Phantasm
Soft staccato lisps of a bush katydid
Rising through cool mists of dusk
In a leftover lonely glacial spruce bog
High in the Appalachian Mountains
Quick insistent chirpings of brown field crickets
Across the moonlit landscape
Of a tiny hill prairie overlooking
Endless Mississippi River bottomlands
Eerily whining buzz of a great green
Grasshopper wafting on the wind
From far across the night-shrouded dunes
Alongside the lake named Michigan
Silvery tinkle of a miniature yellow cricket
Hidden in sun-speckled undergrowth
Of shadowy swamps across
The Everglades of Florida
Crashing synchrony of numberless cicadas
Chorusing deafeningly in the warm brightness
Of a June afternoon in open oak forests
Across the southeastern hills of Ohio
Lazy deep chir-ruping of a solitary beach cricket
Rising ghost-like in crashes of surf
Along a desolate rocky stretch
Of Atlantic coast at midnight
In such atmospheres I have imagined
I am the only human on earth, alone
A thousand centuries ago.
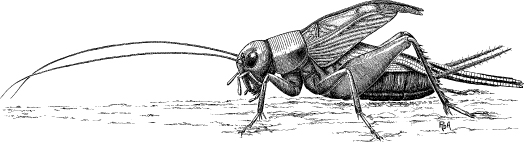
Figure 1.1 Singing male field cricket, courtesy of R. D. Alexander
Dick Alexander once recounted the story of a conversation with his maternal grandfather, Noble Porter Heath II, an Illinois farmer, about his plan to go to Australia to pursue taxonomic studies of the singing Orthoptera. “That’s a long ways to travel on a cricket,” his grandfather quipped. This volume tells of an intellectual voyage that went from a doctoral thesis on the taxonomy, sound production, and classification of field crickets and cicadas, through studies of the social behavior of insects and mole-rats, to seminal influential work on human culture and morality—also a long ways to travel on a cricket.
Alexander’s path-breaking contributions to the study of human evolution did not come out of the blue. They grew from an unusually acute understanding of Darwinian evolution, that is, adaptive evolution by natural selection. He was already a well respected evolutionary biologist—“distinguished” if we can judge by several prominent awards—when he jumped wholeheartedly into the perpetual fray of speculation about human evolution with a 1969 lecture on “The search for an evolutionary philosophy of man” (Alexander 1971). His ability to see the adaptive significance of major patterns in human behavior owes to his preparation for this as a pioneer in Darwinian studies of nonhuman behavior. So looking at his earlier work is important for understanding his innovative thinking about the social behavior of humans and other animals.
The pioneer aspect of Alexander’s work, and the beginnings of a broad interest in evolution, were evident in his graduate research in entomology at Ohio State University. As a student of Donald Borror, Alexander was introduced to insect sounds as tools for the identification of species. But Borror used the characteristics of insect songs as if they were morphological characters with no attention to their functions or evolution, the aspects of most interest to Alexander even then: “Function – behavior, and what it accomplished for the animal—was my first interest” (RDA unpbl ms Autobiography IIIE, 2003).
Systematics has many virtues for understanding evolution. Among them is the need for close attention to phenotypic variation and to the importance of genetics, in particular reproductive (genetic) isolation, for producing the observed variation. Taxonomic research and fieldwork also promotes deep understanding of a particular group of organisms, including its morphology, behavior, and natural history. Alexander’s earliest publications included articles on taxonomy that revealed large numbers of cryptic species, and of previously unappreciated morphological characters, based on studies of their acoustical communication (1957a), as well as papers on sound production itself (1957b) and arthropod communication in general (1960; 1964; 1967). The early publications grew increasingly broad in scope, as they treated an expanding list of behavioral and life history phenomena as factors in speciation (e.g., Alexander and Moore 1962; Alexander and Bigelow 1960) and evolution (e.g., Alexander and Brown 1963).
