INTRODUCTION
The coronavirus disease-2019 (COVID-19) pandemic has caused a momentous economic, healthcare, and social disorder worldwide since early 2020. This coronavirus disease is declared as a pandemic by the World Health Organization (WHO), and it exhibited human to human transmission with rapid spread across the globe [1]. However, it is still unknown to define proper measures to control or treat COVID-19. In December 2019, the COVID-19 epidemic was initially reported in Wuhan, China, responsible for 80 904 confirmed cases as of August 31, 2020, China, with 24,854,140 cases reported worldwide 838924 mortalities. Upon the onset of this epidemic, the Chinese Government immediately has taken strict measures to control its transmission to other cities by completely lockdown Wuhan city, and many countries followed this practice. The number of COVID-19 cases is still increasing with few exceptions whatever the measures have been taken. A huge literature has been reported on the weather as an important factor in transmitting infectious diseases, especially influenza and severe acute respiratory syndrome (SARS). A remarked change in ambient temperature is linked with a higher risk of SARS [2], while influenza is linked with dry, hot, or cold air [3]. In Northern Europe, less temperature and low solar ultraviolet radiations (UVR) are examined to be linked with maximum activity and transmission of the influenza virus [4]. Based on the previous observations and reports, it is assumed that the COVID-19 epidemic can be reduced or even completely controlled as soon as the summer approaches. Seasonal variation (Temperature, humidity, and UVR) is important and is key to the spread of several infectious diseases [5-7]. Previously, the sunlight-driven nitric oxide (NO) mechanism was described as reducing blood pressure and a lower frequency of myocardial infarctions [8-10]. Most outcomes of COVID-19 are linked with dysfunctions of the vascular system, especially in the lungs.
Deadly infections are causing human morbidity and mortalities for centuries of known human history. Novel pathogenic conditions arise by the end of 2019, the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [11], and spread globally by early 2020. Certain secure, potent, and effective therapies are a dire need to minimize this SARS-CoV-2 pandemic unless a verified vaccine came into the market. Many studies indicate the shielding function of UVR in human health and for the disinfection of routine utensils. UVR is further divided into ultraviolet A (UVA) (320-400 nm), ultraviolet B (UVB) (280-320 nm), and ultraviolet C (UVC) (100-280 nm). UVR has long been recognized to pose strong anti-microbial effects. Among UVR, UVC is widely used to decontaminate and disinfect environmental objects and surfaces but is unsafe for human DNAs [12-15].
UVA radiation is a major environmental threat, but at the same time, certain therapeutic benefits have also been reported, and recently its potential use to minimize or reduce COVID-19. UVA (365 nm) is well known for inducing cellular signaling when irradiated in the physiological dose range. Solar UVR (UVA and UVB) is FDA-approved for various dermatological conditions such as skin lymphoma, psoriasis, and eczema [12, 16-18]. Still, we will elaborate on UVA light in the current draft only due to its least damaging effects for mammalian cells [13, 19]. Recent advancements in light-emitting diodes (LEDs) make it more versatile for the therapeutic application of lights for various internal organs [20]. FDA approves the only external application of the UVA light in therapeutic dosage range, but recently in vivo intraluminal UVA irradiations have produced no discernible endoscopic, histologic, or dysplastic changes in mice tissues [12]. Moreover, specific doses of UVA had strong inhibitory effects on coronavirus-229E and have proven a possible, safe, and quite effective treatment regimen for infectious diseases of internal viscera, but still demands clinical studies to define the safety, accuracy, and efficiency of UVA therapy in humans against COVID-19.
The current study aimed to propose the consideration of UVA therapy to control the cure for COVID-19 patients. The reduction of coronavirus replication in the lungs, modulation of NO in skin cells following UVA exposures, and this NO influx inside the bloodstream to deliver into lungs endothelial cells need to be evaluated. It incurs cytoprotection to lung alveolar cells shall be studied. Structural complexity, origin, classification, and molecular structure shall also be elucidated herein. Moreover, it is also proposed for direct application of therapeutic doses of UVA light inside the lungs to modulate the production of NO in lung endothelial cells, which will diffuse into the bronchi and lungs to leave bronchodilatory and vasodilatory effects. Moreover, how NO reduces inflammatory burst and ROS in the lungs' alveolar cells shall also be discussed.
