12
Application of Atomic and Molecular Spectroscopic Techniques in Food Forensics
The electromagnetic radiation methods in foods are very common. These methods can be used either as part of the food processing train or as analytical methods in providing evidence in food forensic cases. Both types of radiation (ionizing and non‐ionizing) have found application in food forensic analyses and/or food processing procedures. Ionizing radiation includes gamma‐rays, which can be emitted from sources including radioisotopes, for example 137Cs, high energy electrons, X‐rays, etc. Non‐ionizing radiations, on the other hand, include ultra violet‐visible radiation, nuclear magnetic resonance (NMR), infrared, Raman, and microwaves, are also used in food forensics. In most cases, these methods complement each other to enable more reliable information to be deduced from the sample under investigation. Non‐ionizing spectroscopic techniques that will be discussed as far as this topic is concerned include ultra violet‐visible, infrared (near, mid and far/terahertz), Raman, and microwaves. Gamma rays and X‐rays will be covered in the next chapter.
Introduction
Atomic spectrometry techniques of interest for this chapter are mainly atomic absorption spectrometry techniques and atomic emission spectrometry techniques.
Atomic Absorption Spectrometry (AAS)
Atomic absorption spectrometry (AAS) is a spectroscopic‐based analytical technique for the determination of elements based on the absorption characteristics when their free atoms in gaseous state are exposed to optical radiation in the ultraviolet region. For AAS to be used for quantitative measurements, it requires the use of standards with known concentrations that will provide the means to come up with the possibility to correlate the analyte concentration with the corresponding measured absorbance according to Lambert–Beer’s law.
AAS requires that elements be converted to gaseous atoms and for this reason, several techniques are available to provide atomization of the elements, which include flame (mainly air‐acetylene flame and nitrous oxide‐acetylene flame); and electrothermal (also known as graphite tube atomization). Other atomization methods include glow‐discharge atomization, hydride atomization (for hydride formers such as arsenic, selenium, antimony) and cold‐vapor atomization (for mercury species). Flame atomization techniques work best for analytes contained in liquids/solutions (dissolved samples), while electrothermal AAS can analyze any kind of sample (solid, liquid, and gaseous samples). ET AAS results in much lower detection limits as compared to FAAS.
Atomic Emission Spectrometry: Inductively Coupled Plasma Techniques
Inductively coupled plasma optical emission spectrometry (ICP‐OE and ICP‐MS) is another analytical technique used for the measurements of trace metals, which makes use of inductively coupled plasma for both atomization as well as for ionization of sample analytes to produce atoms and ions that when exposed to a specific electromagnetic radiation (light), will produce a signal that is characteristic and specific to that particular element. For quantitative measurements, standards are normally employed such that the intensity of this emission is directly proportional to the concentration of the element present in the sample. In addition to trace metal determination, ICP‐MS can also be used for the determination of isotopic concentrations in food samples. ICP is more attractive than AAS‐based methods, because it gives much lower detection limits, wide‐range linearity, superior sensitivity, high efficiency, and it is also a multi‐elemental technique capable of the simultaneous multi‐element determination of the majority of elements from a single sample in one run.
Applications of Ion Chromatoraphy and Atomic Spectrometry in Food Forensics
Ion chromatography and atomic spectrometry techniques are widely used for mineral and trace element determination in food samples, as well as for the verification of authenticity and also for the indication of the geographical origin of foods.
The Relationship Between Mineral Composition in Foods, Fruit Bearing Plants, Flowers, Vegetables/Vegetable Derived Products, and Soil Structure
The geological set up of a place determines the type and composition of soils, as well as the quality and quantity of mineral elements. This, together with factors such as pH, mineralogical patterns, clay type, and composition and humic complexes of the soil, determines the bioavailability of the minerals, both major and trace (Alloway, 1995). Since plants that form the source of food and foodstuffs, including fruits, drinks derived from fruits, corn, etc., their mineral composition can be taken as a reflection of the element composition of the soils in which the plant grows. This fact can thus justify the relationship between mineral composition in the soils of a particular locality to the mineral composition in plants growing there and to the fruits, oils, etc. derived from those plants.
For this reason, plants grown in different geographical locations would be expected to exhibit different patterns and profiles of trace elements due to the differences in the chemistry of the soils, which is patterned by different climatic and weather agents, such as weathering of rocks that differs from place to place. It should be noted that different geographical locations may have similar precursors of rocks, which may result in similarities in the types of soils, thus mineral and trace element patterns; however, scientific and geological data suggest that it is highly unlikely for the trace element geochemistry and precursor‐mineralogy in different geographical locations with similarities in rock types to be equivalent, due to the fact that each of these areas tend to be subjected to a wide variety of geological processes that will give uniqueness to each location (Kabata‐Pendias and Pendias, 1984). Moreover, the chemistry of a particular place will tend to influence the uptake of minerals by plants different from another location, thus resulting in a unique uptake of trace elements and minerals by those plants (Kabata‐Pendias and Pendias, 1984). Therefore, it is very scientific to link, discriminate, and classify different soils and crops (fruits and vegetables) that grow there, by using analytical techniques capable of single and multi‐elements analysis such as AAS and ICP (Ariyama and Yasui, 2006).
Atomic Spectrometry (AAS, ICP‐OES, ICP‐MS)
Ground samples are subject to dissolution procedures and analysis for solution‐based ICP‐MS and ICP‐OES, or AAS and IC. Similar dissolution and analysis procedures were used for all sample types investigated in the studies detailed in this chapter. However, pork samples were treated differently as these were dissolved without drying.
Dissolution can be done using nitric acid (appropriate volume per unit weight of the sample) and by leaving the sample in an oven at about 90 °C overnight. Add more nitric acid (a volume of about 10% of the original volume used for dissolution), put on hot plates at 160 °C for 2 hrs, then evaporate the nitric acid and the samples should be allowed to dry. Then digest samples using 4:1 nitric/perchloric acid mixture and place them on hot plates at 180 °C for 3 hrs. The temperature can be reduced to 100 °C and the samples are allowed to remain at this temperature overnight to allow sufficient time for the reaction (digestion to set free elements from the matrix). Then evaporate nitric/perchloric acid to dryness to obtain the required residue and to this solution add an appropriate volume of 20% nitric acid with gentle warming.
Alternatively, the nitric acid/hydrogen peroxide procedure can be adopted where the case is related to protein samples. The nitric acid/hydrogen peroxide can be used to digest the protein matrix, with the hydrogen peroxide playing the role of destroying fat residues in the samples.
NB: For complete procedures, the interested parties may consult relevant literature, which is available in many databases.
The sample solutions can then be analyzed by IC (for inorganic anions), AAS (FAAS or GFAAS), or ICP (OES/MS, solution or laser ablation for solid samples) techniques, depending on the technique that is available or depending on the parameters that are required.
Soils also contain inorganic anions that are characteristic of a particular geographical location in terms of types and ratios and this can be used to identify plants and their derived products with the types of soils from which they originate. The inorganic anions can be determined by many techniques, but the most common is ion chromatography (IC). Examples of the application of IC in ion analysis include the analysis of toxic ion species, such as azide, arsenite, arsenates, cyanide, sulfide, iodide, and bromide, etc., which are normally detected using various detectors, such as conductivity or UV detector (Annable and Sly, 1991 ; Bond et al., 1982 ; Gailer and Irgolic, 1994 ; Larsen et al., 1993 ; Rockline and Johnson, 1983 ; Rockline, 1991 ; Sheppard et al., 1992 ; Williams, 1983).
Sample Preparation for Elemental Composition Analysis in Foods
Type of samples for trace element composition analysis are plant materials (fruits, flowers, pollen, etc.), and animal products from animals that graze or feed on plants growing in the different geographical localities earmarked for provenance or authenticity investigation.
For fruits, whole fruit samples can be used, for plants, relevant parts, for example flowers, pollen, roots, stem, leaves, etc. can be collected and for soils, representative samples should be collected. For soil, or if water used for irrigation is required, then a representative sample (volume) must be collected from the area under investigation.
Sample Preparation (Typical Example of the Procedures)
- Soil samples: air‐dry the bulky soil samples, grind to obtain a fine earth;
- Roots: ensure that the roots are cut from the plant with the soil tightly adhering to the root surface (rhizo soil); then separate by gently shaking and scraping; air‐dry, grind to powder;
- Stems and leaves: hand‐clean then oven‐dry at 55 °C for 48 hrs. then grind to powder;
- Fruits: hand‐clean, freeze, and then freeze‐dry at –40 °C for 48 hrs, then grind to powder;
- Meat samples: no need for drying; and
- For all these samples (soils and plant materials), measure the moisture by weighing after drying at 105 °C for 16 hrs.
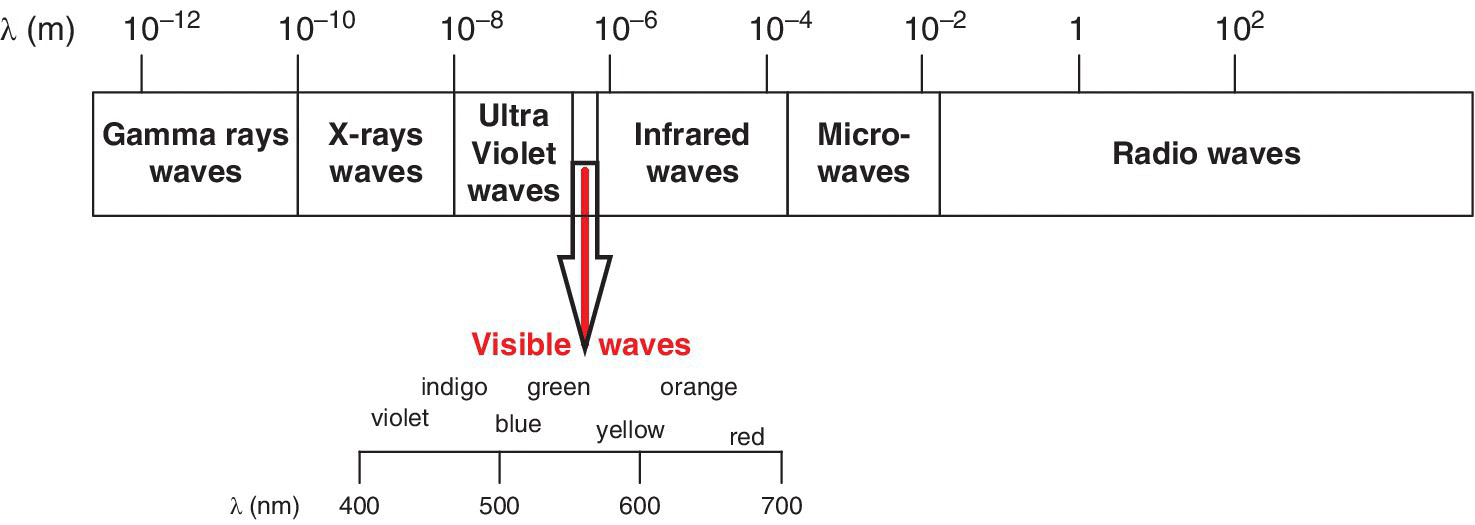
Figure 12.1 Positions of selected spectroscopic bands within the electromagnetic radiation.
Ultraviolet‐visible (UV‐Vis) Spectroscopic Methods for Food Forensics
The UV‐Vis region of the electromagnetic radiation spectrum covers a wavelength range from 190 nm to about 800 nm. Irradiation of UV‐Vis to sample molecules triggers the promotion of electrons to higher energy levels, which in turn provides information about the presence of conjugated p systems and the presence of double and triple bonds. UV‐Vis is attractive for applications in food forensics due to its non‐destructive properties, and the methods are known to involve simple operations, are economically viable, and can therefore be afforded by most laboratories. The principle for the detection is based on the ability of samples to be absorbed in a characteristic wavelength region of UV‐Vis. Any sample with chromophores can be analyzed using this technique. Food samples that contain molecules that are either unsaturated, conjugated, or with aromaticity can be good candidates for UV‐Vis analysis. UV‐Vis can be used for a large number of samples daily to ascertain the concentrations of biomolecules such as proteins and DNA, oligonucleotide analysis, peroxide value determination, food color additives, authentication, and detection of illicit/surrogate food products.
For example, UV‐Vis spectroscopy can be employed in the food industry to ascertain and verify the quality of edible oil products, based on the measure of a number of parameters including:
- the anisidine value that is related to the magnitude of fats oxidation by ascertaining the magnitude of aldehyde oxidation, which may occur during the oxidation of fat, and the anisidine value obtained can be used to assess the quality of edible oils. In measuring the anisidine value, UV‐Vis spectroscopy measures the absorbance of the sample solution of fat foods that contain aldehydes that come from lipid oxidation (mainly dienals or alka‐2‐enas) after they react with p‐anisidine. Since the anisidine value of edible oils is regulated (it should not exceed 8), the measure obtained from UV‐Vis spectroscopy can be used in food forensic investigation in cases where there is no compliance;
- another parameter that can be monitored by UV‐Vis spectroscopy is the total oxidation value, which can indicate the quality of edible oils in terms of total fat deterioration;
- the peroxide value is another parameter that is used to ascertain the quality of edible oils after exposure to extreme physical conditions of temperature, oxygen, or daylight, which may cause generation/production of oxidation products of fatty acids, mainly peroxides, and hydroperoxides. The peroxide value is also regulated and the guidelines stipulate that the maximum permissible level in edible oils is 10 meq O2 kg−1 for oil produced through cold press extraction and 5 meq O2 kg−1 for a refined oil;
- another parameter of edible oil used to ascertain quality is general color, which is normally investigated by measuring the saturation of pigments, mainly chlorophyll and carotenoid pigments. It should be noted that carotenoid pigments are known to have antioxidant activity and thus are used as food additives and therefore foods with higher contents of carotenoids are classified as more nutritious than the ones with higher contents of chlorophylls. The UV‐Vis measurement is normally carried out at wavelengths of 460 nm for carotenoid and 666 nm for chlorophyll.
Application of Spectroscopic Methods in the Determination of Food Colors
Food legislation and regulations always require the food processing industry, food suppliers, and distributors to strictly adhere to the labeling requirements regarding food ingredients, including food coloring agents (dyes). Under certain circumstances, food color may change, fade, or become diluted during food production. For example, when certain foods are exposed to oxygen or light for a prolonged period of time, there may be a change in food color concentration. For this reason, food manufacturers need to ensure that such changes do not take place, as this implies changes in the quality of the food. To test food colors, spectroscopic techniques (Figure 12.2) are very central.
