[The concept of geologic time] makes you schizophrenic. The two time scales—the one human and emotional, the other geologic—are so disparate. But a sense of geologic time is the important thing to get across to the non-geologist: the slow rate of geologic processes—centimeters per year—with huge effects if continued for enough years. A million years is a small number on the geologic time scale, while human experience is truly fleeting—all human experience, from its beginning, not just one lifetime. Only occasionally do the two time scales coincide.
—ELDRIDGE MOORES, AS QUOTED BY JOHN MCPHEE IN ASSEMBLING CALIFORNIA
It is perhaps a little indelicate to ask of our Mother Earth her age, but Science acknowledges no shame and from time to time has boldly attempted to wrest from her a secret which is proverbially well guarded.
—ARTHUR HOLMES, THE AGE OF THE EARTH
IMPASSE
James Hutton wrote that the earth had “no vestige of a beginning,” and after the first volume of Charles Lyell’s Principles of Geology was published in 1830 almost all geologists agreed that the earth was immensely old. But just how old? How could we put a number on the age of the earth?
The problem was a hard one, but it failed to daunt scientists, who tried all sorts of ingenious solutions. The most common method was to add up the maximum thicknesses of all the sedimentary rocks on Earth, calculate how long it would have taken to deposit those layers, and use that as a minimum age. For example, they compiled the data for the maximum thickness of the Cambrian anywhere on earth, the maximum thickness of the Ordovician, and so on, then estimated how long the Cambrian or Ordovician might have lasted given typical rates of sediment accumulation. Most such estimates came to about 100 million years since the Cambrian, which we now know is off by a factor of almost 50. Why? As in all these early methods, there was a faulty assumption built into the calculation. The biggest factor was that they did not account for erosional gaps in the record, known as unconformities, when there is no rock representing a time interval. Later studies have shown that the rock record is full of time gaps and is actually “more gaps than record.” Some geologists at the time suspected that there might a problem with unconformities, but no one could know how big a problem it really was.
Then there was the famous estimate by Irish physicist John Joly. He tried to calculate how long it would take for the oceans to go from freshwater to their current salinity, knowing the rate at which salt enters the oceans from the world’s rivers. He also came to estimates of 80–100 million years, which we now know to be off by more than a factor of 50. What went wrong? Again, the problem was his faulty assumptions: that the oceans have been constantly adding salt since they formed. It turns out that the salt content of the oceans does not change much through time, because much of it gets locked into salt deposits in the earth’s crust and seawater salinity is in equilibrium and stays very stable.
But the most famous and influential estimate was made by the renowned physicist William Thomson (later known by his title, Lord Kelvin). Kelvin made huge discoveries in the field of physics, especially in thermodynamics. The Kelvin temperature scale is named after him, since he pioneered the concept of absolute zero temperature (now known as 0 K—in case you were wondering, the unit of temperature is the kelvin, not the “degree” Kelvin, so there is no degree symbol with this unit). He was also a great inventor and helped create the transatlantic cable system that enabled telegraph and then telephone communications between Europe and North America. Thus, he was a giant among scientists of his time, and few people dared disagree with him.
In 1862 Kelvin attacked the problem of the age of the earth using thermodynamics. He assumed that the earth had started as a molten ball at the same temperature as the sun and that it had cooled off at rates that we can measure from the heat coming up from the earth’s interior. From this method, he estimated the earth was only 20 million years old, much younger than most geologists were willing to accept. It was also a problem for Charles Darwin, who knew that the earth had to be immensely old for his newly proposed concept of evolution to work. Kelvin’s estimate didn’t seem to offer enough time.
Through the rest of the nineteenth century, physicists and geologists were at an impasse. Neither group could comprehend the arguments of the other side or see the flaws in its own estimates. By the late 1800s, geologists began to give in and fudge their estimates a bit from the original values of 80–100 million years to numbers closer to Kelvin’s 20 million years. Physics envy was just as powerful then as it is now! But the problem with Kelvin’s estimate was just like the others: faulty assumptions. Kelvin made his calculation of the cooling of the earth by assuming that the heat was from the original solar system and no other heat sources were involved. We now know this is wrong. The earth does have an additional heat source.