Solar Light and Ultraviolet Radiation System
UV exposure is a key environmental factor that may affect skin functions significantly. Sunlight is an important environmental factor, which is further divided into various electromagnetic radiations, e.g., UV light that spanned 100-400 nm of the solar spectrum. UVR can be classified into further sub-classes such as UV light with wavelengths of 100-280 nm is the UVC, which absorbs at the upper stratospheric layers and have no chance to get its entry into the biosphere. While wavelength between 280-320 nm are further split into two groups 280-311 nm (broadband), and 311-313 nm (narrowband) [21-23], and the longest wavebands in the UV region (320-400) is torn up to UVA2 (320-340 nm) and UVA1 (340-400 nm), which can make their way through the ozone layer and approach biosphere. Despite the various harmful effects of UVA and UVB, many different skin conditions, particularly psoriatic lesions and eczema, are being treated with these UV radiations [24]. A huge amount of literature claimed the differential effects/outcomes of both UVA and UVB in the human skin. Due to the deep penetration of UVA into the dermis and subcutaneous, but UVB affects the epidermal tissues. Hence the outcomes of both remained different [25, 26]. The exit has an inverse relationship with the energy and wavelength of UVs, i.e., the longer the wavelength the less energy is, and vice versa. UVB radiations are five times more potent than UVA radiations. However, the former accounts for only 5% but later comprised 95% of the total UV light reaching the earth [3-7], and both have differential penetration in the skin, but overall UVs effects are the same [27, 28]. Contemporary, UVR is also very useful to our skin in a way that, it synthesizes activated form of vitamin D3, immunomodulatory responses, reduces blood pressure, promotes healing of wounds, lessen pains, pigmentation of the skin, and production of NO [22, 24, 25, 29]. Short exposure to UVA light may protect the tissues from being irradiated [30].
Long term Environmental Exposure of Ambient Solar UVA Radiation and COVID-19
The sunlight is a source of energy in the biosphere in visible light, infrared radiation-producing heat, and invisible but harmful UVR. Fortunately, naturally, the ' 'Earth's atmosphere is shielding us from the harmful and damaging effects of UVR. Still, at the same time, our bodies need intermittent exposure to solar light to form skin vitamin D [31]. The ambient UVR in the biosphere is comprised of UVA (>95%) wavelengths, and the remaining is the UVB. This proportion of UVA to UVB may vary during different times of the day, season, latitude, and altitude. The peak proportion of UVB radiation is observed during midday time during summers at the low latitude and high altitude areas. Moreover, the cloud cover or haze may potentially act on the variation of the UVA to UVB proportion. From 10 a.m. to 4:00 p.m, solar radiations have the maximum exposure for UVs. Still, it's more important for proper protective measures all year round because UVs are always strong even it's cloudy or haze, bright or sunny days, winter or summer, especially at altitudes [32]. In the invisible UVR, UVB (280-320 nm) parts are shorter but having more energy that easily enters through the outermost epidermis of the skin and produces burns, certain chemicals, inflammatory mediators, damage to the genetic materials in the cells leading to cancers even. Longer exposures affect the immune system by damaging the self-rebuild system of the tissue, activating melanocytes to provide melanin for filtration and protection of skin tissue under exposure [33].
UVA shows strong antiviral activity for positive sense, single-stranded RNA viruses such as coxsackievirus group B and coronavirus-229E [12]. It is assumed that UVA is involved in the alteration of the antiviral signaling mechanisms, especially the increased expression of mitochondrial antiviral signaling protein (MAVS), which is a major component of cellular antiviral immunity helping to suppress cellular viral invasion [34]. This suggests that UVA is involved in activating MAVS and later helps enter into an antiviral state without initiating antiviral apoptotic cell death.
On the other hand, UVA (320-400 nm) are longer waves, but less energetic than UVB may also have certain chronic damage [22]. UVA has far more penetration in the skin to dermal tissues, attacks cellular membranes, DNA damage, affects cellular signaling for transcriptional and translational levels mainly by the production of ROS and RNS [35]. UVR as a whole is linked with carcinogenesis, sagging of the skin, immune suppression, and sunburns, etc. Prolonged or chronic exposures to UVA may lead to dilated tiny blood vessels in the skin. Despite all the damaging effects of UVR, the therapeutic doses of UVA and UVB have useful effects on the human body. Specifically, a loose therapeutic dose of UVA may be used to boost immune response, regulate blood pressure, and adjust the redox balance of the cells [36]. Moreover, the UVA-mediated production of NO gets its entry to the blood vessels, and provides a protective and relaxing role in the lung tissues, and poses cytoprotective roles.
Sunlight has extensively been reported to inactivate SARS-CoV-2, a major cause of the COVID-19 under certain controlled studies. How increased aerosols help spread and transmit COVID-19 remained a big question for scientists. The aerosol is thought to be the possible way of transmitting COVID-19, and the whole phenomenon depends on multiple environmental conditions, especially the sunlight [37]. Moreover, simulated sunlight and matrix significantly affected the virus's decay rate, suggesting sunlight as a crucial factor that influences the risk of aerosol-mediated transmission of COVID-19 [37]. Sneezing, coughing, breathing, and talking can help generate aerosols in the respiratory tract [38, 39]. Coronaviruses HKU1, OC43, and NL63 genetic material have also been recovered from aerosols of exhaled breath of COVID-19 infected individuals. The genetic material of SARS-CoV-2 was also observed in upper respiratory tract specimens [10, 11].