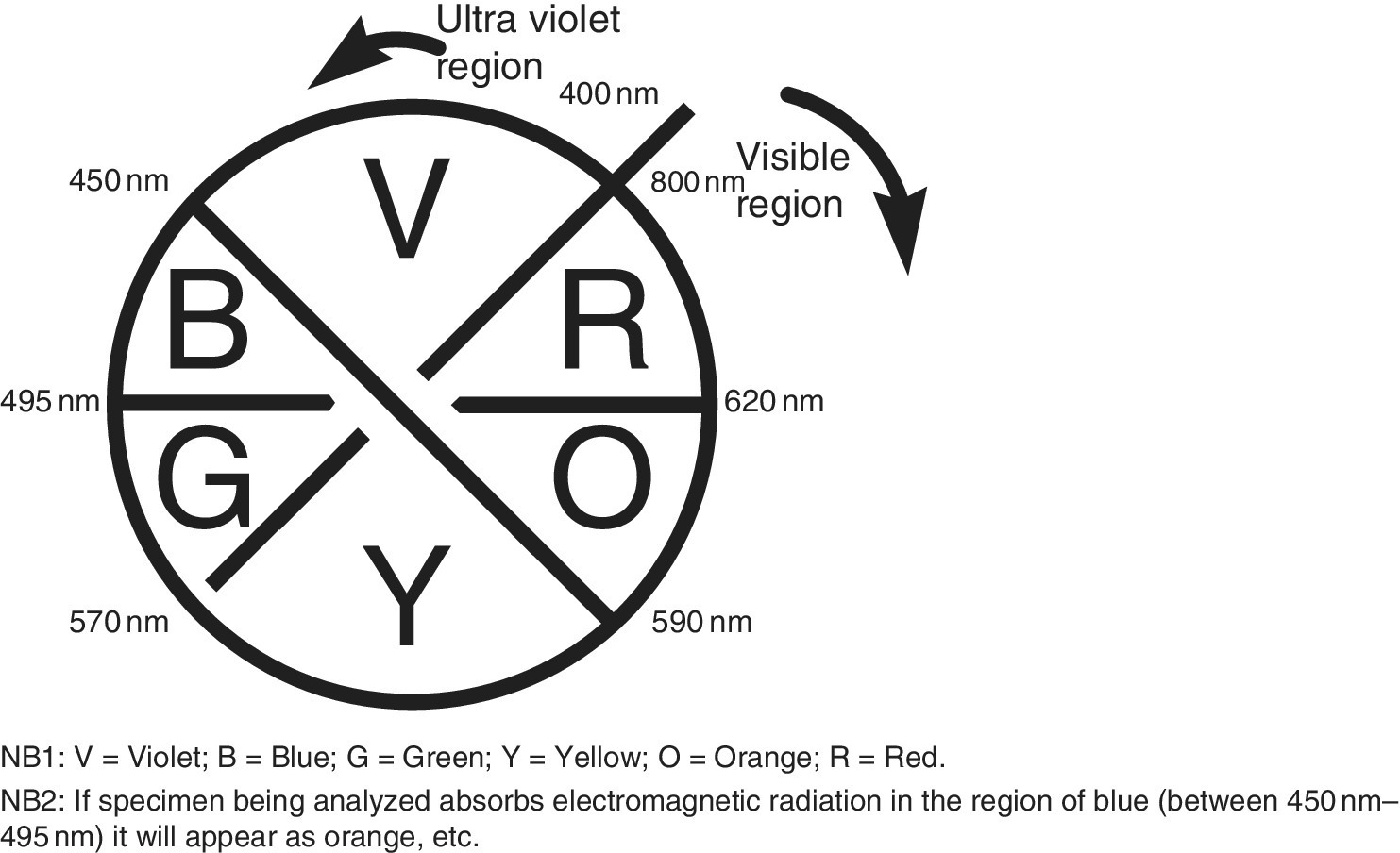
Figure 12.2 UV‐Vis in combination with HPLC and CE in food forensics.
Application of Molecular Spectroscopy Methods in Food Forensics: Fluorescence Spectroscopy
Fluorescence occurs when electrons have undergone excitation to higher energy levels and then returns to the ground state such that the emitted light has energy equal to the difference between energies of ground and excited states. The attractive features associated with fluorescence‐based techniques include high sensitivity and specificity and due to these attributes, food analysis has exploited the characteristic advantages of fluorescence spectroscopy, especially in food forensic cases. The use of two kinds of spectra (excitation spectra and emission spectra) explains the reason for high specificity of fluorescence‐based methods, but the high sensitivity of fluorescence‐based techniques arises as a result of measuring radiation against absolute darkness.
Fluorescence spectroscopy presents a number of attractive features as a technique with great potential in food analysis and food forensics. These attractive features include its high sensitivity and selectivity relative to other spectroscopic methods.
The advancements in technology that have application in fluorescence spectroscopy together with the increase in the use of chemometric techniques has accelerated the use of fluorescence spectroscopy in food research, including food forensics. Moreover, the use of fluorescence in food analysis and food forensics is plausible due to the fact that most foods contain intrinsic fluorophore moieities. Examples of foods with fluorophores include proteins that are made up of amino acids such as tryptophan, tyrosine, and phenylalanine residues. Fluorescence spectroscopy will generate spectral data and unique information about these aromatic amino acids and their respective chemical and biological environment in food or biological samples and such data can reveal the nature of the structure of proteins and how they interact with other proteins or other biomolecules (Herbert et al., 2000 ; Luykx et al., 2004). The uniqueness of the spectral data and the use of some specific fluorescence spectroscopic techniques such as front‐face fluorescence spectroscopy can make possible the discrimination of protein‐based foodstuffs based on their respective geographical origin (Dupuy et al., 2005 ; Karoui et al., 2004, 2005).
Fluorescence Coupled Techniques for Application in Food Forensics
Despite the fact that fluorescence‐based techniques are very sensitive and specific, they cannot be used alone to provide a complete set of the quality data or measurements needed for all food samples.
It is therefore imperative to synchronize fluorescence spectroscopy (with other detection systems, e.g. spectrofluorimeter, electrophoresis equipped with laser‐induced detector, high performance liquid chromatography fluorescence detector, fluorescence polarization (FP) immunoassay) and this makes it useful in providing appropriate measurements that may provide evidence in food forensic cases, such as food poisoning, for example issues related to toxic fungal metabolites poisoning (mycotoxins), which contain strong fluorophores, with the exception of G1 and G2, which do not exhibit fluorescence. Another application of fluorescence spectroscopy is in the authentication and adulteration of edible oils, such as olive oils, of which the market price is normally governed by the quality of olive oils. Unscrupulous distributors and suppliers tend to mix quality olives with those of inferior quality.
Nuclear magnetic resonance spectroscopy (NMR) makes use of radiofrequency waves of the electromagnetic radiation by bombarding these waves to atomic nuclei with non‐zero spins in a strong magnetic field (Ibañez and Cifuentes, 2001). NMR causes the excitation of the nucleus of atoms through radiofrequency irradiation, thus providing the information about molecular structure and atom connectivity of the sample under investigation. The presence of atoms in the surroundings does have an effect on the absorption of the atomic nuclei, as these atoms tend to cause minute but significant local modifications to the external magnetic field, thus enabling the analyst to obtain detailed characteristic data and information about the molecular structure of the food sample under investigation.
The physical principle on which NMR is based can be explained by the fact that some of the atomic nuclei have nuclear spin, for example hydrogen‐1 (spin = 1/2), carbon‐13 (spin = 1/2), nitrogen‐15 (spin = 1/2), oxygen‐17 (spin = 5/2), fluorine‐19 (spin = 1/2), and phosphorous‐31 (spin = 1/2) (Table 12.1) and this spinning charge creates a magnetic moment, such that these nuclei can be considered as tiny magnets. When these nuclei with spin are introduced into a magnetic field, they will tend to either line up with or against the field by either spinning clockwise or anti‐clockwise. Depending on the strength of the magnetic field, normally the alignment with the magnetic field (assigned as α) is associated with lower energy than the alignment that is against the magnetic field (ß) (Figure 12.3).
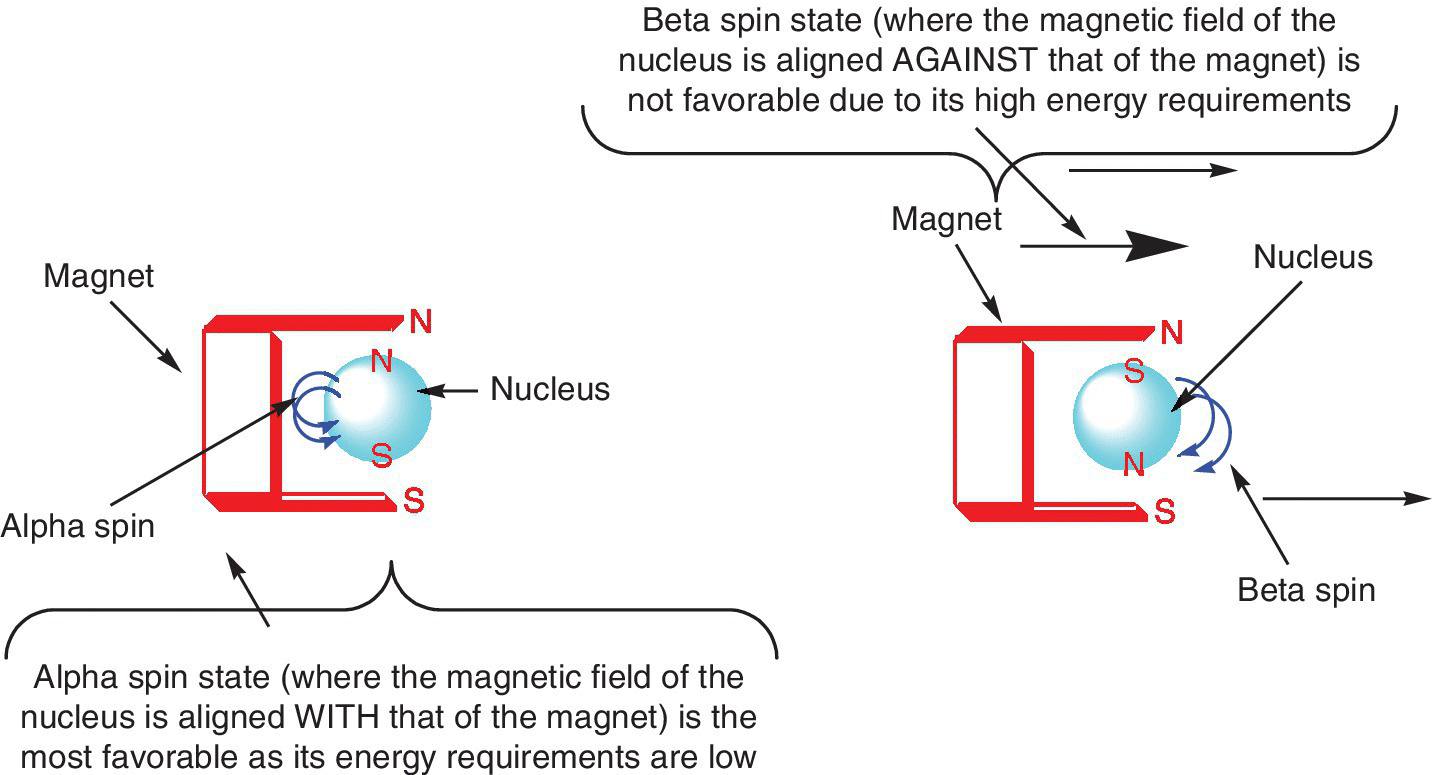
Figure 12.3 An illustration of alignment of nuclei with spin on a magnetic field.
Table 12.1 ½ Spin nuclei (Harris, 1983).
| Nucleus | Chemical shift parameter range (ppm) |
| 3H | 13 |
| 1H | 13 |
| 19F | 400 |
| 31P | 530 |
| 13C | 250 |
| 15N | 1700 |
There are several NMR experiments that are normally used concurrently to enable structure elucidation and these are proton NMR (1H NMR), 13C NMR, and its various experiments such as distortionless enhancement by polarization transfer (DEPT). The proton NMR spectra, for example, can generate information about the following data:
- Chemical shift data: this can provide information about what kinds of protons are present in the sample being analyzed;
- Integrals: provide the information about the ratio of each kind of proton in the sample; and
- 1H–1H coupling: provides information about protons that are near other protons.
On the other hand, the data from 13C spectra can be useful in providing information about each carbon present in the sample, the type of carbons in the sample, the number of carbons in the sample, and the assignment of different types of carbons (primary, secondary, tertiary carbons… etc.), which can be done by performing DEPT 45, DEPT 90 or DEPT 135 experiments.
It should be noted that in case of nuclei that do not have spin, such as 12C, they present no difference in terms of energy between alignments in a magnetic field, because they are not magnets and therefore it is impossible to analyze them using NMR spectroscopy.
Monakhova et al. (2011) recently reported the successful application of the UV‐Vis spectroscopic method and 1H NMR in the determination of surrogate alcohol products. Surrogate alcoholic products are contraband products, which are legally banned as not fit for human consumption. However, such products are sold and consumed illegally and in such cases the consumer and seller are considered to be transgressing the law and thus subject to forensic investigation. because surrogate alcohols are known to have caused poisoning to consumers due to their composition, which may include disinfectants, medicinal alcohols, and perfumes (Monakhova et al., 2011). In their report, they developed and validated spectroscopic‐based techniques, mainly 1H NMR and UV‐Vis for the determination of diethyl phthalate (DEP) and polyhexamethylene guanidine (PHMG), components that may be found in surrogate alcohol. The analysis using UV‐VIS spectrophotometry was performed after derivatization with Eosin Y, while samples for 1H NMR spectroscopy were analyzed after extraction with dimethylsulfoxide (DMSO).
