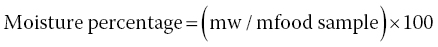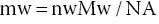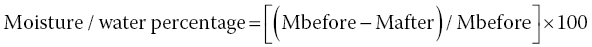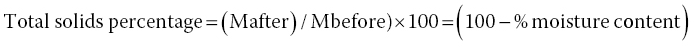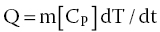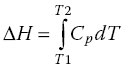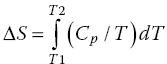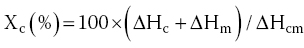18
Application of Thermal Methods in Food Forensics
Thermal methods of analysis provide a physical property measure of food samples as a function of temperature at the same time as when the food sample is being subjected to a controlled temperature program regime. The changes that are monitored include mass/weight (loss or gain), density, transition temperatures/energy, heat capacity, phase transitions, visco‐elasticity/gelation, modulus, rheology, and dimensional properties. The magnitude of the change associated with physical properties is essential, as it provides information about the quality, authenticity, or possible adulteration of the food involved in the tests.
Introduction
Thermal methods of analysis are highly useful in food forensic analyses, because most foods are susceptible to variations in their physical parameters that eventually alter the chemistry of food constituents that are associated with the quality of foods, including texture, taste, aroma, stability, and taste. Moreover, parameters such as temperature may change during processing (cooking, freezing, pasteurization, etc.), transportation and storage, and will affect food properties such as mass/weight, density, rheology, and heat capacity. This may alter the composition and ratio of ingredients and thus lead to wrong labeling. Moreover, chemical reactions that occur during various stages and steps of food processing, preparation, storage, and consumption, such as hydrolysis and redox reactions, may be triggered in foods, thus causing changes in the food’s physical properties in terms of, for example, evaporation, melting, crystallization, aggregation, or gelation. Thus, these changes will affect the overall natural properties of the food on the one hand, but may also be used to indicate where there is a misnomer in terms of food quality/composition.
The Relationship Between Temperature and Food Properties
There is a direct relationship between the physical changes of food components and the variations of the temperature during processing, preparation, or storage of foods. The physical changes that are related to temperature variation include density, phase transition, and gelation. Normally, solid food materials have a higher magnitude of density than liquid foods. When solid foods change phase into the liquid phase through melting processes or when liquid foods crystallize into a solid state, the change in density that accompanies such a phase transition is significant and therefore the variation of density with temperature. For pure materials that normally do not involve any phase transition processes, for example melting (solid‐to‐liquid), crystallization (liquid‐to‐solid), evaporation (liquid‐to‐gas), condensation (gas‐to‐liquid), sublimation (solid‐to‐gas), and glass transitions (glassy‐to‐rubbery), their densities tend to decrease with increase in temperature due to the fact that the space between atoms in such materials increases when the atoms absorb energy, thus increasing their kinetic energy causing them to move randomly and vigorously. For materials that undergo phase transition, there is a drastic variation that is observed with density as the temperature varies. On the other hand, the mass of materials does not have any relationship with the variation in temperature as long as processes such as condensation or evaporation do not take place. In cases where the volume is dependent on temperature, then there will be an inverse relationship between density and volume.
Phase transition brings about a change in the overall properties of foods and thus techniques capable of measuring physical properties such as molecular structure, molecular mobility, density, rheology, and heat capacity of a material that change with phase transition, need to be employed to monitor such changes.
Gelation, on the other hand, is a property that enables food materials to form gels under certain conditions of temperature. Gel structures refer to the three‐dimensional assemblage of biopolymer aggregates or colloids that have attracted and gathered water around them. Gels may have different types of appearances such as opaque or they may be transparent, depending on the composition of their aggregates. The composition of aggregates also determines other properties of gels, such as stability and rheology. Certain foods are known where gelling affects their overall properties greatly and these include yoghurt, eggs, and jellies. The dependence of gelling on temperatures comes from the fact that in some foods, gelling is observed only when heat treatment has been applied. These types of foods (e.g. egg white) or food‐gels are commonly called heat‐setting gels, while those that form when the cooling process has been applied, are known as cold‐setting gels. All these gel types (cold‐setting and heat‐setting) may either be reversible or irreversible and since they are all temperature dependent, they may then be termed as either thermos‐reversible (if they are reversible) or thermos‐irreversible (for those which are irreversible). To investigate gelling behavior of foods and their relationship with changes in physical parameters such as temperature, techniques are normally employed for such purposes. Such properties may indicate cases of adulterations, authenticity, and food quality.
Generally, thermal analysis‐based techniques play an important part in the field of food characterization, as they generate useful experimental data that explains how various food components behave when subjected to heat treatment. Different food types can be characterized by using thermal methods of analysis. For example, they can be used to study protein behavior upon heat treatment related to their conformation changes, thermal/freezing denaturation, and stability; and food polymers (e.g. starch, polysaccharides, fats, and oils) behavior upon treatment (gelling properties, phase transitions crystallization, stability changes, and decomposition). For frozen foods, thermal analysis techniques may be used to study their thermal stability, glass transition, and lyophilization and for food microorganisms these techniques are instrumental in establishing the heat killing food microorganisms, microbial growth, and microbial metabolism.
The thermo‐analytical methods that are of interest in food forensics and food analysis include thermal gravimetric analysis (TGA), which provides a measure related to the change in weight (gain or loss) and/or rate of change in weight as a function of temperature; differential thermal analysis (DTA), which measures the amount of heat evolved or absorbed; differential scanning calorimetry (DSC), which provides a measure related to the differential temperature or heat flow either to or from the sample against the reference material (DSC and DTA are all calorimetric based methods); and thermomechanical analysis (TMA), which is useful for measuring the penetration, expansion, contraction, or extension properties of materials as a function of temperature.