IT’S RADIOACTIVE!
In 1896 Henri Becquerel in France discovered radioactivity, and in 1903 Marie and Pierre Curie showed that radioactive materials like radium produced a lot of heat. At the same time, New Zealander Ernest Rutherford was England’s foremost authority on this new source of energy. In 1904 he was getting ready to address the Royal Institution of Great Britain about this new discovery, when he suddenly realized that the 80-year-old Lord Kelvin was in the audience. The young Rutherford was about to challenge the world’s most famous physicist’s estimate of the age of the earth! As Rutherford wrote later:
I came into the room which was half-dark and presently spotted Lord Kelvin in the audience, and realised that I was in for trouble at the last part of my speech dealing with the age of the Earth, where my views conflicted with his…To my relief, Kelvin fell fast asleep, but as I came to the important point, I saw the old bird sit up, open an eye and cock a baleful glance at me. Then a sudden inspiration came, and I said Lord Kelvin had limited the age of the Earth, provided no new source [of heat] was discovered. That prophetic utterance referred to what we are now considering tonight, radium! Behold! The old boy beamed upon me.
Kelvin’s estimate had been based on the faulty assumption that there were no other sources of heat beyond the earth’s original heat when it cooled from a molten mass and that it would cool in no more than 20 million years. But radioactivity provides that additional heat. In fact, radioactivity provides so much heat that it is now the only source of heat that we measure coming from the earth’s interior. The original heat from the cooling of the earth Kelvin thought he was measuring dissipated billions of years ago, maybe even during the 20 million years since the earth first formed 4.6 billion years ago.
GEOLOGIC TIME
Becquerel, the Curies, and Rutherford had pioneered the physics and chemistry of radioactivity, and their discoveries showed that Kelvin’s assumption of no additional heat was wrong. But they were not geologists interested in determining the earth’s age. It was two other scientists, Bertram Boltwood and Arthur Holmes, who realized that radioactivity provided a solution not only to Kelvin’s dilemma of where the heat came from, but also the answer to the question: How old is the earth?
The method is a simple one, but widely misunderstood. There are just a handful of elements in nature that are radioactive and spontaneously decay from a parent atom (such as uranium-238, uranium-235, and potassium-40) to a corresponding stable daughter atom (lead-207, lead-206, and argon-40, respectively) at rates slow enough to use in geologic dating. This rate of decay is precisely known, so if we can measure the amount of both the parent atom and the daughter atom in a sample, their ratio is a measure of how long that decay has been ticking away.
Of course, there are lots of complications with real rocks, so very special conditions have to be met. The decay is measured from the time the decaying parent atoms are first locked into a crystal, so it primarily works on igneous rocks that cool from a magma, like lava flows, volcanic ash layers, and intruding bodies of magma forming dikes. Geochronologists (specialists in radiometric dating) try to get the freshest crystals possible to ensure there is no leakage and contamination of parent or daughter atoms leaving or entering the crystal that might distort the ratio. There are all sorts of laboratory procedures designed to eliminate expected problems in advance and to correctly calibrate the instrument used (known as a mass spectrometer, since it separates and measures the different isotopes by mass) to give reliable ages. Finally, every radiometric date comes with an error estimate based on how reproducible each result from the machine can be. So, for example, if they cite an age of 100 millions of years ago ± 5 million years (the unit for millions of years ago is Ma), they are saying there is a 95 percent probability that the true age lies somewhere between 95 Ma and 105 Ma.
But none of this was known in 1900 when radioactivity was just beginning to be understood. Physicists had been trying to date rocks by measuring the helium given off by uranium decay, but it was almost impossible to capture all the helium gas. Instead, it was Yale chemist Bertram Boltwood who discovered that uranium decayed to lead through radioactive breakdown. Following a suggestion from Rutherford, Boltwood noticed that the rocks he knew to be older had more lead in them than those that he knew to be younger. Unfortunately, he was using the very primitive understanding of the uranium-lead system that prevailed at the time. He didn’t realize, for example, that there are two different radioactive isotopes of uranium, uranium-238 and uranium-235, each with a different decay rate and a different daughter isotope of lead. Nonetheless, he analyzed the samples he had, and in 1907 he obtained dates on samples ranging from 400 million years to as old as 2.2 billion years. This was the first evidence that the earth was indeed billions of years old, as geologists had long suspected, and that Kelvin’s estimate was way off. Unfortunately, in his later life Boltwood suffered from severe depression, and his research came to a standstill. He committed suicide in 1927.
