I do remember an apothecary—And hereabouts he dwells—which late I noted In tatter’d weeds, with overwhelming brows, culling of simples. . . . Noting this penury, to myself I said, ‘And if a man did need a poison now, Whose sale is present death in Mantua, Here lives a caitiff wretch would sell it him.’
—William Shakespeare, Romeo and Juliet
IN 399 B.C. the Athenian gadfly Socrates was sentenced to death for subverting the youth of Athens by encouraging them to question their elders. He carried out the sentence himself by drinking a cup of poison hemlock. As described by witnesses to his death, he expired quite peacefully on his couch.
The next poisoning of historical significance was also self-inflicted. In 31 B.C. Cleopatra, the queen of Egypt, held an asp to her bosom.
The earliest-known professional poisoner was the Roman matron Locusta, a former slave who became an instrument of imperial policy. When Agrippina, wife of the emperor Claudius, decided to do away with her husband, she sought advice about poisons. As the historian Tacitus tells us in The Annals of Imperial Rome, Agrippina needed something subtle that would upset the emperor’s faculties but defer his death. An expert in such matters was located—Locusta. Her special skills would reprieve her from a recent sentence of death and fit her for a long career of imperial service. After two attempts the desired result was achieved, and in 54 A.D. Agrippina’s son Nero became the sixth emperor of Rome. He rewarded his mother by ordering her murder.
In mid-seventeenth-century Europe, poisoning became a popular hobby among the gentry. This was particularly the case among women, for whom traditional methods of slaughter—those requiring physical strength and mastery of edged weapons—were infeasible. The advantages of poison were obvious: it required neither strength nor dexterity, and could be administered in comparative anonymity.
In the 1650s there was a considerable increase in the number of young, rich widows in the larger cities of Europe. Many of them confessed to their priests that they had poisoned their husbands. Bound by the seal of the confessional, the priests could take no action against the offenders. But as they compared notes, the sheer number of these confessions frightened them. In 1659 a delegation of priests informed Pope Alexander VII of the problem.
The Pope, who had temporal as well as spiritual authority over the city of Rome, began an independent investigation. His agents found that a group of young wives, some from among Rome’s first families, met regularly at the house of Hieronyma Spara, a reputed witch and fortune-teller. Then, as described by Charles Mackay in Extraordinary Popular Delusions and the Madness of Crowds:
A lady was employed by the government to seek an interview with them. She dressed herself out in the most magnificent style; and having been amply provided with money, she found but little difficulty, when she had stated her object, of procuring an audience of La Spara and her sisterhood. She pretended to be in extreme distress of mind on account of the infidelities and ill-treatment of her husband, and implored La Spara to furnish her with a few drops of the wonderful elixir, the efficacy of which in sending cruel husbands to “their last long sleep” was so much vaunted by the ladies of Rome. La Spara fell into the snare, and sold her some of her “drops” at a price commensurate with the supposed wealth of the purchaser.
The liquor thus obtained was subjected to an analysis and found to be, as was suspected, a slow poison—clear, tasteless, and limpid.
As a result of these investigations, Spara, her assistant Gratiosa, and three young wives were hanged. More than thirty women were whipped in the streets, and several women whose social or political positions made it inexpedient to punish them openly were banished from the city. Over the next few months, nine more women were hanged and another group flogged.
The practice of poisoning was not restricted to Rome. In 1679 two poison wholesalers, Mesdames Lavoisin and Lavigoreux, were arrested in Paris. Between thirty and fifty of their retailers, mostly women, were also apprehended in Paris and in other principal cities of France. The pair were burned alive in the Place de Greve on February 22, 1680, and their retail agents hanged. This malevolent band had been the teachers, instigators, and suppliers for hundreds of poisonings performed overwhelmingly by or on behalf of women who wished to be rid of their husbands (though there was a sprinkling of men who sought to eliminate rich fathers or uncles). The poison used, probably a salt of arsenic, was called by those in the know “inheritance powder.”
This wave of seventeenth-century poisonings was attributed to a cabal of women, invariably described as witches or fortunetellers, who possessed the necessary skills to manufacture the tasteless, undetectable poisons. These women first became the confidantes of their prospective clients, then whispered suggestions in their not-altogether-unwilling ears. That so many women were apparently eager to dispose of their husbands may speak to the impossibility at the time of escaping an unhappy marriage by any means except murder. The husband in a bad marriage could effectively ignore his wife and do just about as he chose. The wife, however, was deprived of her rights by law and custom, and was subject to her husband’s command as long as he lived.