Application of Thermal Gravimetric Analysis Techniques in Food Forensics
Thermogravimetric techniques are attractive and useful in food analysis due to their capabilities to continuously and simultaneously measure the mass of a food sample as it is being heated at a controlled rate of temperature. Normally water molecules are expelled first from the food materials and depending on the food molecular environment, free water molecules tends to be expelled at lower temperatures as compared to bound water molecules The technique can thus be used to estimate the amount of water (free and/bound) that is contained in the different molecular environments of the food.
Moisture Content and Water of Crystallization of Foods Using Thermogravimetry: Measure of Food Authenticity, Quality Control and Quality Assurance
Being one of the parameters that are controlled and which may be involved in food forensic investigations because it is regulated, food moisture content of foodstuffs is always monitored, because there are guidelines and legal limits that must be adhered to and for this reason, the labeling must be verified. This stems from the fact that the water content in foods may encourage microbial growth unless foods are dried to the level that is below specific critical moisture of that particular foodstuff. Moreover, the majority of characteristics that determine the quality of foods such as taste, appearance, texture, and stability are all highly dependent on the amount of moisture contained in that particular foodstuff. In some instances, some unscrupulous business entities try to adulterate foods by adding excessive amounts of water for economic gain and this is an obvious crime that calls for forensic investigation.
By definition, the percentage of moisture content in food samples is given as the ratio of the mass of the water (mw) to that of the food sample (mfood sample) multiplied by 100 (Equation 18.1):
NB: The mass of water in the food sample can be worked out from the data related to its molecular weight (MW), i.e. 18 g per mole (nW), as well as the Avogadro number (NA), i.e. one mole of a substance contains 6.02 × 1023 units (atoms, ions, molecules), such that the mass of water can be calculated as follows (Equation 18.2):
Moisture as water molecules in food samples exists in different forms/environments, depending on how they interact with specific matrices in foods and they (water molecules) become imparted with different chemistries that can be categorized in different groups, including bulky water that can be contained in foods which is not interacting with the food matrix. This category of water will thus possess all the common properties of water such as density, boiling point, etc. Moisture or water in foods may be present as trapped capillary water that is trapped and held in tiny channels by capillary forces. There is also what is known as chemically bound water in foods, representing a water fraction that has chemical interactions with food matrices. An example is the water of crystallization, which may exist in forms of water of hydration that are characterized by relatively stronger bonds as compared to ordinary water molecules and therefore they possess different chemical and physical properties as compared to ordinary water, such as boiling point, lower melting point, etc. Also, they have higher density and heat of vaporization than water, but lower compressibility and different electromagnetic absorption frequencies. Apart from chemically bound water, there is also physically bound water in foods, which represents a fraction of the water in foods that is not entirely surrounded by only water molecules but also adsorbed to other biomolecules present such as sugars/carbohydrates, amino acids/proteins, or inorganic species such as salts and minerals. For this reason, this fraction of water possesses different and unique properties from that of normal water.
Practically, foods are very heterogeneous in the sense that they consist of different categories (chemically bound, physically bound, bulky, capillary water) and water of different forms (liquid water, solid water, and vapor) in different ratios and proportions. To be able to correctly determine the different categories or forms requires a proper choice of the method and technique and also the expertise and experience of the analyst.
There are thus a number of methods and techniques that are used to measure the moisture content of foods, which differ in terms of their specificity, level of accuracy, and sensitivity. Therefore the choice of analytical method will only depend on the nature of the food being analyzed and the required information. However, sample introduction to these techniques is always preceded by sample preparation steps, which will thus be discussed prior to the instrumental analytical techniques for the measurement of moisture/water in food samples.
Sampling and Sample Preparation Methods in the Analysis of Moisture/Water Content in Foods
Sampling is a crucial step that needs to be handled professionally and carefully, as it ensures a proper selection of a representative sample and also ensures that no unwanted changes in terms of the properties of the food sample will take place prior to analysis, in order to avoid unnecessary analytical errors that may compromise with the process of food analysis. There are also crucial precautions that need careful observance when analyzing food moisture content, in order to eliminate the possibility of introducing errors due to either any loss or gain of water from the surroundings. These precautions include ensuring that the food sample is protected from exposure to the atmosphere and also the food is protected from any excessive temperature fluctuations. An example of the strategies that are normally used to minimize loss or gain of water is to ensure that the storage containers for food are filled to the brim, allowing no appreciable headspace.
There are traditional wet methods for measuring moisture content and total solids in foods. These methods do not involve expensive instrumentation and do not require highly skilled personnel to handle them. They are therefore attractive, cheap, and affordable to many laboratories.
Among the traditional wet methods are the evaporation methods that are based on the fact that water in foods has a lower boiling point as compared to the other ingredients and constituents present in the food, such as biomolecules (sugars, salts, proteins, etc.). In these approaches, the amount of water/moisture is worked out by considering the difference in terms of weight before and after the evaporation process, according to Equation 18.3:
The total solid fraction in foods is related to moisture in foods, because it represents a portion of the material that remains after the evaporation of water from foodstuffs (Cai and Chen, 2008) and it can be mathematically expressed according to Equation 18.4:
One challenge that an analyst has to overcome is the difficulty in eliminating all the moisture in the food sample without tampering with other constituents of the food, including those that are volatile. There are therefore standard techniques that are normally used and which are known to result in reproducible and accurate data for moisture content in foods, because they employ a standardized temperature and time during the evaporation processes.