The global impact of COVID-19 is numerous and uncountable. Still, precise forecasting of the coronavirus spread solely depends on historical data (total confirmed cases, number of deaths, and recoveries) may provide a chance to stop further spread. But at the same time, no forecast is certain as the future rarely repeats itself in the same way as the past because predictions rely on the source of the data, vested interests, and variables to be undertaken. Similarly, psychological factors significantly affect how people perceive and respond to the disease threats and internal fear that may affect badly [40].
Coronavirus
Coronaviruses (CoVs) are positive-sense, non-segmented single-stranded RNA-enveloped viruses that belong to the Coronaviridae family of the order Nidovirales [41]. Human CoVs (hCoV) were initially identified during the 1960s in patients with the common cold. Four known genera in this family are well recognized viz. α-coronavirus (α-CoV), β-coronavirus (β-CoV), γ-coronavirus(γ -CoV), and ∆-coronavirus (∆-CoV). The α-CoV and β-CoV can only infect mammals, including bats, cats, pigs, and humans. The γ-CoV mostly infects birds while ∆-CoV infects both mammals and birds. Six CoVs are known to be responsible for causing fatal infections in humans viz. human-CoVNL63 (hCoV-NL63) and human-CoV229E (hCoV-229E) of the genera α-CoV; human-CoVHKU1 (hCoV HKU1) and human-CoV OC43 (hCoV-OC43) in β-CoV lineage A are frequently involved in common cold symptoms in immunocompetent people. SARSr-CoV of β-CoV lineage B, and MERS-CoV belonging to β-CoV lineage C [42]. These are also known as zoonotic viruses due to their capacity for movement across the trans-species and well adaptation in various animals. CoVs involved in human diseases includes SARS, MERS, other respiratory infections, or gastrointestinal infections to attack birds and mammals [43]. SARS-CoV was initially identified during SARS outbreaks in 2002-03 in Guangdong province, China [44, 45]. MERS-CoV is responsible for causing severe respiratory disease outbreaks during 2012 in the Middle East [46]. These viruses are turned into a pandemic, and SARS caused over 8096 infections in 26 countries with almost 800 deaths, while MERS was identified in 2500 confirmed cases of infection in 27 different countries, which leads to 860 mortalities [47, 48].
The emergence potential of CoVs is mainly linked with its mutation and recombination, allowing the generation of new viral strains with higher susceptibility for new hosts [49-55]. The novel SARS-CoV-2 causing 2019 novel COVID-19 first appeared in Wuhan, Hubei province, China, in December 2019. COVID-19 causes severe respiratory infections, including pneumonia. Initially, unidentified sources of pneumonia was later found associated with the seafood and wet animal wholesale market in Wuhan, Hubei Province of China [56]. Later on, deep sequencing analysis of lower respiratory tract pathological samples from infected individuals indicated a novel coronavirus strain that was later designated as SARS-CoV-2. However, SARS-COV-2 spread in over 185 countries around the globe, including countries in almost every continent, caused around 19,462,112 infections and 722,285 deaths [58]. Initially, the mortality rate of COVID-19 in China was 2.3% compared with other viruses, viz. MERS (34.4%) and SARS (9.6%) announced by WHO [57]. Due to the daily increase in cases and death globally, make it hard to conclude about the exact fatality rate of the disease. But the latest data showed a sudden increase in Italy, the Islamic Republic of Iran, and the Republic of Korea during March 2020 [58]. Initial clinical studies reported the laboratory, clinical, radiological characteristics, and possible epidemiological features of such viruses [59].
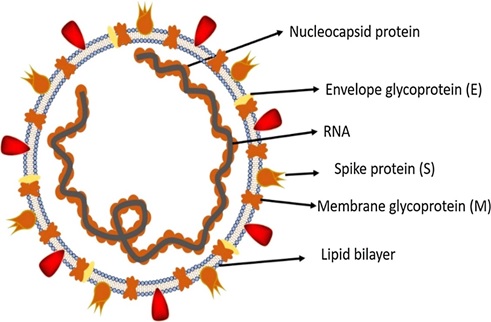
Fig. (1))Structure of SARS-CoV-2. Fig. (2))
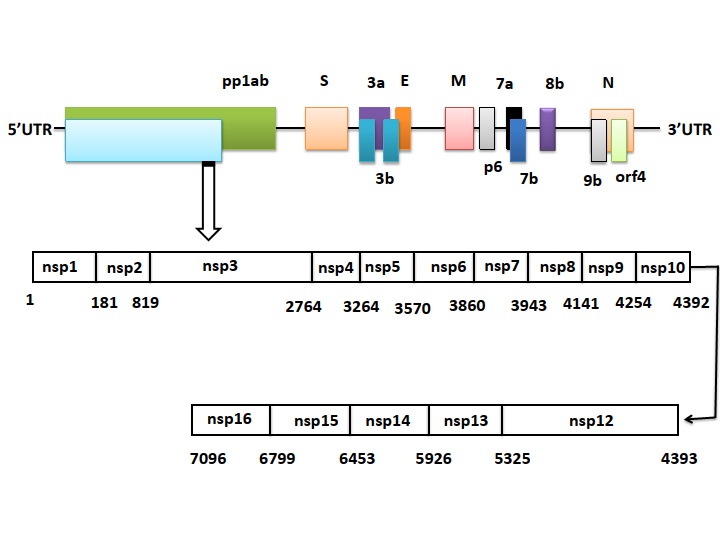
Details of the genome of SARS-CoV-2 that comprised of approximately ~30 kb, which encodes both structural and non-structural proteins.