Unless the poison was violently corrosive, caused clear physical symptoms that could be observed in the dying person, or remained noticeable on the corpse, there were no techniques at the time for detecting the presence of poison in a body. Those who were caught had probably purchased their potent powders from a supplier who had brought attention on herself. Since torture was still a popular tool of criminal investigation, they were soon identified.
In order to protect society from poisoners, a means had to be found to detect poisons in the body for some time after death. Although the “subtle poison undetectable by science” has long been a staple of detective stories, such a substance is virtually nonexistent today. But the development of reliable means of detection has been a long, slow process.
Dr. Hermann Boerhaave (1668–1738) was the first to describe a practical, if possibly dangerous, way to identify suspected poisons. He advised placing the material in question on red-hot coals. The smoke rising from it was then to be cautiously smelled. A trained nose could probably detect most simple chemical poisons this way, but how safe or reliable this method was is not known. At any rate, it did not catch on.
In Elementa medicinae et chirurgiae forensis, Joseph Jacob Plenck (1739–1807) pointed out that the only positive proof of death by poisoning is the chemical identification of the poison itself within the organs of the body. It would be another half-century before this theory was generally accepted.
Modern toxicology, the study of poisonous substances, originated in 1814 with the publication of the first volume of A Treatise on Poisons Derived from the Mineral, Vegetable and Animal Kingdoms, or General Toxicology Considered with Respect to Physiology, Pathology and Legal Medicine by Dr. Matthieu Joseph Bonaventura Orfila. It was followed by a second volume a year later. Originally published in French, the book was translated into English, Spanish, and German within only a few years and remained a standard toxicology text for more than a century. Still a student in Paris when he began to catalog poisons, by 1819, when he was only thirty-two years old, Orfila was a professor of legal medicine and within a few years became head of the chemistry department of the University of Paris. In 1840 Dr. Orfela presented evidence at the trial of Marie Lafarge for the poisoning of her husband, one of the most celebrated crimes of the nineteenth century.
Orfela was assisted in his forensic labors by Marie Guillaume Alphonse Devergie, who went on to develop the use of microscopic analysis in forensic pathology. In 1835 Devergie published his findings in his landmark book Medecine légale, theorique et pratique.
The classification of objects is often a problem, representing as it does a human need for order rather than an intrinsic relationship of the objects under consideration. So it is with poisons. Authorities may agree that there are three types of poisons, but may then disagree as to what these three types are. One tome may divide poisons by their source—mineral, vegetable, or animal. Another will classify them according to their effects—corrosive, irritating, or systemic. Still another will classify poisons as those which act by attacking the gastrointestinal canal, those which attack the central nervous system, and those which depress the nerves and circulatory systems.
The definition of poison is itself subjective. As the Merck Manual says, almost any substance may be toxic if inhaled, ingested, or otherwise taken into the body in the right proportions and under the right circumstances. Water, salt, and even air can be deadly. Some substances, such as the nerve poison curare, are toxic in small quantities and yet are used medicinally in even smaller quantities. Medicines used to cure or ameliorate one condition can kill if prescribed for a different condition. Botulism toxin, the second-deadliest poison known, is now injected into the facial muscles of members of our aging population to eliminate wrinkles. Insulin, taken daily by diabetics to replace the natural insulin no longer manufactured by their bodies, will kill if administered in a large dose. Several chemicals used as anesthetics during surgery will kill subtly, quickly, and painlessly. These break down in the body so as to be essentially undetectable at autopsy unless their use is suspected. Even then they are very difficult to detect. So whether or not a substance is a poison sometimes depends on the intent of the user.
The medical examiner today has a variety of sensitive machines available to help determine if poison is present in a sample of tissue or fluid. But every body cannot be routinely examined for the presence of poison. Many poisons leave no outward sign, and the signs left by others mimic the appearance of natural causes of death. Still, poisoners often give themselves away through overconfidence, greed, and a tendency to murder again at the slightest provocation. It is true that poisoners often suffer from a fatal hubris. Unfortunately the poisoner’s hubris is usually fatal to several innocent people before he himself is caught.
The subject of poisons and poisoning has been rife with misconceptions and myths. For instance, it was once believed that boiling a food suspected of containing poison and then dropping a silver coin into it would remove the poison. Alternatively, it was thought that the coin would turn black, thus indicating the presence of poison. This is incorrect. Silver turns black in the presence of sulfur, which is not usually used as a poison. There was also a common belief that the corpse of a person who had been poisoned would turn black and blue, appear spotted, or smell bad—a description of the normal processes of decomposition.