It should be noted that there are factors that control the rate and extent of water removal from food samples. These include the sample size, fineness, and shape of the sample. For samples that have been ground to a very fine size, they will possess greater surface area, which will imply that much of the sample is subjected to the drying environment and thus the sample will dry at a faster rate than those with a smaller surface area. Moreover, as discussed above, the type or category of water that is contained in the food will determine the ease of the drying process through evaporation. For example, free water can be more easily evaporated than chemically bound water and this will imply that for chemically or physically bound water, relatively more stringent temperature conditions may be required to expel all the moisture from food samples. The same consideration goes also with the water portion of food, which contains some solutes because the solutes tend to decrease the partial pressure of the water, a condition that will need an increased temperature to elevate the vapor pressure to be equal to the atmospheric pressure for the boiling to occur, making it possible for moisture to escape in the form of vapor.
However, by increasing the temperature or drying for an unusually prolonged time, care should be taken not to cause thermal decomposition, degradation, and denaturation of food constituents such as saccharides, carbohydrates, or proteins. For example, at high temperatures, disaccharides such as sucrose tend to break down to their monomeric sugar components (monosaccharides, i.e. glucose and fructose), a reaction which occurs when water is present. In this instance, water will be used through this reaction and it may cause serious errors as to the actual water content that was present in that particular foodstuff. In some other instances, food components such as carbohydrates at high temperatures, tend to give decomposition products that include water and this may cause errors in the estimation of the water/moisture content present in that particular foodstuff. In the case of foods that contain volatile components, such as flavoring/aroma constituents and/or odor constituents that may volatilize easily, even at normal temperature needed for evaporation, care should be taken to avoid any errors that may result in the decrease of weight/mass of food materials and where necessary an alternative drying method may be considered. For samples that may be adulterated through the addition of water (e.g. milk, juices), a multi‐stage drying procedure may be considered, because heating such foods may cause spattering as well as excessive accumulation of vapor. These phenomena are undesirable as spattering may cause loss of food constituents, especially in cases where ovens are used for drying. An example of a multiple drying stage that can be considered includes steam‐bath drying before oven drying. This will mostly depend on the mode in which the thermal energy is being transferred to the food sample, whether directly or indirectly. An example of direct heat transfer may be the use of an oven and an example of indirect heat transfer may be the use a microwave, which uses electromagnetic energy to heat the food sample through the mechanism that involves the absorption of the microwave energy by water molecules present in the food.
Modern Instrumental Methods and Techniques for the Analysis of Moisture Content in Foods
TGA has been widely used in the characterization of moisture content, as well as water of crystallization of foods. Normally foods contain moisture and the characterization of this moisture/water content is important for assessing end use properties. The moisture/water levels of foods can have an impact on important characteristics including stability, shelf life times, stickiness, visual appearance, and ability to dissolve in water. Even small differences in the level of water can have a significant effect on the properties of foods.
Apart from TGA, calorimetric methods, for example differential scanning calorimetry (DSC) and differential thermal analysis (DTA), are capable of providing a measure related to changes in terms of either the amount of heat absorbed or that which is released by a food sample or other materials such as food packaging as its temperature is varied at a controlled rate. These techniques can also provide a measure of water (free or bound) contained in a particular food sample, simply by making use of a measure of the sample’s melting point of water, which is largely dependent on its molecular environment. Normally free water tends to melt at a higher temperature than bound water and therefore if the enthalpy change of the food sample is measured with temperature or time, one can easily deduce from the data the amount of water that is contained in that particular food material under different molecular environments, whether free or bound.
During the measurement of moisture content, which provides the magnitude of the difference in terms of the weight of the food sample that takes place simultaneously during the heating process, the food sample is weighed before the heat treatment and also when the heating process has attained a steady state mass subsequent to drying. Since in thermogravimetry the rate of mass/weight loss properties is measured as a function of time or temperature, it is thus possible to use this technique to obtain valuable data on the composition, which may indicate evidence on possible adulteration, thermal stabilities, oxidative stability properties of foods, and also lifetimes, which may show the actual expiry date that may indicate any fraudulence in labeling.
Many reports have shown successful moisture measurements of different types of foods using gravimetric methods. For example, Tomassetti et al. (1987) employed TGA in the measurement of moisture in ten types of commercial food flours (mainly wheat meal, hard corn meal, semolina, rye flour, ground rice, corn flour, potato starch, chick pea flour, soya flour, and powdered chestnuts). In this work, the data obtained for both moisture and ash content was correlated to standard literature data for verification purposes.
Moisture content measurement is also important in authenticity tests for stored food products such as cereal grains, peas, beans, oil‐seeds, copra, cocoa beans, and also many other spices used in foods, because the measure of moisture content can be used to differentiate between oily grains and non‐oily grains. Tomassetti et al. (1986) analyzed for authentication purposes the amount of water in vegetable seeds, such as basil, cabbage, parsley, carrot, tomato, onion, and lettuce.
The authentication of hydrophilic biopolymers, such as poly(hydroxystyrene) and its derivatives, which are known to be biodegradable and thus useful as food packaging materials, can be done by measuring the bound‐water content using thermogravimetry (Hatakeyama et al., 1988). The analysis of the amount of bound water to biopolymers using thermogravimetry is made possible due to the fact that thermogravimetry will give a measure of heat of melting or heat of crystallization of water, and this measure can be used to work out the bound water content and in addition to these the thermogram curves will give an indication of the heat of vaporization for the biopolymers that will provide the magnitude of bound water for all the biopolymer components of the food (Hatakeyama et al., 1988).
