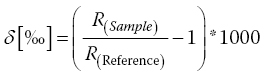16
Application of Hyphenated Techniques in Food Forensics
Chromatography – Mass Spectrometry Hyphenated Techniques for Food Forensics
Mass spectroscopy, also called mass spectrometry, is a scientific method that analyzes a sample of material to determine its molecular makeup. By ionizing a sample, a scientist can cause it to separate into its individual ions. This allows him to analyze and categorize those ions to determine the sample’s composition. Mass spectrometry has become a valuable tool in forensic science, where it can provide clues from the barest traces left by a suspect. Mass spectrometry in combination with chromatography (a technique which can separate mixtures) can provide a powerful means to confirm the presence of adulterants in foods or identity/authenticity of foods.
Hyphenated techniques can be used for targeted analysis for pre‐defined components in food sample, whereby foods can be analyzed by either liquid chromatography‐mass spectrometry (LC‐MS) or gas chromatography‐mass spectrometry (GC‐MS), with further confirmation of the structures of the components using nuclear magnetic resonance spectroscopy (NMR). Targeted analysis can provide the means to resolve food forensic cases involving deliberate contamination of foods using banned food coloring agents, such as Sudan dyes normally incorporated into spices such as chilli as a flavor enhancer, in which case LC‐API‐MS/MS can be employed (http://www.scientistlive.com/content/14486). LC‐API‐MS/MS can also be used to verify the labeling presented on food labels regarding additives such as the authenticity of meat bindings, composition, quantities and ratios of all the ingredients and whether they comply to standards, regulations and guidelines regarding ethical and religious norms.
Additives such as meat binding (glue) are permitted to be used for meat binding of off‐cuts and meat trimmings; however, the same meat bindings have been reported in fraud cases where offenders have tried to incorporate them for the purpose of deliberately increasing the weight of the meat content stated on the labeling (Grundy et al., 2007, 2008). Some other illegal practices have involved the use of blood plasma fibrin protein together with thrombin to bind meat, a practice which may involve mixing blood thrombin fibrin protein from different animals (e.g. bovine, porcine, etc.). Hyphenated techniques such as LC‐API‐MS/MS can point to the exact origin of the blood or the issue of mixing species (Grundy et al., 2007, 2008). LC‐API‐MS/MS can differentiate the fibrin protein‐thrombin blood clots used in the binding procedures, because of the specificity of the peptides that are released during the blood clotting process related to the binding agent technology used. The fibrio‐peptides from different species vary and are species specific in terms of their molecular masses, bearing in mind that in LC‐API‐MS/MS one can generate fragments up to MS3 or more to ensure that even similar peptides will have differences in terms of the fragments in a certain MS‐MS experiment.
LC‐MS/MS in combination with NMR can also be employed to verify cases of adulteration in other foods and food products, such as monofloral‐honey due to the presence of unique and very specific biomarkers in the nectar of plant flowers that the bees used. Examples of the biomarkers which are unique and specific to the nectar of some plant species include kynuric acid found specifically in chestnut honey. Manuka honey, which is well‐known for its health beneficial bioactive compounds, has a specific and unique nectar obtained from the Leptospermum scoparium plant species (Donarski et al., 2010).
Generally, all compounds that can be analyzed using HPLC or GC can also be analyzed by LC‐MS and GC‐MS respectively.
GC‐MS for Food Forensics
GC‐MS has been instrumental in providing evidence in many food forensic cases. For example, there have been a number of deliberate food poisoning cases using toxins such as pesticides (Ochiai et al., 2005), dioxins and phthalate acid ester plasticizers (Shen, 2005), scopolamine (Chu et al., 2006), and veterinary drugs and growth promoters in animal husbandry (Ramos et al., 2003).
These deliberate food poisoning scandals have even prompted import bans by some countries. The monitoring and analysis of such molecules has been made possible by such techniques as GC‐MS. GC–MS is an appropriate and perfect analytical tool for forensic analysis of molecules like dioxins, pesticides, etc. in foods because of its simplicity, sensitivity and effectiveness in separating and identifying components.
In GC‐MS hyphenation, the GC separates components of the sample mixtures in the chromatography column based on their differences in terms of chemical properties such as boiling points, molecular weights, etc. The MS ionizes the individual separated species on the basis of their mass‐to‐charge ratio, which will be discriminated in the mass analyzer part of the MS and then detected by the MS detector.
Compound Specific Isotope Analysis (CSIA) and On‐line Combustion Gas Chromatography Coupled to Stable Isotope Ratio Mass Spectrometry (GC‐C‐IRMS) for Food Forensics
The technique, known as compound‐specific isotope analysis (CSIA) using isotope ratio mass spectrometry (IRMS) coupled to on‐line combustion (C) – gas chromatography (GC), is one of the newly introduced techniques that have also found applications in food forensics. The attractive feature of this technique can be realized in its application related to the authenticity of foods as well as food provenance issues. This is due to the fact that CSIA‐GC‐C‐IRMS has the capability to determine and analyze isotope distribution at natural abundance levels with great accuracy and high precision.