Many of Alexander’s early papers appealed broadly to students of behavior and evolution, including especially graduate students. My favorite is the now-classic and still valuable monograph on cricket behavior, “Aggressiveness, territoriality, and sexual behavior in field crickets (Orthoptera: Gryllidae)” (Alexander, 1961). That paper documents the effects of male isolation, history of wins and losses, age, size, and copulatory success on social rank, and it describes comparative studies of mating behavior and sound communication, illustrating these phenomena with 63 figures, including photographs of behavior and audiospectographs of the calling, fighting, and courting sounds of eight different species. The monograph ends with a discussion of the evolution of social behavior in insects (see especially p. 212), something that is surprising given the title of the paper, but not given the author. It specifies how phenomena observed in crickets—evolutionary change in length of adult life, and the transfer to females of communication morphology that originated in males—could affect the evolution of sociality in general: “Comparison of the behavior of different species of field crickets and related Gryllinae suggests some of the probable intermediate stages in the evolution of social behavior in insects through an initial isolation of breeding pairs and their attachment to and modification of particular localities” (p. 217).
Dick Alexander was a leader in promoting behavior as a taxonomic tool, but went beyond that to emphasize the importance of behavior for understanding adaptive evolution in general: “To paraphrase an old adage, it is not what one has that counts in evolution, it is what one does with what one has—and what one does is not always entirely clear from what one apparently has,” meaning morphology alone (Alexander 1962a). He once brought an antique apple peeler to class and asked what we guessed its function might be. The gadget had a number of puzzling complicated parts, but their functions were clear once an apple was attached and the handle turned.
A 1962 article in Evolution on “Evolutionary change in cricket acoustical communication” (Alexander 1962b) could serve as a model of how insightful, detailed comparative study of phenotypes—behavior, ontogeny, sex differences and ecology—can be used to make deductions regarding evolution without fossils and without explicit genetic data, using indirect evidence of genetic divergence. These early publications emphasized the use of behavior as the secret to understanding adaptive significance, and the search for adaptive significance as the secret to understanding behavior. It is obvious that such lines of thinking were important for developing ideas about human evolution. They show how Alexander’s bold breach of the boundary between biology and the social sciences grew from a background in taxonomy, a field where orderly and essentially conservative practices prevail and expertise is often deep but circumscribed within what has been called the “comfortable separateness” of a specialized field (Anonymous, 2011). Notable for reaching beyond such boundaries, the 1962 paper on “The Role of Behavioral Study in Cricket Classification” (Alexander 1962a), presented at a 1961 meeting of the American Association for the Advancement of Science, won the AAAS Newcomb Cleveland Prize for an “outstanding contribution to science.”
The scientific values that grew out of Dick Alexander’s early work deeply influenced his later research and that of his students, who, like me, took those values as their own. One of them is a consuming passion for work on a particular group of organisms, with a determination to learn all that is known about it—a commitment to taxon-centered research whose merits I have discussed elsewhere (West-Eberhard 2001). Concentrating initially on evolutionary questions in a particular group of organisms always reveals something of general interest. And it quickly makes a beginner into an expert ready to tackle larger questions, with a concrete basis for critical thinking on almost any major topic in evolutionary biology. It also fosters confidence in one’s ability to make an original contribution of some importance. In Dick’s case the originality and breadth of his comparative studies of the singing insects made him quickly recognized as an expert on the evolution of animal communication and its role in systematics and speciation. From there it is not so difficult to embark on comparably original work on the evolution of humans.
Still, one might wonder: What would motivate a scientists trained as an entomologist to turn from successful work on six-legged creatures to confront the complex and controversial world of research on human evolution? This question has been answered by Alexander himself, in a biographical essay (Alexander 2009, p. 23):
How and why did I make the transition, initiated around 1967, from studying the singing insects as a systematist and behaviorist to eventually writing some 50 articles and two books about how evolution applies to humans and, in particular, human behavior? I decided in 1954, the day after I passed the written and oral preliminary examinations for the doctorate, that I wanted to be an evolutionary biologist. A few years later I realized I would like to think of myself (grandly!) as trying to falsify the hypothesis that everything about life is a result of evolution,… The approach I set for myself would require that I proceed eventually to the most difficult of all traits (behavior) and the most difficult of all species (humans).