Until about two hundred years ago it was widely believed that tomatoes were poisonous, possibly because they are a member of the deadly nightshade family of plants. There is an unverified story that a British agent once tried to poison General Washington by feeding him cooked tomatoes.
Incidentally, the received wisdom that poison is a woman’s weapon is not borne out in practice. Poison is an equal-opportunity murder weapon.
Arsenic, a metallic element, has been known in its various forms for more than two thousand years. It was used in ancient China as an insecticide and in Rome as a medicine. It is ubiquitous in soil, in water, and in the human body, usually in amounts that are small enough to be considered safe (though there is growing opinion among health experts that no amount of arsenic is truly safe—it is now believed to be a potent carcinogen).
From Roman times on, the custom of eating small quantities of arsenic persisted in various parts of the world. People ate it to improve their complexions as well as their overall health, stamina, and sex drive. They fed it to their horses to increase their endurance and add luster to their coats. Whether arsenic actually conferred any of these benefits is doubtful. A possible exception is arsenic’s effects on the complexion. Apparently arsenic ruptures the blood vessels that lie just beneath the skin, imparting a “healthy” ruddiness to the cheeks.
During the earliest years of the Joseon dynasty of Korea (1392–1910), compounds of arsenic and sulfur were used in making sayak, a deadly beverage that was used for the official executions of high-ranking court officials and members of the royal family.
Fowler’s Solution, an arsenic compound of potassium arsenite and lavender water, was devised by Dr. Thomas Fowler in the 1780s as a remedy for whatever ailed you. The usual dose was twelve drops, three times a week, in a glass of wine or water. It was recommended internally for the treatment of ague, periodic neuralgia, rheumatism, and epilepsy, and as a possible cure for dropsy, syphilis, palsy, nervous tremor, chorea, scrofula, tetanus, and snakebite. Externally, as a paste, it was used for malignant ulceration, lupus, noli me-tangere (a cancerous ulceration of soft tissue), fungal infection, and gangrene. It was not recommended for use against fleas, lice, and other bodily pests because the necessary concentrations might prove fatal to the user.
Easy access to arsenic was certainly one of the reasons for its popularity as a “remover of obstacles.” Another was that arsenic is odorless and tasteless and thus undetectable in food. As a bonus to poisoners, the symptoms of arsenic poisoning mimic a range of common illnesses, from severe stomach upset to cholera. And even if its presence were discovered, there might be several innocent explanations for its ingestion. Arsenic is widespread, after all—it was once even to be found in wallpaper, green wallpaper especially.
Sometime around 1785 Samuel Hahnemann, whose medical observations led him to develop the practice of homeopathy, discovered that when dissolved in a liquid containing hydrochloric acid and hydrogen sulfide, arsenic would form a yellow precipitate. This became the first practical method for detecting the presence of arsenic in foods or in the bodies of the recently deceased.
In 1787 Johann Daniel Metzger, a professor of medicine at the University of Berlin, discovered an easier way to detect white arsenic in solution. When the suspect material was heated with charcoal, arsenious oxide (white arsenic) would vaporize, if present, and form a shiny black deposit on a porcelain plate.
But supposing the arsenic was in some other form? The Swedish chemist Karl Wilhelm Scheele (1742–1786), an early discoverer of oxygen, chlorine, citric acid, oxalic acid, gallic acid, uric acid, and a host of important chemical processes, also devised a method of producing arsine gas from any arsenic compound that happened to be present in, say, someone’s dinner or internal organs. If sulfuric acid or hydrochloric acid is mixed with any liquid containing arsenic, and zinc is then added, the reaction between the zinc and the acid will produce hydrogen, which bubbles through the liquid. The hydrogen will “steal” the arsenic from any arsenic compound present and form arsine gas. If the gas is ignited as a cold porcelain bowl is held to the flame, the shiny black deposit that Metzger discovered will form.
In 1806 Valentine Rose devised a technique that used nitric acid, potassium carbonate, and lime to detect the presence of arsenic in human organs.