What is SARS-CoV-2?
SARS-CoV-2 (Fig. 1) is a single-stranded RNA enveloped β-CoV and efficiently causes acute respiratory syndrome named COVID-19. SARS-CoV-2 genome is comprised of approximately ~30 kilobases, which encodes both structural and non-structural proteins (Fig. 2). The later proteins such as 3-chymotrypsin-like protease, papain-like protease, RNA-dependent RNA polymerase, and helicases are vital enzymes in maintaining the virus particle life cycle within the cell. The pairwise protein sequence analysis of these seven protein conserved non-structural proteins demonstrate that the virus belongs to the species of SARSr-CoV [60]. On the contrary, the structural proteins include spike glycoprotein and its accessory proteins required for the virus to cell receptor interactions during viral entry into the host cell [61]. Therefore, these proteins are documented as potential targets to develop antiviral drugs against SARS and MERS [62], and probably almost mimicking in the case of COVID-19 as revealed by recent genomic data of this novel virus from Wuhan [63]. This on-time identification of receptors and binding proteins will be helpful in vaccine developments and using therapies of the past SARS and MERS outbreak to cure current COVID-19 patients. The evolutionary history of SARS-CoV-2 and those of other CoVs genomes to track its origin. The full-length genome phylogenetic analyses suggested that COVID-19 shares about 79.5% sequence identify SARS-CoV (Fig. 3) [64-66]. This data suggests SARS-CoV-2 as a novel β-CoV from the subgenus Sarbecovirus [64-67].
Fig. (3))A comparative evolutionary history of SARS-CoV-2 with other CoVs genomes to track its origin.
The 21st century has witnessed two pandemics by coronaviruses, MERS-CoV and SARS-CoV [68, 69], which were highly pathogenic. Starting during December 2019, in Wuhan, China [56], a cluster of people with pneumonia from unknown sources and epidemiology was linked to a seafood and wild animal wholesale market. SARS-CoV-2 can infect humans through the mouth and nose mucous membranes and use mucous membranes in the eyes, boosting regional circulation and large-scale spread [70]. Remarkable scientific advancements and united global efforts enable rapid identification of the novel coronavirus as SARS-CoV-2 and help its epidemic prevention. Notably, SARS-CoV-2 was closely related with 88% sequence similarity to two Zhoushan, Eastern China, bat-derived SARS-like coronaviruses, i.e., bat-SL-CoVZXC21 and bat-SL-CoVZC45, but was more distant from MERS-CoV (50% sequence similarity) and SARS-CoV (79% sequence similarity) [63]. Phylogenetically, the 2019 novel CoV originated from the genus β-CoV, which includes coronaviruses (SARS/SARS-like -CoV and others) identified in bats, humans, and few wild animals [71]. The spike (S) proteins, crucial for host tropism and transmission capacity, mediates receptor binding through its domain S1 and plasma membrane fusion through S2. S protein of SARS-CoV-2 shared high sequence similarity (88%) with SARS-CoV suggesting its possible utilization of Angiotensin-Converting Enzyme 2 (ACE2) as a cell receptor [72]. However, several key residues required for the binding of the ACE2 receptor to the coronavirus receptor-binding domain displayed similarly between the receptor-binding domains of SARS-CoV and SARS-CoV-2.
Unlike MERS-CoV and SARS-CoV receptor-binding domain, the SARS-CoV-2 receptor binding domain contains several mutations but still employs the ACE2 binding receptor. This mutation was introduced at Furin protease, which may enhance the transmissibility and significantly improve the efficiency of infecting cells by SARS-CoV-2 than SARS-CoV. The lessons learned through experiences resulted from the outbreaks of CoVs prevalence over the last two decades, tremendous advances, and valuable insight toward unraveling the physiognomies of SARS-CoV-2 have been achieved at an unprecedented speed. It is still might be able to use this binding receptor. Whether mutations in ACE2 affect its binding or change receptor tropism need to explore in detail [63].