Proton (1H) NMR and 13C NMR Spectroscopy for Food Fingerprinting and Food Provenance
Both proton (1H) NMR and 13C NMR in combination with chemometric methods have been employed in the investigation, fingerprinting, and characterization of various profiles of a food products and correlate the food chemical composition to their geographical origin. Generally, 1H NMR has been widely used because it is more sensitive than 13C, 31P, 19F, etc. and its application has been reported in food analysis, fingerprinting, and food provenance, for instance discrimination of various types of foods and food products such as olive oils into their respective regions of origin (Mannina et al., 2001 ; Rezzi et al., 2005). The discrimination of olive oil using 1H NMR was possible, because phenolic extracts from different olives have differences in terms of ratios and abundancies for some of the characteristic phenolic compounds, as well as the ratio of the nuclei (1H and 13C) (Sacco et al., 2000). In the recent past, the use of high resolution nuclear magnetic resonance (HR‐NMR), in its mode known as “site‐specific natural isotope fractionation” (SNIF), has been widely reported in the fingerprinting and identification of the geographical origin of foods and food products (Martin et al., 1999 ; Ogrinc et al., 2001). This implies that by determining the abundance of the natural isotopic ratios of the isotopes of carbon (C), hydrogen (H), or oxygen (O), it can provide the criterion for discriminating food products from different geographical conditions, as they are linked to climatic conditions in those geographical locations/countries. The identification by this NMR technique (SNIF) is based on the fact that nuclei present in the components of these foods differ from those growing or found in one location to those in another locality/country, due to differences in climate in various places (Reid et al., 2006 ; Remaud et al., 1997). Apart from fats and oils, NMR spectroscopy in its various techniques has been reported in the analysis of many different types of foods such as fruits, beverages, vegetables, meat, and meat products. In these foods, the investigation covered chemical characterization, provenance, genetic modification, authentication, and quality control (Spyros and Dais, 2000, 2009).
These NMR techniques can be used to provide information about the time period at which the food was produced, by simply monitoring the disappearances of these nuclei through the isotopic ratio calculations, information about food composition, adulterations, geographical origin, and storage index (how long the food has been kept stored). For example, 1H and 13C NMR techniques can be used to ascertain quality control of foods such as olive oils by monitoring levels and ratio of glycerides. Virgin olive oils contain between 1 and 3% of total glycerides, while fresh olive oils contain 1,2‐diglyceride. The diglyceride ratio in fresh olive oil to that in virgin oil will stand at 0.9 or above, because diglycerides become reduced with time during storage. Therefore, this can be used to give a measure for the ageing index for olive oils.
For solid and semi‐solid types of foods, magic angle spinning (MAS) has been the NMR technique of choice, but it gives broad line spectra due to the immobility of its chemical constituents. This shortcoming can be minimized by turning the rotation of the sample at the magic angle (θ) to 54.7, which will generate narrow line signals similar to those obtained for liquid types of foods (Spyros and Dais, 2000, 2009). The NMR analysis of semi‐solid foods is normally performed using high resolution magic angle spinning (HR‐MAS) and cross‐polarization MAS is used mainly for rigid solid foods such as grains.
Similar kinds of information can be obtained for other types of foods, such as beer (NMR can be used to provide information about the brewing site; the type of beer, whether lager or ale; whether barley or wheat malt was used in brewing; and ageing as well as storage effects). Other foods and foodstuffs such as vegetable oils, tea, coffee, wines, honey, etc. can be analyzed by NMR to provide forensic evidence.
Application of 19F in Food Forensics
The use of 19F nuclei NMR in food forensics has received a lot of attention and popularity, mainly due to its high abundance in nature (99%), relatively high sensitivity when compared to other nuclei NMR used in the analysis of foods, and also the possibility of labeling with fluorine nucleus further increases its sensitivity, allowing the detection and quantification of the oil species, even the minor ones that enhance its sensitivity and the ability to detect analytes even at low concentrations, including by‐products (Petrakis et al., 2008).
There are many biological molecules in foods, plants, or animals that are used as food sources and that are composed of fluorine‐containing compounds of either amino acids, nucleosides, lipids, or sugars.
19F NMR, like 1H NMR, employs a similar approach in terms of obtaining NMR spectral data, except that it uses a variety of reference standards (Table 12.2) as opposed to 1H NMR, which use mostly TMS (tetramethyl silane) as the reference standard, and is virtually the same as that involved in obtaining proton NMR data. Another marked difference between 1H NMR and 19F NMR can be observed from the fact that fluorine is surrounded by nine electrons vs. one for hydrogen and therefore one would expect that the range and magnitude of fluorine chemical shifts as well as sensitivity would be higher in 19F NMR than in 1H NMR (Tables 12.2–12.3). For example, in a given food amino acid moiety, where there is a replacement with a fluorinated analog, the fluorinated analog will tend to exhibit a spectrum that has s more resolved signal as compared to the same amino acid that did not have replacement of its protons with fluorine.
Table 12.2 19F NMR reference standards.
| Compound | Chemical formula | Chemical shift, δ (ppm) vs trichloro‐fluoro‐methane, CFCl3 |
| Trichloro‐fluoro‐methane | CFCl3 | 0.00 |
| Trichloro acetic acid | CF3COOH | –76.55 |
| Hexafluorobenzene | C6F6 | –164.90 |
| Monofluorobenzene | C6H5F | –113.15 |
| Trifluoro‐chloro‐methane | CF‐28.60Cl | –28.60 |
| Elemental fluorine | F2 | +422.92 |
| Monofluoro acetonitrile | CH2FCN | –251.00 |
| Difluoro, tetracholoroethane | CFCl2CFCl2 | –67.80 |
| Trifluoro‐toluene | C6H5CF3 | –63.72 |
| Tetrafluorosilane | SiF4 | –163.30 |
| Sulfur hexafluoride | SF6 | +57.42 |
| Disulfuryl difluoride | S2O5F2 | +47.20 |
| Hexafluoro acetone | (CF3)2CO | –84.6 |
| Para‐difluorobenzene | p‐FC6H4F | –106.00 |
| Boron trifluoride | BF3 | –131 to −133 |
| Hydrogen fluoride (aq) | HF (aq) | –204.00 |
| Carbon tetrafluoride | CF4 | –62.50 |
| Aqueous fluorine | Aq F− (KF) | –125.30 |
NB: (+) values refers to higher frequency (downfield shifts where there is lower shielding) and vice versa for (–) values.
Table 12.3 Fluorine chemical shifts in various solvents (Gerig, 2001).
| Chemical functional group | Chemical shift relative to trichlorofluoromethane (ppm) | Chemical shift relative to trifluoroacetic acid (ppm) | Chemical shift relative to 1‐fluorobenzene (ppm) |
| Substituted fluoromethane containing compounds | –210 | –131 | –96 |
| Substituted difluoromethane containing compounds | –140 | –69 | –26 |
| Mono substituted fluorobenzene containing compounds | –140 | –60 | –26 |
| Substituted 2,2‐difluoroethenone containing compounds | –125 | –46 | –11 |
| Substituted 1,1,1‐trifluoroethane containing compounds | –75 | +4 | +39 |
| Mono substituted 2,2,2‐trifluoroacetaldehyde containing compounds | –81 | –2 | +33 |
| Sulfonyl fluoride containing compounds | +50 | +129 | +164 |
19F NMR can be used to authenticate and identify nucleic acids in biomolecules in plants/animal that provide food sources, in case the nucleic acids contain fluorine or fluorinated compounds or if some of the bases or sugars in the nucleic acids contain fluorine. The same can be said for carbohydrates that are fluorinated. This technique can be employed in the identification of enzyme products that are essential in the formation of essential food molecules. For example, 19F NMR can be used to characterize the regiochemistry of enzymes that play an important role in fatty acid biochemistry (Buist et al., 1996).
Phosphorus NMR and the Application of 31P NMR Spectroscopy in Food Forensics
Phosphorus NMR is one of the NMR techniques known to be both non‐destructive and non‐invasive. This technique makes use of magnetic resonance of a 31P nucleus to identify the chemical species of 31P that are present in the sample to be analyzed. Phosphorus NMR (31P NMR) is attractive in that it can simultaneously identify all speciation forms of phosphorus present in the sample, for example phosphonates, orthophosphate, pyrophosphate, polyphosphate, orthophosphate monoesters, and orthophosphate die‐sters. It has the advantage that all P species can be characterized simultaneously, without the need for complex purification and chromatographic separation procedures (Cade‐Menun, 2004). 31P is an isotope of phosphorus that has a spin 1/2 nucleus with about 100% natural abundance in nature. It has a good natural receptivity as compared to other isotopes, for example, 31P is known to be 391 times larger than 13C and the range of its chemical shifts extend from 500 to –200, with the chemical shift of 0.0 being assigned to 85% H3PO4, which is taken to be the reference 0 (Gorenstein, 1984 ; Quin and Verkade, 1994 ; Spyros and Dais, 2009).
31P NMR spectroscopy has been useful as an NMR technique capable of supplementing 1H and 13C NMR spectroscopies, because in many cases proton and carbon‐13 NMR spectroscopic techniques, when used in food analyses, have resulted in complex spectral patterns with overlapping 1H NMR and also in the case of 13C, the resulting spectra have produced long relaxation times, because 13C nuclei is not that more sensitive and this makes it difficult to deconvolute and interpret the results.
Phosphorus in Foods
Both inorganic and organic phosphorus are known to be important components of the biological materials in both plants and animals (nucleic acids, proteins, adenosine triphosphate, cell membranes, etc.) and are actually part of what are known as essential elements that are involved in various metabolic processes necessary for life. Moreover, phosphorus forms part of skeletal structures (found in bones and teeth) in various complexes, for example as calcium phosphate, etc.
Another important aspect of phosphorus is that it is one ingredient that is normally incorporated in certain food additives, such as ammonium polyphos phosphatides in synthetic lecithins, where they are included to enhance emulsification and food texture. Also phosphates in their inorganic forms have been added in soft drinks as acidity regulators. In meat, inorganic phosphates are normally added to impart water holding capacity for meat and other food items such as cheese or bread.
Some researchers have, however, raised some concerns on the application of inorganic phosphates as food additives, although there has not been any solid scientific base to justify such concerns (Vartanian et al., 2007 ; Uribari and Calvo, 2003).
Assignment of Chemical Shifts for Food Components in 31P NMR
The analysis of food samples that contain pentavalent phosphorus in the form of phosphate, results in a spectrum showing these pentavalent phosphorus species appearing in chemical shifts in the region between 20 and –40 ppm. Trivalent phosphorus needs to be derivatized before 31P NMR analysis (Spyros and Dais, 2000) and have characteristic chemical shifts appearing in the region between 100 and 200 ppm. The derivatization methods and the choice of derivatizing reagents normally target the replacement of the labile hydrogen atoms in the chemistry of the functional groups, such as CHO, COOH, or OH. The examples of the derivatizing reagents may include 2‐chloro‐4,4,5,5‐tetramethyldioxaphospholane (Jiang et al., 1995).
Application of 31P NMR in Plant Based Food Samples
The majority of plant‐based foodstuffs such as potato, sweet potato, maize, wheat, rice, oat, millet, etc., contain a high percentage of starch, because it is a major energy source on which plants rely as it is found in plant seeds, roots, and tubers. The human diet from plants harnesses this important polysaccharide in which phosphorus is present in different chemical forms, which are starch, phosphate monoester, phospholipids, and inorganic phosphate (Srichuwong and Jane, 2007 ; Tester et al., 2004). The different types and speciation forms can enable discrimination of foodstuffs from different geographical origins or starches from different types of foods, such as tubers (potatoes) vs. cereals (maize, wheat), etc. For example, starch from legume sources, such as green peas, lima beans, mung beans, and lentils, as well as waxy starches, have been reported to contain mainly phosphate monoesters, making them easier to discriminate from starches in other foodstuffs of plant origin (Lim et al., 1994).
Other plant originating foods, such as fruits and vegetables, have been reported to be rich in inositol hexaphosphate, which is also known as phytic acid or phytate. However, for this type of phosphate to be able to be digested in the mammalian gut, some specialized enzymes known as phytases present in certain types of foods, fungi, yeast, and some bacterial species are necessary (Vohra and Satyanarayana, 2003). Inositol hexaphosphates are known to have many beneficial effects to humans, including the reported role they play as antioxidants (Wu et al., 1994) and they also have good anticarcinogenic activity (Harland and Morris, 1995). 31P can be used to discriminate between phytate in raw foods vs. cooked/processed foods, or foods consumed in different cultural entities, or between leavened and unleavened bread, because there are lots of phytases in leavened bread products, making phosphate more bioavailable than is the case for unleavened bread (Spyros and Dais, 2009). The technique has also been used successfully in discriminating between different types of phosphorus groups found in different plant oils, such as phosphatidylcholine and lyso‐phosphatidic acid, as well inorganic phosphorus (Bosco et al., 1997).
31P NMR for Foods of Animal Origin
The quality of meat and meat products is normally highly dependent on their water holding capacity, which in turn influences the type and magnitude of metabolic reactions taking place in muscle (Bertram and Andersen, 2007). A number of these metabolic reactions lead to phosphorus‐containing metabolites, which can be characterized using 31P NMR as well as circular polarization – magic angle spinning (solid state NMR), which can generate data useful in the fingerprinting of the animal’s identity in terms of species, age, geographical origin of the animal, level of phosphorylation, muscular type, or in the case of processed meat, storage conditions, levels and variation of soluble muscle metabolites such as adenosine triphosphate, phosphocreatine, phosphomonoesters, as well as inorganic phosphate (Bertram et al., 2004a, b ; Vogel et al., 1985). Moreover, Roberts et al. (1981) and Burt et al. (1976) have reported that 31P NMR measurements for phosphorus‐soluble metabolites such as adenosine triphosphate, phosphomonoesters, etc., which have stable pKa values, can be used reliably to ascertain the measure of intracellular pH of meat muscle by considering their chemical shifts.
31P NMR can also be used to discriminate between the different quality classes of pork meat into their respective groups, which include normal pale soft exudative and dark firm dry, by evaluating the measure of the different types and quantities of soluble phosphorus compounds, as well as a measure of intracellular pH and water‐holding capacity. For example, pork meat that is characterized by high ultimate intracellular pH and high water holding capacity belongs to the dark film dry class, while the one that contains low water holding capacity falls under the pale soft exudative class (Honikel, 2004). Also pork meat that is characterized by high phosphomonoesters and low phosphocreatine concentrations implies that meat falls under the dark firm dry class, while the meat with high content of inorganic phosphorus and at the same time low concentration of phosphomonoesters, belongs to the pale soft exudative‐prone pork meat.
