17
Application of Electromigration Driven Techniques in Food Forensics
Indicative‐species Targeted in Electromigration Methods:
- Hydroxymethylfurfural (HMF), lactoglobulins, casein, furosine – milk;
- Organic acids (benzoic, ascorbic, sorbic, erythorbic, citric, isocitric, malic, tartaric, fumaric, etc.), polyphenols, phenolic amines, flavonoids, DL‐amino acids, aspartame, saccharine, acesulfame, alitame, BHT, BHA, gallate esters, erythrosine, fast green, SF yellow, light green, amaranth, sunset yellow, new coccine, tatrazine, – food additives (e.g. sweeteners, antioxidants, colorants), preservatives, food antioxidants, fruit juices and citrus fruits authenticity;
- Gliadins, glutenins, storage proteins, Glu‐1 genes – differentiation of cereal (e.g. wheat, oats, rye, barley, rice, maize, etc.) cultivars;
- Cations and anionic profiles – fruit juices and beverage authenticity;
- DNA fragments – GMO foods;
- Hemoglobin, myoglobin, actin, myosin, MRM, lysozyme – meat;
- Sarcoplasmic proteins – fish.
Electromigration techniques employ external voltage sources of electrokinetic methods to run the separation process to discriminate components of mixtures. Examples of these methods include electrophoresis and electro‐osmosis, which refers mainly to the volumetric liquid that is flowing in a capillary and which is driven by an electrical field. For a complete (100%) electrophoretic separation to take place, there have to be significant differences in terms of the velocities of the migrating charged species, which move under the electric field that is the driving force. Just like all other analytical separation methods and techniques, there are a number of parameters that control or influence the efficiency of the electromigration procedures that may be associated with the resolution of the mixtures and need to be optimized. Factors with the tendency to control separation of the mixtures in the electromigration driven techniques include electrode polarization, applied voltage, temperature, capillary, background electrolyte, and various additives. For example, a number of factors are known to be responsible in causing the differences in the velocities of the migrating species and include electrophoretic mobility, whereby ionic species separate based on their differences in terms of the charge‐to‐size ratio. It should be noted that the magnitude of the electrophoretic mobility in a particular buffer is characteristic to that buffer system and thus it has a constant value that is unique to that particular ionic species in that particular buffer system. It follows therefore that when buffer properties and characteristics are carefully optimized, it makes it possible to control the resolution (separation) of the migrating ionic species, because it tends to greatly influence their electrophoretic mobility.
Another factor that influences the separation of the migrating ionic species is the electro‐osmotic flow (EOF), which actually refers to the motion of the electrolyte (buffer system) that is inside the capillary tube where silanol groups are exposed. The EOF is highly influenced by the differences in potentials and is triggered by the electric charges. It is also dependent on both the ionization properties of the migrating ionic species that are present inside the capillary tube as well as the adsorption pattern of the migrating ionic species. The pH of the separation buffer also has the potential to cause ionization of the silanol groups inside the capillary tube, thus creating negative charges inside the capillary tube. The silanol ionization effect creates a double layer at the interface between the electrolyte and the inner walls of the capillary tube. The double layer is made of a static/permanent layer and a weak mobile diffusion layer. The migrating ionic species presenting the weakly‐bound diffusion layer have the flexibility to become involved in the EOF due to their susceptibility to exchange phenomena with the ionic species present in the electrolyte.
At the interface of the two layers (diffusion and static/permanent), an electrokinetic potential known as the Zeta potential (ζ) develops. The magnitude of ζ is directly proportional to the charge density, which is also dependent on the pH of the separating buffer. Because of the dependence of ζ to pH, it follows that it has direct control over the EOF, such that when the experimental conditions are highly alkaline (at high pH) where the silanol groups are completely ionized, the EOF is quickest and vice versa under acidic conditions (low pH) where there is minimal dissociation of the silanol functional groups. The magnitude of ζ is also highly dependent on the ionic strength of the electrolyte and is actually inversely proportional to it.
During the optimization process, the performance of these parameters is measured by factors such as migration time, efficiency, selectivity and resolution. Unlike in chromatographic techniques, where the separation is effected in the stationary phase of the column where the flowing phase is forced through using mechanical pumps, in electromigration driven processes, the separation takes place in a buffer, also known as either the electrolyte or background electrolyte, separation buffer or separation electrolyte. The buffer can either be an aqueous solution or it can be a solution based on pure organic solvents or their mixtures (Divall, 1985 ; Kvasnicka, 2000 ; Landers, 2007 ; Righetti et al., 1997 ; Vanhoenacker et al., 2001).