Mode of Transmission of SARS-CoV-2
Since the outbreak of SARS-CoV-2, the level of infections and severity, mode of transmission, and mortality rate appear unclear and controversial till now. In the emergence of the SARS-CoV-2 outbreak, health officials and administrations mostly rely on prevention to use face masks in public places. Whole cities were in quarantine to reduce surge capacity [73]. Emerging evidence supported asymptomatic transmission [74] while numerous ways of transmission are adding up. In the current scenario, a health official Guangfa Wang, with a protective face mask N95 complained about eye redness before the onset of pneumonia was infected with the novel virus in Wuhan [75]. Lu and colleagues [76] confirmed the ocular transmission of this deadly virus almost similar to 2003 SARS-CoV, the primary way it transmits through the mucus membrane [77]. Thus, the ocular transmission of SARS-CoV-19 should not be underestimated. The current study suggests the better protection of health workers is a more effective way to prevent disease amplification.
Additionally, the Government has already warned and instructed the general public about SARS-CoV-19 ocular transmission. More recently, the appalling human-to-human hospitalized transmission was 44 cases (29%) [78]. This makes it more amiable to speculate that there would be more unknown ways of transmission of COVID-19 [79]. Hence certain robust and serious precautions are needed to prevent or check transmissions. Urine and stool samples should be tested to prevent a possible alternative transmission route at this stage [79].
Moreover, researchers speculated sewage transmission in Hongkong and Guangzhou, China, as a viral infection has had been demonstrated in Caco-2 cell lines when co-cultured with samples from infected individuals in Germany [80, 81]. This suggested that, like SARS and MERS, COVID-19 shares a similar transmission mode [82]. This evidence adding up anxiety in the SARS-CoV-2 outbreak and require further in-depth studies of contact patterns on epidemic dynamics, hence certain robust and serious precautions needed to prevent or check transmissions.
Prevention Strategies Adopted
Although SARS-like outbreaks have not been reported over a decade, modeling SARS transmission and control in the healthcare systems can be applied to control COVID-19 outbreaks [83-85]. MERS, SARS, and COVID-19 share certain common features that could be implemented in healthcare systems and display transmission heterogeneity [86]. The lack of an appropriate animal model that mimics the natural history of the disease has slowed down the development of effective pharmaceutical interventions against MERS-CoV [87] and SARS and now for COVID-19. The CDC recommends a set of preventive measures to help check the amplification of respiratory viruses [83, 84].
Like the SARS outbreak, reducing close contact by quarantine people to their houses and a travel ban would be an effective measure to limit the spread of SARS-CoV-2 [88]. Furthermore, due to persons to person, asymptomatic transmissions and incubation period [74, 89] make their way far more vigilant for control measures of the epidemic which seems to be pandemic. One such measure taken by the Chinese Government was the quick isolation of suspected, infected, and close contact individuals. In addition, SARS-CoV and MERS-CoV super spreading events [90] cannot be completely ignored in the current SARS-CoV-2 outbreak. So far, the transmission of COVID-19 occurs either through close contact or aerosol, which seems controversial along with fecal transmission [91]. Health workers should follow SARS and MERS type infection standard procedures while handling infected patients and during sample collection [92].
UVA Mediates Production of NO
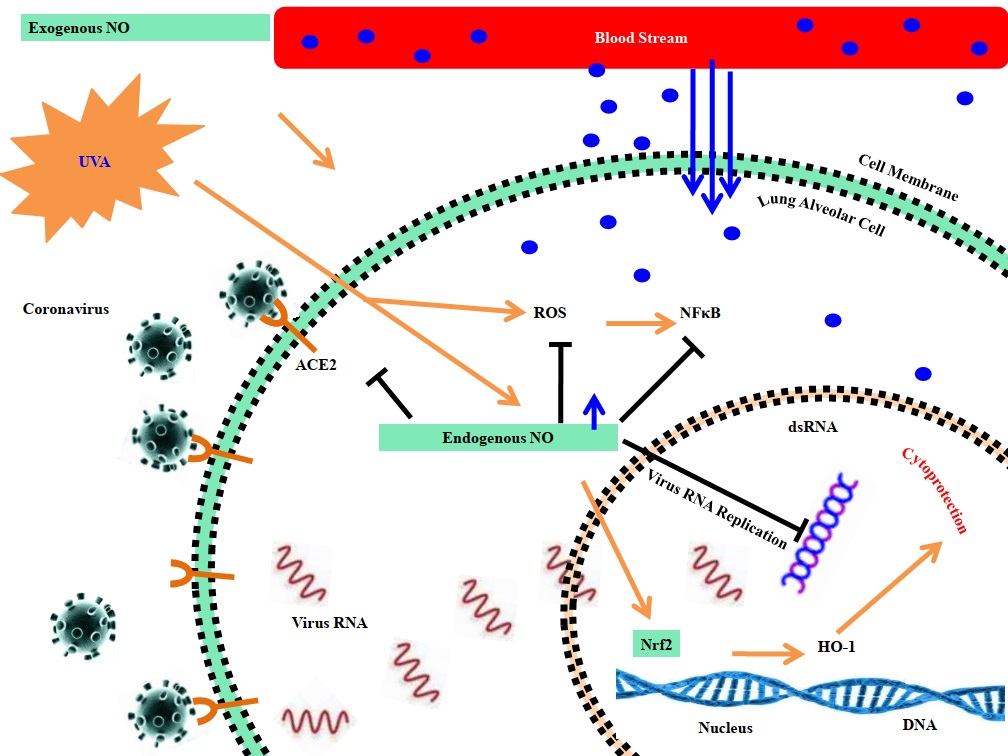
