References
Andrews RP, Baldar NA. Amino acid analysis of feed constituents. Sci. Tools. 1985;32(2):44–48.
Batterham ES. Availability and utilization of amino acids for growing pigs. Nutr. Res. Rev.. 1992;5:1–18.
Belitz H-D, Grosh W, Schiberle P. Food Chemistry. fourth ed. Berlin, Germany: Springer; 2009:8–29.
Benson J, Louie P, Pradshaw R. Amino acid analysis of peptides. Peptides. 1981;4:217–259.
Blackburn S. Sample preparation and hydrolytic methods in amino acid determination. second ed. Methods and Techniques. New York, NY, United States: Marcel Dekker Inc.; 1973: .
Bos KD, Verbeck C, Slump P. Gas-Liquid chromatographic method for the analysis of methionine and of methionine sulfoxide in proteins. J. Agric. Food Chem.. 1983;31(1):63–66.
Butikofer U, Fuchs D, Bosset JO, Gmur W. Automated HPLC-amino acid determination of protein hydrolysates by precolumn derivatisation with OPA and FMOC and comparison with classical ion exchange chromatography. Chromatographia. 1991;31:441–447.
Chang JY. Amino acid analysis in the pico mole range by precolumn derivatization and high performance liquid chromatography. Methods Ezymol.. 1983;91:41–48.
Deglaire A, Moughan PJ. Animal models for determining amino acid digestibility in humans: a review. Br. J. Nutr.. 2012;108:S273–S281.
Deng YL, Zheng LP. Analysis of the relationship between serum homocysteine level and breast tumor. Mod. Prev. Med.. 2012;34:87–89.
Deyl Z. Profiling of amino-acids in body fluids and tissues by means of liquid chromatography. J. Chromatogr.. 1986;379:117–250.
D’Mello JPF. Adverse effects. In: D’Mello JPF, ed. Amino Acids in Human Nutrition and Health. Wallingford, UK: CAB International; 2012:322–353.
Elango R, Ball RO, Pencharz PB. Indicator amino acid oxidation: concept and application. J. Nutr.. 2008;138:243–246.
Elango R, Ball RO, Pencharz PB. Recent advances in determining protein and amino acid requirements in humans. Br. J. Nutr.. 2012;108:S22–S30.
FAO/WHO Ad Hoc Expert, 1973. Energy and protein requirements. WHO Technical Report Series, No. 522, FAO Nutrition Meetings Reports Series 52.
FAO/WHO, 1991. Protein Quality Evaluation: Report of a Joint FAO/WHO/UNU Expert Consultation, FAO Food and Nutrition Paper 51. Rome.
FAO/WHO, 2001. Report of the FAO/WHO Working Group on Analytical Issues Related to Food Composition and Protein Quality. Rome, Italy.
FAO/WHO/UNU, 2002. Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition. World Health Organization, Geneva, Switzerland.
Finley JW. Reducing variability in amino acid analysis. In: Finley JW, Hopkins DT, eds. Digestibility and Amino Acid Availability in Cereals and Oilseeds. St Paul, MN, United States: American Association of Cereal Chemists; 1985:15–30.
Finot PA. Lysinoalanine in food proteins. Nutr. Abstr. Rev.. 1983;53(2):67–78.
Flores G, Negrete-Diaz JV, Carrion M, Andrade-Talavera Y, Bello SA, Sihra TS, Rodriguez-Moreno A. Excitatory amino acids in neurological and neurodegenerative disorders. In: D’Mello JPF, ed. Amino Acids in Human Nutrition and Health. Wallingford, United Kingdom: CABI Publishing; 2012:479–524.
Ghosh Sh, Suri D, Uauy R. Assessment of protein adequacy in developing countries: quality matters. Br. J. Nutr.. 2012;108:S77–S87.
Gilani GS. Background on international activities on protein quality assessment of foods. Br. J. Nutr.. 2012;108:S168–S182.