Capillary Electrophoresis and Food Authenticity in Forensics
Capillary electrophoresis has many potential applications in food forensics. especially in cases related to the authentication of foods. As indicated above, capillary electrophoresis is one of the electromigration (electrokinetic) analytical techniques employed mainly in the separation of compounds in a mixture based on their differences in terms of electrophoretic mobility, phase partitioning, ionic potential (pI), molecular size, etc. There are several variants (modes) of CE, which are all based on similar principles, but operate in different configurations and they are thus used for the analysis of a wide variety of analytes ranging from simple inorganic ions, small organic molecules, peptides, proteins, nucleic acids to viruses, microbes, and particles (Everaerts et al., 1976 ; Foret et al., 1993 ; Righetti et al., 1997 ; Vanhoenacker et al., 2001). These variants of CE techniques include capillary zone electrophoresis (CZE), capillary isoelectric focusing (CIEF), capillary isotachophoresis (CITP), on‐line coupling of CITP and CZE, capillary gel electrophoresis (CGE), and capillary electrochromatography (CEC). For food authenticity testing, fingerprinting, provenance, etc., there are several variants of CE that are normally employed and they include free solution capillary electrophoresis (FSCE) and micellar electrokinetic chromatography (MEKC), which can be used for DNA analysis, for identification and fingerprinting of foods; the same methods can be used for the analysis of proteins as biomarkers for foods.
Free Solution Capillary Electrophoresis (FSCE) and Food Forensics
Free solution capillary electrophoresis (FSCE) provides the possibility of simultaneous separation of both positively and negatively charged species, especially when experimental conditions such as electrophoretic mobility and electro‐osmotic mobility have been optimized. Generally, the order of migration in FSCE is such that ionic species with higher positively charged density and those with smaller radius tend to migrate first.
Despite the advantages of FSCE, the technique is limited when it comes to separating species with either similar (same) charge‐to‐mass ratio such as DNA fragments and sodium dodecyl sulfate complexes. Moreover, with FSCE there are challenges in terms of separating uncharged species. FSCE is also unsuitable for the separation of species that possess high positive electrical charge density, because such species have a tendency to adhere to the inner walls of the capillary tube. Moreover, like all capillary electrophoresis modes, the technique is not suitable for trace analysis.
Micellar Electrokinetic Chromatography (MEKC)
Micellar electrokinetic chromatography (MEKC) is highly suitable for the separation of non‐charged species, although this does not mean that it cannot be used entirely for the separation of charged species. The principle of MEKC is that it incorporates surfactants (usually sodium dodecyl sulfate, SDS) to the separation buffer. The amount of surfactant added is such that it is sufficient to form micelles. The formation of micelles is crucial because they act as another stable phase (actually a pseudo‐stationary phase) where neutral species interact at their optimal specific partition coefficient, and because SDS micelles are negatively charged, they migrate towards the anode (positive electrode).
There are other CE techniques such as agarose gel electrophoresis (AGE) that can be used for authenticity tests in DNA analysis, but these methods are known to suffer from low sensitivity, insufficient resolution and also make use of highly toxic and teratogenic ethidium bromide compounds to enable visualization of the amplicons, and so this makes this technique environmentally and user unfriendly. A good thing is that the bottleneck related to AGE can be addressed by coupling them to molecular biology methods such as a polymerase chain reaction (PCR) (Garcia‐Cañas et al., 1994a,b, 2002).
In the case of protein analysis, normally polyacrylamide gel electrophoresis (PAGE) slabs have traditionally been used for the analysis of protein composition of foods and beverages. Capillary electrophoresis methods are not popular for protein separation because of the challenges that are normally encountered when these techniques (CE) are used for the separation of proteins. These problems arise from the fact that biopolymers such as proteins tend to adsorb on the walls of the fused capillary tubes, thus making the technique less attractive for such applications.
The adsorption of biopolymer analytes onto the fused capillary tube walls is caused by the electrostatic interactions that occur between the positively charged biopolymer residues (i.e. protein residues) and the negatively charged groups of the silanol inside the capillary walls. However, a good thing is that these problems can be circumvented by the incorporation of either highly alkaline or acidic buffers and/or specific polymeric additive materials of high salt concentration in order to protect the negatively charged groups of silanol. Another alternative way to counter the electrostatic phenomena that occur between these oppositely charged species is to coat the inner walls of the capillary with specific adsorbents or to chemically modify the inner walls of the fused capillary tube (Clifuentes, 2006 ; Frazier and Papadopoulou, 2003).
Capillary electrophoresis methods have also been highly useful in the analysis of chiral compounds present in foods, such as amino acids (D and L amino acids). In order to enhance the separation of these chiral compounds in foods, specific chiral selectors, mainly cyclodextrins and their derivatives, have to be included in the separation buffer. In some cases (where necessary), some functional groups in D‐ and L‐amino acids present in foods presented as evidence in food forensics related cases, may need to be derivatized using agents such as fluorescein isothiocyanate (FITC) in order to boost the strength of the fluorophores to obtain more sensitivity and for a better separation of such chiral amino acids, etc.
Considerations During the Application of Electromigration Techniques in Food Forensics
The application of electromigration methods in food forensics targets specific indicative target species that are electrophoretically active, which (indicative species) vary from one type of food to another. These molecules include specific protein classes such as gliadin, glutenins (for cereals like wheat), some organic acids, amino acids, antioxidants (for preservatives), etc. Electromigration methods also require optimization of the buffer systems (composition and type) and the type of capillary (coated or uncoated), the length of the capillary, temperature, voltage and the detection wavelengths in the case where fluorescence or UV‐Vis are used as detectors.