The increasing literature ever portrays the positive effects of solar radiation; especially UVA radiation reduces blood pressure mainly by producing NO in the skin keratinocytes and microvascular endothelial cells [29]. NO is well known for killing or inhibiting the replication of intracellular pathogens, specifically viruses, where NO targets viral proteases necessary for virulence and the life cycle of many viruses [93-98]. NO mainly targets Coxsackievirus proteases 3C, torn the virus polyproteins into individual polypeptides, and S-nitrosylate cysteine residues present in the active-site of protease 3C to inhibit protease activity [93]. COVID-19 pandemic is not finished yet, though some signs of the second phase are striking ears for days, e.g., Hongkong's recent COVID-19 outbreak in the last July 2020, according to WHO. Keeping in view the continuous and vigorous spread of COVID-19, certain risk factors (temperature, humidity, and solar UVR) are seriously considered while attempting to minimize the SARS-CoV-2 [99]. The human skin gets exposed to solar light, UVA light induces the discharge of NO from skin cells making its way to enter the bloodstream (Fig. 4), which dilates blood vessels and minimizes blood pressure, promoting cardiovascular disease and metabolic health [99]. This produces a reduction in the risk of death from COVID19 since heart disease, vascular conditions, and metabolic syndrome are high-risk factors [99].
Fig. (4))Possible molecular mechanisms involved in mediating the beneficial effects of solar ultraviolet radiation.
Moreover, UVA irradiation is also suggested to inactivate the virus in airborne droplets and fomites. The spread rate reduction and the size of the inoculating dose in those coming into contact with infectious material, hence reducing infection levels [99]. It is also said that NO may modulate the expression of proteins of the Bcl-2 family, which appears as the molecular mechanism underlying this cellular protection [100].
Effect of NO on SARS-CoV-2
NO a key signaling molecule between cells, shows an inhibitory effect on some virus infections [101]. Infection caused by SARS-CoV-2 may lead to a diversity of cardiopulmonary and vascular complications, specifically upper respiratory tract issues, severe acute respiratory distress syndrome (ARDS), shock, acute kidney injury, and thromboembolic complications [102, 103]. Various reactive oxygen and nitrogen species play important roles in human physiology, but gaseous NO is quite an important signaling molecule.
In vascular smooth muscle cells showed high expression levels of the canonical receptor (guanylate cyclase) that gets activated after the NO attaches to heme moiety and remarkably increases its enzymatic conversion guanosine-5’-triphosphate to cyclic guanosine monophosphate, which subsequently promotes vasorelaxation. The gaseous NO has distinctive pharmacological properties, especially import into well-ventilated lung units promoting local vasodilatation. The extremely short half-life NO after getting entered into the blood quickly forms a complex with intraerythrocytic Hb and inactivated. It will lead to a strong check on its systemic effects [104]. Gaseous NO preferentially vasodilates pulmonary arterioles in well-ventilated lung units. It decreases the relative blood flow to poorly ventilated lung units and enhances V/Q matching to increase blood oxygenation [105].
Moreover, NO is also said to induce mild bronchodilation and check neutrophil-mediated oxidative burst [105-108]. In SARS patients, inhaled nitric oxide (iNO) is linked with enhanced oxygenation in a severity-matched observational cohort [109]. Both endogenous and exogenous NO showed to inhibit SARS-CoV virus replication [101] (Fig. 5). On the other hand, iNO has significant effects on oxygenation in ARDS patients and hence reduces pulmonary vascular resistance [105]. Such therapeutic activities give an idea that iNO may be used as an early cure during CoVID-19 infection to minimize the demand for invasive mechanical ventilation [104]. Certain recent cases reported the superiority of iNO delivery systems to facilitate patients even at their homes. At the same time, GENOSYL DS (VERO Biotech) has designed a tankless system to deliver at homes [110]. Still, many other systems such as Nu-Med Plus (UT), INOpulse (Bellerophon Therapeutics), and an iridium electric NO generator have also been introduced
due to easy portability. Still, all these iNO therapy systems have certain limitations for their universality.