The excerpts reprinted here are from “Comparative animal behavior and systematics,” (1969), the published version of a 1967 lecture given at an international conference on systematic biology convened by the National Academy of Sciences in Ann Arbor. It represents a clear bridge between the early papers on behavior and systematics and the later ones on humans. This is the paper that Alexander himself recognizes as the turning point in his commitment to put human evolution at the center of his research (Alexander 2009). Even though the lecture was presented at a symposium on the classification of organisms, it is really an essay on adaptive evolution and the urgency of extending modern Darwinian thought to studies of human behavior. Following some brief introductory remarks on behavior and systematics, the paper moves abruptly to a section headed “Behavior and Man’s Evolution,” with a single transitional sentence: “The facts I have outlined above suggest some of the problems and possibilities in using behavior to understand the history of life. I think one of the best illustrations of these problems and possibilities comes from the evolution of man himself” (p. 495).
With that sentence Alexander began more than four decades of research devoted to understanding human behavior and the evolution of sociality. The excerpts reprinted here emphasize the general principles of adaptive evolution that inspired Dick Alexander, and through him others, to give studies of human evolution a solid Darwinian base.
Alexander, R.D. 1957a. The taxonomy of the field crickets of the eastern United States (Orthoptera: Gryllidae: Acheta). Ann. Entomol. Soc. Amer. 50 (6):584–602.
Alexander, R.D. 1957b. Sound production and associated behavior in insects. Ohio J. Sci. 57 (2):101–113.
Alexander, R.D. 1960. Sound communication in Orthoptera and Cicadidae. In: W. E. Lanyon and W.H. Tavolga (eds.), Animal Sounds and Communication, AIBS Publications 7:38–92.
Alexander, R.D. 1961. Aggressiveness, territoriality, and sexual behavior in field crickets (Orthoptera: Gryllidae). Behaviour 17:130–223.
Alexander, R.D. 1962a. The role of behavioral study in cricket classification. System. Zool. 11 (2):53–72.
Alexander, R.D. 1962b. Evolutionary change in cricket acoustical communication. Evolution 16 (4):443–467.
Alexander, R.D. 1964. The evolution of mating behavior in arthropods. Symp. R. Entomol. Soc. Lond. 2:78–94.
Alexander, R.D. 1967. Acoustical communication in arthropods. Annu. Rev. Entomol. 12:495–526.
Alexander, R.D. 1969. Comparative animal behavior and systematics. In: Systematic Biology. Proceedings International Conference on Systematics (Ann Arbor, Michigan, July 1967). National Academy of Sciences Publication 1962: 494–517.
Alexander, R.D. 1971. The search for an evolutionary philosophy of man. Proc. R. Soc. Victoria 84:99–120.
Alexander, R.D. 2009. Understanding ourselves. In L.C. Drickamer and D.A. Dewsbury (eds.), Leaders in Animal Behaviour: The Second Generation. Cambridge: Cambridge University Press, pp. 1–37.
Alexander, R.D. 2011. The Mockingbird’s River Song: Poems, Essays, Songs and Stories, 1946-2011. Manchester, MI: Woodlane Farm Books.
Alexander, R.D. and Bigelow, R.S. 1960. Allochronic speciation in field crickets, and a new species Acheta veletis. Evolution 14 (3):334–346.
Alexander, R.D. and Brown, W.L. 1963. Mating behavior and the origin of insect wings. Univ. Mich. Occas. Pap. 628:1–19.
Alexander, R.D. and Moore, T.E. 1962. The evolutionary relationships of 17-year and 13-year cicadas with three new species (Homoptera: Cicadidae: Magicicada). Univ. Mich. Mus. Zool. Misc. Pub. 121:1–59.
Anonymous, 2011. Editorial statement. Daedalus 140 (1):1.
West-Eberhard, M.J. 2001. The importance of taxon-centered research in biology. In M. J. Ryan (ed.), Anuran Communication. Washington, DC: Smithsonian Institution Press, pp. 3–7.
Behavior is probably the most diverse aspect of the animal phenotype—at least, as William Morton Wheeler (1905) put it, “in the field of possible observation.” On this basis alone, behavior should be fascinating to the systematists because they are always looking for characters. Furthermore, among biologists, systematists are, more than any other group, the real students of diversity. Comparison is their chief method of exploration, and the comparative method, of course, depends upon and thrives upon diversity.
