[1]
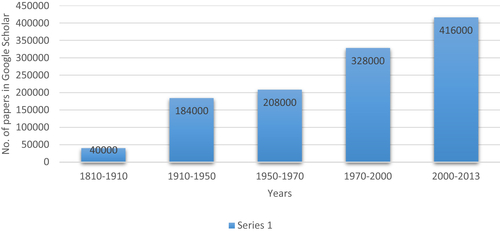
Google Scholar search data for key words “Medicine”, “Ayurveda” and “Traditional Chinese Medicine” [accessed on 11th May 2015] from Pune India.
[2] Hajarizadeh B, Grebely J, Dore G.J. Epidemiology and natural history of HCV infection [Internet]. Nat Rev Gastroenterol Hepatol. 2013;10(9):553–562.
[3] Liu C.J, Kao J.H. Global perspective on the natural history of chronic hepatitis B: role of hepatitis B virus genotypes A to J. Semin Liver Dis. 2013;33(2):97–102.
[4] Mulrow C.D. Rationale for systematic reviews. BMJ. 1994;309(6954):597–599.
[5] Nuzzo R. Scientific method: statistical errors. Nature. 2014;506(7487):150–152.
[6] Cochrane A.L. Effectiveness and efficiency: random reflections on health. London: Nuffield Provincial Hospitals Trust; 1972.
[7]
http://www.cochrane.org.
[8] L’Abbe K.A, Detsky A.S, O’Rourke K. Meta-analysis in clinical research. Ann Intern Med. 1987;107(2):224–233.
[9] Evidence-Based Medicine Working Group. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA. 1992;268(17):2420–2425.
[10] Sackett D.L, Rosenberg W.M.C, Gray J.A.M, Haynes R.B, Richardson W.S. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71–72.
[11] Balshem H, Helfand M, Schünemann H.J, Oxman A.D, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406.
[12] Feinstein A.R, Horwitz R.I. Problems in the “evidence” of “evidence-based medicine.” Am J Med. 1997;103(6):529–535.
[13]
www.handbook.cochrane.org.
[14] Smith G.C.S, Pell J.P. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ. 2003;327(7429):1459–1461.
[15] Raha S. Foundational principles of classical Ayurveda research. J Ayurveda Integr Med. 2013;4(4):198–205.
[16]
http://www.compmed.umm.edu/cochrane_about.asp.
[17] Narahari S.R, Aggithaya M.G, Suraj K.R. A protocol for systematic reviews of Ayurveda treatments. Int J Ayurveda Res. 2010;1(4):254–267.
[18] Shang A, Huwiler-Müntener K, Nartey L, Jüni P, Dörig S, Sterne J.A, et al. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo-controlled trials of homoeopathy and allopathy. Lancet. 2005;366(9487):726–732.
[19] When to believe the unbelievable. Nature. 1988;333(6176):787.
[20] Maddox J, Randi J, Stewart W.W. “High-dilution” experiments a delusion. Nature. 1988;334(6180):287–291.
[21] Calabrese E.J. Hormesis and homeopathy: introduction. Hum Exp Toxicol. 2010;29(7):527–529.
[22] Calabrese E.J, Iavicoli I, Calabrese V. Hormesis: Its impact on medicine and health. Hum Exp Toxicol. 2013;32(2):120–152.
[23] Bell I.R. All evidence is equal, but some evidence is more equal than others: can logic prevail over emotion in the homeopathy debate? J Altern Complement Med. 2005;11(5):763–769.
[24] Shaw D.M. Homeopathy is faith healing without religion. Focus Altern Complement Ther. 2014;19(1):27–29.
[25] Beecher H.K. The powerful placebo. J Am Med Assoc. 1955;159(17):1602–1606.
[26] Hróbjartsson A, Gøtzsche P.C. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2010(1) CD003974. 10.1002/14651858.CD003974.pub3.
[27] Thangapazham R.L, Gaddipati J.P, Rajeshkumar N.V, Sharma A, Singh A.K, Ives J.A, et al. Homeopathic medicines do not alter growth and gene expression in prostate and breast cancer cells in vitro. Integr Cancer Ther. 2006;5(4):356–361.
[28] Clausen J, Albrecht H, Mathie R.T. Veterinary clinical research database for homeopathy: placebo-controlled trials. Complement Ther Med. 2013;21(2):115–120.
[29] Ernst E. Homeopathy: what does the “best” evidence tell us? Med J Aust. 2010;192(8):458–460.
[30] Evans D. Placebo: the belief effect. London: HarperCollins; 2003.
[31] Singh S.E.E. Trick or treatment? Alternative medicine on trial. UK: Bentham Press; 2008.
[32] Pert C.B. Molecules of emotion: why you feel the way you feel. Scribner Book Company; 1997.
[33] Ciaramella A, Paroli M, Poli P. An emerging dimension in psychosomatic research: the nocebo phenomenon in the management of chronic pain. ISRN Neurosci. 2013:574526. doi: 10.1155/2013/574526.eCollection 2013.