Gilani GS, Xiao C, Lee N. Need for accurate and standardized determination of amino acids and bioactive peptides for evaluating protein quality and potential health effects of foods and dietary supplements. J. AOAC Int.. 2008;91(4):894–900.
Greenfield H, Southgate DAT. Food composition data: production management and use. New York, NY, United States: Springer US; 1992.
Humayun MA, Elango R, Moehn S, Ball RO, Pencharz PB. Application of the indicator amino acid oxidation technique for the determination of metabolic availability of sulfur amino acids from casein versus soy protein isolate in adult men. J. Nutr.. 2006;137:1874–1879.
Jonker R, Engelen MPKJ, Deutz NEP. Role of specific dietary amino acids in clinical conditions. Br. J. Nutr.. 2012;108:S139–S148.
Kabaha K, Taralp A, Cakmak I, Ozturk L. Accelerated hydrolysis method to estimate the amino acid content of wheat (Triticum durum Desf.) flour using microwave irradiation. J. Agric. Food Chem.. 2011;59:2958–2965.
Katsanos CS, Madura JA, Roust LR. Essential amino acid ingestion as an efficient nutritional strategy for the preservation of muscle mass following gastric bypass surgery. Nutrition. 2016;32(1):9–13.
Levesque CL. Metabolic amino acid availability in foods of plant origin: implications for human and livestock nutrition. In: D’Mello JPF, ed. Amino Acids in Higher Plants. Wallingford, United Kingdom: CAB International; 2015:497–507.
Levesque CL, Elango R, Ball RO. Metabolic availability of amino acids in food protein: new methodology. In: D’Mello JPF, ed. Amino Acids in Human Nutrition and Health. Wallingford, United Kingdom: CAB International; 2012:256–266.
Lin J, Lee IM, Song Y, Cook NR, Selhub J, Manson JE. Plasma homocysteine and cysteine and risk of breast cancer in women. Cancer Res.. 2010;70:2397–2405.
Lin XY, Yu HJ, Mei X. An improved method for the determination of tryptophan in foods. Acta Nutr. Sin.. 1985;7(1):67–72.
Liu T, Chang J. Hydrolysis of protein with p-to-luene sulfonic acid. Determination of trypthophan. J. Biol. Chem.. 1971;246:2842–2848.
Miller JW, Beresford SA, Neuhouser ML, Cheng TY, Song X, Brown EC. Homocysteine, cysteine, and risk of incident colorectal cancer in the Women’s Health Initiative observational cohort. Am. J. Clin. Nutr.. 2013;97:827–834.
Millward DJ. Amino acid scoring patterns for protein quality assessment. Br. J. Nutr.. 2012;108:S31–S43.
Millward DJ. Identifying recommended dietary allowances for protein and amino acids: a critique of the 2007 WHO/FAO/UNU report. Br. J. Nutr.. 2012;108:S3–S21.
Moehn S, Bertolo RFP, Pencharz PB, Ball RO. Development of the indicator amino acid oxidation technique to determine the availability of amino acids from dietary protein in pigs. J. Nutr.. 2005;135:2866–2870.
Moughan PJ. Preface: dietary protein for human health. Br. J. Nutr.. 2012;108:S1–S2.
Otter D. Standardised methods for amino acid analysis of food. Br. J. Nutr.. 2012;108:S230–S237.
Palego L, Betti L, Rossi A, Giannaccini G. Tryptophan biochemistry: structural, nutritional, metabolic, and medical aspects in humans. J. Amino Acids. 2016;2016:8952520.
Pellet PL, Young VR. Nutritional Evaluation of Protein Foods. Tokyo, Japan: United Nations University; 1980: .
Penke B, Ferenczi R, Kovacs K. A new acid hydrolysis methods for determining tryplophan in peptides and proteins. Anal. Biochem.. 1974;60:45–51.
Pienjacek D, Grabarek Z, Rakowska M. A Quantitative determination of the content of available methionine and cyslieine in food proteins. Nutr. Metab.. 1975;18:16–22.