On the other hand, to some extent the diversity of behavior results from its being, in general, more directly and probably more complexly related to the genotype than to any other aspect of the phenotype. This particular feature discourages the systematists. They are not interested in getting involved with phenotypic variations that might be due solely to variations in the developmental environment. After all, morphology is troublesome enough in that regard.
Behavior has some other special features. In general, it is more strongly selected—or perhaps I should say more directly selected—than morphology or physiology. By this I mean that in any representation of the chains of cause-effect relationships between gene action and selective action in animals, behavioral characteristics nearly always would be placed directly next to selective action.1
The systematist’s concern with adaptation should prevent him from passing this off too lightly. On the other hand, behavior is often difficult to document or to communicate to others. As Dr. Wagner stressed in his paper, repeatability is the essence of science, and to many taxonomists this traditionally has meant that morphology alone is sacred. Very little behavior is evidenced by preserved specimens or fossils.
The facts I have outlined above suggest some of the problems and possibilities in using behavior to understand the history of life. I think one of the best illustrations of these problems and possibilities comes from the evolution of man himself. We surely would all agree that the most important thing we could possibly discover about man’s transition from the nonhuman state to the human state would be how he behaved during that period—the details of what he did and how he lived while he was evolving into a man. We know positively that he did make the transition from ape to man. What we do not know is precisely how he did it. By that I mean we do not know what the selective forces were, and, for example, why such forces seem to have been relatively strong and unidirectional for a while—at least in regard to changes in size of the brain case—and then to have slacked off, perhaps rather abruptly, some tens of thousands of years ago. We speak (vaguely, I think) of tools and communication, and of growing food and fighting off predators, but the truth is we still have no really good notion how and why men with bigger brains once outreproduced those with smaller brains and then stopped doing so.
A wide range of possibilities still exists, and the answers could very well turn out to be more startling than most of us might suppose. As one example, we do not really know what kinds of predators, if any, might have been involved in the steady increase in man’s brain size, and, as much as we may dislike the idea, I believe the possibility still exists that man himself is the only one that could have done the job.
Perhaps I can explain what I mean, and demonstrate some of our ignorance about man’s evolution, by posing a question. Intraspecific competition, in connection with natural selection, may be said to occur in three possible forms. Sometimes different individuals simply compete indirectly, without direct interactions, for whatever food, mates, shelter, or other commodities may be in short supply. In other cases, some kinds of individuals may partially or completely exclude others from the best sources of food, mates, and shelter through territoriality of one sort or another. There is another possibility, less often recognized. Superior individuals might sometimes actually pursue and destroy competitors, or potential competitors, thus removing them and their descendants from the possibility of competing. Such a superior individual might, in addition to removing competition, actually derive direct benefit from the slaughter, through cannibalism. Which of these three kinds of intraspecific competition operated during the evolution of humans from nonhuman primates, and how significant was each? The question has certainly not been answered; I do not think it has even been clearly posed before. Yet the different possibilities could scarcely fail to produce widely different attitudes among men trying to understand themselves and their behavior through knowledge of history. [Since submission of this manuscript the ideas involved here have been discussed and extended in a book review coauthored by D. W. Tinkle (Bioscience 18:245–248).]
Sometimes I have thought that to understand the selective action that made a nonhuman primate into a man could be the most important question in all of biology. It could change man’s attitude toward nearly everything he does or tries to do—in education, politics, religion, and all the rest—for it could tell him more precisely what he is, and therefore why, in one sense, he persists in doing some of the things he does, and why he still fails to accomplish some of the things he seems to want to do. Any adult who has tried to explain to a child the pre-eminence of things sexual in so much of human affairs (as well as in the lives of other organisms) without using natural selection in his explanation surely will understand what I mean. To use another example, it is possible that we should be taking the history of selective action upon man much more directly into account in our attempts to deal with overpopulation and its consequences.
In other words, we cannot learn how man became a man, and therefore, in a sense, what a man really is, without knowing some things about the history of his behavior. Yet, it seems that the only thing we can do about this problem is to dig and scrape around at a few fossils that reflect his morphology and represent a few indirect traces of his behavior.