Considerations Needed to be Taken into Account During Data Interpretation from TGA Curves
- The analyst needs to understand the atmosphere (air? nitrogen? oxygen?) in which the reaction takes place;
- The analyst must take note of the initial decomposition temperature (IDT) (the onset point) where the sample material begins to decompose. This point gives a measure of the thermal stability of the sample material;
- The point which denotes a maximum rate of decomposition (MRDT) gives an indication of the regime at which most of the sample component is undergoing decomposition;
- The D(1/2), i.e. d‐half, denotes the temperature at which the half‐weight percent of the sample material has undergone decomposition;
- The FR point (or final residue) gives a measure of the amount that remains after the end of the heating program and this point is useful in providing the final composition of the sample material, while the area under the derivative curve determines qualitatively the amount of sample component that has been decomposed;
- In case the results from the curves have either very close or similar magnitudes of the area under the main decomposition stage, it will imply that the sample component is decomposing as a function of the temperature.
Protein Identification/Authentication and Species Differentiation Using Differential Scanning Calorimetric (DSC) Methods
Differential scanning calorimetry (DSC) provides a measure of the energy required to keep a zero temperature difference between a test sample and a reference sample by either heating or cooling the sample at a controlled rate. When the sample is subjected to DSC analysis, it will undergo phase transitions and when this happens it implies that it has either absorbed or released energy (heat). The DSC can be used to keep the temperature of both the sample and that of the reference at an equivalent amount of energy (heat) by giving the magnitude of the energy that needs to be supplied to either the test sample or the reference sample. Normally, the DSC data is reported as the rate of energy absorption (Q) by the test sample relative to the reference sample with respect to the external temperature. In some cases, DSC can provide data related to the change in the heat released by the test sample as a function of time (instead of temperature) under isothermal (constant temperature) conditions.
In food forensics, differential scanning calorimetry (DSC) has proved to be very useful in the determination of energy changes in samples that are subjected to thermal treatment. Researchers and scientists can make use of DSC when investigating phase transitions that involve energy changes for the sample in question. Examples of such transitions include endothermic denaturations (endothermic: crystalline melting (heat of fusion), dehydration); exothermic denaturations/decompositions (exothermic: crystallization (ordering or freezing) polymerization, etc.); and glass transitions or melting behavior. Differential scanning calorimetry is based on measuring temperature as well as the spontaneous/compensating heat fluxes (Bershtein and Egorov, 1994).
In food forensics, physico‐chemical thermal methods such as DSC can be useful in species identification and authentication of foods from different species. This is because both the structural similarities and structural differences between targeted specific proteins from different plant/animal species can be revealed using thermal DSC techniques that are capable of providing the contrast in terms of the patterns and changes that are related to the protein thermal behavior of different species. DSC can provide information about the denaturation temperatures (Td) and transition enthalpies (ΔH) that can be matched/compared to the protein in various species in each of the DSC processing steps (Murray et al., 1985). DSC can also be used to provide information about protein thermal stability, protein overall conformation, the (apparent) thermal (endothermic/exothermic) transition midpoint (Tm) values, profile similarity values, and protein domain folding integrity that is characteristic to individual species (Ibarra‐Molero et al., 2016 ; Wen et al., 2012; Xiong et al., 1987).
The similarities or differences in terms of protein profiles under DSC can be explained by the fact that the evolution of proteins in each species is associated with specific mutations in certain sequences of amino acids and peptides in some of the proteins to enable the proteins to adapt better to the environment, to make them more suitable for some specific functions, or make the organism successful in the population in terms of passing on its genes. In this way the same protein becomes highly conserved for all members of the same species that contain the protein. Since mutations are known to be very rare and they also occur randomly, these highly conserved proteins so specific to species may not have more than one chance for them to occur. For this reason, all organisms that possess the same protein (conserved protein), the chances are high that they belong to the same species and those organisms in which the target conserved protein is not there, belong to different species. Different proteins will have different patterns under DSC and they can thus be differentiated.
Application of DSC in the Analysis of Protein Thermal Stability
Normally biological molecules such as proteins, when they are in solutions, tend to exist in equilibrium between their native (folded) and denatured (unfolded) conformations. Thermal phase transitions can occur in such molecules, which accompany either absorption or release of heat energy. The protein stability will depend on the magnitude of the thermal transition midpoint (Tm), such that the higher the Tm, the more stable the biological molecule is. DSC is a physicochemical thermal method that provides a measure of a number of parameters, including the enthalpy (∆H) of unfolding that is related to heat‐induced denaturation, the change in heat capacity (ΔCp) of denaturation, and also factors that govern the folding behavior and stability of native biomolecules such as proteins.
When protein samples are subjected to DSC for thermal stability analysis while the protein sample is in a dilute solution environment, the observations that may be expected include those that involve changes in the specific partial molar heat capacity of the protein at constant pressure (ΔCp). The specific heat capacity is an essential parameter for the food industry, as it provides indication and guidance for the estimation of the measure of the amount of energy required to either be introduced or removed from the sample, so as to increase or decrease its temperature by a certain quantity. The importance of the knowledge of the magnitude of specific heat capacity of a given food material lies in the fact that it is used in the design of processes, for example chilling, freezing, warming, sterilization, and cooking. Thermal techniques such as DSC and DTA can all be useful to provide a measure of the specific heat capacities of foods. For example, when DSC is used, the measure or magnitude of the specific heat capacity (CP), can be worked out using Equation 18.5:
where Q = heat flow per unit time; m = sample mass; CP = specific heat capacity of the material; and dT/dt = the rate of change of the external temperature.
Generally, whenever there is a change in the magnitude of heat capacity of any sample, it implies that the sample possesses the tendency to absorb heat. Different substances possess specific measures of heat capacity that is characteristic to them. For example, if the specific heat capacity of water is compared to that of many organic compounds including proteins, it will be found that water possesses a higher value of specific heat capacity than protein due to the presence of an increased measure of hydrogen bond network. Water molecules are also found forming matrices in proteins and those water molecules that are found in protein matrices have a highly ordered chemistry in terms of their molecules being tightly held near and around the hydrophobic environment found on the protein’s surface, and due to the fact that water molecules cannot form hydrogen bonding with hydrophobic molecules, it makes the formation of hydrogen bonding between water molecules highly optimal (Shinoda, 1977). During the DSC heating program and as the temperature is increased, the ordered water fraction on the surface of the protein becomes distorted and starts to behave like the bulk water and this results in an increased heat capacity of protein aqueous solutions.