In 1832 James Marsh, a chemist at the Royal British Arsenal in Woolwich, was asked by the justice of the peace in nearby Plumstead to take time out from his research into a recoil brake for naval guns to see if he could somehow detect arsenic in a coffee pot. John Bodle, a local farmer, was suspected of poisoning George Bodle, his eighty-year-old grandfather. It was known that John had recently bought some arsenic, supposedly to kill rats, from a pharmacist on Powis Street. This was a week before George sickened after breakfast with a sudden onset of vomiting, cramps, diarrhea, and weakness in his limbs. George’s wife, daughter, granddaughter, and the serving girl, all of whom had breakfasted with him, also developed cramps and stomach pain but recovered. George died.
When Sophia Taylor, the serving girl, told Mr. Slace, the justice of the peace, that John had uncharacteristically volunteered to take the kettle to the well that morning, Slace locked up the coffee pot, ordered an autopsy on Bodle, and sent the coffee and Bodle’s intestines to James Marsh, the only qualified chemist around. Marsh, who had no interest in forensic chemistry, had to look up the method for detecting arsenic; he was unaware of the work of either Metzger or Scheele. He used the Hahnemann technique. If arsenic was present, a yellow precipitate of arsenic trisulfide would result. It did. Unfortunately (or fortunately for John Bodle), by the time of the trial the exhibit had deteriorated and was difficult to explain. The British jury found all the messing with acids and gases and yellow precipitates and such to be too much hocus-pocus to be believed, and Bodle was found innocent.
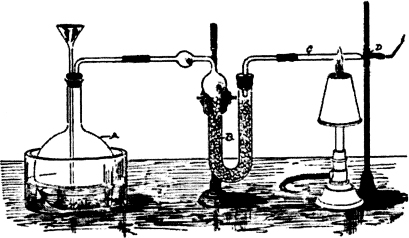
James Marsh found this annoying. He was particularly upset when, sometime later, John Bodle confessed to murdering his grandfather. So Marsh set out to devise a test for arsenic that was both infallible and showy enough to convince a jury. When he read of Scheele’s method, he knew this was the place to start. Marsh set out to create an apparatus that would test for arsenic in solution in one step.
First, he took a glass bottle and stoppered it with a two-hole rubber stopper. Then, through one of the holes he inserted a thistle tube—a hollow glass rod with a bulge at one end—to allow liquids to be poured into it. It was inserted thistle side up with the other end extending to the bottom of the bottle. A U-shaped glass tube was then inserted in the other hole, and the bottle partly filled with zinc and sulfuric acid. This generated hydrogen, which could then be released from the U-shaped tube and ignited. If a mixture containing arsenic was added through the thistle, it would combine with the hydrogen, and the resulting arsine gas would form a dark brown spot on a porcelain bowl. If antimony was present, it would also react with the hydrogen and leave a spot on the bowl that was pitch black and easily differentiated from arsenic.
The Marsh test apparatus, designed to test for the presence of arsenic.
Several refinements have been made to the Marsh test over the years, and some technicians have found these to be useful—the Berzelius-Marsh method and the Gutzeit test, for example. Today a well-equipped lab within commuting distance of a nuclear irradiating facility will use neutron-activation analysis to determine the percentages of arsenic (and eighty or so other elements) in the sample.
The 1840 trial of Marie-Fortunée Lafarge for the murder of her husband Charles Pouch-Lafarge, known throughout France as l’affaire Lafarge, remained a cause célèbre for half a century. As a matter of public fascination it was not eclipsed until the 1894 trial for treason of Captain Alfred Dreyfus.
There were those who thought the beautiful Marie must be innocent; there were those who thought her guilty simply because man is defenseless against the insidious machinations of a supposedly loving wife. There were those who believed she was probably guilty but that Charles deserved it—the brute!
Marie Capelle, the daughter of one of Napoleon’s favorite officers, a colonel of the Old Guard, was twenty-three when she married Charles Lafarge. She realized immediately that she had made a mistake. He was a coarse, vulgar twenty-eight-year-old with a rat-infested old house in the province of Correze—and, if not a mountain of debt, at least a sizable hill. As soon as they returned from their honeymoon Marie locked herself in her room and slipped a note under the door protesting that she loved another and wanted out of the marriage. That didn’t work (and the absent lover was imaginary anyway).
Marie seemed to reconcile herself to her situation, but still the house was filled with rats, and this was too much to bear. She went to the village druggist and bought a large supply of arsenic rat poison. When Charles went to Paris on business for a month, she mailed him a cake to remind him of home. After a few bites he began to throw up. (One of the unexplained facts of the story is that in the letter that accompanied the cake, Marie said she was sending “some little cakes.” But what arrived was one large cake. A small point, perhaps, but worthy of consideration according to the “she didn’t do it” crowd.)