Sample Preparation, Derivatization and Isotopic Calibration for CSIA‐GC‐C‐IRMS in Food Forensics
There are important considerations that are crucial when preparing samples for CSIA‐GC‐C‐IRMS, which include that all steps involved during sampling and derivation must be thoroughly optimized to ensure that there are no phenomena associated or related to isotopic fractionation of the analyte of interest. Another consideration is for the incorporation of an internal standard, which takes care of the process of the fractionation of the analyte of interest. An ideal internal standard is the one that should not require any derivatization procedure and it must have a known isotopic composition. Moreover, an ideal internal standard must be proven to be highly chemically stable, must be of high purity, and be soluble in high purity solvents. It must have low vapor pressure at standard temperatures and pressures. It must be environmentally rare, must not co‐elute with the analyte of interest in the chromatographic column, and must be compatible to both LC and GC techniques.
In cases where derivatization steps for the sample are involved in IRMS procedures, account should be taken of the associated effect, because derivatization tends to add 13C tracer dilution, which therefore requires a corrective step to compensate for changes related to 13C. Moreover, apart from derivatization having effects on the changes of 13C, it also has serious effects on the GC separation and volatilization of CO2 and N2 species. For example, derivation procedures that make use of silylation, especially where apolar derivatization reagents (trimethylsilyl‐TMS, ter‐butyldimethylsilyl‐t‐BDMS) are used, also results in the potential to jeopardize a proper resolution of the mixture in the GC column. In addition, such derivatives are known to be associated with high carbon loads, which have the potential to cause incomplete combustion, thus affecting the accuracy of the isotopic analysis. Other derivatization reagents, such as those in the class of trifluoroacetates (TFAs), are also not recommended because they are associated with non‐quantitative sample conversion phenomena. Heptafluorobutylated (HFB) derivatization reagents are also problematic, as they tend to form refractory (stable) fluorine derivatives, which when they react with copper and nickel negatively affect the efficiency of combustion for CuO/NiO. Another reason for avoiding the use of HFB derivatization reagent is that fluorine tends to foul the combustion catalyst platinum (Meier‐Augenstein, 1997).
CSIA‐GC‐C‐IRMS for Adulteration Tests, Authenticity and Adulteration of Foods
This hyphenated technique can be useful to trace the origin and authenticity of a variety of foodstuffs such as sweeteners, flavors, flavor enhancers and fragrances among others, and its advantage is that it enables both the identification and isotopic ratios (Berneuther et al., 1990). For example, the authenticity of fruit juices such as apricot and peach has been investigated using CSIA‐GC‐C‐IRMS by means of measuring 13C/12C ratios of food grade flavor ingredients, mainly γ‐decalactone (Figure 16.1) and then using d13C to determine the authenticity (Berneuther et al., 1990).
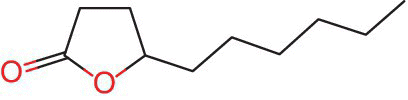
Figure 16.1 Chemical structure of γ‐decalactone.
Together with δ13C or δ15N measurements in various foods, the enantioselectivity measurements versus measurements of GC‐C‐IRMS for the γ‐decalactone has been proved to provide further evidence of the authenticity test for fruit juices (Mosandl et al., 1990) (Equation 16.1):
GC‐C‐IRMS can also be used to provide evidence in cases of food adulteration; for example, cases involving addition of sugar or vitamin C to fruit juices and wines using low‐quality grade corn syrup. CSIA‐GC‐C‐IRMS can in such cases be used to provide measurements of δ13C of either glucose or bulky carbon, which can point to the availability of corn syrup glucose. Also, the presence of biogenetic biomolecules, such as L‐ascorbic acid, L‐malic acid and L‐tartaric acid in wines, whey and fruit juices, is key for the application of the CSIA‐GC‐C‐IRMS technique to point to cases of possible adulteration or authenticity of these foodstuffs and beverages (Gensler et al., 1995 ; Jamin et al., 1997 ; Weber et al., 1997a, b). In these cases, measurements of δ13C can be taken and correlated to their corresponding sugar molecules. In the same way glycerol, which is normally sourced from natural sources and incorporated into wines, can be differentiated with artificial glycerol adulterated in wine products (Weber et al., 1997). CSIA‐GC‐C‐IRMS can also be instrumental in resolving food forensic cases related to vegetable oil fraud and adulteration such as partial or total substitution of high‐quality oil for low‐quality ones. In such cases, δ13C values of fatty acids can be evaluated to show the possibility of adulteration or provide evidence of the true geographical origin of the vegetable oil.
Ion Chromatography (IC) and Atomic Spectrometry Methods in Food Forensics: Verification of Food Authenticity, Adulteration, Provenance and Isotopic Fingerprinting of Foods
The inorganic anions, minerals and trace element content in foods can be used in forensic cases to provide evidence for food authenticity and indication of the geographical origin of that particular foodstuff.