In order to work out the real measure of the protein’s specific heat capacity calorimetrically (the protein’s measure of its specific partial Cp), the subtraction of a scan of a buffer blank sample from the protein sample profile data has to be done. The magnitude of partial Cp also represents the contribution of the effects of the protein in the aqueous solution matrix, in addition to it providing a measure of the heat capacity of the protein that is being analyzed for its thermal stability properties (Bruylants et al., 2005 ; Freire, 1995). During the heating process, there will be a phenomenon that is associated with raising the baseline and as the heat starts to be absorbed by the protein, it will reach a certain characteristic temperature range where the protein starts to thermally unfold, which will result with the observation of an endothermic peak. The temperature range at which the protein becomes thermally unfolded is characteristic of different types of proteins and is also specific from proteins from different species (e.g. muscle proteins from different species). This means that the technique can be used for differentiation of species, authenticity tests, food adulteration cases, etc. The magnitude of the enthalpy (ΔH) can be worked out by integrating the data for the heat capacity of the protein and plotting this value against the corresponding temperature values used during the DSC run (Equation 18.6):
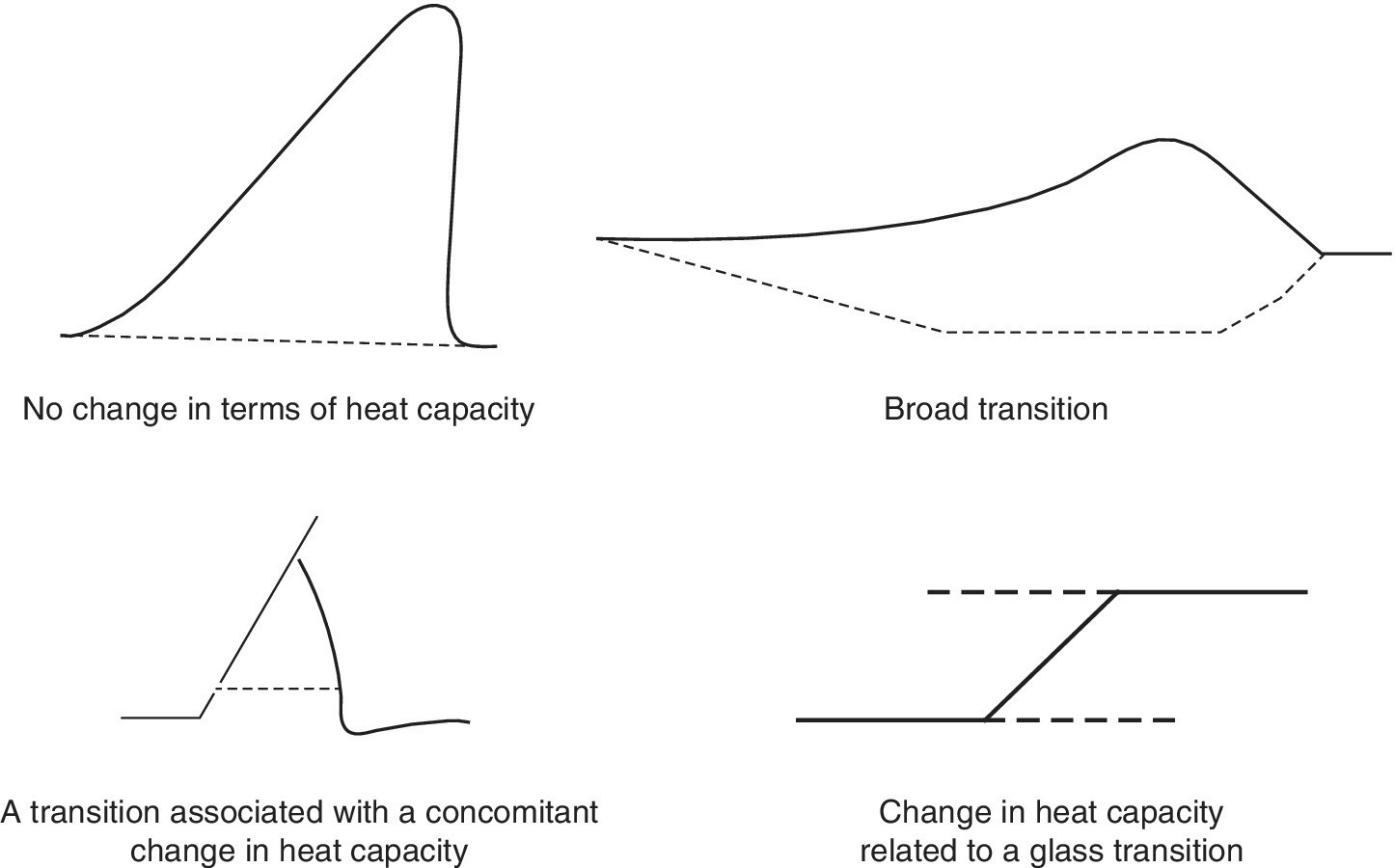
From the plot drawn using Equation 18.6, a trend will be observed, showing a shift in baseline before and after the transition and this is important as from it the change in heat capacity (ΔCp) of the protein (and associated water) due to the unfolding phenomena can be deduced. Another important point that can be deduced from the graph is the transition midpoint (Tm), which is also known as the melting temperature, and which represents the point where half of the protein molecules are folded and the other half are unfolded. The area under the curve plot (Cp/T vs. T), obtained using Equation 18.7, is important for providing information regarding the magnitude of entropy (ΔS) (Bruylants et al., 2005 ; Marky and Breslauer, 1987).