One of the food products that is of animal origin is milk, an important food source of dietary phosphorus. Milk and other milk‐derived products such as concentrated milk, whey, skimmed milk, casein, etc. contain different species of phosphorus in terms of types and ratios, such that 31P NMR can be used to identify the presence of these phosphorus species, for example inorganic phosphates and phosphoserine residues, where the normal position of casein or glycerophosphorylcholine can be identified mainly in normal milk and skimmed milk (Spyros and Dais, 2009). 31P NMR can also be used to authenticate milk products from various species by investigating the distribution of phosphorus compounds in milk samples, because the distribution pattern is species specific. Moreover, phospholipids contents in milk samples from different animal species tend to vary with the type of feed in terms of types, amounts, distribution pattern, and ratios, making it possible to categorize milk from different animals (Spyros and Dais, 2009). Sphingolipid content and composition in the milk samples from different animal species can also be analyzed using 31P NMR to authenticate the milk or species from which it originated (Byrdwell and Perry, 2006).
Application of 31P NMR for Seafood Forensics
31P can be used to discriminate fish within and between species, by studying their phosphate metabolism and observing their responses in terms of muscle physiology, biochemical point of view, or their responses toward exposure to certain environmental stresses such as hypoxia, anoxia, etc. (VanDenThillart and VanWaarde, 1996 ; VanGinneken et al., 1995 ; VanWaarde et al., 1990). This technique can also be applicable in cases of determining the extent of freshness of fish and effects of heat during preservation and how different fish species can be differentiated in terms of their responses to freshness after being preserved at a particular temperature (Chiba et al., 1991; Yokoyama et al., 1996a, b). 31P NMR can also be used to characterize and discriminate fish species based on their phospholipid contents, as well as the degree of formation and ratios of phosphorylated metabolites in different fish species (Gradwell et al., 1998).
Application of 31P NMR for Food Additives Forensics
A number of food additives contain phosphorus/phosphates in their chemical compositions. For example, phospholipid extracts (lecithins) from both animal and plant sources are incorporated in foodstuffs such as breads, baby foods, chocolates, etc., where they are used as emulsifiers or dispersing agents due to their unique surface‐active properties (Diehl and Ockels, 1995 ; Diehl, 2001). 31P NMR can be used to classify and identify various brands of these lecithin‐containing foodstuffs, based on the phospholipid content, type/class, or give an emulsification signature/properties of lecithins for different types of foodstuffs where it is being used as an additive (Helmerich and Koehler, 2003). 31P can also be used to identify lecithins obtained through different extraction procedures or from different geographical locations (Spyros and Dais, 2009).
Other food additives, especially those that play roles as stabilizers and gelling/foam agents contain peptide components (proteins) that impart textural properties to foods due to their characteristic surface‐active functionalities (Weder and Belitz, 2003). There are naturally‐occurring food stabilizer compounds, such as those found in milk and eggs, which are used in drinks and beverages such as beer; some are used in desserts, sweets, breads, and other baked products. 31P NMR can be used to differentiate and authenticate protein‐containing additives based on the stability, type, and quantity of phospholipids, phosphatidylcholine, and lysophosphatidylcholine, or how these molecules interact with other biomolecules such as albumin or fatty acids present (Chiba and Tada, 1989 ; Mine et al., 1992, 1993). 31P NMR can also be used to verify whether these food additives have been phosphorylated, as well as the sites that have been phosphorylated (Li et al., 2003).
Other phosphate‐containing food additives include leavening agents used in the bakery industry. The phosphate compounds used for this purpose include the oligophosphates (inorganic), cyclic phosphates, and linear phosphates, as well as those that play important roles in the enhancement of water‐holding capacity in meat and meat products, and as stabilizers in milk (both ultra‐high temperature and sterilized brands) (Spyros and Dais, 2009). 31P NMR can be used to identify at which point or step during the food processing the additive containing such a phosphate was added. It can also be used to track the stability of these food additives under various storage conditions (e.g. effect of temperature, freezing, etc.) (Belloque et al., 2000).
Where the presence of organophosphorus agrochemical residues in foodstuffs involve deliberate negligence or poisoning, 31P NMR can be used for both qualitative and quantitative purposes to identify the phosphate‐containing moieties for both the parent compounds and their metabolites.
Nitrogen NMR in Food Forensics
There are many nitrogen‐containing analogs in foods, such as proteins, nucleic acid bases, etc. Therefore, nitrogen NMR should have a special place in terms of different types of applications in the science of foods.
14N and 15N NMR Techniques in Food Forensics
There are two NMR active isotopic nitrogen nuclei, which are 14N and 15N and of the two, the 15N nucleus is characterized by poor or inadequate sensitivity, even though it yields sharp line signals as compared to 14N, which is characterized by providing medium sensitivity and weak and broader line signals due to quadrupolar interactions. Both 14N and 15N share some common properties in that they both produce signals that make it possible for characteristic assignment to nitrogen found in various chemical/biological environments and that the two nitrogen isotopic nuclei have the same chemical shift ranges and that CH3NO2 (90% in deuterated chloroform (CDCl3)) and also liguid ammonia (NH3, – under pressure) are used as chemical shift standards, with the latter being used more frequently, especially for 15N. In order to convert 15N chemical shifts to those obtained when CH3NO2 is used as a standard in 15N measurements, 380.5 ppm should be subtracted, while 381.6 ppm should be subtracted for measurements involving 14N.
The chemical shifts for nitrogen isotopic nuclei are very wide (0–900 ppm) (Table 12.4).
Table 12.4 Approximate chemical shift values for nitrogen isotopic nuclei as found in different types of chemical environments when CH3NO2 is used as a standard.
| Nitrogen compounds | Approximate chemical shifts, ppm |
Amines 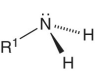
|
0 – 100 |
| Ureas CH4N2O | 20 – 140 |
Amides 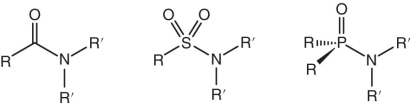
|
30 – 170 |
Terminal azide 
|
100 – 130 |
Nitriles 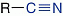
|
170 – 220 |
Indoles 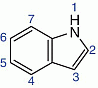 and pyrroles
and pyrroles 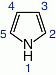
|
180 – 300 |
Azide center, example 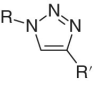
|
220 – 250 |
Diazo 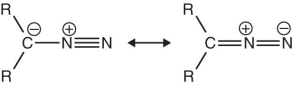
|
200 – 450 |
Pyridines 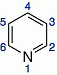
|
220 – 380 |
Nitro 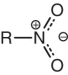
|
320 – 380 |
| Polyheteroaromatics (compounds containing many aromatic heterocycles), eg those found in nucleic acids (DNA, RNA) and enzymes | 250 – 550 |
Azo 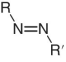
|
520 – 580 |
Nitroso 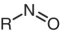
|
550 – 900 |
Multidimensional NMR Techniques
Application of Molecular Spectroscopy in Food Forensics: Rotational‐vibrational Spectroscopy
Rotational‐vibrational spectroscopy covers that region in the electromagnetic spectrum that is useful in providing information for the identification of molecular species that are subject to exposure to rotational‐vibrational radiation or molecular species produced as a result of surface reactions. The techniques that can be employed to deconvolute and study vibrational molecular surface data from either liquid, solid, or gas phase samples, include the infrared (IR), Raman, microwave, etc. Apart from these techniques, there are those techniques that can be employed to deconvolute data from the rotations/vibrations of molecules at interfaces and these include electron energy loss spectroscopy (EELS) and sum frequency generation spectroscopy (SFG).
Infrared Spectroscopy
Infrared techniques can be performed in a number of ways, using different principles depending on the sample characteristics (Cotton and Wilkinson, 1989 ; Hoffman, 1988). In the case of solid samples with high surface area, two IR techniques are normally suitable for use:
- Transmission infrared spectroscopy (TIS): which can be used for solid samples that are infrared transparent; and
- Diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS): which is used for samples that are not transparent enough for TIS.
For low surface area samples, the following infrared techniques are normally used:
- Reflection‐absorption infra‐red spectroscopy (RAIRS): which is normally used for highly reflective samples; and
- Multiple internal reflection spectroscopy (MIR): commonly known as attenuated total reflection (ATR).
Generally, the bombardment of infrared radiation triggers molecular vibrations which provides the most information about the presence or absence of certain functional groups. Infrared radiation is a form of thermal energy which, when exposed to molecules, induces molecular vibrations, especially for those molecules with covalent bonds, causing different types of vibrations such as bending, stretching, etc. (Table 12.5). However, with IR, specific types of bonds absorb or respond to IR radiation of specific wavelengths. Moreover, not all covalent bonds absorb in the IR region; it is only polar bonds that are capable of displaying bands at such wavelengths. The extent and magnitude of the dipole moment related to the polar molecular bond being analyzed will determine the intensity of the band.
Table 12.5 Example of some selected functional groups, their positions, shape and band strengths in IR spectra of bacteria.
Sources: Beekes et al., 2007 ; Helm et al., 1991a, b ; Maquelin et al., 2002 ; Mauer and Reuhs, 2010 ; Naumann, 2000 ; Naumann et al., 1991a,b; Agnieszka Nawrocka and Lamorska, http://dx.doi.org/10.5772/52722; Yu and Irudayaraj, 2005 .
| Type of food component | Functional group | Molecular vibrations of the functional group | Wave number (cm−1) |
| Proteins | N─H | Amide (stretching) in proteins | 3200 |
| Fatty acids | C─H | ─CH3 (asymmetric stretching) in fatty acids | 2955 |
| Fatty acids | C─H | >CH2 (asymmetric stretching) in fatty acids | 2930 |
| Amino acids | C─H | ≥C─H (stretching) of amino acids | 2898 |
| Fatty acids | C─H | ─CH3 (symmetric stretching) in fatty acids | 2870 |
| Fatty acids | C─H | >CH2 (symmetric stretching) in fatty acids | 2850 |
| Lipid esters | ─CO─OR (ester group) | >C ═ O (stretching) of lipid esters | 1740 |
| Nucleic acids | ─CO─OR (ester group) | >C ═ O (stretching) of lipid ester group in nucleic acids | 1715 |
| Carbonic acid | ─CO─OR (ester group) | >C ═ O (stretching) of lipid ester group in carbonic acids | 1715 |
| Proteins | Amide I band components of proteins | 1695–1675 | |
| Proteins | Amide I of alpha‐helical structures of proteins | 1655 | |
| Proteins | Amide I of b‐pleated sheet structures of proteins | 1637 | |
| Proteins | Amide II band of proteins | 1550–1520 | |
| Tyrosine band | 1515 | ||
| Lipid proteins | C─H | >CH2 deformation in lipid proteins | 1468 |
| Proteins, carbohydrates, nucleic acids | C─O─H in‐plane bending | 1415 | |
| Amino acids and fatty acids | C ═ O symmetric stretching of COO─ groups in amino acids and fatty acids | 1400 | |
| Proteins | Amide III band components of proteins | 1310–1240 | |
| Phospholipids | P ═ O asymmetric stretching of phosphoester in phospholipids | 1240 | |
| Polysaccharides | C─O─C; C─O ring vibrations | 1200–900 | |
| Phospholipids and nucleic acids | P ═ O (symmetric stretching) in nucleic acids (DNA/RNA) and phospholipids | 1085 | |
| Fatty acids and proteins | C─H | >CH2 (rocking) in fatty acids and proteins | 720 |
| Fingerprint region | 900–600 |
Vibrational spectroscopic methods are known to be very sensitive, non‐destructive, and are also “first‐pass” techniques that require either very minimal or completely no sample preparation procedures. These are attractive attributes in food forensic investigations, because in most cases specimens presented as evidential materials are low in quantities (concentrations and/or the target ingredient may be highly diluted or contained in the complex matrix of the sample). Infrared spectroscopy can provide spectral data information for all molecule dipole moments, such as those containing polar functional groups (e.g. hydroxyl (–OH) and carbonyl (C ═ O)), whereas homopolar molecules (N ═ N or C ═ C), which are not infrared active, are Raman active due to the fact that Raman spectroscopy is dependent on molecular polarizability effects.
Terahertz, on the other hand, uses the regime in the electromagnetic radiation that is found between the microwave and infrared frequencies. The common feature for the three spectroscopic techniques (infrared, microwave, and terahertz) is that they are all non‐destructive and have the ability to penetrate non‐conducting materials. Terahertz waves are non‐ionizing radiations that cannot penetrate liquid, water, or metal, but can penetrate biological specimens such as tissue with low water content, for example fatty tissue. Terahertz radiation can be useful to provide information about the difference in the amount of water, as well as the density of a particular tissue.
Vibrational Spectroscopy in Food Forensics
There are three main techniques that fall under vibrational spectroscopy, which have been useful in criminal food forensics and these are infrared, Raman, and terahertz (THz) spectroscopy (Figure 12.4). These techniques are useful for forensic fingerprinting and identification of the forensic specimen materials presented as evidence, by probing the molecular structure of these evidential materials as characterized by the pattern of vibrational spectral data obtained, either in terms of the presence or absence of certain functional groups. The spectral data emanating from these techniques can indicate the presence of a particular functional group that is a fingerprint of the suspected forensic material, by using the frequency at which the signal is occurring, shape, signal/band intensity, etc., or by comparison to a matched library pattern of reference spectra populated in a standard library database.
