Chapter 22
Polymer Applications: Adhesives
22.1 Adhesives
Adhesives have been a technologically important application of polymers for thousands of years. Many of the early natural adhesives are still used. These include starch and protein-based formulations such as hydrolyzed collagen from animal hides, hooves, and bones, and casein from milk. As new adhesive formulations based on synthetic polymers (often the same polymers used in other applications) continue to be developed, the range of applications for adhesives has expanded dramatically [1–7].
An adhesive has been defined as a substance capable of holding materials (adherends) together by surface attachment. Adhesives offer a number of significant advantages as a means of bonding. (1) They are often the only practical means available, particularly in the case of small adherends. For example, it is hard to imagine welding abrasive grains to a paper backing to make sandpaper or bolting the grains together to make a grinding wheel. (2) In the adhesive joining of large adherends, forces are fairly uniformly distributed over large areas of the adherend, resulting in low stresses, and holes (necessary for riveting or bolting), which invariably act as stress concentrators in the adherends, are eliminated, thus lowering the possibility of adherend failure. (3) In addition to joining, adhesives may also act as seals against the penetration of fluids. In the case of corrosive fluids, this, coupled with the absence of holes, where corrosion usually gains an initial foothold, can minimize corrosion problems. (4) In terms of weight, it does not take much adhesive to join much larger adherends. Usually, a molecular thickness of the glue is adequate. Hence, it is not surprising that many high-performance adhesives were originally developed for aerospace applications. (5) Adhesive joining may offer economic advantages, often by reducing the hand labor necessary for other bonding techniques.
A detailed treatment of the science of adhesion is beyond the scope of this chapter. Nevertheless, some important generalizations will be drawn [8, 9]. Adhesion results from (1) mechanical bonding between the adhesive and adherend, and (2) chemical forces—either primary covalent bonds or polar secondary bonds—between the two surfaces. The latter are thought to be the more important, and this explains in part why inert nonpolar polymeric substrates, such as polyethylene and polytetrafluoroethylene (PTFE), present challenges to forming adhesive bonds (the process for bonding PTFE to cookware is a closely guarded trade secret of DuPont). To adhere these kinds of polymers to a surface, they must first be chemically treated to introduce polar sites on the surface. To promote mechanical bonding, adherend surfaces are often roughened before joining, but this is sometimes counterproductive. It can trap air bubbles at the bottom of crevices that act as stress concentrators and promote failure in rigid adhesives.
The strength of adhesion can be measured using an automated materials testing system, such as the one described in Chapter 16. By pulling the bond apart (e.g., by applying axial tension), the work of adhesion can be measured. Peel tests are also commonly done, and these largely resemble pulling a piece of tape (or a Band-Aid®) off a surface.
With good bonding between adhesive and adherend, joint failure is cohesive (the adhesive itself or the substrate fails). Where the adhesive is weaker than the substrate, to a good approximation, the properties of the adhesive polymer determine the properties of the adhesive joint, that is, the bond can be no stronger than the glue line. Brittle polymers give brittle joints, polymers with high shear strengths give bonds of high shear strength, heat-resistant polymers produce bonds with good heat resistance, and so on.
To form a successful joint, the adhesive must intimately contact the adherend surface. First this requires that it wet the surface. The subject of wetting is considered in detail in a number of sources on surface chemistry [10, 11]. In general, wetting is promoted by polar secondary forces between adhesive and substrate. This is another reason why low-polarity polymeric adherends such as polyethylene and PTFE are difficult to bond with adhesives. To insure proper wetting and interfacial bonding, it is often necessary to clean the adherend surfaces carefully before joining [12]. Good contact also requires a viscosity low enough under conditions of application to allow the adhesive to flow over the surface and into its nooks and crannies. Once contact has been established, the adhesive must harden to provide necessary joint strength. There are five general categories of organic adhesive that accomplish these objectives in different ways.