Phase transitions such as crystallization, melting, glass transitions, and conformational changes in foods are important, because they provide useful information regarding the temperature at which these transitions take place (Ttr ), the enthalpy change associated with such a transition (ΔHtr ), the type of transition involved whether it is exothermic or endothermic, and the measure in terms of quantity of sample material that undergoes this transition. However, there are certain important considerations that have to be taken into account when using DSC for the measurements of these transitions. One such consideration is that all food components are biologically and chemically complex and thus the phase transitions may occur over a wide range of temperatures. For example, the composition of vegetable edible oils may include different chemistries of different triacylglycerols and each of these may produce a different pattern in terms of the melting point profiles. Moreover, due to the different chemistries of the triacylglycerols in plant‐based edible oil, there is a high possibility that they can have more than one crystalline structure due to their polymorphic nature.
Factors that Affect Thermodynamic Stability Properties of Proteins
There are several factors that govern the stability of a protein and/or its Tm value:
pH of the Environmental Matrix
Among these factors is pH, which can cause specific proteins to be stable at a particular characteristic pH value, and if the pH shifts or is off the range of stability, the protein loses that stability (Privalov and Khechinashvili, 1974).
Concentration of Protein
The concentration of protein can determine its conformational stability, because it is related to protein–protein interactions. It also affects the ionic strength of the solution that can be an indicative measure and role of electrostatic interactions and in addition to all these, the concentration of proteins is proportional to the scan rate (Griko et al., 1995 ; Pfeil and Privalov, 1976 ; Robertson and Murphy, 1997).
One limitation of using DSC for protein analysis is mainly the amount of protein that is normally available for use as the sample is always limited, something which can seriously restrict the application of thermal biophysical methods such as the normal DSC.
Differential Thermal Analysis (DTA) and Food Forensics
Like DSC, DTA measures the changes related to either heat absorbed or released by a test food sample while varying the sample’s temperature at a controlled rate. The changes in the magnitude of heat absorbed/released happen during the DTA processing of the sample, whereby food constituents undergo some specific phase transitions such as crystallization, melting, evaporation, glass transitions, and conformational change, or in some cases there may be chemical reactions such as oxidation, reduction, or hydrolysis that may occur instead of or in addition to phase transitions.
In DTA, the difference in the measure of temperature between the test sample and the reference sample versus time or temperature is recorded simultaneously, as the two samples (test and reference) are subjected to the same temperature treatment whereby the sample cells are either heated or cooled at a controlled rate regime.
From the DTA results, if the sample temperature is more than that of the reference, it means that heat energy was released during the phase transition and thus the process is termed as exothermic when DT > 0 and endothermic when DT < 0 (heat energy absorbed during the phase transition).
Other important information about the sample, for example the type of transitions that have taken place can be deduced from the nature of a peak, whether it is exothermic, endothermic, or it can be deduced from peak shape. In addition, signal (peak) position enables analysts to obtain information regarding the temperature range where the transition takes place. These points are characteristic to species and food types and they can be used to discriminate, classify, and provide authenticity as well as evidence in cases of adulteration.
Isothermal Titration Calorimetry (ITC) Application in Food Forensics
Isothermal titration calorimetry (ITC) is used to provide information about a measure of enthalpy changes that occur due to the interactions between different types of molecules. To perform ITC analysis, one would require having a reference sample, a test sample and an injector solution. The requirement for an ideal reference is that it must not undergo any enthalpy changes when subjected to ITC and the example is distilled water. Then a measure of the energy needed to maintain the test sample and the reference sample at the same temperature is recorded as a function of time. This will generate data that can be plotted as the amount of heat (Q) against time and from the nature of the process whether it involved exothermic or endothermic processes or by investigating the magnitude of the area under the curve and shape, one can deduce information regarding the interactions, whether binding or conformational, which are occurring between molecules in the test sample.
Thermogravimetry and Cooking/Edible Oil Fingerprinting
Cooking or edible oils are important additions to cooked foodstuffs as they improve the taste of foods. There are parameters that are known to define the quality of cooking oil and include the fatty acid types, fatty acid composition and the oil’s resistance to oxidation. In some cases, some additives (mainly antioxidants) are introduced into the oil to enhance its stability to the oxidation processes. Other approaches to control oxidation of oils and increase oil stability such as hydrogenation and modification of fatty acid composition, etc. are known to add to the stability and quality of oils. There are other external factors (apart from fatty acid composition, antioxidants. and water that are intrinsic factors) that contribute to the oil’s lipid oxidation. The external factors that contribute to the lipid oxidation/stability include light, high temperatures, and gases, mainly oxygen (Kamal‐Eldin, 2006 ; Merrill et al., 2008).
Despite the presence of all these factors, in most cases it is the fatty acid composition that is taken as the predictor factor for oil stability and therefore the oil’s shelf life.
The stability of oils can be ascertained using thermo methods of analysis, mainly thermogravimetry (TG), differential thermogravimetry (DTG), and differential scanning calorimetry (DSC), which can also be used to verify the authenticity and differentiation of cooking/edible oils (De Souza et al., 2004 ; Diniz et al., 2008 ; Freire et al., 2009 ; Litwinienko and Kasprzycka‐Guttman, 1998 ; Santos et al., 2002 ; Simon and Kolman, 2001). In food forensic cases related to edible oil products, TG can be employed for the analysis of oils, because it can reveal semi‐quantitative information regarding thermal degradation processes that take place during thermochemical conversion reactions that occur under different atmospheres. This information can be coupled to that from the DTG, in which information regarding the measurement of the rate of weight loss of the oil versus temperature and/or time is obtained. The information from DTG is important as it enables the prediction of the thermal behaviors of the different types of oils.