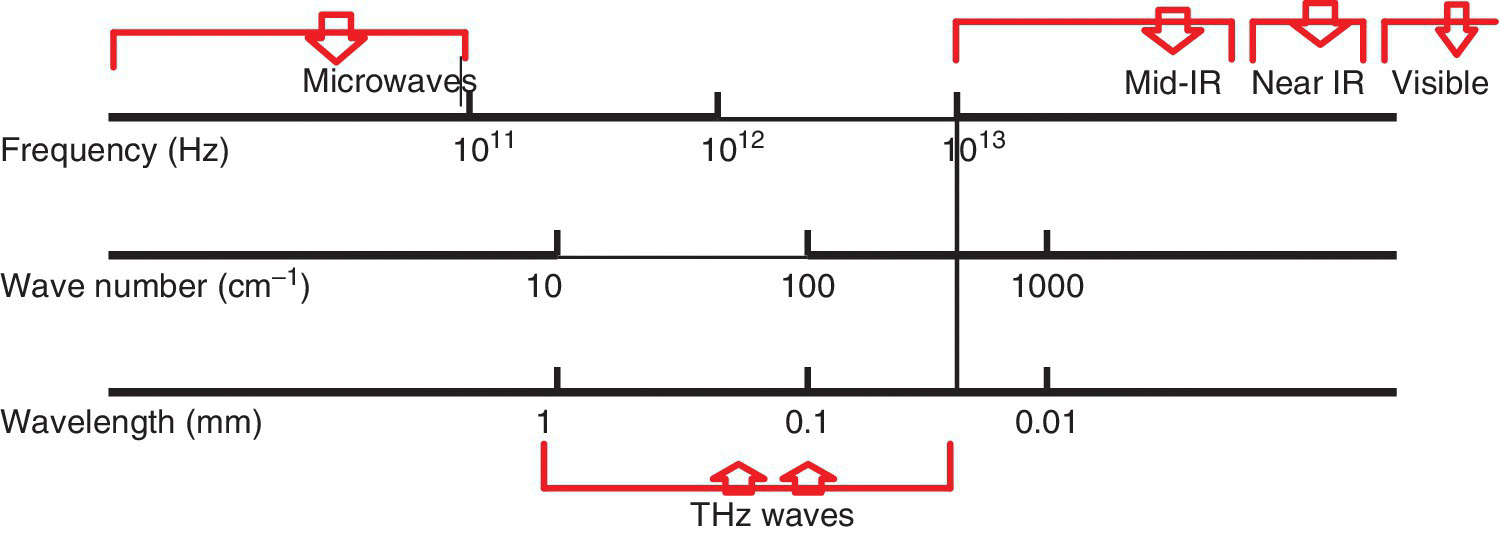
Figure 12.4 Infrared, terahertz and microwave vibrational spectroscopy waves.
The infrared part of the electromagnetic radiation spectrum has three regions, including the near infrared, mid infrared, and far infrared. These regions are located in the electromagnetic radiation in increasing wavelengths, such that the near infrared region covers wavelength bands 12 800–4000 cm−1, the mid infrared covers 4000–400 cm−1, and the far infrared covers wavelength bands 400 to ~10 cm−1. On the other hand, the Raman spectral region covers the wavelength bands between ca. 400 and 5 cm−1 to ~4000 and 3800 cm−1, which also encompasses much of the THz absorption wavelength band region. However, for food forensic specimens, the most plausible region will be the one where the normal modes of vibration of organic molecules normally occur and this region occurs in the mid infrared (4000–400 cm−1) or for Raman, the region for organic molecules is 4000–400 cm−1.
Unlike conventional spectroscopic methods, such as microwaves and X‐rays, electromagnetic waves that fall within the region of THz radiation, or THz waves, are regarded as not only the modern but also the future technology for sensing and imaging. THz waves have been used in many scientific and engineering disciplines, such as the study of matter (solid, liquid, and gaseous) materials (De Lucia, 2003 ; Jacobsen et al., 1996 ; Woolard et al., 1999).
Other applications, which THz has been subjected to, include medical diagnosis, health monitoring, environmental control, chemical and biological identification, and non‐destructive evaluation (Arnone et al., 2000 ; Globus et al., 2003; Markelz et al., 2000 ; Walther et al., 2000; Woolard et al., 2000; Zelsmann and Mielke, 1991), as well as in food and agricultural products inspection.
The many applications of THz spectroscopy stem from the attractive and unique technical features THz waves possess for sensing and imaging applications. These attractive features include the fact that the magnitude of the energy levels of low photon energy, sub‐millimeter wavelengths magnitude (in terahertz band measures between 1 and ~10 meV), is the same as the molecular transition. In addition to this, another important attribute is that large‐scale molecular resonance is found in the THz band. As pointed out previously, THz radiation is non‐ionizing and is well absorbed by polar molecules such as water. Since there are many biological samples (e.g. microorganisms) and chemicals that are active when subjected to THz radiation, this spectroscopic band may be useful in the identification and measurements of such materials. It should be noted that THz radiation waves possess the ability to penetrate various materials, including those used in food packaging with modest attenuation, making it possible for the inspection of food items in their packaging. THz radiations are capable of providing unique spectral signature information about the transitions between rotational states for the intermolecular and intramolecular interactions for even very complex (i.e. microorganisms) and polar molecules.
THz waves can also be used for the investigation of non‐polar food components, non‐polar liquids/fluids (which are transparent when subjected to THz radiation), and non‐metallic materials such as food packaging made of plastic material, due to the fact that these materials are reflective and semi‐transparent at THz magnitudes of between 0.2 and 5 THz. Water and ice behave differently when subjected to THz radiation, because water molecules absorb strongly in the THz range, its rotational motion arising due to its dipole properties, but ice, now a crystal, is transparent because the dipole motions in such a crystal molecule have been frozen.
Application of Vibrational Spectroscopy in Food Forensics
The application of vibrational spectroscopy in food forensics is made possible by the availability of a combined full set of spectral information from the molecular analysis of the food samples or specimens from a particular species. This spectral information reveals the molecular composition, chemical composition, and structural composition, as well as information about molecular interactions for a distinctive target tissue. Such information is normally specific, unique, and characteristic to the target distinctive tissue, because cellular components tend to display a unique and characteristic spectral pattern in terms of the frequency of the vibrational spectrum where they absorb, and the intensity at that particular wavelength and bandwidth. Such spectral patterns, characteristic to a particular tissue, can then be useful in the identification and fingerprinting of a particular tissue in the food stuff or species under investigation.
Vibrational spectroscopy can be used to characterize the functional groups in a distinctive target tissue in food samples presented as evidence in food forensic cases. In addition to this, vibrational spectroscopy can be used to follow the biochemical changes associated with macromolecules (e.g. proteins, lipids, DNA, RNA, etc.) in terms of their levels and composition at various growth stages. Vibrational spectroscopy is also capable of monitoring physical changes in cellular or histological structural changes that involve biomolecules such as lipids, proteins, DNA, RNA, etc. However, for vibrational spectroscopy to be of use in food forensic issues, the band spectral data has to be correctly assigned in terms of the position of the absorption frequency, height of the peak signal, peak area, and peak bandwidth.
Infrared (IR) and Raman are known as complementary vibrational spectroscopic techniques that have been extensively applied to deduce molecular information about the sample being analyzed. The information obtained is about the vibrational states of the functional groups of the molecules in the sample, also the molecular imaging, molecular structure, and molecular composition of the whole sample being analyzed (i.e. the sample information is obtained without manipulation and the sample is analyzed as it is). Moreover, infrared and Raman can provide both qualitative and quantitative data about the sample’s chemical composition and concentration, as well as confirmation (Cakmak et al., 2006 ; Chawla and Thomas, 2004 ; Dogan et al., 2007 ; Nissen et al., 2005). Despite the attractive features of these vibrational spectroscopic methods, they still possess some drawbacks. For example, the mid‐infrared region of the IR spectroscopy, which is highly useful for the analysis of organic samples, suffers water absorption problems because many biological samples contain water, which then absorbs strongly at several wavelengths, such that the signal due to water molecules masks the vibrational signal due to functional groups from the molecules present in the sample of interest. One of the possible remedies for this problem is to replace water (H2O) with heavy water (D2O) during the sample preparation procedures or analyze the sample in the form of a dry film. This may appear to be the solution to the problem, but it creates other different products. For example, by replacing H2O with D2O, the possibility of exchanging hydrogen for deuterium may occur and this will change the pattern of the vibration spectra for the sample. Or if dry film is used, it may imply that the sample integrity may be compromised. A more reliable solution will be to analyze the sample in its aqueous solution state and then subtract the spectral blank (buffer) vibrational signal from the real sample vibrational spectral signal.
With Raman spectroscopy, unlike IR, water does not present a problem, because the Raman spectrum for water is very weak, so cannot interfere or mask the spectra for the sample. However, the only limitation for Raman spectroscopic analysis lies in the fact that the technique itself generates spectra with low probability and therefore to get round this problem, the analyst must ensure that the sample amount (concentration of the analytes in the sample) is high, or otherwise high intensity laser sources have to be employed to enhance the vibrational signal intensity of the sample.
Monitoring of Foods in which Processing has Altered the Composition by Vibrational Spectroscopic Techniques
Some food processing may alter the composition of the final product. This will cause wrong labeling in terms of the presence of various ingredients and their levels and can result in a forensic case, especially if proven that there was unprofessionalism or an intentional or deliberate mishandling in some of the steps involved in food processing.
Application of Mid‐infrared Spectroscopy
The mid‐infrared part of the vibrational spectroscopy, which extends at wavelengths of approximately 4000–400 cm−1 (2.5–25 µm), may be used to monitor and ascertain the structural molecular rotational‐vibrations of food samples, which are suspected to have undergone changes after processing. Processing is meant to improve the quality of food by adding some components at some specified conditions of temperature, pH, etc. For example, irradiation using various sources at an optimized irradiation dosage is normally practiced for disinfestation purposes (eliminate pathogens). For example, Dogan et al. (2007) reported a study where they investigated the radiation‐induced molecular changes in macromolecular components of hazelnut tissues (Corylus avellana L.). In their study, they used gamma‐radiation from a cobalt‐60 source to irradiate hazelnut at two different irradiation dosages, with one low dosage (1.5 kGy) and another higher dosage (10 kGy). The changes in terms of the mid‐infrared position of the signal frequency, signal intensity, and intensity ratio of IR bands were then studied and the results showed that when the hazelnuts were irradiated with 1.5 kGy, the level of the total and unsaturated lipid as well as the ration of lipid to protein went up, but at a higher dose of 10 kGy, there was production of peroxide that was recorded and also the levels for unsaturated lipids seemed to go down. The infrared technique allows for the possibility to follow changes in the magnitude of concentration levels of biomolecules (functional groups of biomolecules) by monitoring the spectral signal intensities of the respective molecular functional groups.
Another observation that was recorded at higher dosage of irradiation was that structural changes in terms of protein composition became evident due to cross‐linking and aggregation of proteins. Infrared can be used to track molecular structural changes, such as the magnitude of chemical/hydrogen bonding, the order and disorder of membrane lipids, etc., by monitoring the magnitude of the shift of spectral band positions that can be assigned using the wave number reading taken at the midpoint of the band wave (at 0.8 × height of the signal). However, for more conclusive results on the effect of irradiation on foodstuffs, IR results need to be complemented with chemometric methods, such as partial least square (PLS) and least squares support vector machines (LS‐SVM).
In another study, Kizil et al. (2002) demonstrated that it was possible to employ mid‐IR spectroscopy in combination with chemometric techniques, such as principal component analysis (PCA), to streamline the massive data obtained, as well as CVA and PLS models to probe and classify the effects of food irradiation on starch (Kizil et al., 2002). Another report by Vlachos et al. (2006) detailed the application of mid‐IR in combination with chemometrics to study the trend of corn oil oxidation after exposure to UV radiation (Vlachos et al., 2006). The trend in the oxidation reactions and progress in this case can be easily followed by monitoring specific wavelengths associated with C–H stretching bonds, which occur in wavelengths of 2850–3100 cm−1 and also for ester’s carbonyl carbon of triglycerides, which occur at around 1745 cm−1.
These reports are among several such publications that show how useful mid‐infrared spectroscopy is in providing explanations in food forensic cases associated with inaccurate food composition labeling, which may be a result of deliberate actions of improper food processing.
Application of Mid‐infrared for Authenticity and Compliance Testing of Food Packaging
Currently, food industries, suppliers, and vendors are highly encouraged to move from the use of plastic food packaging to those that are highly biodegradable, such as those made of cellulose or hemicellulose materials. Normally, consumers and vendors have a tendency to warm foodstuffs using microwave ovens, the food wrapped in its packaging just before consuming. However, microwave energy may have a notable effect on these biodegradable packaging materials. Infrared has been employed to study the stability and effects of exposure of hemicellulose and cellulose‐based food packaging to microwave radiation. After subjecting the hemicellulose materials to microwave energy, mid‐infrared can be employed to probe the lauroylation of the hemicellulose and compare the spectrum profile of the native and lauroylated hemicellulose in terms of peak position shift, increase or decrease of certain peaks, the peak intensity, especially of hydroxyl functional groups that are expected to occur at wavelengths of 3413–3479 cm−1. It should be noted that lauroylation may result in some peaks to disappear or decrease due to the formation of hydrogen bonding.
Application of Mid‐infrared to Monitor the Effects of Processing on the Integrity of Food Components
Processes that are followed in food industries are regulated in terms of the conditions and the type of additives involved in the processing, in order to avoid production of undesirable products or loss of important nutritious ingredients. Care needs to be taken in carrying out of all steps during food processing, for example the optimal temperature or irradiation needed and skilled personnel to be involved. The ingredients may be altered during food processing, resulting in mislabeling that may trigger a forensic case.
Mid‐infrared spectroscopy, in combination with chemometrics, can be a useful method to follow changes (both cellular structural and compositional) that may be associated with different types of treatment (e.g. high heat, low heat, etc.) during food processing. For example, plant‐derived foodstuffs such as fruits and vegetables have their cellular structures enveloped by cell walls. The structure of these cell walls is highly influenced by the structure of various biomolecules in the cells, such as polysaccharides as well as the chemistry of the glycosidic bonding types that characterize the building blocks for polysaccharides, which are mainly simple sugars (monosaccharide), for example, whether the glycosidic linkages are 1,2‐; 1‐3; 1,4‐glycosidic bonds etc.; bond lengths of such glycosidic linkages; the types of monosaccharides that polymerize to form polysaccharides; the nature of polymerization, and whether it forms branched patterns or linear chains.
Many fruits and vegetables have their cell walls composed of pectin polysaccharides, hemicelluloses, and cellulose, which together play important functions in giving specific and unique structural and textural characteristics to these foods. These biomolecules provide a means of classifying and distinguishing between different species of foods, as they are characteristic to a particular species or variety. This is because different polysaccharides have different chemistries in terms of composition and structure of their monomers (monosaccharides), thus imparting different characteristics that are distinctively unique for each food item.