THE DATING GAME
Boltwood had done the first analyses, and his data indicated the ages of some rocks to be as old as 2.2 billion years, but he never followed up on his breakthrough. Thus, it fell to a young British geologist, Arthur Holmes (figure 8.1), to take the budding young field of geochronology and develop it into a rigorous science. Born to a family of modest means in 1890 in the tiny town of Gateshead (near Durham and the Scottish border), Holmes was originally planning to be a physics major at the Royal College of Science (now University College London). But in his second year, against the advice of his tutors, he took a course in geology and found his true calling.
Figure 8.1
A young Arthur Holmes in 1912, as he was beginning his career in geochronology and finishing graduate school. (Courtesy of Wikimedia Commons)
Holmes proved to be a brilliant undergraduate and was soon doing research. He latched onto the hot problem of radioactivity and realized that Boltwood’s 1907 paper on uranium-lead dating held immense promise. For his undergraduate research project, he had a granitic rock from the Devonian of Norway to analyze. He cut his Christmas holiday short and spent his “holiday break” in London, working alone in the silent lab. As his advisor, physicist Robert Strutt, later recalled,
We are at present largely subsisting on loaned apparatus, some of which belongs to other public bodies, such as the Royal Observatory, the Royal Society, etc., while some has been borrowed from private friends. I need hardly say that it seems rather below the dignity of an institution like the Imperial College that its teachers should have to beg apparatus of their personal friends for the purpose of teaching the students.
Holmes worked away in the cold, silent, lonely lab in January 1910, crushing the rock into a powder of mineral grains in a mortar made of agate, fusing the mineral grains in a platinum crucible with borax, dissolving it in extremely caustic hydrofluoric acid (see chapter 11), then boiling it again and again while measuring the radon emissions (an indirect measure of how much uranium was present). The lead content was measured by fusing the powder in a cake, then boiling it, dissolving it in hydrochloric acid twice, then letting it evaporate all its water. Then he heated it in ammonium sulfide to make the lead precipitate out as lead sulfide (known as the mineral galena). The precipitate was collected on a filter, dried, ignited, treated with nitric acid, boiled, treated with sulfuric acid, and heated again. Eventually, as Holmes wrote, “A tiny white precipitate then remained. This was collected on a very small filter, washed with alcohol, dried, ignited, and weighed with the greatest possible accuracy.” Often there were only a few milligrams of material left.
These complicated chemical operations took incredible patience, extraordinary dexterity, and lots of time and often used up nearly all his original sample. Then to top it off, the results had to be verified, so the entire analysis was repeated two to five times, depending on how much original sample he had left. Once he had to discard all his data because radon had been leaking into the room. In other cases, he had to go begging at the British Museum for more sample, because he had used up his original allotment. Eventually, however, all this hard work paid off, and he got a reliable date of 370 Ma on his Devonian granite from Norway. He had greatly improved Boltwood’s original methods and proven that uranium-lead dating could work and that it was possible to generate dates on rocks. The results were published in 1911, soon after he graduated in 1910.
Holmes was so poor after spending years living on a tiny scholarship of £60 a year that he quit school for a while and took a job prospecting for minerals in Mozambique to earn some real money. He spent 6 months there, found nothing, and suffered so extremely from malaria that his colleagues sent a letter home telling his family he was dead. He eventually recovered and managed to get a boat home, where he became a demonstrator (a low-level instructor) at his alma mater, Imperial College London. There he resumed his studies of the uranium-lead dating technique, figuring out that there were two different isotopes of uranium and lead, and this had to be understood in the dating analysis.