In a randomized and placebo-controlled trial of ambulatory patients with fibrotic lung disease requiring long-term oxygen. INOpulse therapy was associated with greater physical activity than placebo. In an acute dose-escalation study of patients with pulmonary hypertension associated with pulmonary fibrosis, iNO delivered through the INOpulse system lead to a 30% reduction in pulmonary vascular resistance, with improvements in Q and pulmonary artery compliance.
Fig. (5))Role of UVA and exogenous NO in lung alveolar cell to inhibit SARS-CoV-2.
NO: A Key Player in the Airways
Various biological and pathological outcomes of NO are due to some deleterious or cytoprotective effects primarily depends on the immediate micro-environment [111, 112] (Fig. 5). For decades, NO remained a major atmospheric pollutant and cause of acid rains [113]. Still, late in the 20th century, NO appeared as a mimicking factor of endothelium-derived relaxing factor (EDRF) [114], performing multiple functions in the human body, especially the respiratory tract. Nitric oxide synthase (NOS) exists in two isoforms, namely inducible (iNOS) and constitutive (cNOS), which help to release endogenous NO into the airways. The constitutive NOS poses protective effects on airways against excessive bronchoconstriction. It inducible NOS modulates airways inflammatory conditions, particularly in asthma [115], platelet aggregation [116], and neurotransmission [117]. A higher NO level is suggested in asthmatic conditions due to overexpression of NOS [118, 119]. Moreover, endothelial and epithelial cells of the lung airways and inflammatory cells are capable of producing NO.
Fig. (6))Schematic representation of UVA-based direct tracheobronchial UVR applications: (A) Appropriate UVA (365 nm) source with a fiberoptic adapter; (B) direct bronchoscopic application of UVA. Note that radiation intensity should be limited to physiological doses and that treatments should be applied every 4-8 h, with at least 24 h of therapy before reassessment. Discontinuation of treatment should be determined by the treating physician after considering: (a) the status of the patient's condition and (b) the safety and efficacy of the treatment.
Many studies reported the negative role of NO in health. Still, minor oxidative stress leads to releasing the redox-active chelate iron pool (CIP) of the cell, which potentially and actively amplifies the cellular ROS led to oxidative stress later to pose multiple pathologies. In macrophages exposed to NO, the CIP gets complexed with NO to form di-nitrosyl iron with thiol-containing ligands (DNICs) [120]. At the cellular level, NO slowed or check H2O2 metabolism that suggests some H2O2 is consumed during cellular iron metabolism to pose cytoprotection against iron toxicity [120]. In severe lung issues specifically, asthmatic individuals, increased levels of NO have been observed. Keeping this fact in mind, we proposed a UVA-based direct tracheobronchial UVR application shown in Fig. (6). It needs an appropriate UVA (365 nm) source with a fiberoptic adapter for the direct bronchoscopic application of UVA. Moreover, UVA radiation application should be limited to physiological doses and applied every 4-8 h, with at least 24 h of therapy before reassessment. The treating physician should determine discontinuation of treatment following observation of the patient's condition and the safety and efficacy of the treatment.
NO Blocks Virus Replication Inside Host Cells
NO is an important signaling molecule between cells that have been shown to have an inhibitory effect on some virus infections. For a long time, NO has been considered a physiological mediator of endothelial cell relaxation, especially in hypertension. Previously, NO was studied extensively as an intracellular messenger and reported as a potent mediator of endothelial cell relaxation that plays a significant role in hypotension. Macrophages, natural killer cells, and neutrophils comprise the innate immune system. It mainly use pattern recognition receptors to identify molecular patterns linked with inhibition of various pathogens, mainly by checking replication and release of numerous effector molecules, particularly NO [121]. iNOS mediated NO potentially inhibits the SARS-CoV replication cycle at some early stages of infection [101, 122]. The diffusion of NO through the cell membrane to reach the virus should be significantly more effective at the very high NO concentration [122]. NO plays certain key roles in pulmonary vascular functions during viral infections and many pulmonary conditions [123]. The lung cells are reported to have higher levels of iNOS when there is a viral attack, and NO checks the replication of the SARS-CoV-1 virus mainly through certain cytotoxic intermediate reactions such as the formation of peroxynitrite [101]. Nitrosation of various surface thiols of red blood cells (RBC) and β-chains of the hemoglobin tetramer help stabilize for hemolysis and oxidative damage, respectively. It depicts the potential of NO to stop SARS-CoV-2 RBC-linked pathogenic processes [124, 125]. SARS-CoV-2 primarily attack to infect lung endothelial cells, which are the main resource for NO production. Hence naturally, NO is holding a better position to respond against virus attacks quickly [123].
