6
Food Forensics Cases Related to Food Bioterrorism Poisoning using Pathogenic Viruses
Viruses are infectious particles (agents) that lack the capability of self‐replicating but can do so when they gain access to the inside of living cells of other organisms such as animal cells, plant cells and even microbial cells such as bacteria and archaea (Koonin et al., 2006).
There are different types of viruses which are grouped based on different criteria. One grouping is based on how the nucleic acid genetic material that is present inside the virus particles replicates. One of the virus types is the RNA viruses, in which the replication of their genetic materials takes place in the cytoplasm. The viruses in which the replication of genetic material takes place in the nucleus of the host cell belong to the type known as DNA viruses. There are several groups within the DNA viruses, with those with the capability to penetrate the host’s cell membrane by diffusion controlled mechanisms through the receptors that may be present on the surface of the cell membrane of the host, and those that penetrate the cell membrane of the host through receptor‐facilitated endocytosis mechanisms. Other types of viruses comprise those with single‐stranded RNA, which are known as reverse transcribing viruses (Staginnus and Richert‐Pöggeler, 2006).
Pathogenic Influenza Viruses and Food Forensics
Influenza viruses are a major concerns because of their potential to be used in food bioterrorism acts. In the 1990s to the 2000s, the world experienced outbreaks of different types of influenza viruses such as avian (e.g. highly pathogenic avian influenza A virus, H5N1, avian influenza A, H7N9 virus, H1N1) flu, swine flu, the virion that caused bovine spongiform encephalopathy (BSE), commonly known as mad cow disease, and when in humans the variant of this disease is known as Creutzfeldt–Jakob disease, etc. (WHO, 2008).
Avian Influenza Viruses and their Potential Application in Food Bioterrorism
Avian influenza viruses are normally grouped into Influenza virus A, Influenza virus B and Influenza virus C. Influenza A viruses are further subdivided into several subtypes based on two proteins or antigens; hemagglutinin (HA) and neuraminidase (NA), which are found on the surface of the virus (Figure 6.1). For example, an influenza virus subtype H7N2 has an HA 7 protein and an NA 2 protein; the subtype H5N1 has an HA 5 protein and an NA 1 protein, etc. These are among the numerous influenza virus subtypes that have been reported to contain a mixture of HA and NA proteins.
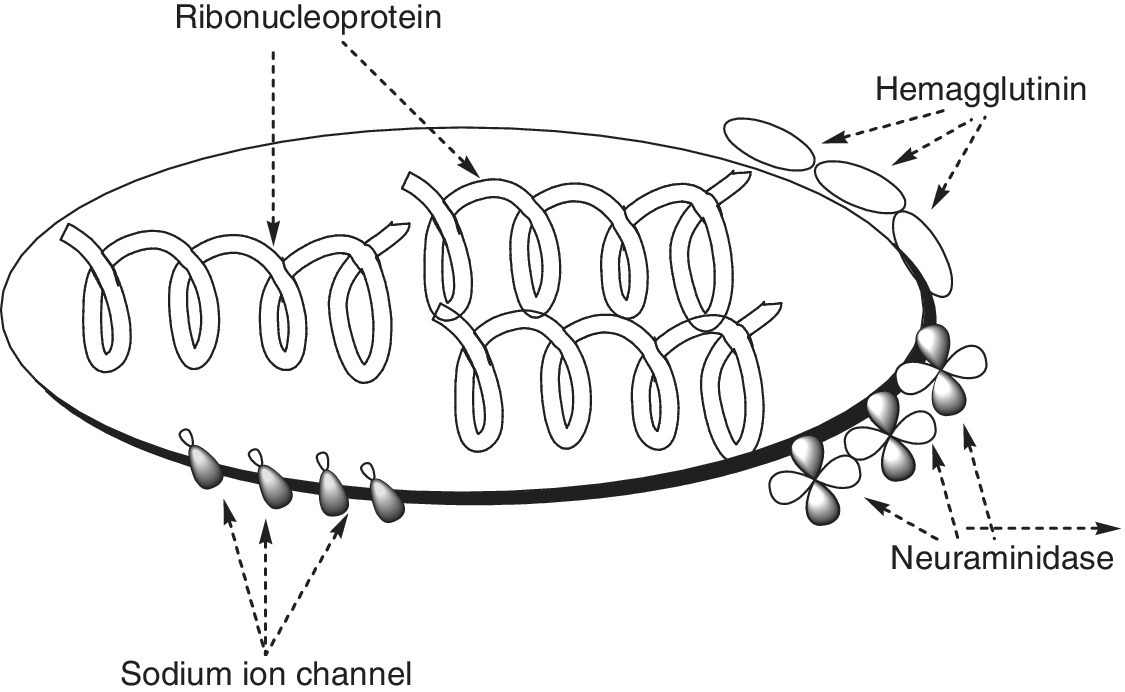
Figure 6.1 Influenza virus
(Source: http://www.cdc.gov/flu/images/virus/fluvirus‐antigentic‐characterization‐large.jpg).