It seems as though this is all we can do; but my theme here is that such an idea about evolution is false. I suggest that we can find out how man’s behavior evolved and the kinds of selective action that were involved. More fossils will help, of course, but we can do it without fossils if we have to; in any case, the most important advances in understanding man’s history may not come from fossil evidence, and I consider it unlikely that satisfactory progress will come from the efforts of humanists who are not simultaneously first-rate evolutionary biologists. I believe that we will make the significant advances in this area in the same way that we eventually would have arrived confidently at the conclusion—even without the help of a single fossil—that man and the other living primates have diverged from common ancestors. We would have done this, of course, through extensive, intensive, and perceptive comparative study over a period of time long enough for us to have developed—on the side, from direct observation and experimentation—an understanding of the steps and the mechanics of the process of evolution.
It should be clear by now that I am not arguing simply about the role of behavior as a tool for taxonomists. I want to argue instead for the establishment of a reasonable relationship between those biologists interested primarily in behavior and those interested primarily in systematics in the broadest sense—a relationship that will result in the kind of reverberating feedback between these fields that both need, and have needed, for a long time. I think the key to this relationship—perhaps the only key—lies in applying the comparative method to behavior on a much wider scale than has been the case. I realize that I am one small voice in a long line of people carrying this particular argument to the zoologists. But I do think the point has not yet been properly made.
To some zoologists—though perhaps not to those here—to argue for a rejuvenation of comparative study must sound a little old-fashioned. Nowadays biologists are calling for precise, quantitative results and for more and more experimentation. Comparative study and the broad-scale, observational-descriptive work that undergirds it are often viewed as outdated, trivial pursuits. The need for more experimental work, however, and the possibility of more precise experimentation do not reduce the need for good, evolutionarily oriented, comparative investigations. Rather, though it may surprise some biologists, the need for comparative study is thereby increased, for it is a central role of comparison to tell us which experiments to do, and which ones to do first.
In his recent book on adaptation and natural selection, Williams (1966a) argued that systematists never will prosecute the study of adaptation the way it ought to be prosecuted. All of us will agree that there is a lot of shortsighted, narrow-minded systematic work going on but, contrary to Williams’ argument, I believe that the methods of systematists represent a great potential contribution to the study of adaptation, beginning at the point where we find ourselves today. And I refer specifically to the comparative study of behavior, which Williams himself employed effectively in his book. Comparative study is the stock-in-trade of the systematist. It has never been the stock-in-trade of any other group of biologists in a very extensive, persistent, or pertinent fashion—least of all, perhaps, the behaviorists.
One of my favorite psychologists argued recently that molecularly oriented biologists are on the wrong track when they believe they can predict everything of significance about the biological world through a knowledge of structure and function at molecular and submolecular levels. He noted that, while theoretically this may be possible, it is unreasonable or impractical unless one knows beforehand what it is that he must be able to predict. I suggest that the same criticism can be leveled at many people studying behavior. They expect to be able to predict from precise, quantitative, laboratory experimentation without having any idea of the complexity, the variety, or even the nature of the things they will have to predict.
Zoologists left behavior largely to the psychologists, long past the time of knowing that psychologists in general do not answer the kinds of questions that zoologists must have answered. And systematists, in turn, have left zoological studies of behavior to the experimental zoologists, despite the fact that certain questions about behavior that are of importance to everyone are not going to be answered for a very long time using the methods generally conceded to experimental biology.
There is nothing mysterious about the comparative method. Yet I am convinced that many systematists and other biologists who use it all the time scarcely know what they are accomplishing with it, are not sufficiently prepared to explain and defend its problem-solving value, and, in any case, could not give a clear exposition of its usefulness to systematics or to biology in general.
So far, I have said nothing about what undoubtedly has been the knottiest problem in the study of animal behavior, and the one responsible more than any other, I suppose, for slowing the advance of comparative study of behavior. If we call this the “problem of instinct,” most people have a good idea what is meant. It would be more descriptive, however, to term it the problem of the extent and nature of hereditary influences in behavioral variations, both between species and among the individuals of each species.