Ion Chromatography and Food Forensics
Ion chromatography is an analytical technique that is used to separate ions (mainly inorganic anions such as NO3 −, NO2 −, SO4 2−, PO4 3−, SiO−, F−, Cl−, etc.) and polar molecules based on their affinity to the ion exchange stationary phases. The ions of interest are separated from other ions in the samples based on their affinity to the column stationary phases and are normally detected using a conductivity detector (Lopez‐Ruizm, 2000; Tamisier‐Karolak et al., 1999).
Conclusions
Hyphenated techniques that involve mass spectrometry are normally taken as confirmatory methods due to the fact that they are very sensitive and selective. Therefore, for a confirmation beyond reasonable doubt of authenticity of foods, evidence of adulteration, etc., these methods are normally the ones that are considered mandatory.
References
- Berneuther, A., Koziet, J., Brunerie, P., Krunmer, G., Christoph, N. et al. (1990) Chirospecific capillary gaschromatography (HRGC) and on‐line HRGC‐isotope ratio mass spectrometry of γ‐decalactone from various sources. Zeitschrift für Lebensmittel‐Untersuchung und ‐Forschung, 191: 299–301.
- Chu, J.X., Xie, Y. and Zhuo, X.C. (2006) Determination of scopolamine in the poisoning case by GC/MS [Article in Chinese]. Fa Yi Xue Za Zhi, 22(4): 285–287.
- Donarski, J.A., Roberts, D.P.T. and Charlton, A.J. (2010) Quantitative NMR spectroscopy for the rapid measurement of methylglyoxal in manuka honey. Analytical Methods, 2: 1479–1483.
- Gensler, M., Rosmann, A. and Schmidt, H.I. (1995) Detection of added L‐ascorbic acid in fruit juices by isotope ratio mass spectroscopy. Journal of Agricultural and Food Chemistry, 43: 2662–2666.
- Grundy, H.H., Reece, P., Sykes, M.D., Clough, J.A., Audsley, N. and Stones, R. (2007) Screening method for the addition of bovine blood‐based binding agents to food using liquid chromatography triple quadrupole mass spectrometry. Rapid Communications in Mass Spectrometry, 21(18): 2919–2925.
- Grundy, H.H., Reece, P., Sykes, M.D., Clough, J.A., Audsley, N. and Stones R. (2008) Method to screen for the addition of porcine blood‐based binding products to foods using liquid chromatography/triple quadrupole mass spectrometry. Rapid Communications in Mass Spectrometry, 22(12): 2006–2008.
- Jamin, E., Gonzalez, J., Remaud, G., Naulet, N., Martin, G.G. et al (1997) Determination of the carbon‐13 content of sugars and pulp from fruit juices by isotope‐ratio mass spectrometry (internal reference method). A European interlaboratory comparison. Analytica Chimica Acta, 340: 21–29.
- Lopez‐Ruiz, B. (2000) Review: Advances in the determination of inorganic anions by ion chromatography. Journal of Chromatography A, 881: 607–627.
- Meier‐Augenstein, W. (1997a) The chromatographic side of isotope ratio mass spectrometry: Pitfalls and answers. Liquid Chromatography–Gas Chromatography, 15: 244–252.
- Meier‐Augenstein, W. (1997b) A reference gas inlet module for internal isotopic calibration in high precision gas chromatography/combustion‐isotope ratio mass spectrometry. Rapid Communications in Mass Spectrometry, 11: 1775–1780.
- Mosandl, A., Hener, U., Kreis, P. and Schmarr, H‐G. (1990) Enantiomeric distribution of a‐pinene, b‐pinene and limonene in essential oils extracts. Part 1: Rutaceas and G Gramineae. Flavour & Fragrance Journal, 5: 193–199/
- Ochiai, N., Sasamoto, K., Kanda, H., Yamagami, T., David, F. et al. (2005) Optimization of a multi‐residue screening method for the determination of 85 pesticides in selected food matrices by stir bar sorptive extraction and thermal desorption GC‐MS. Journal of Separation Science, 28(9–10): 1083–1092.
- Ramos, F., Cristino, A., Carrola, P., Eloy, T., Silva, J.M. et al. (2003) Clenbuterol food poisoning diagnosis by gas chromatography–mass spectrometric serum analysis. Analytica Chimica Acta, 483(1–2): 207–213.
- Shen, H‐Y. (2005) Simultaneous screening and determination eight phthalates in plastic products for food use by sonication‐assisted extraction/GC–MS methods. Talanta, 66(3): 734–739.
- Tamisier‐Karolak, S.L., Le Potier, I., Barlet, O. and Czok, M. (1999) Analysis of anions in aqueous samples by ion chromatography and capillary electrophoresis. A comparative study of peak modeling and validation criteria. Journal of Chromatography A, 852: 487–498.
- Weber, D., Roßmann, A., Schwarz, S. and Schmidt, H.‐L. (1997a) Correlations of carbon isotope ratios of wine ingredients for the improved detection of adulterations I. Organic acids and ethanol. Zeitschrift für Lebensmitteluntersuchung und ‐Forschung A. 205(2): 158–164.
- Weber, D., Rossmann, A, Schwartz, S and Schmidt, H.L. (1997b) 13C‐pattern of natural glycerol: origin and practical importance. Journal of Agricultural and Food Chemistry, 45: 2042–2046.