The Genetics of Influenza Virus Type A
The majority of influenza viruses are known to have the capability to infect birds and poultry, with the exception of two subtypes, which are H17N10 and H18N11 that infect bats and unlike subtypes H1N1 and H3N2, these subtypes (i.e. H17N10 and H18N11) have been reported to be incapable of growing in human cells (Tong et al., 2012).
Based on the viral molecular attributes and the ability to cause disease and mortality in birds, avian influenza type A viruses are further subdivided into two groups, namely: (i) highly pathogenic avian influenza virus A (HPAI); and (ii) low pathogenic avian influenza virus A (LPAI) (Alexander, 2000). The HPAI types cause serious and severe diseases and high mortality in poultry that have been infected, while the infection due to LPAI does not result in any serious disease (Alexander, 2000).
Avian Influenza A Viruses that Infect Animals, Wild Birds, Poultry and Humans
Several subtypes of avian influenza A type H5 including H5N1, which was reported in Asian and Middle‐eastern countries (and some other countries), have caused severe pneumonia with about 60% mortality worldwide; H5N2, H5N3, H5N4, H5N5, H5N6, H5N7, H5N8, and H5N9 are among H5 types that affect both poultry and humans.
Another avian influenza, the influenza A H7 does infect both birds, animals and humans. The H7 subtypes that cause diseases to poultry and humans include H7N1, H7N2, H7N3, H7N4, H7N5, H7N6, H7N7, H7N8 and H7N9. The majority of these H7 subtypes belong to LPAI group of influenza viruses.
Another influenza virus A known to infect animals, wild birds, poultry and humans is H9 and its subtypes are namely H9N1, H9N2, H9N3, H9N4, H9N5, H9N6, H9N7, H9N8 and H9N9. They generally cause mild upper respiratory tract illness.
It should be known that certain subtypes of influenza A virus show preference in terms of the host they infect, because some are very specific to certain species of animals but they are all capable of infecting birds without any exceptions. For example, H1N1 viruses have shown preference to pigs, while H7N7 and H3N8 virus have been more specific to horses.
The genome of influenza A viruses is comprised of up of eight separate single stranded RNA gene segments. The eight gene segments of influenza A virus encode 10 proteins: hemagglutinin (HA), neuraminidase (NA), matrix proteins M2 and M1, nonstructural (NS) proteins NS1 and NS2, the nucleocapsid, and the three polymerases, the PB1 (polymerase basic 1), PB2 and PA (polymerase acidic) proteins (Webster, 1992). In addition, certain influenza viruses contain the PB1 gene, which has been reported to encode for the PB1‐F2 protein (Chen et al., 2001).