22.1.1 Solvent-Based Adhesives
Here the adhesive polymer is made to flow by dissolving it in an appropriate solvent. Thus, the polymers used must be linear or branched to allow solution, and the joints formed will not be resistant to solvents of the type used initially to dissolve the polymer. To get a good bond, it helps if the solvent attacks the adherend also. In fact, solvent alone is often used to “solvent weld” polymers, dissolving some of the adherend surface to make it sticky and form an adhesive on application.
One of the drawbacks to solvent-based adhesives based on rigid polymers is the shrinkage that results when the solvent evaporates. This can set up stresses that weaken the joint. An example of this type of adhesive is the familiar model airplane cement, basically a cellulose nitrate solution, with perhaps some plasticizer. Rubber cements, of course, maintain their flexibility, but cannot support as great a stress. Commercial rubber cements are based on natural, SBR (poly(butadiene-co-styrene)), nitrile (poly(butadiene-co-acrylonitrile)), chloroprene (poly(2-chlorobutadiene)), and reclaimed (devulcanized) rubbers. Examples are household rubber cement and Pliobond®. Rubber cements may also incorporate a curing agent to crosslink the polymer after application and evaporation of the solvent. This greatly increases solvent resistance and strength.
22.1.2 Latex Adhesives
These materials are based on polymer latexes made by emulsion polymerization. They flow easily while the continuous water phase is present and dry by evaporation of the water, leaving behind a layer of polymer. In order that the polymer particles coalesce to form a continuous joint and be able to flow to contact the adherend surfaces, the polymers used must be above their glass transition temperature at use temperature. These requirements are similar to those for latex paints, so it is not surprising that some of the same polymers are used in both applications, for example, styrene-butadiene copolymers and poly(vinyl acetate). Nitrile and neoprene rubbers are used for increased polarity. A familiar example of a latex adhesive is “white glue,” basically a plasticized poly(vinyl acetate) latex. Latex adhesives have displaced solvent-based adhesives in many applications because of their reduced pollution and fire hazards. They are used extensively for bonding pile and backing in carpets.
22.1.3 Pressure-Sensitive Adhesives [13]
These are really viscous polymer that melts at room temperature, so the polymers used must be above their glass transitions. They are caused to flow and contact the adherends by applied pressure, and when the pressure is released, the viscosity is high enough to withstand the stresses produced by the adherends, which obviously cannot be very great. The key property for a polymer used in this application is tack; thus low molecular weight additives, which can compose up to 40 wt% of the adhesive, that increase tack are called tackifiers. Tack basically means that the polymer has a low enough viscosity to permit good surface contact, yet is high enough to resist separation under stress, something on the order of 104–106 cp [14], although elasticity probably also plays a role. Natural, SBR, and reclaimed rubbers are common in this application, although weaker adhesives have found one important use: Post-it® notes. The many varieties of pressure-sensitive tape are faced with this type of adhesive.
Contact cements are a variation in which the rubbery polymer is applied to each adherend surface in the form of a solution or, increasingly, a latex. Evaporation of the solvent or water leaves a polymer film with the tack necessary to grab and hold the adherends when they are pressed together.
22.1.4 Hot-Melt Adhesives
Thermoplastics often form good adhesives simply by being melted to cause flow and then solidifying on cooling after contacting the surfaces under moderate pressure. Polyamides and poly(ethylene-co-vinyl acetate) are used frequently as hot-melt adhesives. Electric “glue guns,” which operate on this principle, are readily available in the consumer market (through hardware and craft stores).
22.1.5 Reactive Adhesives
These compounds are either monomers that are low molecular weight polymers, which solidify by a polymerization and/or crosslinking reaction after application. They can develop tremendous bond strengths and have good solvent resistance and good (for polymers, anyhow) high-temperature properties. The most familiar example of reactive adhesives are the epoxies (Example 2.4O) generally cured by multifunctional amines. These are often sold as two-part epoxies, where the two components are kept in physically separated chambers and contact each other to start the reaction when applied to a surface. Polyurethanes (Example 2.4Q) also make excellent reactive adhesives.