After Charles returned home from his trip, he quickly sickened again and died. Suspicion soon fell on his young wife when servants said they had seen her stirring a white powder into the dinner. She was arrested for murder and scheduled for trial. At the request of the prosecutors, the contents of Charles’s stomach and samples of his uneaten food were examined for arsenic by local doctors. They found arsenic in the food but, curiously, none in his stomach.
At the trial Mme. Lafarge’s lawyers questioned the doctors’ techniques and results and demanded that the food be tested again. The prosecution in turn requested that Lafarge’s body be exhumed and tested for the presence of arsenic in the organs. The body was exhumed, the doctors did the tests, and again no arsenic was found. The food was tested again and, again, more arsenic was found. The French newspapers eagerly followed each new twist in the case and took sides, as did the French public.
With the agreement of both sides, the judge asked Dr. Mathieu Orfila, recognized as the world’s greatest expert in toxicology, to come from Paris and test Charles Lafarge’s body for traces of arsenic. Using the latest version of the Marsh test, Orfila did a thorough job, examining not only the body but the soil around the body, to ensure that any arsenic he might find had not leached in from the surroundings.
Orfila found slight traces of arsenic in the soil and a great deal more in the body tissues. Mme. Lafarge was found guilty and sentenced to death by guillotine, a sentence that was subsequently commuted to life in prison. She was pardoned after twelve years but lived less than a year after being released. Several people wrote books explaining that she was innocent all along, naming a servant as the true killer. But no one disputed Dr. Orfila’s demonstration that there was, indeed, arsenic in Charles Lafarge’s body.
Napoleon Bonaparte, emperor of France and conqueror of most of Europe, died in exile on the island of St. Helena on May 5, 1821. He was fifty-two years old. The official cause of death was stomach cancer, but for almost two centuries a rumor has persisted that he was poisoned with gradual doses of arsenic. The only question was, who had done it—the British, or agents of King Louis XVIII of France, intent on making sure he would never return?
A 1961 analysis of Napoleon’s hair found arsenic in it and seemed to confirm the rumor. But in 2008 scientists conducted a study of samples of Napoleon’s hair taken from different periods of his life, along with samples from Empress Josephine and from Napoleon’s son. The hair was tested at the University of Pavia by a process known as neutron activation, a method that provides extremely accurate results without destroying the sample. The examiners found that the arsenic levels in Napoleon’s hair and in the other samples stayed constant, but that the constant level was one hundred times higher than that found in modern samples. In other words, Napoleon was not deliberately poisoned with arsenic, but there was a lot of it in the environment two hundred years ago, and everyone ingested their share.
In 1860 an American explorer of the Arctic regions, Charles Francis Hall (1821–1871), went to Baffin Island in northern Canada in search of some sign of the Arctic expedition of Sir John Franklin, missing since 1845. He returned two years later with artifacts from the Martin Frobisher expedition of 1578, having discovered Frobisher’s stone house. His book, Arctic Researches and Life Among the Esquimaux, recounts the experience. On his second expedition (1864–1869), Hall did discover traces of the Franklin party.
In 1871 Hall commanded a government-sponsored expedition to the North Pole. His ship, the Polaris, reached 82°11′ north latitude, a new record, before calling it quits.
While his ship was encased in ice for the winter, Hall collapsed as he drank a cup of coffee. For about a week he seemed to recover, but then he relapsed and died. During his delirium he accused several people, including Emil Bessels, the ship’s doctor, of poisoning him.
In 1968 Chauncey C. Loomis, a Dartmouth College professor and biographer of Hall, went to Hall’s grave in Greenland and had his body exhumed. Samples of Hall’s hair and a single fingernail—all that could be collected from the frozen body—were sent to the Toronto Center of Forensic Sciences for neutron-activation analysis. The center’s report found “an intake of considerable amounts of arsenic by C. F. Hall in the last two weeks of his life.” The base of the fingernail, grown out during those last weeks, contained 76.7 parts per million of arsenic—three times the amount found in the tip and certainly enough to kill him.
Dr. Emil Bessels, who had a long, contentious relationship with Hall, is the only logical suspect. There the story must end.
Prussian Blue, one of the first synthetic pigments, was probably created by the paint maker Johann Jacob Diesbach sometime around 1706. By 1709 his “Preussisch blau” was sold all over Europe. The first artist known to use it was Pieter van der Werff in his 1709 painting Entombment of Christ, now hanging in the picture gallery of Sanssouci Palace, Potsdam.