During food processing, especially where heat treatment is involved, heating facilitates some changes such as loss of rigidity (softening) where the cell wall becomes weakened because of the breaking of the glycosidic linkages, which imply that the depolymerization of polysaccharides is taking place. These changes, which arise due to heat treatment, can be followed by using infrared spectroscopy (mid‐infrared). The mid‐infrared technique can show the magnitude at which the side chains of the neutral sugar molecules have been altered during the heat treatment procedures. In such processing, the side chains of the neutral sugars are believed to interact chemically with other hemicellulose components such as xyloglucans and therefore mid‐infrared will indicate the missing functional groups for both the xyloglucans and the side chains of the neutral sugars.
Also mid‐infrared may be used to identify cases that involve mixed foods, where the ratios or types of components are deliberately mislabeled or not disclosed accordingly. For example, mid‐infrared spectroscopy can be used to distinguish between pectic carbohydrates (polysaccharide), which contain low methoxylation from one with high levels of methoxyl composition or distinguish foods based on the extent of esterification. The distinction of these food properties and characteristics is possible by studying the spectral patterns obtained where wave number ranges of 1500–1800 cm−1 are normally correlated to carbonyl groups of esters and carbohydrates, while wave numbers between 850 and 1200 cm−1 are correlated to sugar components based on types and compositional ratios, which provide a fingerprint pattern of a particular species. The fingerprint pattern of sugars can also be used as an indicator for a ripening stage of fruits or vegetables from a particular species. Mid‐infrared spectroscopy can also be useful in the classification of the foodstuffs’ polysaccharide, based on the extent of methoxylation or esterification.
Mid‐infrared and Chemometric Methods
Generally, the spectral information generated by the infrared technique for food samples is normally highly complex, due to the fact that bio(macro)molecules that form food composition contain a diversity of chemistries and functional groups, with numerous possibilities of engaging in chemical interactions, which complicate further the spectral pattern generated. This makes the interpretation of these spectral data a difficult task. For the purpose of making it possible to simplify the interpretation of such data, multivariate chemometric methods have been employed due to their capabilities to compress massive data and reduce the complexity of information generated. Moreover, for more reliable conclusions and for validation of the results, mid‐infrared spectral data are normally complemented with chemometric methods of analyses. These multivariate statistical methods are attractive, because of their capabilities to compress massive data obtained and can also be used to discriminate between different forms of monosaccharides, for example galactose and glucose or disaccharides based on their distinctive characteristic infrared spectral patterns.
The multivariate chemometric tools that have been employed to unpack the complex information generated by infrared spectroscopy, include principal component analysis (PCA), trimmed object project (TOP), outer product analysis (OPA), which is normally useful for unmasking spectral data information, and partial least squares (PLS), etc.
PCA and TOP are both exploratory multivariate chemometric techniques. PCA is a bilinear multivariate chemometric method that is useful in the compression and reduction of complex data and can be used to explain variability of properties for a small set of data (compressed) from a complex matrix. PLS, on the other hand, is used to perform predictions and calibrations that play an important role in explaining the relationship between the signal and the measured parameter, according to the appropriate vectoral regressions, in order to provide information about the sample and predict the magnitude of the parameter of interest for the sample under investigation.
Application of Near Infrared in Food Forensics
Unlike the mid‐infrared spectral patterns that arise as a result of molecular vibrations at particular frequencies after exposure to mid‐infrared radiations, near‐infrared spectral information originates from the overtone in combination with molecular vibrations and for this reason, near‐infrared spectra are complex, making interpretation somewhat of a challenge. Generally, near‐infrared spectroscopic methods are suitable for applications in food forensic issues, both as at‐line, on‐line, and in‐line techniques, especially for the authentication of food and agricultural products.
The attractive features of near‐infrared spectroscopy include the fact that the technique is known to provide a rapid means of analysis; it is a proven economical approach and a non‐destructive method, which is suitable and convenient for applications that need to establish the correct classification of foods or food quality. However, the application of near‐infrared in the probing of the effects of irradiation on foods is not well reported.
A few instances where near‐infrared spectroscopy was used in analysis of foods include a report by Barabassy et al. (1992), where this technique was employed in the investigation of the effects of gamma rays irradiation on paprika powder, which revealed that gamma rays caused significant changes in the structure of water and hydroxyls as a function of time. In another report, Seregely et al. (2006) employed a combination of techniques, which included near‐infrared, chemosensor array, and chemometrics to monitor the effects of subjecting egg white to stresses, including high hydrostatic pressure (400 MPa), gamma rays (2 kGy), and low temperature pasteurization (4°C). This study revealed that irradiation and pressure caused significant changes in terms of the quantity of volatiles, whippability, and foam stability. These changes mean a lot to the quality of food in terms of taste, aroma, and other properties, including color.
It is possible to employ both mid‐ and near‐infrared techniques to investigate food quality or as techniques that can enable proper classification of foods (Reid et al., 2005).
THz‐based Techniques for Food Forensics
Terahertz spectroscopy can find applications in food science, especially in the identification of production area, food process monitoring, and virus and microbial testing in foods and also the analysis of the presence of agrochemicals in foods (Kawase, 2012). These waves are also useful in food forensics cases, which may involve deliberate food quality and standards violations, poisoning, adulterations, and presence of illegal or banned additives. The mode of action for THz radiation involves the interactions of the THz waves with biomolecules, whereby they initiate low‐frequency molecular vibrations, which then promote molecular groupings through the formation of weak hydrogen bonds, van der Waals, and hydrophobic interactions.
There are several THz‐based techniques that find application in food forensics, and these methods are discussed below:
THz‐time Domain Spectroscopy (THz‐TDS)
The instrumentation for THz‐TDS involves the source for the ultrafast femtosecond laser. A splitter is also incorporated such that the laser is directed separately into two portions, with one directed to a pump beam (to illuminate the emitter) and another to a probe beam (to illuminate the receiver). In the set up, an optical delay line has to be included in the probe beam to control the variations of the difference in optical delay within the zero magnitude between the THz pulse that is incoming and the probe laser pulse at the detection system. A measure of the magnitude of the Fourier transforms and comparisons of these pulse shapes will provide information regarding the absorption and dispersion pattern of the sample. In order to obtain good results, the THz‐TDS system has to be operated under room temperature in order to nullify signals arising from noise background noise, which would otherwise mask the THz‐sample signal.
This technique has another advantage, as it offers the possibility to obtain the measurements of both absorption and refractive index of a sample and can also distinguish molecular species or mutants within the same species of organisms. It can also be used to differentiate different molecular conformations (Ferguson et al., 2004 ; Markelz, 2002).
THz‐TDS is therefore a suitable technique for the investigation of biological macromolecules that characterize food components such as proteins and tissues, because these molecules are characterized by low‐frequency motions that can be found within the THz region. The THz signal for these molecules is produced such that it gives a distinct signature pattern for particular molecules analyzed.
THz‐TDS can be used to provide evidential information regarding food materials wrapped in sealed packaging that are transparent to THz radiation. This is possible due to the high specificity of the transitions of the vibrational states that occur for materials that are used in the packaging, which are in most cases crystalline in nature and are triggered by the lattice modes that are unique to particular crystalline materials and are also a fingerprint of the structure of the molecules. Different food materials will have a distinct fingerprint in the THz‐TDS spectral range and therefore they can be recognized when this technique is applied.
THz Pulsed Imaging
THz pulsed imaging refers to an electromagnetic radiation‐based technique that is capable of providing in‐depth information about the measure of either transmission or reflectivity of the sample after it has been exposed to THz radiation. This technique causes the terahertz waves to penetrate dried samples, plastics, papers, and polar and non‐polar organic sample specimens. For the principles of THz imaging for samples that display dielectric properties, their absorption characteristics are controlled by optical phonons as well as other sample properties such as the polarity and the measure of the sample’s optical phonon resonance (Mittleman et al., 1996). This technique can provide crucial absorption spectral or sample’s radiation scattering behavioral information and therefore by analyzing the resultant changes arising from terahertz pulses due to the interaction with samples, one is able to classify and group samples according to how they behave when exposed to THz waves.
THz Continuous Wave Imaging
As compared to THz pulsed imaging, the THz continuous wave imaging technique is inferior in terms of the quality and the depth of either the sample’s frequency‐domain information or the sample’s time‐domain information at any fixed‐frequency source where the same detector has been used. The data that is generated by THz‐continuous wave imaging can suit the intended purposes in all the sample’s imaging applications (Karpowicz et al., 2005). Despite these limitations, this technique has several attractive features, including the fact that it is fast, compact, simple, and relatively economical as compared to pulsed THz (Karpowicz et al., 2005).
THz imaging techniques can be used to detect, fingerprint, and map the quantitative and qualitative composition of the food sample in terms of spatial distribution and concentration.
THz Wave and Molecular Fingerprinting
There is a direct link between the weak bonds that are responsible to make molecules active for THz spectroscopy and various chemical interactions that enable the process of binding of substrates or inhibitors to enzymes, and the same bonding mechanisms are known to have a significant impact to the processes related to the transmission of genetic impulses.
This implies that biomolecules such as microbial cells, and their organelles, as well as nucleic acid materials possess optical characteristics that can make them active for THz spectroscopic analysis and can provide very useful fingerprint information about these biomolecules. The ability of THz waves to sense biomolecules, even in the liquid phase, opens the possibility for the fabrication of THz‐based biosensors.
Food Forensics Application of Microwave Rotational Radiation
Microwave rotational spectroscopy makes use of microwave radiation as part of the electromagnetic radiation, to ascertain the energies of molecular rotational transitions for gaseous samples. Microwave radiations employs energy generated in the microwave region of electromagnetic radiation spectrum, to cause transitions in the rotational energy levels of molecules existing in the gas phase.
Principle of Microwave Spectrometry
When microwave radiation bombards the sample, depending on the orientation and relaxation, one of the following outcomes will occur:
- There may be field reductions caused by the dielectric properties of the sample molecules, because sample molecules are affected differently, depending on the magnitude of the dielectric constant (ε’);
- Alternating polarization phenomena of the sample molecules may be triggered and the sample molecules will be able to store a certain magnitude of the energy, which is then released slowly. The magnitude of the dielectric constant (ε’) is given a unity value when the microwave radiation passes through the vacuum and for dielectric materials this value is normally above unity;
- Heat loss due to phenomenon caused by the friction that occurs amongst molecules, which in turn causes a reduction of the magnitude of the signal of the wave amplitude and can be monitored by the magnitude of dielectric loss (ε”).
When either ε’ or ε” is plotted vs. the frequency at which the measurement has been taken, it results in a spectrum (Walmsley and Loades, 2011).
It should be noted that IR spectroscopy can also be employed to monitor rotational transitions in molecules existing in the gas phase. However, unlike microwave spectroscopy, the rotational transitions in IR spectroscopy are coupled to the vibrational transitions. Raman spectroscopic techniques that are equipped with high sensitivity detectors can also be used to monitor rotational transitions, as they use UV‐visible light scattering to ascertain the molecular energy levels (Harris and Bertolucci, 1978 ; Hollas, 2002). Microwave spectroscopy cannot be applied to study samples in liquid or solid states, because of the hindrance of intermolecular interactions. Moreover, compounds that are active in the microwave region are those that possess a permanent dipole moment (e.g. HCl, etc.) and thus possess the capability to absorb or emit radiation in the microwave transitions to produce a characteristic spectrum. Homonuclear molecular species, such as nitrogen (N2) and oxygen (O2), are inactive to microwave radiation and thus do not show any rotational spectrum, because the display transitions are not accompanied by any change in the dipole moment during the rotation. Moreover, diatomic molecules that are linear tend to be inactive to microwave radiation, due to the fact that their moment of inertia is small. Microwave spectroscopy can be useful in food forensics that contain volatile components, which are characteristic to that particular foodstuff. Examples of such components include those that impart characteristic aroma, flavor, or taste. In addition, microwave spectroscopy can be used to identify characteristic ions and radicals (reactive species, such as reactive oxygen/nitrogen species) in foodstuffs.
Another attractive feature of microwave radiation is that this radiation has the capability to penetrate materials that are characterized by a low dielectric constant, including glass and plastics. The limitations of microwave radiation in terms of its penetrability powers is that it cannot penetrate materials made of metallic substances because with these kind of materials, microwave radiation is reflected.
Different Microwave Techniques for Food Forensics
Non‐contact Reflective Mode Microwaves
The non‐contact reflective mode microwaves technique has been applauded and described as the best suited for the measurements of the moisture content in cereals (Knöchel et al., 2001), measurement of water uptake, fat content, protein, salt, water, and phosphate in meat and meat products (Kent et al., 2001). For example, Jayanthy and Sankarranarayanan (2007) used the microwave technique to detect moisture content in spices, where the dielectric properties of spices were correlated to grain moisture content. The technique has also been used in the characterization of sugar content in yoghurt, measurement of protein, carbohydrates, and smaller organic molecules, which possess dipole moments (Bohigas, 2008).
Guided Microwave Spectrometry (GMS)
The guided microwave spectrometry (GMS) technique has been described as the most suited for ground meat and is attractive due to the fact that 100% of the sample is measured (Hildrum et al., 2006). This technique has been used in the indirect measurements of fat and oils, which are not microwave active, but since there is a relationship between fats/oils and water and proteins, which are microwave active, then it is possible to exploit this relationship and obtain measures of fats and oils. Cataldo et al. (2009) reported the use of microwave in the measurement of quality of vegetable oils. It should be noted that vegetable oils are controlled and regulated due to numerous adulteration cases. When using microwave spectroscopic techniques for oil measurements, one fact that is useful is that different magnitudes of dielectric moments can be associated with different oils. Also, there are certain characteristic frequency ranges that provide the possibility for the permittivity of different types of oils that can be selectively distinguished from others. This makes it possible to use microwave spectroscopy to fingerprint different types of oils and also their sources. Measurements of food samples using microwave spectroscopy are both quantitative and qualitative, because the signal intensities in the generated spectrum are related to the concentration of the food samples. The technique is advantageous in that it has the capability to penetrate droplets of up to several centimeters and is appropriate for heterogenous samples. It is fast, economical, and can provide multi‐parameters in terms of measurements. The limitations of GMS are that the technique is associated with poor sensitivity when measuring particle size or when it is used to differentiate color. Also, it cannot work for frozen food samples or ice. It also does not work when salt is present, as salt occurs as an interferent.