Pineda O, Torun B, Viteri F, Aroyave G. Protein quality in relation to estimates of essential amino acid requirements. In: Bodwell CE, Adkins JS, Hopkins DT, eds. Protein Anality in Humans: Assesment and In vitro Estimation. Westport, CT, United Nations: AVI, Publishing Co.; 1981.
Prolla IRD, Rafii M, Courtney-Martin G, Elango R, da Silva LP, Ball RO, Pencharz PB. Lysine from cooked white rice consumed by healthy young men is highly metabolically available when assessed using the indicator amino acid oxidation technique. J. Nutr.. 2013;143:302–306.
Ribarova, F., Shishkov, S., Baklova, I., 1987. Amino Acid Composition of Bulgarian Food Products. Zemizdat, Sofia (in Bulgarian).
Ribarova, F., Vazelov, E., Shishkov, S., 2003. Nutrition and Chronic Renal Failure, IN.VENTO P, Sofia (in Bulgarian).
Rutherfurd SM. Amino acid analysis of plant products. In: D’Mello JPF, ed. Amino Acids in Higher Plants. Wallingford, UK: CAB International; 2015:481–496.
Rutherfurd SM, Dunn BM. Quantitative amino acid analysis. Curr. Protoc. Protein Sci.. 2011;63: .
Rutherfurd SM, Moughan PJ. Available versus digestible dietary amino acids. Br. J. Nutr.. 2012;108:S298–S305.
Rutherfurd-Markwick KJ. Food proteins as a source of bioactive peptides with diverse functions. Br. J. Nutr.. 2012;108:S149–S157.
Sarwar GA. Available amino acid score for evaluating protein quality of foods. J. Assoc. Anal. Chem.. 1984;67(3):623–626.
Sarwar GA, Christensen AJ, Hackler LR, Pellet PL. Inter and intralaboratory ariation in amino acid analysis of food proteins. J. Food. Sci.. 1983;48(2):526.
Simpson R, Ncubcrger M, Lin T. Complete amino acid analysis of proteins from a single hydrolyzate. J. Biol. Chem.. 1976;251(7):1936–1940.
Spackman D, Stcin W, Moore S. Automatic recording apparatus for use in the chromatography of amino acids. Anal. Chem.. 1958;30:1190–1205.
Thiele B, Stein N, Oldiges M, Hofmann D. Direct analysis of underivatized amino acids in plant extracts by LC-MS-MS. Methods Mol. Biol.. 2012;828:317–328.
Tome D. Criteria and markers for protein quality assessment: a review. Br. J. Nutr.. 2012;108:S222–S229.
Torun B, Pineda O, Viteri F, Arroyave G. Use of aminoacid composition data to product protein nutritive value of children with specific reference to new estimates of essential amino acid requirements. In: Bodwell CE, Adkins JS, Hopkins DT, eds. Protein Anality in Humans: Assesment and In Vitro Estimation. Westport, CT, United States: AVI Publishing Co.; 1981.
Turnell DC, Cooper JDH. Rapid assay for amino acids in serum or urine by precolumn derivatization and reversed phase liquid chromatography. Clin. Chem.. 1982;28(3):527–531.
Vazelov E, Ribarova F. Dinamic changes in plasma glycine and serine in patients on hemodialysis treatment. Arch. Balkan Med. Union. 2013;48(1):66–69.
Vazelov E, Ribarova F, Shishkov S. Plasma levels of tryptophan and large neutral amino acids (LNAA) in patients on chroniohemodialysis. C. R. Acad. Bulgare Sci.. 1999;LII(1–2):133–136.
Vries J, Koski C, Larson P. Comparison between a spectrophotometric and HPLC method for determining tryptophan in food products. J. Agric. Food Chem.. 1980;28:886–898.
Wang LY, Ma L, Su JR. Initial investigation of serum homocysteine as cancer biomarker. Int. J. Surg.. 2012;39:752–755.