Different food types may require specific electromigration techniques and experimental conditions will also depend on all these factors (food type, the technique and the target indicative analyte species). This is evidenced by a number of reports that are found in the literature. Morales and Jimènez‐Pèrez (2001) reported the application of MEKC to investigate the heat load of milk where hydroxymethylfurfural (HMF) was the target‐indicative molecule. In this report, an uncoated fused silica capillary was used with the buffer system composed of 50 mM phosphate buffer (pH 2.4) and 100 mM SDS, the voltage was set at 20 kV, while the wavelength for the UV detector that was used was set at 280 nm.
The use of MEKC for the detection of food additives and preservatives has been widely reported. For example, Pant and Trenerry (1995) employed this technique making use of organic acids, mainly benzoic acid and sorbic acids as analyte indicative species. The separation buffer for this was composed of 20 mM sodium phosphate buffer and 50 mM SDS in an uncoated fused silica capillary. A UV detector was used and the detection wavelength was set at 230 nm, while the detection voltage was set at 25 kV.
The application of MEKC‐LF in food forensics has also been reported in instances involving the geographical origin of orange juice, whereby DL‐amino acids were analyzed as target analyte indicative species (Simo et al., 2002). In this report, they used uncoated fused silica capillary and the separation buffer was made up of 100 mM sodium tetraborate, 30 mM SDS (pH 9.4) and 20 mM beta cyclodextrin (beta CD). The detection wavelengths (LIF) were set at 488 nm (excitation) and 520 nm (emission).
The application of MEKC for the investigation of antioxidants in foods has been reported by Boyce and Spicket (1997). In this application, uncoated fused silica was used with 20 mM sodium borate, 50 mM sodium cholate, 15 mM SDS and 10% methanol, at 2°C and 18 kV. The wavelength for the UV detector was set at 254 nm and also at 214 nm. In other reports, sweeteners and preservatives were detected using MEKC, whereby aspartame, saccharine, acesulfame, alitame, benzoic acid and sorbic acid were used as target indicative molecules (Thompson et al., 1995), while in a report by Frazier et al. (2000), in which they determined sweeteners, coloring compounds and preservatives, they used caffeine, aspartame, brilliant blue, green S, benzoic acid, sorbic acid, saccharine, sunset yellow, acesultame‐K, quinolone yellow, carmoisine, ponceau 4R and black PN as target analyte indicative species. In a report by Thompson et al. (1995), uncoated fused silica capillary was used (at 25°C) and the buffer system was made up of 100 mM sodium borate, 50 mM sodium deoxycholate and 10 mM potassium dihydrogen phosphate, at a volatage of 20 kV and UV wavelength detection set at 220 nm. The conditions in a report by Frazier et al. (2000) involved the use of uncoated fused silica and the composition of the separating buffer was 20 mM sodium hydrogen carbonate and 2 mM SDS (pH 9.2), the voltage used was 20 kV and the detection wavelength ranged between 190 and 600 nm.
Another electromigration technique, known as capillary zone electrophoresis (CZE), has been widely used in the differentiation of cultivars. A number of reports have exploited the advantages of CZE in food forensic identification of cultivars and their differentiation within and between species. In cultivar differentiation, the technique (CZE) targets various analyte‐indicative species, for example gliadin proteins and glutenin proteins (Bietz and Schmalzried, 1995), which was used as indicative analyte species in the experiment for the differentiation of wheat cultivars. In this report, the buffer system that was used was composed of boric acid (30–60 mM), sodium hydroxide (pH8–9), acetonitrile (0–40%) and SDS (0.1–10%). Uncoated fused silica capillary was used (3–60 mm length; diameter 0.05–0.07 mm). Temperature varied between 20 and 50°C at a constant voltage of 10 kV. The detection wavelength was 200 nm.
The glutenins used as analyte indicative species are actually a group of protein aggregates comprised of both high‐molecular‐mass (HMW) and low‐molecular‐mass (LMW) subunits with molar masses that are characterized by a variety of intermolecular linkages and interactions such as disulfide linkages, hydrophobic bonding, etc. In wheat products and in the bakery industry, glutenins play an important role in strengthening and providing the needed elasticity of the dough (Belitz et al., 2004). Gliadins, on the other hand, are a class within gluten proteins that are found in a number of cereals such as wheat, oats, rye, etc. and they play an important role in making the bread rise during the baking process.
Structurally, gliadins exist as monomeric entities and based on their chemistry, they can be subdivided into alpha‐gliadins, beta‐gliadins, gamma‐gliadins and omega‐gliadins. This chemistry can be the basis of differentiating cereal species using electromigration methods, as the proteins are ionic in nature, thus active under electrophoresis conditions. Genetical differences can also be exploited to differentiate cereals using electromigration methods. There are several genes that code for omega‐ and gamma‐gliadins in cereals, mainly wheat. These genes are located at the Gli‐1 loci found on the short arms of group‐1 chromosomes. In the case of alpha‐ and beta‐gliadins, the genes that encode them are found on the short arms of group‐6 chromosomes. In the case of low molecular weight glutenin protein aggregates, they are encoded by genes located at the Glu‐3 loci, which are linked to the Gli‐1 loci. The most important feature that is used to differentiate species comes from the fact that each gene located at the Glu‐1 locus consists of genes that encode for two different types of high molecular weight glutenins and they are the x‐type and y‐type. The permutations at this locus provide for the polymorphism that provides the possibility for a number of different alleles, thus the criteria for differentiating species in cereals. Some, for example, wheat, lack the y‐type genes at the Glu‐A1 locus and therefore this difference can be exploited to classify or differentiate wheat from other cereals or the criteria for the quality of the dough.
