We have all heard the terms greenhouse gases, global warming and climate change.
Greenhouse gases trap heat near Earth’s surface. As they increase in the atmosphere, the extra heat they trap leads to global warming. This warming in turn places pressure on Earth’s climate system, and can lead to climate change.
There is a difference between weather and climate. Weather is what we experience each day. Climate is the sum of all weathers over a certain period, for a region or for the planet as a whole.
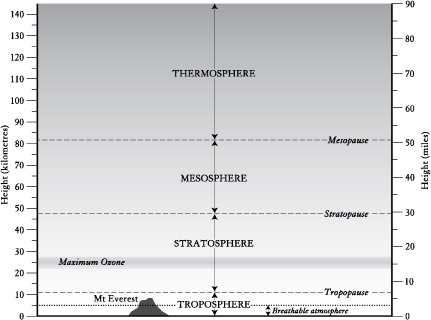
The atmosphere has four distinct layers, which are defined on the basis of their temperature and the direction of their temperature gradient.
The lowest part of the atmosphere is known as the troposphere. Its name means the region where air turns over, and it is so called because of the vertical mixing of air that occurs there.
The troposphere extends on average to twelve kilometres above the Earth’s surface, and it contains 80 per cent of all the atmosphere’s gases. Its bottom third is the only part of the entire atmosphere that is breathable.

The four major parts of the atmosphere, and their associated boundaries. Only a small part of the troposphere is breathable air.
The key thing about the troposphere is that it is ‘upside down’—it is warmest at the bottom, and cools by 6.5°C per vertical kilometre travelled. And it is the only portion of the atmosphere whose northern and southern halves (divided by the equator) hardly mix. That’s why the inhabitants of the Southern Hemisphere don’t breathe the polluted air that limits horizons and dulls panoramas in the more populated north.
The next layer of the atmosphere, known as the stratosphere, meets the troposphere at the tropopause. The stratosphere gets hotter as one rises through it. It is distinctly layered, and fierce winds circulate within it.
Some fifty kilometres above the surface of Earth lies the mesosphere. At -90°C it’s the coldest portion, and above it lies the final layer, the thermosphere, which is a thin dribble of gas extending far into space. There temperatures can reach 1000°C, yet because the gas is so thinly dispersed it would not feel hot to the touch.
The great aerial ocean is composed of nitrogen (78 per cent), oxygen (20.9 per cent) and argon (0.9 per cent). These three gases comprise almost all—over 99.95 per cent—of the air we breathe.
The atmosphere’s capacity to hold water (H2O) depends on its temperature: at 25°C water vapour makes up 3 per cent of what we inhale. But it’s the minor elements, which scientists refer to as ‘trace’ gases—the remaining one-twentieth of 1 per cent—that spice the mix, and some of them are vital to life on this planet.
Take, for example, ozone. Its molecules are composed of three oxygen atoms. Ozone makes up just ten molecules of every million tossed about in the currents of the great aerial ocean. Yet without the shielding effect of that 0.001 per cent, we would soon go blind, die of cancer or succumb to any number of other problems caused by ultra-violet radiation.
We are so small, and the great aerial ocean so vast, it seems impossible that we could do anything to affect it. But if we were to imagine Earth as an onion, our atmosphere would be no thicker than its outermost parchment skin. Its breathable portion does not even completely cover the surface of the planet—which is why climbers on Mt Everest must wear oxygen masks.
The atmosphere looks big because it is made of gas, but if we could compress that gas to liquid we would discover that the atmosphere is only one five-hundredth the size of the oceans. That’s why humanity’s major environmental problems—the ozone hole, acid rain, and climate change— result from air pollution.
And yet the atmosphere is dynamic. The air you just exhaled has already spread far and wide. The CO2 from a breath last week may now be feeding a plant on a distant continent, or plankton in a frozen sea.
In a matter of months all of the CO2 you just exhaled will have dispersed around the planet.
The atmosphere is also telekinetic, which means changes can occur in it simultaneously in different regions. It can transform itself from one climatic state into another instantly. This allows storms, droughts, floods or wind patterns to alter on a global level, at more or less the same time.