By 1913 he had so many new results and so many improvements on the method that he was able to write his groundbreaking book, The Age of the Earth, while he was still a graduate student. In it, he not only explained the basic principles of geochronology and discussed the problems with earlier methods of dating the earth, but also finally laid Lord Kelvin’s mistaken estimate to rest. He had dates on some of the oldest rocks in Britain of 1.6 billion years old, although he refused to speculate on the age of the earth. Later editions included results from analyses of older and older samples, until by the 1950s, he had dates of 4.6 billion years, which is our present estimate. His early research earned him his doctorate from University College London in 1917. But World War I was raging in Europe, and it was hard to make a living on the paltry salary of a demonstrator. To make money for his family, he decided to try a career as an exploration geologist again, this time for an oil company in Burma in 1920. However, the oil company went bankrupt, and Holmes returned to England in 1924 flat broke. This was not his only tragedy: his 3-year-old son had contracted dysentery shortly after arriving in Burma and died there.
Luckily, after his return in 1924, his earlier reputation and research landed him a job as a “reader” in geology at Durham University, close to his birthplace. There he spent the next 18 years teaching geology and adding to and refining the database of radiometric dates from around the world. His work so dominated the field that he became known as the “father of geochronology” or the “father of the geologic time scale.” In 1943 he moved north of the border to the University of Edinburgh, where he spent the last 13 years of his career until his retirement at age 66 in 1956.
A PIONEER OF PLATE TECTONICS
Meanwhile, through all those years teaching geology at the college level, Holmes accumulated the experience to write an introductory geology textbook. First appearing in 1944, Holmes’s Principles of Physical Geology became the standard textbook for generations of British geology students and went through many editions. But this book was not entirely conventional. In the last chapter of his first edition, he fully embraced the controversial idea of continental drift espoused by German meteorologist Alfred Wegener back in 1915. This idea was roundly rejected by most geologists of the time, but Holmes had seen the evidence—rocks in Africa that matched those in South America.
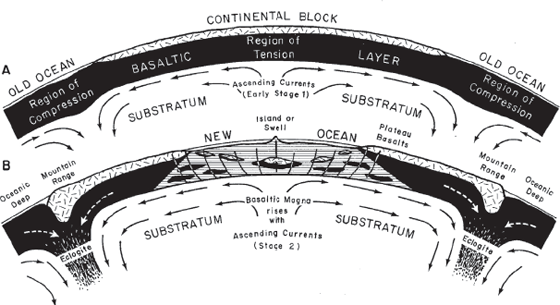
Holmes went even further. Using his understanding of how radioactivity drove the heating of the earth’s interior, he solved the mystery of Hutton’s idea of “Earth’s great heat engine.” In a paper published in 1931 (figure 8.2), Holmes was the first to suggest that this heat drives great convection currents in the mantle that shift the continents above them. He even postulated the notion that the seafloor must spread apart, decades before any evidence of seafloor spreading emerged in the late 1950s.
Figure 8.2
Holmes’s diagram of mantle convection moving the continents around. From Arthur Holmes, Principles of Physical Geology (London: T. Nelson, 1944).
Late in life, he began to receive honor after honor for almost single-handedly solving the problem of geologic dating. He was elected a fellow of the Royal Society in 1942 and won the Geological Society of London’s Murchison Medal in 1940 and Wollaston Medal in 1946. He was given the Penrose Medal, the highest award of the Geological Society of America, in 1956. And in 1964, a year before he died, he received the Vetlesen Prize, sometimes called the “Nobel Prize of Geology,” for his contributions not only to geochronology but also to the newly ascendant field of plate tectonics.
FOR FURTHER READING
Dalrymple, G. Brent. The Age of the Earth. Stanford, Calif.: Stanford University Press, 1994.
Hedman, Matthew. The Age of Everything: How Science Explores the Past. Chicago: University of Chicago Press, 2007.
Holmes, Arthur. The Age of the Earth. London: Harper and Brothers, 1913.
Lewis, Cherry. The Dating Game: One Man’s Search for the Age of the Earth. Cambridge: Cambridge University Press, 2002.
Macdougall, Doug. Nature’s Clocks: How Scientists Measure the Age of Almost Everything. Berkeley: University of California Press, 2008.