Lookhart and Bean (1996) also reported the use of CZE for differentiation of wheat cultivars using wheat gladins and wheat reduced gluteins as specific analyte indicative species. The difference with Bietz and Schmalzried (1996) was the experimental conditions, such that Lookhart and Bean (1996) used 100 mM sodium phosphate (pH 2.5), a non‐ionic surfactant hydroxypropylmethylcellulose (HPMC, 0.05%) and methanol/acetonitrile/2‐propanol/ethylene glycol (EG) at concentrations ranging between 0 and 20%. Uncoated fused capillary was used at a voltage of 15 kV and the detector wavelength was set at 200 nm. In another report, Bean and Lookhart (2000) employed CZE for the study of cultivar differentiation for cereals, mainly wheat, rye, oats, barley and rice, in which cereal storage proteins were specific analyte indicative species. The buffer system used was composed of 50 mM iminodiacetic acid together with acetonitrile (20%), HPMC (0.05%) and uncoated fused silica capillary at temperatures of 45°C and voltage of 30 kV. The detector wavelength was set at 200 nm.
CZE has also been used in food forensic milk differentiation, where casein proteins were used as specific analyte indicator species (Molina et al., 1999). In this study, hydrophilic coated fused silica capillary was used, with a buffer system made up of 320 mM citric acid, 20 mM sodium citrate (HPMC + 6 M urea, 0.05%), a pH of 8.6, and detector wavelength set at 214 nm and 25 kV.
The CZE fish species differentiation study, using sarcoplasmic proteins patterns as target indicative species, was reported by Gallardo et al. (1995). Uncoated fused silica capillary was used together with a buffer system made up of sodium phosphate (pH 2.4) at concentrations between 75 and 100 mM, voltage between 10 and 20 kV, while the wavelength for the UV detector was set at 214 nm.
Hyphenated Techniques Involving Capillary Electrophoresis
A combination of CZE and reversed phase high performance liquid chromatography has been reported for applications in food forensics related to the fingerprinting of wheat cultivars, as well as for the differentiation of genetically closely related wheat cultivar lines (Bean and Lookhart, 1997). In their report, Bean and Lookhart (1997) used the CZE of gliadins and glutens as the analyte indicative species. The buffer system was composed of 0.1 M phosphate buffer (pH 2.5), 20% acetonitrile, 0.05% HMPC, the voltage was tuned at 12.5 kV and the temperature was set at 45 °C, while the detection wavelength was set at 200 nm. Uncoated fused silica was used for all the experiments.
In another report, Salmanowicz and Moczulski (2004) employed a combination of CZE and PCR in the study to investigate the selection of the quality wheat genotypes that may result in the best quality breads. In this work, 89 mM (Tris base and boric acid), 2 mM EDTA, and 1% hydroxyethyl cellulose (HEC) were used as a buffer system (pH 8.5), at 10 kV. Uncoated fused silica with a reversed LIF detector was used. The analyte indicative species were the DNA fragments obtained from Glu‐1 gene high molecular weight (HMW) glutenin subunits encoded by the Glu‐1 genes. Other molecules that have been reported as analyte‐indicative species in cases of food authenticity or fingerprint tests include organic acids in fruits or fruit juices. Examples of these organic acids include citric, isocitric, malic, tartaric, fumaric acid, etc. The associated electromigration techniques that have been used for these organic acids include CZE (Saavenra et al., 2001), where orange juice authenticity was being tested. In this report, the buffer system was composed of 200 mM sodium phosphate (pH 7.5), at a voltage of 14 kV, polyacrylamide coated fused capillary was used and the detection wavelength was set at 200 nm.
Capillary isotachophoresis (CITP) in combination with CZE has been reported by Kvasnička et al. (2002) and also by Kvasnička and Voldřich (2000). In the report by Kvasnička et al. (2002), citrus juice authenticity was tested in which conductometric detection was employed. The buffer system was composed of:
- Leading electrolyte: 6 mM HCl, 3–8 mM BisTrisPropan (BTP), 2 mM CaCl2 and 0.05% hydroxypropyl methylcellulose (HPMC);
- Terminating electrolyte: 5 mM morpholinethanesulfonic acid (MES) and 1 mM BTP.
In a previous report by Kvasnička and Voldřich (2000), the authenticity of apple juice and also the addition of synthetic malic acid was tested, in which case malic acid was used as analyte indicative species.