Of these 10 genes in the genome of influenza virus A, segment 1 encodes for the polymerase proteins: basic polymerase 2 (PB2), segment 2 encodes for basic polymerase 1 (PB1), while segment 3 encodes for the acidic polymerase (PA). These proteins are encoded by segments 1, 2 and 3 and together they form the RNA‐dependent RNA polymerase complex that drives the processes responsible for transcription and replication of the viral genome. In addition to this, segment 2 does encode for another protein, namely PB1‐F2, which is known to induce cell death. Segment 4 encodes for the viral surface glycoprotein HA. The HA plays a crucial role in the process of binding to sialic acids (SAs), the viral receptors on host cells as well as the process of fusion between the viral and host cell membranes upon endocytosis. Segment 5 encodes for the nucleocapsid protein (NP), which is responsible for the binding to viral RNA. Moreover, the NA and polymerase proteins together form the ribonucleoprotein complexes (RNPs). Segment 6 encodes for the neuraminidase (NA), a sialidase, which is crucial for cleaving SAs from host cells and virus particles. Segment 7 encodes for the protein M1, which is actually a viral matrix structural protein as well as another protein M2, which is an ion‐channel protein that is normally found incorporated in the viral membrane. Segment 8 encodes for the NS1 protein, a nonstructural protein, which is crucial as an antagonist protein of host innate immune responses and plays an important role as it interferes with host gene expression. The same segment 8 also encodes for the nucleic export protein (NEP), which facilitates processes that take part in the nuclear export of RNPs in the cytoplasm prior to the virus assembly.
One of the mechanisms that leads to the creation of even new influenza viruses is known as the antigenic shift, in which the segmented influenza virus genome mixes influenza A viruses from different species through the re‐assortment of the genetic information process to replicate and thus create a new virus in cases where influenza A viruses from two different species infect the same individual or animal. This takes place mostly with influenza virus A subtypes, in which the majority of the population possess very limited or no immunity at all against it. This can happen, say in the case where a person is co‐infected with an avian influenza A virus and at the same time with a human influenza A virus or an animal like the swine/pig which has co‐infection due to: i) human influenza A virus; and ii) avian influenza A virus simultaneously, the re‐assortment process will produce a new replicating virus that will be the result of a mixture of the existing genetic information such that the newly created virus will tend to possess most of the genes from the human virus, but a hemagglutinin gene and/or neuraminidase gene and other genes from the avian virus. This will therefore result in a new virus with much enhanced capability and virulence to infect humans and spread easily from person to person and its virulence will be further enhanced due to the fact that it will now have different surface proteins (hemagglutinin and/or neuraminidase).
Mechanism of Action of Avian Influenza Viruses in Bird‐to‐Human Transmission, Pathogenesis and Host Restrictions of Interspecies Transmission
According to scientific research reports, the viral and host factors that control victims’ barrier or restriction are determined by the viral HA and NA genes, several internal genes including the nucleoprotein, the PB2 genes and a combination of other diverse multiple viral genetic factors (Malik Peiris et al., 2007). Differences in the interspecies transmission mechanisms are depicted differently among different species. For example, the HA of human influenza viruses binds to cellular sialic acid that is directly linked to sugar molecule galactose via a α‐2,6 bond (SA α2,6 Gal), but the avian viruses bind to the sialic acid linked to galactose by the α‐2,3 bond (SA α2,6 Gal) (Rogers et al., 1983). It should be noted that the epithelial cells in the human trachea contain SAα2, 6Gal molecules on their surface, but they do not contain SA α‐2,3 Gal molecules.
This observation in terms of the differences in the receptor specificity in different species plays an important role in the control of factors that govern the species restrictions that may prevent avian viruses from infecting humans easily and/or straightforwardly (Malik Peiris et al., 2007). Cases of interspecies transmission (e.g. birds to humans) may be aided by mutations that may occur and which could result in a change in affinity from SA a‐2,3 and SA a‐2,6 receptors (Yamada et al., 2006). In human respiratory tissues, the SAα2, 6Gal oligosaccharides are present largely on the epithelial cells of the nasal mucosa, paranasal sinuses, pharynx, trachea and bronchi, while upon viral infection to humans, the SAα2, 3Gal oligosaccharides are normally found located on non‐ciliated cuboidal bronchiolar cells at the junction between the respiratory bronchiole and alveolus, and also on cells lining the alveolar wall.