In about 1783 Karl Wilhelm Scheele added another discovery to his impressive list. Following the lead of the French chemist Pierre Macquer, Scheele was able to derive pure hydrogen cyanide (HCN) from Prussian Blue pigment. It came off as a gas but could be dissolved to some extent in water. This liquid, known as prussic acid, was lethally poisonous. The word “cyanide,” from the Greek word for blue, hints at HCN’s derivation from Prussian Blue.
Within a dark and dismal cell
In anguish I do lie,
Methinks I hear the solemn knell,
Say Tawell you must die . . .
—English broadside ballad, 1845
On New Year’s Day 1845 Sarah Hart of Salt Hill, Buckinghamshire (now a part of Berkshire), was visited at her home by a man “dressed like a Quaker.” He was observed coming and going from the house twice. That evening several people heard Hart screaming. They ran to her house where they found her in bed and suffering from terrible cramps and pain. One of them gave her water, but foam came to her lips when she tried to drink it. She later died.
The vicar of Upton-cum-Chumley, who had responded to her cries, got a description of the absent visitor from a neighbor, then rushed off to the Slough train station to try to intercept the man.
Unfortunately the train had just left. But the station had one of those new telegraph apparatuses, and the stationmaster sent a message ahead to Paddington Station:
A murder has just been committed at Salt Hill and the suspected murderer was seen to take a first-class ticket to London by the train that left Slough at 7.42 pm. He is in the garb of a Kwaker [the telegraph instrument had no “Q”] with a brown great coat on which reaches his feet. He is in the last compartment of the second first-class carriage.
Shortly a reply arrived:
The up-train has arrived, and a person answering, in every respect, the description given by telegraph came out of the compartment mentioned. I pointed the man out to Sergeant Williams. The man got into a New Road omnibus, and Sergeant Williams into the same.
Sergeant Williams followed his suspect to his lodgings in Scott Yard and arrested him the next morning. His name was John Tawell.
The autopsy surgeon, H. Montague Champneys, smelled “the odor of prussic acid” when he opened the body. He bottled the contents of the stomach and sent them along with a bottle of beer found at the scene to Mr. Cooper, an analytical chemist and lecturer on medical jurisprudence. Cooper added potassium ferrosulfate to the stomach contents and observed that the mixture turned a deep Prussian Blue, indicating that it contained prussic acid. So did the beer.
When the police found that Sarah Hart had been Tawell’s lover for years, that Tawell had recently married a Quaker widow, and further that Tawell had purchased two drams of Scheele’s prussic acid from a chemist in London on the day of the murder “to use for his varicose veins,” they had all they needed. Tawell was brought to trial.
Tawell’s defense counsel, Fitzroy Kelly, attempted an ingenious explanation for the presence of the poison at the scene: apple pips contain prussic acid, and there had been a barrel of apples in Sarah’s room. This set off a scramble by medical experts on both sides to see how much prussic acid could be distilled from apple pips. The prosecution found that the amount in fifteen apples would be harmless, while the defense found that the same amount might be toxic.
The jury found Tawell guilty. He was hanged before a crowd of ten thousand on March 28, 1845. Fitzroy Kelly, who went on to have a distinguished career, was thenceforth and forever known to his intimates as Apple Pip Kelly.
John Sadleir (1813–1856) was born into a Tipperary banking family and seemed to have a knack for the business. Starting out in small Irish banks, he worked his way up and eventually moved to London where he was appointed chairman of the London and County Joint Stock Banking Company in 1848. Sadleir then expanded his interests beyond banking. He financed railways in Italy and France, and started a newspaper, the Weekly Telegraph, in Dublin. He bought the Earl of Glengall’s estate at Cahir. He owned a stable at Watford from which he hunted with the Gunnersbury hounds. He kept his shareholders happy with a 6 percent dividend when most others were paying 4. He was elected to Parliament. He was appointed a junior lord of the treasury.
In 1853 it all began to unravel. After accusations of fraud, Sadleir was forced to resign his seat in Parliament. He was found to be borrowing money from his own bank, whose declared reserves of £17,000 were found to be nonexistent. He had embezzled some £200,000 from the Tipperary Bank and issued £150,000 worth of phony stock in the Swedish Railway. He could not steal fast enough to keep up.