iNOS mediated NO being an important inter/intracellular molecule, has shown an increased level in various microbial infections with appreciable effects for the protozoans and pathogenic bacterial strains, but also shows inhibitory effects on certain virus infections well [101]. An in vitro study, where NO donor S-nitroso-N-acetylepenicillamine checks the replication of the SARS CoV in a concentration-dependent fashion both at translational and transcriptional levels [101]. Some studies reported the suppression of virus replication, where the infected murine macrophages start releasing iNOS-mediated NO without an accessory protein C (4C(-)) from the Sendai virus (SeV). The underlying mechanism in the virus replication is an increased expression of IFN-β and activation of NFκB, and the former modulates the induction of iNOS via the JAK-STAT pathway [126]. NF-κB is a key regulator of transcription of the iNOS gene modulate by 4C(–) infection. It plays a vital role in inducing NO production, and iNOS expression significantly decreased in the cells that express influenza virus NS1 protein constitutively. So, it sequesters double-stranded (ds)RNA, which is an essential factor to trigger signaling pathways leading to activation of NF-κB and IRF3. C protein is marked as suppressing NF-κB activation to inhibit iNOS expression leading to NO production, possibly by limiting dsRNA generation in the context of viral infection [126].
NO Reduces the Inflammatory Influx and Oxidative Stress
The available reports showed that severe patients of COVID-19 had been found with higher levels of pro-inflammatory cytokines such as interleukin (IL)-6 [127]. Higher IL-6 levels show poor prognosis in COVID-19, moreover, excessive infiltration of pro-inflammatory cells viz. T-helper 17 cells and macrophages have been observed in lung tissues of COVID-19 patients. It is assumed that “cytokine burst” may aid in the mortality of COVID-19. SARS-Cov-2 selectively induces a high level of IL-6 and results in the exhaustion of lymphocytes [127].
Both neutrophils and the following monocytes get involved during the inflammations where the former helps to kill pathogenic microbes and ultimately undergoes apoptosis. Monocytes turned to macrophages at the inflammatory tissue, other phagocytes help clear the apoptotic neutrophils and reduce the inflammation.
NO is recently reported to be involved in regulating the inflammatory burst, immune responses and retards the growth of the microorganisms [128]. Moreover, NO has a pivotal role in alleviating oxidative stress by neutralizing ROS (Fig. 5). In certain lungs, asthma and some viral infections, higher levels of NO are examined. Alveolar macrophages (AM) and bronchoalveolar lavage fluid (BALF) in patients with asthma showed a higher level of inflammatory cytokines are being modulated by the presence of NO [129]. Paranasal sinuses continuously generate NO as a ROS agent. It gets diffused into the bronchi and lungs to leave bronchodilatory and vasodilatory effects [130]. NO may lead to initiate an array of reactive nitrogen oxide species [131]. Still, it goes to react with different targets [3], either some free radicals (dioxygen, superoxide, etc.) or free cellular iron (Fe2+) [132] to stabilize the unpaired electron on NO [133], and hence the free radicals get neutralized. It is well established that iNOS-mediated NO modulates the production of various chemokines, which are involved in the neutrophils and monocytes infiltration during inflammations [134]. The levels of NO are the key players in identifying the balanced production of such chemokines. Moreover, the production of pro-inflammatory cytokines appears to be actively suppressed by TGF-β and NO. Phagocytes produce them upon interaction with apoptotic cells [134]. NO is playing a key role in inflammations and, therefore, is considered a potent candidate for developing therapeutics for inflammatory diseases, particularly in COVID-19.
CONCLUSION
The outbreak of COVID-19 is still a big challenge for global health. It is obvious after discussion of the bi-phasic mechanism of UVA radiations that can trigger vasodilatory effects. The UVA as a potential therapeutic source could be used in various conditions. NO production can evidence that nitrites in skin cells are the major source of UV-mediated NO release. The reduction of coronavirus replication can be made by directly applying therapeutic doses of UVA in lungs using a fiberoptic adapter through the modulation of NO. Similar effects can be achieved by exposing the skin cells to UVA radiations that produce the influx of NO inside the bloodstream to deliver into lung endothelial cells to incur cytoprotection to alveolar lung cells. Moreover, it is also being proposed that UVA-mediated production of NO in lung endothelial cells can easily diffuse into the bronchi. It leaves the lungs with bronchodilatory and vasodilatory effects that reduce inflammation to burst and produce ROS. The application of physiological doses of UVA (365 nm) every 4-8 hours for 24 hours before reassessment, while discontinuation of direct UVA application can be done following physician's observations about the status of the patient's condition and the safety and efficacy of the treatment.