Receptor specificity is made possible due to the chemistry and biology of the amino acids that are present in the receptor binding pocket of HA protein together with certain amino acids, mainly glutamine or leucine, which are found at position 226 and also amino acids glycine or serine, which are found at position 228, thus characterizing the specificity for either SAα2, 3Gal or SAα2, 6Gal, respectively. Moreover, in the case of H5N1 virus, there is an introduction of the human‐type amino acid residues at positions 226 and 228 of the HA protein, a property that can be used to investigate and detect SAα2, 6Gal oligosaccharides in addition to SAα2, 3Gal oligosaccharides.
Generally it has been shown experimentally that restriction and pathogenicity of the influenza viruses has been demonstrated mainly with only a few proteins out of those 10 encoded by the 8 genes. These proteins include hemagglutinin (HA), PB2 (polymerase basic 2), NS1 (non‐structural 1) and neuraminidase (NA).
As explained above, the HA protein (antigen) plays a crucial role that enables the influenza virus to attach and also aid its fusion to the cell membrane of the host. Moreover, the HA protein is necessary for the establishment of the pathogenicity of avian influenza viruses. Normally the HA protein is synthesized as a single polypeptide (HA0), then the cellular enzymes (mainly proteases) cleave it into HA1 and HA2 molecules to expose the hydrophobic component of the amino terminus HA2, which actually plays the fusion role and it is therefore the HA2 that is known as the fusion peptide, as it mediates the fusion between the envelope of the virus and the endosomal membrane.
For the HPAI group of viruses, the HA proteins contain certain basic amino acids at the cleavage site, characterized by the presence of ubiquitous proteases, mainly furin and also proprotein convertase 6, which actually cause systemic viral infections. For the LPAI group of viruses, the HA protein is characterized mainly by the presence of single arginine and they are normally cleaved when they are in either respiratory or intestinal organs, implying that they only cause localized infection that in most cases is without any noticeable symptoms or very mild if any.
Another protein out of the 10 that are encoded by the 8 genes of the influenza virus A and which happen to demonstrate restriction and pathonogenecity, is the PB2 protein. This protein is actually a component of the viral RNA replication complex that plays an important role in the recognition and binding to type I cap structures of cellular mRNAs. Moreover, the PB2 segment is known to be involved in several restrictions of pathogenic avian influenza viruses in cell lines. The replication of a human virus containing an avian virus PB2 gene in mammalian cells is highly dependent on lysine, which is only present in human associated viruses. It is not dependent on glutamic acid, an amino acid mainly found in avian species at position 627 of PB2.
NS1 is another protein that is encoded by the eight genes of the influenza virus A and which also demonstrates restriction and pathogenicity. It plays a crucial role as an interferon‐antagonist, targeting the production of interferon‐β and also the activation of interferon induced antiviral genes, thus playing an important role in enhancing the efficiency in virus replication in interferon‐competent hosts. The NS1 protein of the H5N1 viruses demonstrates resistance to the antiviral effects of interferon and at the same time induces elevated levels of pro‐inflammatory cytokines, including the tumor necrosis factor α (TNFα). For example, influenza viruses that contain the H5N1 NS1 gene and which also happen to be most pathogenic in pigs, were found to enhance the transcription of TNFα and that of interferon β, which are present in the primary monocyte‐derived macrophages in humans. In the past, it was observed that the influenza virus interfered with the expression of interferon‐regulated genes more than other viruses. In the case of the H5N1, it has also been established that the activation of cytokines and chemokines is regulated through cellular signaling pathways, including the mitogen‐activated protein kinase (MAPK) pathway. This implies that the viruses that belong to the HPAI group may be responsible for the activation of p38 MAPK and these observations suggest that NS1 of HPAI may be responsible for the cytokine imbalance (cytokine dysregulation) in cases of viral infection that results in a reactive hemo phagocytosis, which is a cytokine‐driven disorder.
Neuraminidase (NA) protein is another protein encoded by the eight genes of the influenza virus A and demonstrates restriction and pathenogenicity. The activity of the enzyme sialidase of neuraminidase plays an important role of removing sialic acid from sialyloligosaccharides of HA, and the cell surface, thus simplifying the release of the virus and the removal of sialic acid from the mucin layer and enabling the virus to get to the surface of the host’s epithelial cells.