In February 1856 his cousin received a letter from him in which he confessed to “numberless crimes of a diabolical character,” which had caused “ruin and misery and disgrace to thousands—ay, tens of thousands.” On Sunday morning, February 16, his body was found on Hampstead Heath. By his side was a silver cream pitcher containing a solution of prussic acid. Dickens used Sadleir as a model for the despicable Mr. Merdle in Little Dorrit. James O’Shea, who wrote the 1999 book Prince of Swindlers, John Sadleir M.P., said of him, “He was well-liked and well-trusted and robbed everybody that he could lay his hands on.”
The Nux Vomica, or strychnine tree, is an evergreen that grows wild in Southeast Asia. The seeds of its round, green-to-orange fruit are the main sources of the alkaloids strychnine and brucine. Strychnine is intensely bitter and highly poisonous. It was introduced to Europe in the sixteenth century as a rodenticide and is still used on occasion to kill small animal pests. Thirty-two milligrams is a lethal dose for humans, but people have been known to die from as little as five milligrams. It is one of the adulterants used to cut street drugs such as amphetamine and cocaine. Brucine, related to strychnine, is not quite as poisonous and is used in minute quantities in several heart medications.
Dr. William Palmer (1824–1856) had a lust for life and a fondness for horse races, gambling, and women. He also had a tendency to steal when he had the chance and to kill when threatened. When still an intern at Stafford Infirmary he was accused of trying to poison a friend. The charges went nowhere, but the infirmary tightened its procedures for access to the dispensary. Palmer moved back to his hometown of Rugeley in Staffordshire and set himself up as a doctor. In 1847 he married Ann Brookes, and they had their first child the next year. Ann had four other children over the next few years, but they all died in infancy.
People who were close to Palmer had a habit of dying. His mother-in-law passed on, as did at least two people to whom he owed money. In 1854, after he took out a £13,000 insurance policy on his wife Ann, she promptly died. The death certificate read “cholera.” Nine months later Eliza Tharme, his housemaid, bore him a son, Alfred. In 1855 Palmer’s brother Walter suddenly died, but the insurance company refused to pay Palmer on that one. Palmer, desperate for money, took out an insurance policy on a friend, George Bates, who then conveniently died. A boot boy claimed to have seen Palmer pouring something into Bates’s drink just before Bates took sick.
In November 1855 Palmer’s friend John Cook won a large sum of money betting on the Shrewsbury handicap stakes. Palmer invited Cook to come with him to Rugeley, and, on November 17, Cook took a room at the Talbot Arms. On that same day Alfred, Palmer’s five-month-old son, died. The next day Cook became very ill, and Palmer went to London to collect his winnings for him. At 1 A.M. on November 18 John Cook died.
Cook’s stepfather came to Rugeley and demanded an autopsy. Palmer, as a doctor and a friend of the deceased, attended. During the proceedings Palmer was spotted trying to leave the autopsy room with the jar containing Cook’s stomach. His bizarre behavior, combined with public sentiment in Rugeley, led to his arrest on suspicion of murder. The bodies of his wife and brother were exhumed and examined for signs of poison, but nothing definite was found.
A special act of Parliament was passed to allow the trial to be moved to London, as public outrage in Rugeley would preclude Palmer’s getting a fair trial there. He was tried only for the murder of Cook. The science at that time was not up to the task of finding strychnine in Cook’s body, but witnesses described the manner of his death, including severe vomiting, spasms, and powerful convulsions. Medical experts testified that only strychnine poisoning could cause these symptoms. The jury took a little more than an hour and a half to find Palmer guilty.
Palmer was hanged at Stafford Prison on June 14, 1846, with an estimated thirty thousand people in attendance. As he stepped onto the trap door, he allegedly turned to the hangman and asked, “Are you sure it’s safe?” There is a persistent story that after Palmer’s death the town of Rugeley petitioned to change its name, fearing that it would be forever associated with “Palmer the poisoner.” The prime minister agreed, with the proviso that they rename the town after him. His name was Palmerston. The town chose to remain Rugeley.
Castor beans are processed around the world to make castor oil. The mash that is left after the beans are ground contains ricin, the third deadliest poison after plutonium and botulism toxin. The compound was discovered in 1800 by Hermann Stillmark as he was developing methods of extracting the oil from the beans. It is believed that in the 1980s ricin was used as an agent of warfare by Saddam Hussein in Iraq’s war with Iran.
A very small amount of ricin—three micrograms—can kill if inhaled, ingested, or injected into the body. Death occurs within thirty-six to forty-eight hours. If the patient manages to hang on for as long as five days, he will probably recover. There is no known antidote for ricin, and a reliable test to detect its presence has only recently been developed by scientists at the Albert Einstein College of Medicine at Yeshiva University. A vaccine is now being licensed by the United States Army.