Other food components that have been used as analyte indicative components in citrus juice authenticity tests include amino acids, polyphenols, phenolic amines, flavonoids and ascorbic acid (vitamin C) (Cancalon and Bryan, 1993). The same indicative species have also been reported by the same authors for use in the detection of pulp wash additions to citrus juices. In the work by Cancalon and Bryan (1993), CZE was used and the electrolyte system was composed of sodium phosphate (50 mM, pH 6.8), and/or borax‐boric acid (50 mM, pH 7.6–9.2) and/or 50 mM borax‐sodium hydroxide (pH 10). Uncoated fused silica capillary was used and the detection wavelength was set between 200 and 500 nm.
Inorganic cations and anions have also been reported as CZE target analyte indicative species for orange juice authenticity (Jezek and Suhaj, 2001 ; Weston et al., 1992). Weston et al. (1992) used potassium, sodium, calcium and magnesium as analyte indicative species, while Jezek and Suhaj (2001) used the anionic profile of the orange juice. Ammonium species (NH4 ‐), potassium, sodium, calcium and magnesium were used in another work by Kvasnička (2000) for citrus juice authenticity testing using the CITP technique.
Capillary gel electrophoresis (CGE)–LIF (laser induced fluorescence) in combination with PCR (polymerase chain reaction), utilizing DNA fragments of maize has been reported by Garcia‐Canas et al. (2004), for the detection of GMO in maize flour. The detection was performed using an LIF Ar with laser. The excitation wavelength was 488 nm, while the emission wavelength was set at 520 nm.
Another electromigration hyphenation technique, SDS‐CE, was reported in the application directed toward cultivar differentiation in wheat, where wheat glutenins and Glu‐1 HMW‐glutenin subunits of wheat were targeted as analyte indicative molecules (Bean and Lookhart, 1999 ; Lookhart and Bean, 1996). In another report by Day and Brown (2001), results on the use of SDS‐CE in the detection of mechanically recovered/reclaimed meat (MRM) were published, where hemoglobin, myoglobin, actin and myosin were identified as analyte indicative species. MRM is also synonymously known as either mechanically separated meat (MSM) or mechanically deboned meat (MDM). It is refers to a paste‐like meat product obtained by forcing either pureed or ground meat using high pressure through a sieve to separate the bone from the edible meat tissue.
Other target analyte indicative molecules, such as carbohydrates and organic acids, have been reported in the CZE studies on juice composition (Soga and Serwe, 2000). In this report, the supporting electrolyte was made up of 20 mM, 6‐pyridinedicarboxylic acid, 0.5 mM cetyltrimethylammonium hydroxide and sodium hydroxide (pH 12.1). Uncoated fused silica was used and the voltage was set at –12 kV (reverse EOF), while the indirect detection was done at 350 nm.
In another report, lactoglobulins were used as target analyte indicative species in a study involving differentiation of milk (Cartoni et al., 1999 ; Herrero‐Martinez et al., 2000). In the report by Cartoni et al. (1999), a methyl deactivated fused silica capillary was used and the electrolyte was made up of sodium borate (50–120 mM, pH 9.2), temperature and voltage were set at 25°C and 4–6 kV respectively, while the wavelength for the UV detector was set at 200 nm. The study by Herrero‐Martinez et al. (2000) utilized uncoated fused silica capillary and the buffer system composed of 50 mM iminodiacetic acid, 0.1% HMPC (Tween 20 + 6 M urea, 0.1–10%), and pH 3.1. The voltage and the detection wavelength were 10–15 kV and 214 nm respectively.
Lysozyme has also been reported for use as a target analyte indicative molecule in the CE‐MS (capillary electrophoresis coupled to mass spectrometry) study of meat differentiation, where ethylpyrrolidine methacrylate‐N,N‐dimethylacrylamide coated silica capillary was used. The electrolyte used in this work was made up of 75 mM ammonium acetate/acetic acid (pH 5.5), and the voltage used was 25 kV. The mass range selected was between m/z = 800 – 2200 (the target mass was m/z = 1500).
Vallejo‐Cordoba et al. (2004) reported the use of furosine (Figure 17.1) (epsilon‐N‐(2‐furoyl‐methyl)‐L‐lysine 2HCl) as target analyte indicative molecules in the CZE study of the quality of dairy products (Vallejo‐Cordoba et al., 2004), while the use of caseinomacropeptide (CMP) as the target analyte indicative molecule in the study of food adulteration involving the addition of whey rennet was reported by Cherkaoui et al. (1997) and erythrosine, fast green, SF yellow, light green, amaranth, sunset yellow, new coccine and tatrazine were reported as target analyte indicative molecules in the detection of food additives, mainly sweeteners, colorants and preservatives (Razee et al., 1995).
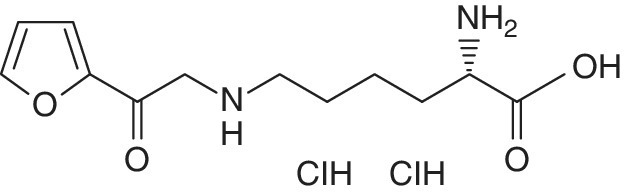
Figure 17.1 Chemical structure of furosine.