[34] McCall M.C, Ward A, Roberts N.W, Heneghan C. Overview of systematic reviews: yoga as a therapeutic intervention for adults with acute and chronic health conditions. Evid Based Complement Altern Med. 2013;2013:945895.
[35] Froeliger B.E, Garland E.L, Modlin L.A, McClernon F.J. Neurocognitive correlates of the effects of yoga meditation practice on emotion and cognition: a pilot study. Front Integr Neurosci. 2012;6:48. doi: 10.3389/fnint.2012.00048.
[36] Rastogi S. Effectiveness, safety, and standard of service delivery: a patient-based survey at a pancha karma therapy unit in a secondary care Ayurvedic hospital. J Ayurveda Integr Med. 2011;2(4):197–204.
[37] Shetty P. Medical tourism booms in India, but at what cost? Lancet. 2010;376(9742):671–672.
[38] Patwardhan B. Ayurveda: finding place in own house. J Ayurveda Integr Med. 2012;3(3):109–110.
[39] Nisula T. In the presence of biomedicine: Ayurveda, medical integration and health seeking in Mysore, South India. Anthropol Med. 2006;13(3):207–224.
[40] Chaudhary A, Singh N, Kumar N. Pharmacovigilance: boon for the safety and efficacy of Ayurvedic formulations. J Ayurveda Integr Med. 2010;1(4):251–256.
[41] Patwardhan K. Medical education in India: time to encourage cross-talk between different streams. J Ayurveda Integr Med. 2013;4(1):52–55.
[42] Patwardhan B, Joglekar V, Pathak N, Vaidya A. Vaidya-scientists: catalysing Ayurveda renaissance. Curr Sci. 2011;100(4):476–483.
[43] Milden S.P, Stokols D. Physicians’ attitudes and practices regarding complementary and alternative medicine. Behav Med. 2004;30(2):73–82.
[44] Anantha N.D. Approaches to pre-formulation R & D for phytopharmaceuticals emanating from herb based traditional Ayurvedic processes. J Ayurveda Integr Med. 2013;4(1):4–8.
[45] Bhutani K.K. Natural products: bench to bedside, an Indian perspective. Planta Med. 2012;78(5) OP20.
[46] Valiathan M.S. Towards Ayurvedic biology, decadal vision document. Bangalore: Indian Academy of Sciences; 2006.
[47] Chandra S. Status of indian medicine and folk healing. Part I. New Delhi: Department of AYUSH, Government of India; 2012.
[48] Chandra S. Status of indian medicine and folk healing. Part II. New Delhi: Department of AYUSH, Government of India; 2013.
[49] Singh R.H. Perspectives in innovation in the AYUSH sector. J Ayurveda Integr Med. 2011;2(2):52–54.
[50] Beryl P, Vach W. Methodological considerations in evidence-based indian systems of medicine – a systematic review of controlled trials of Ayurveda and Siddha. J Altern Complement Med. 2014;20(5):A137.
[51] Sanson-Fisher R.W, Bonevski B, Green L.W, D’Este C. Limitations of the randomized controlled trial in evaluating population-based health interventions. Am J Prev Med. 2007;33(2):155–161.
[52] Van der Greef J, Hankemeier T, McBurney R.N. Metabolomics-based systems biology and personalized medicine: moving towards n = 1 clinical trials? Pharmacogenomics. 2006;7(7):1087–1094.
[53] Patwardhan B, Vaidya A.B.D. Ayurveda: scientific research and publications. Curr Sci. 2009;97(8):1117–1121.
[54] Patwardhan B. European Union ban on ayurvedic medicines. J Ayurveda Integr Med. 2011;2(2):47–48.
[55] Saper R.B, Phillips R.S, Sehgal A, Khouri N, Davis R.B, Paquin J, et al. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the Internet. JAMA. 2008;300(8):915–923.
[56] Thatte U.M, Rege N.N, Phatak S.D, Dahanukar S.A. The flip side of Ayurveda. J Postgr Med. 1993;39(4):179–182.
[57] Valiathan M.S. Putting house in order. Curr Sci. 2006;90(1):5–46.
[58] Lakhotia S.C. Neurodegeneration disorders need holistic care and treatment–can Ayurveda meet the challenge? Ann Neurosci. 2013;20(1):1–42.
[59] Patwardhan B. AyuGenomics – integration for customized medicine. Indian J Nat Prod. 2003;19:16–23.
[60] Furst D.E, Venkatraman M.M, Krishna Swamy B.G, McGann M, Booth-Laforce C, Ram Manohar P, et al. Well controlled, double-blind, placebo-controlled trials of classical Ayurvedic treatment are possible in rheumatoid arthritis. Ann Rheum Dis. 2011;70(2):392–393.