Because communication around the planet is now instantaneous, our global civilisation is telekinetic as well, which is why it is such a powerful force. But its telekinesis also explains why regional disruptions—such as wars, famines and diseases—can have dire consequences for humanity as a whole.
The atmosphere blocks out most forms of radiative energy. Many of us imagine that daylight is the only energy we receive from the Sun, but sunlight—visible light—is only a small part in a broad spectrum of radiation that the Sun shoots our way.
The greenhouse gases especially block the forms of radiative energy we call heat. By doing this, however, these gases become unstable and eventually release the heat, some of which radiates back to Earth. Greenhouse gases may be rare, but their impact is massive. They warm our world and, by trapping more heat near the surface, account for the ‘upside down’ troposphere.
Some idea of the power greenhouse gases have to influence temperature can be gained from other planets. The atmosphere of Venus is 98 per cent CO2, and its surface temperature is 477°C.
If CO2 made up 1 per cent of the atmosphere, it could bring the surface temperature of our planet to boiling point.
If you want to feel how greenhouse gases work, visit New York in August. The unhealthy heat and humidity leave you in a lather of sweat in a crowded environment of concrete, parched bitumen and sticky human bodies. And the worst of it comes at night, when humidity and a thick layer of cloud lock in the heat. I recall tossing and turning between sweat-soaked sheets in a room in a rundown neighbourhood known for its drug-dealers and addicts. As my eyes became gritty and my skin began to crust up, I could smell the grime of the city’s 8 million human bodies.
I longed to be in a desert—a dry, clear desert where, no matter how hot the day, the clear skies of night bring blessed relief. The difference between a desert and New York City at night is a single greenhouse gas—the most powerful of them all—water vapour. Remembering that water vapour retains two-thirds of all the heat trapped by all the greenhouse gases, I cursed the clouds overhead.
But clouds have a saving grace too. Unlike the other greenhouse gases, water vapour in the form of clouds blocks part of the Sun’s radiation by day, keeping temperatures down.
So how do CO2 and water vapour interact? As the concentration of CO2 increases, it warms the atmosphere just a little, which allows it to retain more water vapour. This in turn magnifies the original warming. You can think of CO2 as the lever that shifts our climate—or the match that lights the climate change firestorm.
CO2 is produced whenever we burn something or when things decompose. So how do we measure it? In the 1950s, a climatologist named Charles Keeling climbed Mt Mauna Loa in Hawaii to record CO2 concentrations in the atmosphere. From this he created a graph, known as the Keeling curve, that is one of the most wonderful things I’ve ever seen. In it you can see our planet breathing.
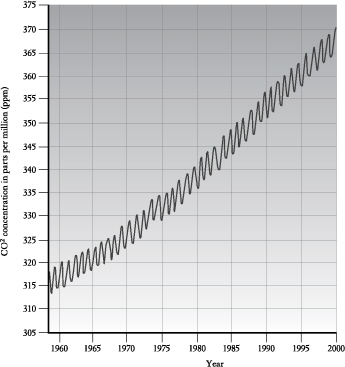
The Keeling curve shows the concentration of CO2 in the atmosphere as measured atop Mt Mauna Loa, Hawaii, between 1958 and 2000. The saw-tooth effect results from the seasonal changes in northern forests, but the inexorable rise is due to the burning of fossil fuels.
Every northern spring as the sprouting greenery extracts CO2 from the atmosphere our Earth begins a great inspiration, which is recorded on Keeling’s graph as a fall in CO2 concentration. Then, in the northern autumn, as decomposition generates CO2, there is an exhalation which enriches the air with the gas.
But Keeling’s work revealed another trend. He discovered that each exhalation ended with a little more CO2 in the atmosphere than the one before. This innocent rise in the Keeling curve was the first definitive sign that we might have to pay a price for our fossil-fuel-addicted civilisation.
Try extending the graph’s trajectory forward into the twenty-first century. Unless we change the way we do things, the concentration of CO2 in the atmosphere will double— from three parts per 10,000 to six.
This increase has the potential to heat our planet by around 3°C, and perhaps as much as 6°C.