Wang XN, Ye P, Cao RH, Yang X, Xiao WK, Zhang Y, Bai YY, Wu HM. Plasma homocysteine is a predictive factor for arterial stiffness: a community-based 4: 8-year prospective study. J. Clin. Hypertens.. 2015;17(8):594–600.
Webb GP. Nutrition: A Health Promotion Approach. third ed. London, United Kingdom: Hodder Arnold; 2008: .
Williams AP. General problems associated with the analysis of aminoacids by automated Ion-exchange chromatography. J. Chromatogr.. 1986;373:175–190.
Zhang SH, Liang ZJ, Huang FW. The value of serum homocysteine in primary hepatocarcinoma. Mod. Oncol.. 2012;20:2580–2581.
Zhang D, Wen X, Wu W, Guo Y, Cui W. Elevated homocysteine level and folate deficiency associated with increased overall risk of carcinogenesis: Meta-analysis of 83 case-control studies involving 35,758 individuals. PLoS One. 2015;10(5):e0123423.
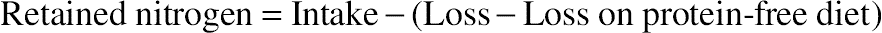
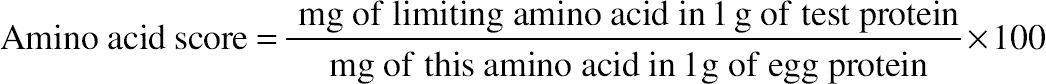
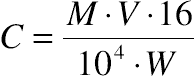
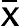
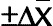
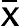
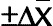
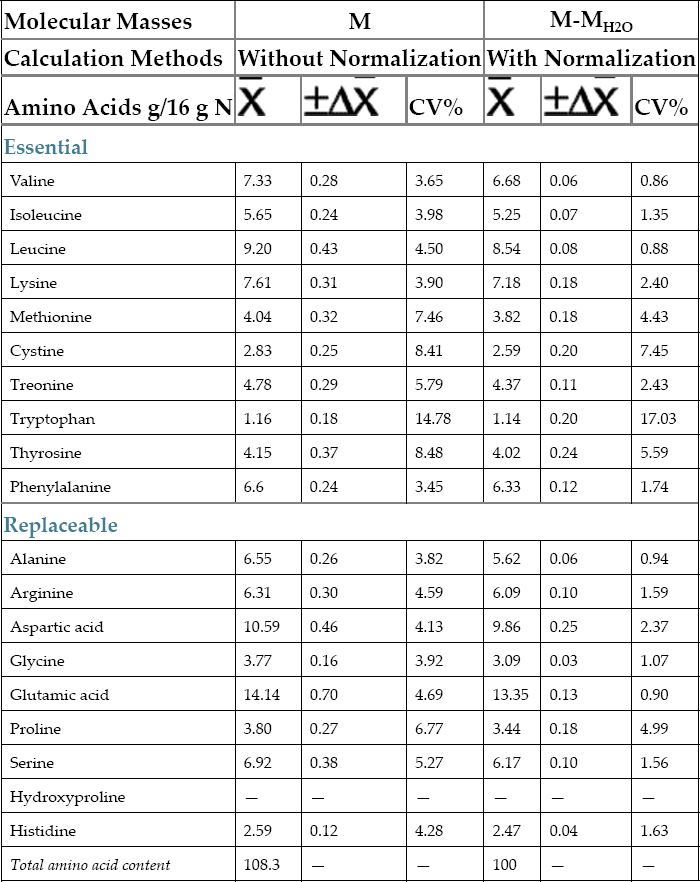
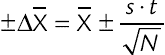 , confidence interval of the value; S, standard deviation; t, Fisher’s coefficient; N, number of tests;
, confidence interval of the value; S, standard deviation; t, Fisher’s coefficient; N, number of tests; 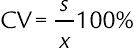 , coefficient of variation (relative standard deviation).
, coefficient of variation (relative standard deviation).