Protein‐bound 3‐methylhistidine as an analyte indicative molecule has been reported in the CITP study of lean‐meat content (Kvasnička, 1999). In this study, the electrolyte that was used had the following composition:
- Leading electrolyte: 5 mM ammonium hydroxide, 10 mM 2‐(N‐morpholino)ethanesulfonic acid (MES).
- Terminating buffer: 10 mM 6‐aminocaproic acid, 5 mM acetic acid.
- A conductometric detector was used for the detection of the analytes of interest.
In another report, Chu et al. (1993) did a study based on the MEKC detection of collagen in muscle tissues, where phenylthiohydantions of hydroxyproline were used as the target analyte indicative molecules. The composition of the separating buffer reported in this study included 50 mM sodium malonate, 75 mM SDS, pH 5, voltage of 15 kV and the detection wavelength was set at 254 nm.
Isoflavones, mainly daidzein and genistein, were reported as target analyte indicative species in the CZE study that involved the detection of soy and lupin proteins in meat products (Melienthin and Galensa, 1999). Uncoated fused silica capillary was used in this study and the experimental temperature was set at 25 °C with the electrolyte being 200 mM boric acid and sodium hydroxide, pH 8.6. The voltage used ranged between 25 and 30 kV and two detection wavelength lines (260 nm and 270 nm) were used.
Food forensic studies involving the testing of wine quality have been widely reported. For example, Pazourek et al. (2000) reported their CZE study on the differentiation of Canary Islands wine products, where the profiles of polyphenols fingerprinting were tested. They used an uncoated fused silica capillary and the composition of the electrolyte included 25 mM sodium tetraborate, pH 9.5 and the voltage used was 20 kV at 25 °C and the detection wavelength was 305 nm.
In another development, a combination of CITP and CZE was reported in the study of wine quality utilizing phenolic compounds such as gallic and caffeine, as well as vanillic acids such as rutin, quercetin and myricetin as analyte indicative species (Hamounovà et al., 2004). The leading electrolyte used was composed of 10 mM HCl, Tris, 0.2% hydroxyethyl cellulose (HEC) and pH 7.2, while the termination electrolyte composition included 50 mM boric acid, barium hydroxide and pH 8.2.
The background electrolyte (BGE) was composed of 25 mM (N‐Tris[hydroxymethyl]methyl‐3‐aminopropanesulfonic acid) (TAPS) – a zwitterionic buffer/(3‐(N‐morpholino)propanesulfonic acid) (MOPS), which is a buffer that is a structural analog to MES; 50 mM Tris, 15–40 mM boric acid and catechins.; 5 mM beta‐cyclodetrin, 20% methanol, 0.2% HEC, and pH 8.5–8.7. The detection wavelength was set at 254 nm.
Another variant of the CZE technique (CZE on Chip) was employed in the study of wine quality, in which chlorogenic, vanillic, gentisic and ferulic acids were targeted as analyte indicative species (Scampicchio et al., 2004). The glass microchip CE separation channel was employed in this study, with the electrolyte being composed of 15 mM sodium borate, pH 9.5, the electrolyte injection voltage was 1.5 kV, separation voltage was set at 2 kV and the amperometric detection potential was +1.0 V.
In a report by Gu et al. (2000), MEKC was used in the study of wine quality, whereby cis/trans‐resveratrol was used as an analyte indicative species. Uncoated fused silica capillary was used, the experimental temperature was set at 25 °C and the separation buffer was made up of 25 mM sodium phosphate, 25 mM sodium borate, 75 mM SDS, pH 9 and the detection wavelength was 310 nm.
Nunez et al. (2000) used CZE in the study which investigated the differentiation of Spanish red wines utilizing inorganic species, mainly potassium (K), sodium (Na), calcium (Ca), manganese (Mn) and lithium (Li) as analyte indicative species. Uncoated fused silica capillary was used with the buffer system composed of 5 mM Cia‐Pack UV‐Cat1 buffer, 6.5 mM alfa‐hydroxyisobutyric acid, 2 mM 18‐crown‐6‐ether, voltage was set at 22 kV and the indirect UV detection at wavelength of 214 nm was used.
Conclusions
Electromigration methods are normally used in certain molecules that are mostly charged, such as proteins, etc. Therefore, for such molecules, the analyst may opt to use them. They are powerful and reliable.
References
- Bean, S.R. and Lookhart, G.L. (1997) Separation of wheat proteins by two‐dimensional reversed‐phase high‐performance liquid chromatography plus free zone capillary electrophoresis. Cereal Chemistry, 74: 758–780.
- Bean, S.R. and Lookhart, G.L. (1999) Sodium dodecyl sulfate capillary electrophoresis of wheat proteins. 1: uncoated capillaries. Journal of Agricultural and Food Chemistry, 47(10): 4246–4255.
- Bean, S.R. and Lookhart, G.L. (2000) Ultrafast capillary electrophoretic analysis of cereal storage proteins and its applications to protein characterization and cultivar differentiation. Journal of Agricultural and Food Chemistry, 48(2): 344–353.