Adaptation is a result of selective action on alternative genetic phenomena. Therefore, it is critical for my topic to determine which behavioral variations correlate with genetic variations. Few people challenge the idea that certain species differences, such as frog and insect calls, firefly flashes, or other behavioral characteristics identified as reproductive isolating mechanisms in any kinds of animals, have genetic bases. And, we know from hybridization experiments that insect and anuran call differences do indeed have genetic bases. In fact, results from crossing experiments on crickets and frogs probably are cited more frequently in reviews concerning transmissibility of behavioral variations than are any experiments with other kinds of animals.
The systematist wants to know more about this. He wants to know whether he can be sure that he is not examining some behavioral difference that has nothing to do with hereditary differences. Some systematists and other biologists have gotten involved in long, bitter, and futile arguments about whether heredity or environment has greater influence in determining the characteristics of particular behavior patterns.
Concerning this topic, we are pursued now by a whole string of admonishments: “To ask how much a given aspect of behavior depends upon genetic factors and how much upon environmental factors is like asking how much of the area of a field depends upon its length and how much upon its width”; “Nothing is inherited but the genotype and a little cytoplasm”; “Heredity is particulate; but development is unitary”; “Instead of speaking of this or that trait as genetic or environmental, the correct way is to ask yourself which, and the extent to which, differences in characters are due to environment on the one hand and to heredity on the other.”
Konishi (1966) has recently written a paper that, I think, clarifies some issues Involved in this problem. He points out that, as one of our shortcomings, we have acted as though it is always true that, in behavior, stereotypy = species specificity = inheritance = central coordination = spontaneity = self-differentiation. These factors are not strictly correlated and, as with learning, what has been called “instinctive” behavior really is not a single phenomenon, and it should not be treated as if it were.
But not all these issues are of great or immediate concern to the systematist or evolutionary biologist interested in behavior. What is of concern is predictability. And it is very likely that significant increases in predictability, in many cases, can be attained sooner by insightful, properly directed, broad-scale (even superficial) comparisons than by detailed studies of development of specific patterns of behavior in individual animals or species. The comparative anatomists, as many ethologists have emphasized, have already shown this to be true. We systematists seem to have allowed ourselves to be overly concerned about precisely how individual patterns of behavior develop. We do not know a great deal about the development of morphology in a wide variety of animals; but we do know a very great deal about speciation, adaptation, and phylogenetic history—all of which knowledge was gained directly, almost solely, from comparisons of those very features of anatomy whose development we still do not understand.
When we will have carried out broad-scale comparative studies of behavior similar to those available in anatomy, and when we begin to acquire the glimmers of understanding that will come from predictiveness based on such studies, then those investigators concerned chiefly with the developmental bases of phenotypic differences will, indeed, have something to think about and work with. Because the question then would concern how much genetic variation is involved, a considerably sharper focus should be provided for the investigations of many biologists now skeptical that broad-scale comparisons can be made in the absence of extensive information on developmental pathways and stimuli for particular behavioral units.
This remark may raise some eyebrows, but I suggest that behavioral variations that at first glance appear useful to systematists—particularly to those working at and above the species level—rarely lack correlation with specific genetic variations. For example, is anyone here in a position to describe a species difference in behavior—any species difference in behavior—that he has cause to suspect does not have a genetic basis?2
Further, and of great importance, the extent and nature of correlations between behavioral variations and genetic variations—or their absence—is predictable to a large extent.
One aspect of such predictability can be exemplified by cricket calls. Examination of cricket biology soon reveals that in most temperate species only the eggs pass the winter, and that the auditory organs are not functional until maturation or near-maturation. With this information alone we can predict confidently that (at least usually) selection favors insulation from influences by environmental sounds in the establishment of the pattern of the call (R. D. Alexander, “Arthropods” in T. Sebeok, Animal Communication, to be published by Indiana University Press), for there can be no appropriate sounds available to copy.
On the other hand, even a limited knowledge of passerine-bird biology allows the reverse prediction: that most young birds probably have evolved specific ways of being influenced by their parents’ song patterns. There are at least two reasons: the overlap of young and adults in each generation, and an apparent premium on individuality in song pattern; the latter is associated with the presence of specialized parental behavior and tendencies toward monogamy and is promoted by having part of the pattern learned.