Thermogravimetry and Food Packaging and Food Contact Substances Authenticity and Compliance
Food packaging provides a limiting barrier that separates and protects food from exposure to any potential contaminants, oxygen, moisture, etc., which may be present around it or in the immediate external environment. The ideal food packaging requires qualities that will ensure hygiene and the ability to minimize the risk of food contamination and where possible, the materials should have the advantage of extending the shelf life of foods. In addition, food packaging must be accompanied by labeling that contains all the necessary and useful information about the food product (ingredients), instructions for use, storage, processing before consumption, expiry date, and details on recycling. Normally all food packaging is required to adhere and comply with specific legal requirements.
In food industries, different materials are normally used as packaging to keep the food safe and if possible fresh so that it can be used at a later date. The types of food packaging materials that are used are in most cases single/multi‐layer films, blends/composites of biodegradable biopolymers, and homopolymers, as well as copolymers.
Biodegradable Biopolymers Used in Food Packaging and Food Contact Substances
Biodegradable biopolymers are normally used for making food packaging and other food‐contact substances including straws, stirrers, disposable cutlery, disposable drinking cups and their lids, disposable plates, water bottles, food overwraps, and food lamination films.
The source of these biodegradable biopolymers can be from renewable resources, which can thus be recycled and composted but are made such that they possess comparable optical, physical, and mechanical properties to their synthetic counterparts, which are normally made from oriented polystyrene (OPS) and polyethylene terephthalate (PET) materials.
There are mainly three major classes of biopolymers used in food packaging and food contact substances and they include (i) polyester biopolymers, (ii) polyhydroxyalkanoate (PHA) biopolymers and(iii) starch‐based biopolymers. The classification is based on the production process that may be used and the source of the raw materials.
Polyester biopolymers can be sourced from natural biomass such as polysaccharides or they can be synthesized using starting materials such as polyetilene terephthalate (PET) containing aliphatic–aromatic copolymers and aliphatic polyesters, which are a polycondensation reaction product of glycol and aliphatic dicarboxylic acid (ADA), polylactide aliphatic copolymer (CPLA), as well as bio‐based monomers, for example poly(lactic acid) and oil‐based monomers such as polycaprolactones. The CPLA is a product of lactide and aliphatic polyesters such as dicarboxylic acid or glycol, with hard materials such as polyester (PS) and soft flexible materials such as polypropylene (PP), at ratios that can be determined by the content of aliphatic polyester in the blend. In addition to these, polyesters can be sourced polymeric materials, such as polyhydroxyalkanoates that can be obtained from natural sources, mainly microorganisms.
The bio‐based polymers such as poly(lactic acid) (PLA), a copolymer between poly(l‐lactic acid) and poly(d‐lactic acid) and which can be prepared by the depolymerization procedure involving lactic acid monomers as sourced from corn and other similar products, have of late been highly promising for use in food packaging. Its attractive features include the ease in recyclability and biodegradability. An oil‐based biopolymer such as polycarolactone (PCL) is used in food contact substances or in food packaging only when mixed with starch, where it is mainly used for trash bags. PCL‐starch blends are thus attractive due to the fact that the polymer blend is highly biodegradable (with very short biodegradation time) and has excellent chemical resistance to solvents such as water, oil, and also chlorine. In addition, it is known to have low viscosity, and is easy to prepare and process.
Other natural materials that are used in food packaging and food contact substances include polyhydroxyalkanoates (PHA) biopolymers, which are produced in nature by microbial fermentation of sugar and lipids. PHA may exhibit properties of either thermoplastic or of elastomeric materials and may be used with or without synthetic plastic or starch in order to form the desired packaging films of the desired/specific qualities (Siracusa et al., 2008). An example of PHA, which does not include any blending polymer, can be the polyhydroxybutyrate (PHB) that is obtained directly from the polymerization of 3‐hydroxybutyrate monomers, while an example of the blend can be the copolymer polyhydroxybutyrate‐valerate (PHBV).
There are also starch‐based biopolymers that are obtained from mixing of oil‐based monomers and different quantities of starch, as well as other additives which play different roles such as flame‐retardants, coloring, etc. An example of this can be low‐density polyethylene film (LDPE)‐starch blend.
When manufacturing food packaging, certain considerations regarding the properties of the materials are needed for specific usage or the required physico‐chemical and thermal properties in order to predict environmental effects as well as the impact modifications that may be incorporated. Other properties related to heat of fusion, glassy state relaxations, tan δ data, secondary transitions and storage modulus may be desirable in order to match the compatibility of various polymer composites/blends.
All these properties can be used to define and give an indication with regard to the compliance in accordance to the legal requirements for food packaging. Different materials and their blends as well as the additives incorporated into packaging give different packaging uniqueness and can also be used to determine the quality and level of compliance.
The thermal technique is one of the many techniques that can be employed to ascertain the compliance, quality, and authenticity of food packaging. For example, DSC can be employed to investigate a number of properties including the glass transition temperature (Tg), melting temperature (Tm), and crystallinity of the polymeric material, which is normally worked out using Equation 18.8:
where ΔHc is the exothermic enthalpy of cold crystallization; ΔHm is the endothermic enthalpy of fusion; and ΔHcm is the endothermic heat of melting of purely crystalline sample polymer under investigation.
Another thermal based technique, thermo‐gravimetric analysis (TGA), can be used to analyze food packaging and provide useful information regarding the decomposition temperature. The breakdown and biodegradability properties of these food packagings can be investigated using DSC and TGA.