- Bietz, J.A. and Schmalzried, E. (1995) Capillary electrophoresis of wheat gliadin: Initial studies and application to varietal identification. Lebensmittel‐Wissenschaft & Technologie (LWT) – Food Science and Technology, 28(2): 174–184.
- Belitz, H.‐D., Grosch, W. and Schieberle, P. (2004) Food Chemistry, 3rd Edition. Springer, Heidelberg, Germany.
- Boyce, M.C. and Spicket, E.E. (1997) Separation of food grade antioxidants (synthetic and natural) using mixed micellar electrokinetic capillary chromatography. Journal of Agricultural and Food Chemistry, 47(5): 1970–1975.
- Cancalon, P.F. and Bryan, P.R. (1993) Use of capillary electrophoresis for monitoring citrus juice composition. Journal of Chromatography A, 652(2): 555–561.
- Cartoni, G., Coccioli, F., Jasionowska, R. and Masci, M. (1999) Determination of cows' milk in goats' milk and cheese by capillary electrophoresis of the whey protein fractions. Journal of Chromatography A, 846(1–2): 135–144.
- Cherkaoui, S., Doumenc, N., Neeser, J.R. and Veuthey, J.L. (1997) Development of a capillary zone electrophoresis method for caseinoglycomacropeptide determination. Journal of Chromatography A, 790(1–2): 195–205.
- Chu, B.T., Evans, M.G. and Zeece, M. (1993) Quantitative separation of 4‐hydroxyproline from skeletal muscle collagen by micellar electrokinetic capillary electrophoresis. Journal of Chromatography B, 692, 293–301.
- Clifuentes, A. (2006) Recent advances in the application of capillary electromigration methods for food analysis. Electrophoresis, 27: 283–303.
- Day, L. and Brown, H. (2001) Detection of mechanically recovered chicken meat using capillary gel electrophoresis. Meat Science, 58: 33–37.
- Divall, G.B. (1985) The application of electrophoretic techniques in the field of criminology. Electrophoresis, 6: 249–258.
- Everaerts, F.M., Beckers, J.L. and Verheggen, T.P.E.M. (1976) Isotachophoresis: Theory, instrumentation and applications. Elsevier, Amsterdam.
- Foret, F., Křivnkov, L. and Boček, P. (1993) Capillary Zone Electrophoresis. VCH, Weinheim.
- Frazier, R.L. and Papadopoulou, A. (2003) Recent advances in the application of capillary electrophoresis for food analysis. Electrophoresis, 24: 4095–4105.
- Frazier, R.A., Inns, E.L., Dossi, N., Ames, J.M. and Nursten, H.E. (2000) Development of a capillary electrophoresis method for the simultaneous analysis of artificial sweeteners, preservatives and colours in soft drinks. Journal of Chromatography A, 876(1–2): 213–220.
- Gallardo, J.M., Sotelo, G., Pineiro, C. and Perez‐Martin, R.I. (1995) Use of capillary zone electrophoresis for fish species identification. Differentiation of flatfish species. Journal of Agricultural and Food Chemistry, 43: 1238–1244.
- Garcia‐Cañas, V., Gonzalez, R. and Clifuentes, A. (2002) Highly reproducible capillary gel electrophoresis (CGE) of DNA fragments using uncoated columns. Detection of genetically modified maize by PCR‐CGE. Journal of Separation Science, 25: 577–583.
- Garcia‐Cañas, V., Gonzalez, R. and Clifuentes, A. (2004a) The combined use of molecular techniques and capillary electrophoresis in food analysis. Trends in Analytical Chemistry, 23: 637–643.
- Garcia‐Cañas, V., Gonzalez, R. and Clifuentes, A. (2004b) Detection of genetically modified organisms in foods by DNA amplification techniques. Critical Reviews in Food Science and Nutrition, 44: 425–436.
- Garcia‐Canas, V., Gonzalez, R. and Clifuentes, A. (2004c) Sensitive and simultaneous analysis of five transgenic maizes using multiplex polymerase chain reaction, capillary gel electrophoresis, and laser‐induced fluorescence. Electrophoresis, 25: 2219–2226.
- Gu, X., Chu, Q., O’Duyer, M. and Zeece, M. (2000) Analysis of resveratrol in wine by capillary electrophoresis. Journal of Chromatography A, 881(1–2): 471–478.
- Hamounová, R., Urbánek, M., Pospišilová, H. and Polášek, M. (2004) Assay of phenolic compounds in red wine by on‐line combination of capillary isotachophoresis with capillary zone electrophoresis. Journal of Chromatography A, 1032(1–2): 281–287.
- Herrero‐Martinez, J.M., Simò‐Alfonso, E.F., Ramis‐Ramos, G., Gelfi, C. and Righetti, P.G. (2000) Determination of cow's milk in non‐bovine and mixed cheeses by capillary electrophoresis of whey proteins in acidic isoelectric buffers. Journal of Chromatography A, 878(2): 261–271.
- Jezek, J. and Suhaj, M. (2001) Application of capillary isotachophoresis for fruit juice authentication. Journal of Chromatography A, 916(1–2): 185–189.