In precocious birds both the critical periods of song learning and the imprinting of following behavior are predictable on the same general basis. Even the indiscriminateness of suitable stimuli for imprinting of following behavior is predictable, for the situation is such that unsuitable stimuli are not likely to be available, and selection, therefore, will have no chance to focus on a restricted group of stimuli. Likewise, we would predict that different populations of birds within a given species should sometimes have song differences that lack genetic bases, as is the case with human languages.
The psychologists who chastised ethologists for erecting dichotomies with regard to learned and unlearned behavior were right, for many ethologists had their dichotomies out of focus. But this argument became a source of confusion rather than clarification when it took the form of rejecting all implications of important dichotomies in the way behavior patterns develop. An important dichotomy can be identified in the examples I have just given: Does the selection favor the use of a given stimulus (sound, for example) in the establishment of a pattern in the same modality as the stimulus or does it specifically favor insulation from all stimuli in that particular modality? This is a dichotomy as to direction of selection, and it leads to extreme differences in certain relationships between genetic phenomena and behavioral characteristics. We identify it and discover its significance by studying adaptation and natural selection in relation to behavioral development.
I suggest that selection acting consistently on any kind of behavioral variation, regardless of its original basis, will usually result in the presence, ultimately, of genetic variation that relates directly to the behavioral variation. This would mean that the more ancient a behavioral difference, the more likely it is to have some genetic basis. By this I do not mean to imply any special kind of selection, I simply mean that selection will work on both genetic and non-genetic variations, but evolution will occur only when genetically based variations become available.
No broad-scale attempt has been made to study adaptation in behavioral terms by the kind of comparison and prediction that I have just described. Yet, if what I have argued is true, such attempts would be fruitful, even those dealing with the behavior of man, of all organisms the most “labile” in the functioning of his phenotype.
I will use a simple example cited by Williams (1966a), who notes that the females of many kinds of animals usually are described as being more “coy” or/ discriminating or reluctant in copulation than the males (or one could turn it around and say that the males are more “aggressive” in courtship), and he notes that this is predictable because in each copulation or fertilization the female invests a greater proportion of her total reproductive potential than the male invests of his. If this argument is correct, then, as Williams points out, the situation should be reversed in parental animals in which the male is solely responsible for the zygotes, or more involved in parental behavior. Such reversals have been reported in pipefishes in the genus Syngnathus, in which the males carry the fertilized eggs (Fiedler, 1954), though not in all such fish (Breder and Rosen, 1966; Straughan, 1960), and also in such birds, as some tinamous and phalaropes, in which the males incubate the eggs and protect the young (Bent, 1927; Tinbergen, 1935; Höhn, 1967).
Similar reversals as to which sex behaves territorially, fights off intruding individuals, and courts more aggressively have been reported in the ornate tinamou (Pearson and Pearson, 1955), red phalarope, northern phalarope, and Wilson’s phalarope (Tinbergen, 1935; Höhn, 1967; Bent, 1927). Polyandry is more likely to be prominent in such animals, and strict polygyny ought to be rare, although both polygamy (or promiscuity on the part of both sexes) and monogamy have been reported (Lancaster, 1964; Höhn, 1967). Polygyny, on the other hand, is prominent among species in which the females carry most or all of the parental responsibility, and polyandry is almost nonexistent. Some of the disagreements in the literature (e.g., see Höhn, 1967) may result from differences in sex ratios among demes studied by different investigators. In some cases, what happens when sex ratios are locally or temporarily uneven may be important in understanding how selection has operated.
The relationship between reproductive effort and proportion of reproductive potential involved in any circumstance or event can be extended to include not only the proportion of eggs or sperm used per copulation but also the proportion of the breeding season used per clutch or pregnancy and the proportion of the total probable reproductive life involved in each season, as Dr. Tinkle demonstrated on this program. Such considerations of proportions must include also the likelihood of changes in reproductive possibility—such as improvements through learning about one’s mate or about the food and predators in one’s territory—and the likelihood of improvement in weather conditions. Williams (1966b) and Lack (1966) have pointed out that this means that longer juvenile lives will correlate roughly with longer reproductive lives, and that clutch sizes will increase with age. We should expect, especially in long-lived, monogamous animals with specialized parental behavior, that selection continually will maximize the slope of a line depicting the increasing reproductive ability of individuals and pairs.