Food Forensics Application of Multi‐dimensional Raman Spectroscopic Pattern Signatures
As discussed above, infrared as well as other methods have their limitations, both qualitatively and quantitatively, and so does Raman spectroscopic methods of analysis. Raman spectroscopic methods are based on the inelastic scattering of laser radiation when it is bombarded and thus interacting with vibrating molecules. Just as with the infrared spectroscopic technique, Raman spectroscopic methods are known to be non‐destructive, require minimal or no sample preparation, and are fast. They are regarded as confirmatory methods for identification of analytes and are very sensitive. When standard Raman spectroscopic methods are employed for the identification of an unknown analyte, the confirmation is based on either the presence of certain characteristic peaks or a comparison of the sample that generated the spectra to other known/certified reference spectra or from known databases. However, most of food forensic samples have a complex composition present in a complex matrix, such that it is most likely that the matrix constituents may cause masking of the signals due to the analytes of interest or cause overlapping that will complicate the interpretation of the spectra. To overcome this bottleneck, multivariate chemometric methods have been complemented to Raman methods and in addition to this, multi‐dimensional Raman spectroscopic approaches (those that are capable of providing a set of spectra that represent major unique variations of the sample) have also been employed to provide spectral pattern signatures that offer advantages that minimize the shortcomings of the traditional Raman methods. In this case, the possibilities of encountering false‐negative or positive observations are greatly minimized (Sikirzhytskaya et al., 2012; Sikirzhytski et al., 2012; Virkler and Lednev, 2009, 2010a,b).
Chemometric methods form an important component to complement Raman methods, because food samples are composed of biological macromolecules such as nucleic acids, chromophores, amino acids, etc., which are known to result in a complex pattern of spectra, making it difficult to deconvolute. A mixture of functional groups in all these macromolecules will provide a complex and overlapping of spectra, covering many regions of the electromagnetic radiation, for example, UV, Vis, IR, and even NIR.
Conclusions
Spectroscopic methods (atomic and molecular) rely on the behavior of organic and inorganic molecules as they are bombarded by specific regions in the electromagnetic radiation spectrum (light). Some molecules will absorb light from a certain region (e.g. UV‐Vis) and they will therefore present a particular signal at that wavelength, and some will vibrate (IR), etc. Therefore, these methods are dependent on the property of the molecule. The instrumentation for spectroscopic analysis ranges from moderately expensive to very expensive (e.g. high resolution NMR) and the skills required for the analysis follows the same pattern, medium to highly skilled personnel.
References
- Alloway, B.J. (ed.) (1995) Heavy Metals in Soils. Springer Science + Business Media, Dordrecht, p. 368.
- Annable, P.L. and Sly, L.A. (1991) Azide determination in protein samples by ion chromatography. Journal of Chromatography, 546: 325–334.
- Ariyama, K. and Yasui, A. (2006) The determination technique of the geographic origin of welsh onions by mineral composition and perspectives for the future. Japan Agricultural Research Quarterly, 40(4): 333–339.
- Arnone, D., Ciesla, C. and Pepper, M. (2000) Terahertz imaging comes into view. Physics World, 13(4): 35–40.
- Barabassy, S., Farkas, J. and Kaffka, K.J. (1992) Study of the gamma‐irradiation effect on paprika powder by NIRS. In: Making Light Work: Advances in Near Infrared Spectroscopy (eds I. Murray and I.A. Cowe). VCH Publishers, New York, pp. 415–424.
- Beekes, M, Lasch, P. and Naumann, D. (2007) Analytical applications of Fourier transform‐infrared (FT‐IR) spectroscopy in microbiology and prion research. Veterinary Microbiology, 123: 305–319.
- Belloque, J., de la Fuente, M.A. and Ramos, M. (2000) Qualitative and quantitative analysis of phosphorylated compounds in milk by means of P‐31‐NMR. Journal of Dairy Research, 67: 529–539.
- Bertram, H.C. and Andersen, H.J. (2007) NMR and the water‐holding issue of pork. Journal of Animal Breeding and Genetics, 124(s1): 35–42.
- Bertram, H.C., Whittaker, A.K., Andersen, H.J. and Karlsson, A.H. (2004a) The use of simultaneous 1H and 31P magic angle spinning nuclear magnetic resonance measurements to characterize energy metabolism during the conversion of muscle meat. International Journal of Food and Science Technology, 39: 661–670.
- Bertram, H.C., Hu, J.Z., Rommereim, D.N., Wind, R.A and Andersen, H.J. (2004b) Dynamic high‐resolution 1H and 31P NMR spectroscopy and 1H T2 measurements in postmortem rabbit muscles using slow magic angle spinning. Journal of Agricultural and Food Chemistry, 52(9): 2681–2688.
- Bohigas, X., Amigo, R. and Tejada, J. (2008) Characterization of sugar content in yoghurt by means of microwave spectroscopy. Food Research International, 41: 104–109.
- Bond, A.M., Heritage, I.D., Wallance, G.G. and McCormick, M.J. (1982) Simultaneous determination of free sulfide and cyanide by ion chromatography with electrochemical detection. Analytical Chemistry, 54: 582–585.
- Bosco, M., Culeddu, N., Toffanin, R. and Pollesello, P. (1997) Organic solvent systems for 31P nuclear magnetic resonance analysis of lecithin phospholipids: applications to two‐dimensional gradient‐enhanced 1H‐detected heteronuclear multiple quantum coherence experiments. Analytical Biochemistry, 245(1): 38–47.
- Buist, P., Marecak, D., Dawson, B. and Black, B. (1996) Use of 19F NMR as a direct probe of D9 desaturase cryptoregiochemistry: A feasibility study. Canadian Journal of Chemistry, 74: 453–456.
- Burt, C.T., Glonek, T. and Barany, M. (1976) Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. Journal of Biological Chemistry, 251(9): 2584–2591.
- Byrdwell, W.C. and Perry, R.H. (2006) Liquid chromatography with dual parallel mass spectrometry and 31P nuclear magnetic resonance spectroscopy for analysis of sphingomyelin and dihydrosphingomyelin. I: Bovine brain and chicken egg yolk. Journal of Chromatography A, 1133: 149–171.
- Cade‐Menun, B.J. (2004) Using phosphorus‐31 nuclear magnetic resonance spectroscopy to characterize phosphorus in environmental samples. In: Organic Phosphorus in the Environment (eds B.L. Turner, E. Frossard and D. Baldwin). CABI Publishing, Oxford, UK, pp. 21–44.
- Cataldo, A., Piuzzi, F., Cannazza, G., Bennedette, E.D. and Tamicone, L. (2009) On the use of dielectric spectroscopy for quality control of vegetable oils. ISBN 978‐963‐884‐10‐0‐10 2009. MECO.
- Cakmak, G., Togan, I. and Severian, F. (2006) 17 b‐Estradiol induced compositional, structural and functional changes in rainbow trout liver, revealed by FT–IR spectroscopy, a comparative study with nonylphenol. Aquatic Toxicology, 77: 53–63.
- Chawla, S.P. and Thomas, P. (2004) Identification of irradiated meat using electron spin resonance spectroscopy: Results of blind trials. International Journal of Food Science Technology, 39: 653–660.
- Chiba, A., Hamaguchi, M., Kosaka, M., Tokuno, T., Asai, T. and Chichibu, S. (1991) Quality evaluation of fish meat by 31P NMR. Journal of Food Science, 56: 660–664.
- Chiba, K. and Tada, M. (1989) Study of the emulsion stability and headgroup motion of phosphatidylcholine and lysophosphatidylcholine by 13C‐ and 31P‐NMR. Agricultural Biological Chemistry, 53: 995–1001.
- Cotton, F.A. and Wilkinson, G. (1989) Advanced Inorganic Chemistry, 5th Edition. John Wiley & Sons, Chichester, UK, pp. 58–62.
- De Lucia, F.C. (2003) Spectroscopy in the terahertz spectral region. In: Sensing with Terahertz Radiation (ed. D. Mittleman). Springer, Berlin.
- Diehl, B.W.K. (2001) High resolution NMR spectroscopy. European Journal of Lipid Science Technology, 103(12): 830–834.
- Diehl, B.W.M. and Ockels, W. (1995) Quantitative analysis of lecithin: Phospholipid analysis with 31P NMR spectroscopy. In: Phospholipids: Characterization, Metabolism and Novel Biological Application (eds G. Cevc and F. Paltauf). AOCS Press, Champaign, pp. 29–32.
- Dogan, A., Siyakus, G. and Severcan, F. (2007) FTIR spectroscopic characterization of irradiated hazelnut (Corylus avellana L.). Food Chemistry, 100: 1106–1114.
- Dupuy, N., Le Dréau, Y., Ollivier, D., Artaud, J., Pinatel, C. and Kister, J. (2005) Origin of French virgin olive oil registered designation of origins predicted by chemometric analysis of synchronous excitation–emission fluorescence spectra. Journal of Agricultural and Food Chemistry, 53: 9361–9368.
- Ferguson, B.S, Liu, H., Hay, S., Findlay, D., Zhang, X‐C. and Abbott, D. (2004) In vitro osteosarcoma biosensing using THz time domain spectroscopy. Proceedings of SPIE – The International Society for Optical Engineering, 5275: 304.
- Gailer, J. and Irgolic, K.J. (1994) The ion‐chromatographic behavior of arsenite, arsenate, methylarsonic acid and dimethylarsinic acid on the Hamilton PRP‐X100 anion‐exchange column. Applied Organometallic Chemistry, 8: 129–140.
- Gerig, J.T. (2001) Fluorine NMR, Department of Chemistry. University of California, Santa Barbara, CA.
- Gorenstein, D.G. (ed.) (1984) Phosphorus‐31 NMR: Principles and Applications. Academic Press, New York.
- Gradwell, M.J., Fan, T.W.M. and Lane, A.N. (1998) Analysis of phosphorylated metabolites in crayfish extracts by two‐dimensional 1H‐31P NMR heteronuclear total correlation spectroscopy (hetero TOCSY). Analytical Biochemistry, 263: 139–149.
- Harland, B.F. and Gurleen Narula, M.S. (1995) Food phytate and its hydrolysis products. Nutrition Research, 19(6): 947–961.
- Harris, D.C. and Bertolucci, M.D. (1978) Symmetry and Spectroscopy. University Press, Oxford, UK.
- Harris, R.K. (1983) NMR Spectroscopy: A Physicochemical View. Pitman Publishers, London, UK.
- Helm, D., Labischinski, H. and Naumann, D. (1991a) Elaboration of a procedure for identification of bacteria using Fourier Transform IR spectral libraries: A stepwise correlation approach. Journal of Microbiological Methods, 14: 127–142.
- Helm, D., Labischinski, H., Schallehn, G. and Naumann, D. (1991b) Classification and identification of bacteria by Fourier‐transform infrared spectroscopy. Journal of General Microbiology, 137: 69–79.
- Helmerich, G. and Koehler, P.J. (2003) Comparison of methods for the quantitative determination of phospholipids in lecithins and flour improvers. Journal of Agricultural and Food Chemistry, 51(23): 6645–6652.
- Herbert, S., Riou, N.M., Devaux, M.F., Riaublanc, A., Bouchet, B. et al. (2000) Monitoring the identity and the structure of soft cheeses by fluorescence spectroscopy. Lait, 80: 621–634.
- Hildrum, K.V., Peterwold, J., Segman, V.H., Ramou, J.P. and Dufour, E. (2006) Techniques for Online Monitoring of Meat Quality: Advanced technologies for meat processing. CRC Press, Taylor & Francis Group, Boca Raton, FL.
- Hoffman, R. (1988) Solids & Surfaces: A chemist’s view of bonding in extended structures. Wiley VCH, pp. 71–74.
- Hollas, M.J. (2002) Basic Atomic and Molecular Spectroscopy. Royal Society of Chemistry, Cambridge.
- Honikel, K.O. (2004) Conversion of muscle to meat – Glycolysis. In: Encyclopedia of Meat Sciences (ed. W.K. Jensen). Elsevier, Oxford, pp. 314–318.
- Ibañez, E. and Cifuentes, A. (2001) New analytical techniques in food science. Critical Reviews in Food Science and Nutrition, 41(6): 413–450.
- Jacobsen, R.H., Mittleman, D.M. and Nuss, M.C. (1996) Chemical recognition of gases and gas mixtures with terahertz waves. Optical Letters, 21(24): 2011–2013.
- Jayanthy, T. and Sankaranarayanan. (2005) Measurement of Dry Rubber Content in Latex Using Microwave Technique. Measurement Science Review, Volume 5, Section 3, 50.
- Jiang, Z.‐H., Argyropoulos, D.S. and Granata, A. (1995) Correlation analysis of 31P NMR chemical shifts with substituent effects of phenols. Magnetic Resonance in Chemistry, 33: 375–382.
- Kabata‐Pendias, A. and Pendias, H. (1984) Trace Elements in Soils and Plants. CRC Press Inc., Boca Raton, FL.
- Karoui, R., Dufour, E., Pillonel, L., Picque, D., Cattenoz, T. and Bosset, J.‐O. (2004) Fluorescence and infrared spectroscopies: A tool for the determination of the geographic origin of Emmental cheeses manufactured during summer. Lait, 84: 359–374.
- Karoui, R., Martin, B. and Dufour, E. (2005a) Potentiality of front‐face fluorescence spectroscopy to determine the geographic origin of milks from the Haute‐Loire department (France). Lait, 85: 223–236.
- Karoui, R., Bosset, J.‐O., Mazerolles, G., Kulmyrzaev, A. and Dufour, E. (2005b) Monitoring the geographic origin of both experimental French Jura hard cheeses and Swiss Gruyère and L’Etivaz PDO cheeses using mid‐infrared and fluorescence spectroscopies: A preliminary investigation. International Dairy Journal, 15: 275–286.
- Karpowicz, N., Zhong, H., Xu, J., Lin, K‐I., Hwang, J‐S. and Zhang, X‐C. (2005) Comparison between pulsed terahertz time‐domain imaging and continuous wave terahertz imaging. Semiconductor Science Technology, 20: S293–S299.