Conclusions
Thermal methods are useful for certain types of foods and food packaging authentication. The analyst must be conversant with the principles involved in these methods so as to correctly choose the appropriate method of analysis for a particular sample. The knowledge of data interpretation from the generated curves is very essential.
References
- Bershtein, V. and Egorov, V. (1994) Differential Scanning Calorimetry of Polymers, Physics, Chemistry, Analysis, Technology. Ellis Horwood Ltd., West Sussex, UK.
- Bruylants, G., Wouters, J. and Michau, C. (2005) Differential scanning calorimetry in life sciences: Thermodynamics, stability, molecular recognition and applications in drug design. Current Medicinal Chemistry, 12: 2011–2020.
- Cai, J and Chen, S. (2008) Determination of drying kinetics for biomass by thermogravimetric analysis under non‐isothermal conditions. Drying Technology, 26: 1464–1468.
- De Souza, A.G., Santos, J.C.O., Conceiç, Ã.O.M.M, Silva, M.C.D. and Prasad, S. (2004) A thermoanalytic and kinetic study of sunflower oil. Brazilian Journal of Chemical Engineering, 21(2): 265.
- Diniz, Z.N., Bora, P.S., Neto, V.Q. and Cavalheiro, J.M.O. (2008) Sterculia striata seed kernel oil: Characterization and thermal stability. Grasas Y Aceites, 59(2): 160.
- Freire, E. (1995) Thermal denaturation methods in the study of protein folding. Methods in Enzymology, 259: 144–168.
- Freire, L.M.S., Bicudo, T.C., Rosenhaim, R., Sinfronio, F.S.M., Botelho, M.J.R. and Carvalho Filho, J.R. (2009) Thermal investigation of oil and biodiesel from Jatropha curcas L. Journal of Thermal Analysis and Calorimetry, 96: 1029–1033.
- Griko, Y.V., Freire, E., Privalov, G., Van Dael, H. and Privalov, P.L. (1995) The unfolding thermodynamics of c‐type lysozymes: A calorimetric study of the heat denaturation of equine lysozyme. Journal of Molecular Biology, 252: 447–459.
- Hatakeyama, T., Nakamura, K. and Hatakeyama, H. (1988) Determination of bound water content in polymers by DTA, DSC AND TG. Thermochimica Acta, 123: 153–161.
- Ibarra‐Molero, B., Naganathan, A.N., Sanchez‐Ruiz, J.M. and Muñoz, V. (2016) Modern analysis of protein folding by differential scanning calorimetry, Chapter 12. Methods in Enzymology, Calorimetry, 567: 281–318.
- Kamal‐Eldin, A. (2006) Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. European Journal of Lipid Science Technology, 58: 1051–1061.
- Kelly, J.W., Chemmalil, A.L., Muzammil, S., Gabrielson, J. and Jiang, Y. (2012) Applications of differential scanning calorimetry for thermal stability analysis of proteins: Qualification of DSC. Journal of Pharmaceutical Sciences, 101(3): 955–964.
- Litwinienko, G. and Kasprzycka‐Guttman, T. (1998) A DSC study on thermoxidation kinetics of mustard oil. Thermochimica Acta, 319: 185–191.
- Marky, L.A. and Breslauerm, K.J. (1987) Calculating thermodynamic data for transitions of any molecularity from equilibrium melting curves. Biopolymers, 26: 1601–1620.
- Merrill, L.I., Pike, O.A., Ogden, L.V. and Dunn, M.L. (2008) Oxidative stability of conventional and high‐oleic vegetable oils with added antioxidants. Journal of the American Oil Chemists’ Society, 85: 771–776.
- Murray, E.D., Arntfield, S.D. and Ismond, M.A.H. (1985) The influence of processing parameters on food protein functionality. II: Factors affecting thermal properties as analyzed by differential scanning calorimetry. Canadian Institute of Food Science and Technology Journal, 18(2): 158–162.
- Pfeil, W. and Privalov, + P.L. (1976) Thermodynamic investigations of proteins. III: Thermodynamic description of lysozyme. Biophysical Chemistry, 4: 41–50.
- Privalov, P.L. and Khechinashvili, N.N. (1974) A thermodynamic approach to the problem of stabilization of globular protein structure: A calorimetric study. Journal of Molecular Biology, 86: 665–684.
- Robertson, A.D. and Murphy, K.P. (1997) Protein structure and the energetics of protein stability. Chemical Reviews, 97: 1251–1267.
- Santos, J.C.O., Dos Santos, I.M.G., De Souza, A.G., Prasad, S. and Santos, A.V. (2002) Thermal stability and kinetic study on thermal decomposition of commercial edible oils by thermogravimetry. Journal of Food Science, 67(4): 1393–1398.
- Shinoda, K. (1977) “Iceberg” formation and solubility. Journal of Physical Chemistry, 81: 8069–8072.
- Simon, P. and Kolman, L. (2001) DSC study of oxidation induction periods. Journal of Thermal Analysis and Calorimetry, 64: 813–820.
- Siracusa, V., Rocculi, P., Romani, S. and Rosa, M.D. (2008) Biodegradable polymers for food packaging: A Review. Trends in Food Science & Technology, 19: 634–643.
- Tomassetti, M., Campanella, L. and Delfini, M. (1986) Determination of water in plant samples: A comparative thermogravimetric and NMR study on different species of seeds. Thermochimica Acta, 105: 179–190.
- Tomassetti, M., Campanella, L., Delfini, M. and Aureli, T. (1987) Determination of moisture in food flours. A comparative thermogravimetric and NMR study. Thermochimica Acta, 120: 81–95.
- Xiong, Y.L., Brekke, C.J. and Leung, H.K. (1987), Thermal denaturation of muscle proteins from different species and muscle types as studied by differential scanning calorimetry. Canadian Institute of Food Science and Technology Journal, 20(5): 357–362.