- Kvasnicka, F. (2000) Application of isotachophoresis in food analysis. Electrophoresis, 21: 2780–2787.
- Kvasnička, F. (1999) Isotachophoretic determination of 3‐methylhistidine. Journal of Chromatography A, 838: 191–195.
- Kvasnička, F. and Voldřich, M. (2000) Determination of fumaric acid in apple juice by on‐line coupled capillary isotachophoresis‐capillary zone electrophoresis with UV detection. Journal of Chromatography A, 891(1): 175–181.
- Kvasnička, F., Voldřich, M., Pys, P. and Vins, I. (2002) Determination of isocitric acid content in fruit juices – Detection of authenticity. Journal of Food Composition and Analysis, 15: 685–691.
- Landers, J.P. (ed.) (2007) Handbook of Capillary and Microchip Electrophoresis and Associated Microtechniques. CRC Press, Boca Raton, FL.
- Lookhart, G.L. and Bean, S.R. (1996) Improvements in cereal protein separations by capillary electrophoresis: resolution and reproducibility. Cereal Chemistry, 73(1): 81–87.
- Melienthin, O. and Galensa, R. (1999) Analysis of polyphenols using capillary zone electrophoresis and HPLC: Detection of soy, lupin, and pea protein in meat products. Journal of Agricultural and Food Chemistry, 47(2): 594–602.
- Molina, E., Martin‐Alvarez, P.J. and Ramos, M. (1999) Analysis of cows’, ewes’ and goats’ milk mixtures by capillary electrophoresis: Quantification by multivariate regression analysis. International Dairy Journal, 9: 99–105.
- Morales, F.J. and Jiménez‐Pérez, S. (2001) Hydroxymethylfurfural determination in infant milk‐based formulas by micellar electrokinetic capillary chromatography. Food Chemistry, 72(4): 525–531.
- Nunez, M., Pana, R.M., Herrero, C. and Garcia‐Martin, S. (2000) Analysis, 25: 432–437.
- Pant, I. and Trenerry, V.C. (1995) The determination of sorbic acid and benzoic‐acid in a variety of beverages and foods by micellar electrokinetic capillary chromatography. Food Chemistry, 53(2): 219–226.
- Pazourek, J., Gonzalez, G., Sevilla, A.L. and Havel, J. (2000) Separation of polyphenols in Canary Islands wine by capillary zone electrophoresis without preconcentration. Journal of Chromatography A, 874(1): 111–119.
- Razee, S., Tamura, A. and Masujima, T. (1995) Improvement in the determination of food additive dyestuffs by capillary electrophoresis using β‐cyclodextrin. Journal of Chromatography A, 715: 179–188.
- Righetti, P.G., Gelfi, C. and Conti, M. (1997) Current trends in capillary isoelectric focusing of proteins. Journal of Chromatography B, 699: 91–104.
- Saavedra, L., Rupères, F.J. and Barbas, C. (2001) Capillary electrophoresis for evaluating orange juice authenticity: A study on Spanish oranges. Journal of African Food Chemistry, 49(1): 9–13.
- Salmanowicz, B.P. and Moczulski, M. (2004) Multiplex polymerase chain reaction analysis of Glu‐1 high‐molecular‐mass glutenin genes from wheat by capillary electrophoresis with laser‐induced fluorescence detection. Journal of Chromatography A, 1032(1): 313–318.
- Scampicchio, M., Wang, J., Mannino, S. and Chatrathi, M.P. (2004) Microchip capillary electrophoresis with amperometric detection for rapid separation and detection of phenolic acids. Journal of Chromatography A, 1049: 189–194.
- Simo, C., Barbas, C. and Clifuentes, A. (2002) Sensitive micellar electrokinetic chromatography − laser‐induced fluorescence method to analyze chiral amino acids in orange juices. Journal of Agricultural and Food Chemistry, 50(19): 5288–5293.
- Soga, T. and Serwe, M. (2000) Determination of carbohydrates in food samples by capillary electrophoresis with indirect UV detection. Food Chemistry, 69(3): 339–344.
- Sutton, K.H. and Bietz, J.A. (1997) Variation Among High Molecular Weight Subunits of Glutenin Detected by Capillary Electrophoresis. Journal of Cereal Science, 25(1): 9–16.
- Thompson, C.O., Trenerry, V.C. and Kemmery, B. (1995) Micellar electrokinetic capillary chromatographic determination of artificial sweeteners in low‐Joule soft drinks and other foods. Journal of Chromatography A, 694(2): 507–514.
- Vallejo‐Cordoba, B., Mazorra‐Manzano, M.A. and Gonzalez‐Cordova, A.F. (2004) Tequila volatile characterization and ethyl ester determination by solid phase microextraction gas chromatography/mass spectrometry analysis. Journal of Agricultural and Food Chemistry, 52(18): 5787–5790.
- Vanhoenacker, G., van den Bosch, T.D., Rozing, G. and Sandra, P. (2001) Recent applications of capillary electrochromatography. Electrophoresis, 22: 1103–4064.