketamine and Midazolam [23]
| Variable | N | Mean (SD) |
|---|---|---|
| Age (years) | 30 | 2.3 (1.0) |
| Weight (kg) | 30 | 1.17 (0.36) |
| HR (bpm) | 29 | 273 (31) |
| IVSd (cm) | 30 | 0.36 (0.07) |
| IVSs (cm) | 30 | 0.48 (0.11) |
| LVIDd (cm) | 30 | 0.88 (0.15) |
| LVIDs (cm) | 30 | 0.59 (0.15) |
| LVWd (cm) | 30 | 0.42 (0.11) |
| LVWs (cm) | 30 | 0.58 (0.99) |
| FS (%) | 30 | 33 (14) |
| EF (%) | 27 | 69 (19) |
| RVWd (cm) | 27 | 0.12 (0.03) |
| RVIDd (cm) | 28 | 0.38 (0.10) |
| LA diameter (cm) | 26 | 0.71 (0.18) |
| AO diameter (cm) | 25 | 0.53 (0.10) |
| LA : AO | 26 | 1.33 (0.27) |
| PA diameter (cm) | 27 | 0.48 (0.9) |
| Doppler HR (bpm) | 29 | 280 (32) |
| AOmax (m/s) | 25 | 0.89 (0.20) |
| AO VTI (cm) | 25 | 5.15 (1.45) |
| AO CSA (cm2) | 25 | 0.23 (0.08) |
| AO SV (mL) | 21 | 1.19 (0.60) |
| AO CO (mL/min) | 21 | 330 (185) |
| AO CI (mL/min/kg) | 21 | 261 (117) |
| PAmax (m/s) | 29 | 1.10 (0.14) |
| PA VTI (cm) | 29 | 6.70 (1.05) |
| PA CSA (cm2) | 27 | 0.19 (0.06) |
| PA SV (mL) | 27 | 1.22 (0.5) |
| PA CO (mL/min) | 26 | 346 (145) |
| PA CI (mL/min/kg) | 26 | 296 (124) |
| Mitral E (m/s) | 15 | 0.70 (0.10) |
| Mitral A (m/s) | 15 | 0.52 (0.11) |
| Mitral E : A | 15 | 1.38 (0.32) |


AVD is characterized by thickening and increased echogenicity of any of the valves in older ferrets. The aortic and mitral valves are most commonly affected. The diastolic dimension of the left ventricle is increased, but the systolic dimension is normal to slightly increased, indicating normal myocardial contractility. This is due to the permissive effect of the mitral valve regurgitation, allowing the ventricle to eject against low atrial pressures while the aortic valve is closed. FS is normal to increased in AVD due to low-pressure regurgitation across AV valves. Regurgitation is best demonstrated using Doppler (color flow or pulse wave) methods.
Dehydrated ferrets may have an increased diastolic left ventricular interventricular septum (IVSd) and free wall thickness exceeding normal limits. Dehydrated ferrets may have a decreased LVIDd and LVIDs which can produce end-systolic cavity obliteration in ferrets. Altered hydration status may produce echocardiographic changes in ferrets as seen in cats and can lead to the erroneous diagnosis of cardiomyopathy [26].
Ferrets require little preparation for echocardiographic examination. Probes need to have a small footprint and be 7–10 MHz frequency. Image quality is often compromised by rib shadowing, rapid respiratory, and heart rates. At times, only a quick qualitative assessment of the ventricular contractility and wall thickness can be achieved in a ferret in CHF or in a fractious patient. Sedation is neither required nor desirable except in very uncooperative patients. If sedation is employed, the potential influence of the drug(s) on heart rate, chamber dimensions, and ventricular motion must be considered in the interpretation. Ideally, hair is clipped over the left and right precordial transducer locations. Satisfactory images can almost always be obtained by parting the hair coat on the sides of the chest and by applying a liberal quantity of coupling gel or by wetting the area with water or alcohol and then applying coupling gel. Ferrets can be examined in dorsal recumbency, scruffed, and held vertically or in laterally recumbent positions without substantial alteration of examination technique. However, the image quality is enhanced by positioning the animal in lateral recumbency on a table with an opening that allows transducer manipulation and examination from beneath the animal. This position results in the heart contacting a larger area of the lateral thorax and creates a larger US window for examination.
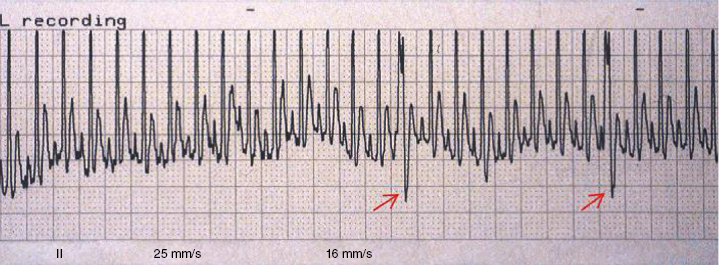
Electrocardiograph (ECG) is used primarily to determine abnormal rhythms and electrical conduction/propagation disturbances. Electrocardiographic data from four studies of clinically normal ferrets are presented in Table 18.4 [1,24,27,28]. However, in one study, experimentally induced right ventricular hypertrophy and corresponding right mean electrical axis deviation in ferrets were not detected by ECG [29]. S-T segment elevation is seen in normal ferrets and in ferrets with cardiac disease. Other changes in the QRS-T complex are seen in normal ferrets and in ferrets with cardiac disease, but the significance of some of these changes remains unknown and requires additional sudy. Generally, the ferret QRS complex resembles that of the dog (tall R waves), and the P waves are small as in the cat (Fig. 18.13). Right lateral recumbency is the standard position for ECG recording. Sternal positioning changes mean electrical axis, P wave amplitude, R wave amplitude in leads I and II, and Q (or S) wave amplitude in lead I [28]. Often, only a single lead (lead II) is needed to identify and characterize an arrhythmia. With cardiac disease, sinus tachycardia is the most common rhythm (Fig. 18.14); occasionally premature atrial contraction (PAC) and PVC occur. Atrial fibrillation has not been reported in the ferret. Second- and third-degree heart (AV) blocks are the most common conduction disturbances and can cause life-threatening bradycardia (Fig. 18.15 and Fig. 18.16). Severe bradycardia (<80 bpm) carries a poor prognosis, and ferrets often die in a month or two of CHF. Ferrets with severe bradycardia can be treated with isoproterenol, pseudoephedrine, metaproterenol, or with an implantable pacemaker (Fig. 18.15 and Fig. 18.16) [16,30]. It appears that an intrinsic rate of 50–80 bpm must be treated to prevent progression to CHF. If a ferret with bradycardia is not weak, inactive, or in CHF, there may be little improvement with treatment. Ferrets with a second-degree heart block and presenting heart rates 100–160 bpm may not warrant positive chronotrope treatment (Fig. 18.16) or pacemaker implantation. Arrythmias can also be seen in the context of anesthesia (Chapter 12).
Table 18.4. Electrocardiographic Data from Clinically Normal Ferretsa [1,21,25,26]