- Kawase, M. (2012) Application of terahertz waves to food science. Food Science and Technology Research, 18(5): 601–609.
- Kent, M., Knöchel, R., Daschner, F. and Berger, L.J.K. (2001) Composition of foods including added water using microwave dielectric spectra. Food Control, 12: 467–482.
- Kizil, R., Irudayaraj, J. and Seetharaman, K. (2002) Journal of Agricultural and Food Chemistry, 50(14): 3912–3918.
- Knöchel, R., Daschner, F. and Tante, W. (2001) Resonant microwave sensors for instantaneous determination of moisture in foodstuffs. Food Control, 12: 447–458.
- Larsen, E.H., Pritzl, G. and Hansen, S.H. (1993) Speciation of eight arsenic compounds in human urine by high‐performance liquid chromatography with inductively coupled plasma mass spectrometric detection using antiomonate for internal chromatographic standardization. Journal of Analytical Atomic Spectrometry, 8: 557–563.
- Li, C.P., Salvador, A.S., Ibrahim, H.R., Sugimoto, Y. and Aoki, T. (2003) Phosphorylation of egg white proteins by dry‐heating in the presence of phosphate. Journal of Agricultural and Food Chemistry, 51: 6808–6815.
- Lim, S.T., Kasemsuwan, T. and Jane, J.L. (1994) Characterization of phosphorus in starch by P‐31‐nuclear magnetic‐resonance spectroscopy. Cereal Chemistry, 71: 488–493.
- Luykx, D.M.A.M., Casteleijn, M.G., Jiskoot, W., Westdijk, J. and Jongen, P.M.J.M. (2004) Physicochemical studies on the stability of influenza haemagglutinin in vaccine bulk material. European Journal of Pharmaceutical Sciences, 23: 65–75.
- Mannina, L., Patumi, M., Proietti, N., Bassi, D. and Segre, A.L. (2001) Geographical characterization of Italian extra virgin olive oils using high‐field 1H NMR spectroscopy. Journal of Agricultural and Food Chemistry, 49: 2687–2696.
- Maquelin, K., Kirschner, C., Choo‐Smith, L.P., van den Braak, N., Endtz, H.P. et al. (2002) Identification of medically relevant microorganisms by vibrational spectroscopy. Journal of Microbiological Methods, 51: 255–271.
- Markelz, A. (2002) THz time domain spectroscopy of biomolecular conformational mode. Physics in Medicine & Biology, 47: 3797–3805.
- Markelz, A.G., Roitberg, A. and Heilweil, E.J. (2000) Pulsed terahertz spectroscopy of DNA, bovine serum albumin and collagen between 0.1 and 2.0 THz. Chemical Physics Letters, 320: 42–48.
- Martin, G.J., Mazure, M., Jouitteau, C., Martin, Y.‐L., Aguile, L. and Allain, P. (1999) Characterization of the geographic origin of Bordeaux wines by a combined use of isotopic and trace element measurements. American Journal of Enology and Viticulture, 50(4): 409–417.
- Mauer, L.J. and Reuhs, B.L. (2010) Mid‐infrared sensors for the rapid analysis of select microbial food borne pathogens. In: Wiley Handbook of Science and Technology for Homeland Security (ed. J.G. Voeller). John Wiley & Sons, Hoboken, NJ.
- Mine, Y., Kobayashi, H., Chiba, K. and Tada, M. (1992) 31P NMR Study on the interfacial adsorptivity of ovalbumin promoted by lysophosphatidylcholine and free fatty acids. Journal of Agricultural and Food Chemistry, 40: 1111–1115.
- Mine, Y., Chiba, K. and Tada, M. (1993) Effect of phospholipids on conformational change and heat stability of ovalbumin. Circular Dichroism and Nuclear Magnetic Resonance Studies. Journal of Agricultural and Food Chemistry, 41: 157–161.
- Mittleman, D.M., Jacobsen, R.H. and Nuss, M.C. (1996) T‐ray imaging. IEEE Journal of Selected Topics in Quantum Electronics, 2: 679–692.
- Monakhova, Y.B., Kuballa, T., Leitz, J. and Lachenmeier, D.W. (2011) Determination of diethyl phthalate and polyhexamethylene guanidine in surrogate alcohol from Russia. International Journal of Analytical Chemistry, Article ID 704795, doi:10.1155/2011/704795
- Msagati, T.A.M. (2012) The Chemistry of Food Additives and Preservatives. Wiley‐Blackwell, Chichester, UK.
- Naumann, D., Helm, D. and Labischinski, H. (1991a) Microbiological characterizations by FT‐IR spectroscopy. Nature, 351: 81–82.
- Naumann, D. (2000) Infrared spectroscopy in microbiology. In: Encyclopedia of Analytical Chemistry (ed. R.A. Meyers). John Wiley & Sons, Chichester, UK, pp. 102–131.
- Naumann, D., Helm, D., Labischinski, H. and Giesbrecht, P. (1991) The characterization of microorganisms by Fourier‐transform infrared spectroscopy (FT‐I). In: Modern Techniques for Rapid Microbiological Analysis (ed. W.H. Nelson). VCH, New York, pp. 43–96.
- Nawrocka, A. and Lamorska, J. Chapter 14; Determination of Food Quality by Using Spectroscopic Methods; http://dx.doi.org/10.5772/52722
- Nissen, L.R., L. Mansson, L., Bertelsen, G., Haynh‐Ba, T. and Skibsted, L.H. (2000) Protection of Dehydrated Chicken Meat by Natural Antioxidants as Evaluated by Electron Spin Resonance Spectrometry. 48(11): 5548–5556.
- Petrakis, P.V., Agiomyrgianaki, A., Christophoridou, S., Spyros, A. and Dais, P. (2008) Geographical characterization of greek virgin olive oils (cv. Koroneiki) using 1H and 31P NMR fingerprinting with canonical discriminant analysis and classification binary trees. Journal of Agricultural and Food Chemistry, 56: 3200–3207.
- Ogrinc, N., Košir, I.J., Kocjančič, M. and Kidrič, J. (2001) Determination of authenticity, regional origin, and vintage of Slovenian wines using a combination of IRMS and SNIF‐NMR analyses. Journal of Agricultural and Food Chemistry, 49: 1432–1440.
- Quin, L.D. and Verkade, J.G. (eds) (1994) Phosphorus‐31 NMR Spectral Properties in Compound Characterization and Structural Analysis. Wiley‐VCH, New York.
- Reid, L.M., Woodcock, A., O’Donnell, C.P., Kelly, J.D. and Downey, G. (2005) Differentiation of apple juice samples on the basis of heat‐treatment and variety using chemometric analysis of MIR and NIR data. Food Research International, 38: 1109–1115.
- Reid, L., O’Donnell, C.P. and Downey, G. (2006) Recent technological advances for the determination of food authenticity. Trends in Food Science & Technology, 17: 344–353.
- Remaud, G.S., Martin, Y.‐L., Martin, G.G., Naulet, N. and Martin, G.J. (1997) Authentication of mustard oils by combined stable isotope analysis (SNIF‐NMR and IRMS). Journal of Agricultural and Food Chemistry, 45: 1844–1848.
- Rezzi, S., Axelson, D.E., Héberger, K., Reniero, F., Mariani, C. and Guillou, C. (2005) Classification of olive oils using high throughput flow 1H NMR fingerprinting with principal component analysis, linear discriminant analysis and probabilistic neural networks. Analytica Chimica Acta, 552: 13–24.
- Roberts, J.K.M., Wade‐Jardetzky, N. and Jardetzky, O. (1981) Intracellular pH by P‐31 NMR. Influence of factors other than pH on chemical shifts. Biochemistry, 20: 5389–5394.
- Rockline, R.D. (1991) Detection of ion chromatography. Journal of Chromatography, 546: 175–187.
- Rockline, R.D. and Johnson, E.L. (1983) Determination of cyanide, sulfide, iodide and bromide by ion chromatography with electrochemical detection. Analytical Chemistry, 55: 4–7.
- Sacco, A., Brescia, M.A., Liuzzi, V., Reniero, F., Guillou, G. et al. (2000) Characterization of Italian olive oils based on analytical and nuclear magnetic resonance determinations. Journal of the American Oil Chemists’ Society, 77(6): 619–625.
- Samuels, A.C. (2003) THz‐Spectroscopy of biological molecules. Journal of Biological Physics, 29: 89–100.
- Seregely, Z., Furkas, J., Tuboly, E. and Dalnadi, I. (2006) Investigating the properties of egg white pasteurised by ultra‐high hydrostatic pressure and gamma irradiation by evaluating their NIR spectra and chemosensor array sensor signal responses using different methods of qualitative analysis. Chemometrics and Intelligent Laboratory Systems, 82(1–2): 115–121.
- Sheppard, B.S., Caruso, J.A., Heitkemper, D.T. and Wolnik, K.A. (1992) Arsenic speciation by ion chromatography with inductively coupled plasma mass spectrometric detection. Analyst, 117: 971–975.
- Sikirzhytskaya, A., Sikirzhytski, V. and Lednev, I.K. (2012a) Raman spectroscopic signature of vaginal fluid and its potential application in forensic body fluid identification. Forensic Science International, 216(1–31): 44–48.
- Sikirzhytskaya, A., Sikirzhytski, V. and Lednev, I.K. (2012b) Raman spectroscopic signature of sweat and its potential application to forensic body fluid identification. Analytica Chimica Acta, 718(9): 78–83.
- Spyros, A. and Dais, P. (2000) Application of 31P NMR spectroscopy in food analysis. 1: Quantitative determination of the mono‐ and diglyceride composition of olive oils. Journal of Agricultural and Food Chemistry, 48: 802805.
- Spyros, A. and Dais, P. (2009) 31P NMR spectroscopy in food analysis. Progress in Nuclear Magnetic Resonance Spectroscopy, 54: 195–207.
- Srichuwong, S. and Jane, J.L. (2007) Physicochemical properties of starch affected by molecular composition and structures: A review. Food Science Biotechnology, 16(5): 663.
- Tester, R.F., Karkalas, J. and Qi, X. (2004) Starch composition, fine structure and architecture. Journal of Cereal Science, 39: 151–165.
- Uribari, J. and Calvo, M.S. (2003) Hidden sources of phosphorus in typical American diet: Does it matter in nephrology? Seminars in Dialysis, 16(3): 186–188.
- Van Den Thillart, G. and Van Waarde, A. (1996) Nuclear magnetic resonance spectroscopy of living systems: Applications in comparative physiology. Physiological Reviews, 76: 799–837.
- Van Ginneken, V., Van Den Thillart, G., Addink, A. and Erkelens, C. (1995) Regulatory, integrative and comparative physiology. American Journal of Physiology, 268: R1178.
- Van Waarde, A., Van Den Thillart, G., Erkelens, C., Addink, A. and Lugtenburg, J. (1990) Functional coupling of glycolysis and phosphocreatine utilization in anoxic fish muscle. I: An in vivo 31P NMR study. Journal of Biological Chemistry, 265(2): 914–923.
- Vartanian, LR., Schwartz, M.B. and Brownell, K.D. (2007) Effects of soft drink consumption on nutrition and health: A systematic review and meta‐analysis. American Journal of Public Health, 97: 667.
- Virkler, K. and Lednev, I.K. (2009) Raman spectroscopic signature of semen and its potential application to forensic body fluid identification. Forensic Science International, 193(1–3): 56–62.
- Virkler, K. and Lednev, I.K. (2010a) Forensic body fluid identification: the Raman spectroscopic signature of saliva. Analyst, 135(3): 512–517.
- Virkler, K. and Lednev, I.K. (2010b) Raman spectroscopic signature of blood and its potential application to forensic body fluid identification. Analytical and Bioanalytical Chemistry, 396(1): 525–534.
- Vlachos, N., Skopelitis, Y., Psaroudaki, M., Konstantidou, V., Chatzilazarou. A. and Tegou, E. (2006) Applications of Fourier transform‐infrared spectroscopy to edible oils. Analytica Chimica Acta, 573–574: 459–465.
- Vogel, H.J., Lundberg, P., Fabiansson, S., Ruderus, H. and Tornberg, E. (1985) Post‐mortem energy metabolism in bovine muscles studied by non‐invasive phosphorus‐31 nuclear magnetic resonance. Meat Science, 13: 1–18.
- Vohra, A. and Satyanarayana, T. (2003) Phytases: Microbial sources, production, purification, and potential biotechnological applications. Critical Reviews in Biotechnology, 23(1): 29–60.
- Walmsley, A.D. and Loades, V.C. (2011) Determination of acetonitrile and ethanol in water by guided microwave spectroscopy with multivariate calibration. Analyst, 126: 417–420.
- Weder, J.K.P. and Belitz, H.‐D. (2003) Protein: Functional properties. In: Encyclopedia of Food Science and Nutrition (ed. B. Caballero). Elsevier, Amsterdam, The Netherlands, pp. 4835–4841.
- Williams, R.J. (1983) Determination of inorganic anions by ion chromatography with ultraviolet absorbance detection. Analytical Chemistry, 55: 851–854.
- Woolard, D., Kaul, R., Suenram, R., Walker, A.H., Globus, T. and Samuels, A. (1999) Terahertz electronics for chemical and biological warfare agent detection. In: IEEE MTT‐S International Microwave Symposium Digest, Anaheim, CA, pp. 925–928.
- Wu, K., Zhang, W., Addis, P.B., Epley, R.J., Salih, A.M. and Lehrfeld, J. (1994) Antioxidant properties of wild rice. Journal of Agricultural and Food Chemistry, 42: 34–37.
- Yokoyama, Y., Azuma, Y., Sakaguchi, M., Kawai, F. and Kanamori, M. (1996a) 31P NMR study of bioenergetic changes in carp muscle with cold‐CO2 anesthesia and non‐destructive evaluation of freshness. Fisheries Science, 62: 267–271.
- Yokoyama, Y., Azuma, Y., Sakaguchi, M., Kawai, F. and Kanamori, M. (1996b) Non‐destructive phosphorus‐31 nuclear magnetic resonance study of postmortem changes in oyster tissues. Fisheries Science, 62(3): 416–420.
- Yu, C. and Irudayaraj, J. (2005) Spectroscopic characterization of microorganisms by Fourier transform infrared microspectroscopy. Biopolymers, 77: 368–377.