[61] Chopra A, Saluja M, Tillu G, Sarmukkaddam S, Venugopalan A, Narsimulu G, et al. Ayurvedic medicine offers a good alternative to glucosamine and celecoxib in the treatment of symptomatic knee osteoarthritis: a randomized, double-blind, controlled equivalence drug trial. Rheumatology (Oxford). 2013;52(8):1408–1417.
[62] Dieppe Paul, Marsden D. Managing arthritis: the need to think about whole systems. Rheumatology (Oxford). 2013;52(8):1345–1346.
[63] Manohar P.R, Eranezhath S.S, Mahapatra A, Sujithra M.R. DHARA: digital helpline for Ayurveda research articles. J Ayurveda Integr Med. 2012;3(2):97–101.
[64] Narahari S.R, Ryan T.J, Aggithaya M.G, Bose K.S, Prasanna K.S. Evidence-based approaches for the Ayurvedic traditional herbal formulations: toward an Ayurvedic CONSORT model. J Altern Complement Med. 2008;14(6):769–776.
[65] Tillu G. Workshop on a CONSORT statement for Ayurveda. J Ayurveda Integr Med. 2010;1(2):158–159.
[66] Witt C.M, Michalsen A, Roll S, Morandi A, Gupta S, Rosenberg M, et al. Comparative effectiveness of a complex Ayurvedic treatment and conventional standard care in osteoarthritis of the knee–study protocol for a randomized controlled trial. Trials. 2013;14:149. doi: 10.1186/1745-6215-14-149.
[67] Narahari S.R, Ryan T.J, Bose K.S, Prasanna K.S, Aggithaya G.M. Integrating modern dermatology and Ayurveda in the treatment of vitiligo and lymphedema in India. Int J Dermatol. 2011;50(3):310–334.
[68] Narahari S.R. Collaboration culture in medicine. Indian J Dermatol. 2013;58(2):124–126.
[69]
http://spokensanskrit.de/.
[70] Brar J.S, Chengappa K.N. Does one shoe fit all? J Ayurveda Integr Med. 2011;2(2):91–93.
[71] Smith R, Rennie D. Evidence-based medicine—an oral history. JAMA. 2014;311(4):365–367.
[72] Greenhalgh T, Howick J, Maskrey N. Evidence based medicine: a movement in crisis? BMJ. 2014;348:g3725.
[73] Mongomery K. How doctors think: clinical judgment and the practice of medicine. Oxford University Press; 2006.
[74]
McNutt R., Hadler N.M. How clinical guidelines fail both doctors and patients. Scientific American Blog Network. Available from: http://blogs.scientificamerican.com/guest-blog/2013/11/22/how-clinical-guidelines-can-fail-both-doctors-and-patients/
[75] Gigerenzer G, Gaissmaier W, Kurz-Milcke E, Schwartz L.M, Woloshin S. Helping doctors and patients make sense of health statistics. Psychol Sci Public Interest. 2008;8(2):53–96.
[76] Phil A. Absence of evidence is not evidence of absence: we need to report uncertain results and do it clearly. BMJ. 2004;328(7438):476–477.
[77] Phillips C.V, Goodman K.J. The missed lessons of Sir Austin Bradford Hill. Epidemiol Perspect Innov. 2004;1(1):3.
[78]
Goodman Amy GJ. A daily independent global news hour. Democracy now; Available from: http://www.democracynow.org/2010/12/17/pharmaceutical_drug_industry_tops_defense_industry.
[79] John B. Corporate crime in the pharmaceutical industry. Routledge & Kegan Paul; 2013 Available from: http://www.ivantic.net/Ostale_knjiige/Zdravlje/Braithwaite-John-Corporate-Crime-in-the-Pharmaceutical-Industry.pdf.
[80] Bero L. Industry sponsorship and research outcome: a Cochrane review. JAMA Intern Med. 2013;173(7):580–581.
[81] Lundh A, Sismondo S, Lexchin J, Busuioc O.A, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2012 Dec 12;12 MR000033. 10.1002/14651858.MR000033.pub2.
[82] Lehrer J. The truth wears off. New Yorker. 2010:1–10.
[83] Harremoes P, Gee D, Malcom M, Andy S, Jane K, Brian W, et al. The precautionary principle in the 20th century: late lessons from early warnings. Routledge; 2013.
[84] Marino M.J. The use and misuse of statistical methodologies in pharmacology research. Biochem Pharmacol. 2014;87(1):78–92.
[85] Egger M, Bartlett C, Juni P. Are randomised controlled trials in the BMJ different? BMJ. 2001;323(7323):1253–1254.
[86] Smith R. Medical journals are an extension of the marketing arm of pharmaceutical companies. PLoS Med. 2005;2(5):e138.
[87] Smith R, Gøtzsche P.C, Groves T. Should journals stop publishing research funded by the drug industry? BMJ. 2014;348:g171. doi: 10.1136/bmj.g171.