Since man’s plasticity in behavior seems for a long time to have been a major reason for our reluctance to discuss the general problem of behavior in relation to heredity and, therefore, a major reason for the reluctance of systematists and other biologists to use behavior in comparative work, it is appropriate that I conclude by referring to the possibility of heredity in an example of variation in man’s behavior. I will use the previously mentioned theory of female “coyness,”; or difference between male and female, and suggest that what we frequently and sometimes jokingly refer to as the “double standard” in man’s sexual behavior is, in part, a reflection of differences in selective action on male and female behavior during man’s evolutionary history.
In general, in both polygynous and monogamous animals with specialized parental behavior, selection should favor females that promote monogamy and should favor males that promote polygyny. Even in evolutionary lines in which monogamy is never actually realized, tendencies toward it in females would be favored consistently if the male’s cooperation in any way promoted the female’s reproductive success. Likewise, even in a monogamous line polygynous tendencies in males often would be favored because in a species in which the female is responsible for the fertilized eggs a male is much more likely to benefit from, shall we say, “stealing” copulations with his neighbors’ females than is the female who indulges in the same kind of behavior. Tendencies toward polyandry in man are evidently rare, but tendencies toward polygyny are not nearly so rare.
Is it reasonable to argue that there likely are no genetic correlates underlying even subtle intraspecific differences of this sort in an organism as plastic as man, when selection on man’s breeding system has probably been consistent in the ways I have described all through man’s evolutionary history? I think not.
I have dealt in this paper with a few points that I believe will be useful in making the analysis of behavior more important in systematic work than it has been in the past, in searching for both similarities and differences among organisms. To bring these two fields into closer cooperation, I believe we need, chiefly, to be (1) more aware of the role of the comparative method in biology and in behavioral analysis, (2) more thoughtful in our searches for behavioral variations likely to be correlated with genetic differences, and (3) as systematists, more cognizant than we have been of the significance of studying adaptation directly, both by experimentation and by comparison.
I wish to thank Daniel Otte, Ann Pace, and Mary Jane West, graduate students at the University of Michigan, for assistance in developing the ideas presented here and for critical examination of the manuscript at various stages.
Alexander, R.D. and Bigelow, R. 1960. Allochronic speciation in field crickets, and a new species Acheta veletis. Evolution 14:334–336.
Bent, A.C. 1927. Life Histories of North American Shore Birds. Part 1. Washington, DC: US National Museum Bulletin 142.
Breder, C.M. and D.E. Rosen, 1966. Modes of reproduction in fishes. Neptune City, NJ: T.F.H. Publications.
Fiedler, K. 1954. Vergleichende verhaltenstudien an seenadeln, schlangennadeln und seepferdchen (Syngnathidae). Z. Tierpsychol. 11:358–416.
Höhn, E.O. 1967. Observations on breeding biology of wilson’s phalarope (Steganopus tricolor) in central Alberta. Auk. 84:220.
Konishi, M. 1966. The attributes of instinct. Behaviour 27:316–327.
Lack, D. 1966. Population Studies of Birds. Oxford: Clarendon Press.
Lancaster, D.A. 1964. Life history of the Boucard Tinamou in British Honduras. Part II: Breeding Biology. Condor 66:253–276.
Pearson, A.K., and Pearson, O.P. 1955. Natural history and breeding behavior of the tinamou, Nothoprocta ornata. Auk 72:113–127.
Straughan, R. P. L. 1960. 100 seahorses spawn. Aquarium J. 31:302–308;325–326.
Tinbergen, N. 1935. Field Observations of East Greenland Birds. I. The Behaviour of the Red-Necked Phalarope (Phalaropus lobatus L.) in Spring. Ardea 24:1–42.
Wheeler, W. M. 1905. An interpretation of the slave-making instincts in ants. Bull. Am. Mus. Nat. Hist. 21:1–16.
Williams, G.C. 1966a. Adaptation and Natural Selection. Princeton, NJ: Princeton University Press.
Williams, G.C. 1966b. Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am. Natur. 100:687–690.