Georgi Markov, a noted novelist and playwright, defected from Bulgaria in 1969 after writing The Man Who Was Me, a play that the Communist government of Bulgaria disapproved of. Markov moved to London and began writing scripts, mostly based on his memoirs, for the BBC and Radio Free Europe. Because of his former access to the upper levels of Bulgarian society, he knew and wrote about many things that the Bulgarian president, Todor Zhivkov, would just as soon not have had aired.
Over the next few years the Bulgarian government made several unsuccessful attempts to assassinate Markov—one in Munich and one on the island of Sardinia. The third attempt, in London, was successful. On September 7, 1978, as Markov waited for a bus on Waterloo Bridge, he felt a stinging sensation in his right thigh as a heavily built man carrying an umbrella bumped into him. The man mumbled “Sorry,” and got into a passing taxi. By that evening Markov had developed a high fever and was admitted to St. James Hospital in Balham. Four days later he was dead.
Forensic pathologists did a thorough autopsy of Markov’s body at the request of Scotland Yard. They found a tiny pellet that was 90 percent platinum and 10 percent iridium imbedded in his thigh. The pellet measured 1.52 millimeters across—almost exactly the size of a pinhead. Two holes, each .35 millimeters wide, were drilled through the pellet, leaving an X-shaped cavity in the center. Experts from Britain’s Defence Science and Technology Laboratory at Porton Downs found that the pellet had been filled with ricin and the holes stopped with a “sugary substance” that was designed to melt at body temperature. Once inside the human body, the substance melted and the ricin was released. At that point nothing could save the victim.
If you happen to have a source of neutrons around the house, say a small nuclear reactor, you have access to one of the best quantitative and qualitative forensic analysis systems yet devised. Neutron-activation analysis involves sticking a sample of just about anything in a small chamber, bombarding it with neutrons, then measuring the gamma rays emitted by the irradiated sample. This method will detect the presence and relative amount of any of about seventy different elements. Best of all, if you’re a forensic scientist, the process is nondestructive—the sample is still there and available for other tests or for waving before a jury.
In June 1965 radio personality René Castellani, the “Dizzy Dialer” of station CKNW, Vancouver, sat in a car perched atop the world’s largest freestanding electrical sign—a ten-story-high pole on the Bowell Maclean car lot—vowing not to come down until the very last car on that lot had been sold. As he sat there his wife, Esther, was hospitalized with a recurrence of the serious gastric upset that had bothered her for several months. After two weeks, René came down. One month later, in early July, Esther Castellani suffered a severe relapse and returned to the hospital. René hurried to her side from a promotion in a toy store, still wearing his gorilla suit (the zipper had broken). He was just in time to be with her when she died.
Several months after Esther was buried—the cause of death was listed as “a viral infection and heart attack”—a woman approached the authorities and claimed that Esther had been poisoned. She would tell all, she said, if she were given immunity. She was insistent enough and her story provocative enough that Esther’s body was exhumed and tested for arsenic. And it was found—a lot of it. Possible sources of contamination—the ground she was buried in and the embalming fluid—were eliminated, and it became clear that Esther had been deliberately poisoned. The informant claimed that she and René were lovers, and that René had poisoned Esther for the insurance money.
René Castellani was brought to trial for the murder of his wife. That she had been poisoned with arsenic was not in dispute. But the defense suggested that the lover had done it to eliminate her rival, and the prosecution would have to prove otherwise.
Samples of the victim’s hair were given to forensic chemist Norm Erickson who cut them into precise lengths corresponding to the amount that hair grows in one week, two weeks, and a month. When the lengths were subjected to neutron-activation analysis, a very interesting fact appeared: all the hair showed a concentration of arsenic. Esther had been fed small doses of it regularly. But during one two-week period the hair was nearly arsenic-free. During this period she had not ingested any of the poison because her husband René had been in a car atop the world’s largest freestanding electrical sign.
René Castellani was convicted of the murder of his wife and sentenced to twenty-five years in prison.
Newer and more subtle poisons are now available. Some of the drugs used as anesthetics will kill quickly with no outward sign, and will disappear from the body completely in three days or less. If the victim suffered from a serious ailment, suspicion would not be aroused. The greater worry, if you’re inclined to worry about such things, is the lack of suspicion among medical professionals. After all, most people aren’t poisoned. Or so we like to think.