Neuraminidase protein of several influenza viruses has been linked to pathogenicity, as it has been found to be central to neurovirulence. Moreover, it has been observed that the absence of a carbohydrate chain at position 146 of neuraminidase (N2 numbering) and the presence of an amino acid lysine at the C‐terminus, enables the neuraminidase protein to bind to and sequester plasminogen, which is a plasmin precursor and therefore assists in the cleavage of the HA protein causing virus pathogenicity.
Analytical Strategy for the Identification of Influenza Virus A Infection Cases
Where there are suspected cases of influenza virus A infection, specimens that are normally collected from patients include respiratory specimens, blood and serum, as well as allantoic fluid. The extraction of viral RNA has been achieved mainly using specialized molecular kits, while the amplification of the gene is normally done using reverse transcription–PCR; the amplified gene is then sequenced and identification can be achieved. Purification techniques, which aim at separating monocytes and macrophages from blood samples, eliminating unwanted matrices, etc., are normally performed prior to identification using either molecular biology techniques (to identify the RNA genes), specific assays (e.g. microneutralization and horse red‐blood‐cell hemagglutinin inhibition assays, etc.) or specific analytical mass spectrometry (LC‐MALDI‐TOF‐MS) for the analysis mainly of proteins associated with influenza virus A infection, as described above.
Conclusions
The possibility of using pathogenic viruses as bioweapons remains high. Bioterrorism may seek to eradicate lives en masse and one of the possible bioweapons to be considered by criminals may be pathogenic viruses. It may be tricky to multiply these agents for reasons that may be explained from a biological point of view (viruses can only replicate when they are in a living medium). However, with the spread of knowledge, it may be possible to encounter bioterrorism incidences that may try to make use of pathogenic viruses. This calls for preparation by having the infrastructure in place to prevent the possibility of anyone trying to use such agents as food bioweapons.
References
- Alexander, D.J. (2000) A review of avian influenza in different bird species. Veterinary Microbiology, 74: 3–13.
- Chen, W., Calvo, P.A., Malide, D., Gibbs, J., Schubert, I. et al. (2001) A novel influenza A virus mitochondrial protein that induces cell death. Nature Medicine, 7: 1306–1312.
- Koonin, E.V., Senkevich, T.G. and Dolja, V.V. (2006) The ancient virus world and evolution of cells. Biology Direct, 1: 29.
- Malik Peiris, J.S., de Jong, M.D. and Guan, Y. (2007) Avian Influenza Virus (H5N1): A threat to human health. Clinical Microbiology Reviews, April: 243–267.
- Rogers, G.N. and Paulson, J.C. (1983) Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 haemagglutinin based on species of origin. Virology, 127: 361–373.
- Staginnus, C. and Richert‐Pöggeler, K.R. (2006) Endogenous pararetroviruses: Two‐faced travelers in the plant genome. Trends in Plant Science, 11(10): 485–491.
- Tong, S., Li, Y., Rivailler, P., Conrardy, C., Danilo, A. et al. (2012) A distinct lineage of influenza A virus from bats. PNAS, 109(11): 4269–4274. Available online at: www.pnas.org/cgi/doi/10.1073/pnas.1116200109
- Webster, R.G., Bean, W.J., Gorman, O.T., Chambers, T.M and Kawaoka, Y. (1992) Evolution and ecology of influenza A viruses. Microbiology Reviews, 56: 152–179.
- WHO (2008) Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO http://www.who.int/csr/disease/avian_influenza/country/cases_table_2008_04_02/en/: accessed 1 August 2016.
- WHO. WHO guidelines for investigation of human cases of avian influenza A. Available online at http://www.who.int/csr/resources/publications/influenza/WHO_CDS_EPR_GIP_2006_4r1.pdf; Writing Committee of the Second WHO Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus. Update on avian influenza A (H5N1) virus infection in humans. New England Journal of Medicine, 358: 261–273.
- Yamada, S., Suzuki, Y., Suzuki, T., Le, M.Q., Nidom, C.A. et al. (2006) Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human‐type receptors. Nature, 444: 378–382.