The α-alkyl cyanoacrylate “super glues” (“one drop holds 5000 lbs”) are now a familiar part of the consumer market. Originally, the monomers had extremely low viscosities and so could crawl into narrow crevices and wet the adherend surfaces rapidly. On the other hand, they would not fill gaps and were absorbed into porous adherends, giving poor bonds. Newer versions are available with higher viscosities to overcome these drawbacks. Cyanoacrylates can polymerize in seconds by an anionic addition reaction believed initiated by hydroxyl ions from water adsorbed on the adherend surfaces:
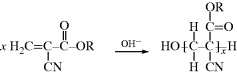
Unfortunately (or fortunately, if you stick your fingers together), being linear and polar, they have poor resistance to polar solvents (acetone is a good solvent), and they are subject to hydrolysis and so have poor environmental stability. Octyl cyanoacrylate, where the R group is C8H17, is better known as Dermabond®, and has been used in medicine to seal wounds with a strong bond while also creating a barrier to bacteria that may infect a wound during the healing process. Here, the adhesion bonds are meant to slowly dissolve or wear away, replaced by the healed tissue. Other reactive surgical glues, such as fibrin glues, are based on natural proteins that are part of the blood coagulation cascade, and can be used to seal internal wounds.
Phenolic and other formaldehyde condensation polymers are also important reactive adhesives. Powdered phenolic resin is mixed with abrasive grains and the mixture is compression molded to form grinding wheels. A B-stage phenolic (see Chapter 18.4 on polymer additives, curing agents) in a solvent is used to impregnate tissue paper. The solvent is evaporated and the dry sheets are placed between layers of wood in a heated press, where the resin first melts and then cures, bonding the wood to form plywood. Similarly, sheets of paper impregnated with a B-stage melamine–formaldehyde resin are laminated and cured to form the familiar Formica countertops.
Unlike the previous examples of reactive adhesives, the phenolics and other formaldehyde condensation polymers evolve water as they cure. If trapped in the joint, this can result in serious weakness, which limits their adhesive applications.
Note that all these examples of reactive adhesives are highly polar polymers that often possess side groups with strong dipoles. It is largely the polarity and secondary bonds that account for their good bonding capabilities.
1. Temin, S.C., Adhesive compositions, p. 547 in Encyclopedia of Polymer Science and Engineering, 2nd ed., Vol. 1, J. Kroschwitz (ed.), Wiley, New York, 1985.
2. Patrick, R.L., Chemistry and technology of adhesives, Chapter 34 in Applied Polymer Science, J.K. Craver and R.W. Tess (eds), American Chemical Society, Washington, DC, 1975.
3. Shields, J., Adhesives Handbook, 3rd ed., Butterworths, London, 1984.
4. Hartshorne, S.R. (ed.), Structural Adhesives: Chemistry and Technology, Plenum, New York, 1986.
5. Wake, W.C., Adhesion and the Formulation of Adhesives, 2nd ed., Applied Science, London, 1982.
6. Wake, W.C. (ed.), Synthetic Adhesives and Sealants, Wiley, New York, 1987.
7. Landrock, A.H., Adhesives Technology Handbook, Noyes, Park Ridge, NJ, 1985.
8. Gent, A.N. and G. R. Hamed, Adhesion, p. 476 in Encyclopedia of Polymer Science and Engineering, 2nd ed., Vol. 1, J. Kroschwitz (ed.), Wiley, New York, 1985.
9. Meyer, F.J., Bonding, p. 518 in Encyclopedia of Polymer Science and Engineering, 2nd ed., Vol. 1, J. Kroschwitz (ed.), Wiley, New York, 1985.
10. Adamson, A.W., Physical Chemistry of Surfaces, 5th ed., Wiley, New York, 1990.
11. Hiemenz, P.C., Principles of Colloid and Surface Chemistry, 2nd ed., Dekker, New York, 1986.
12. Wegman, R.F., Surface Preparation Techniques for Adhesive Bonding, Noyes, Park Ridge, NJ, 1989.
13. Sates, D.(ed.), Handbook of Pressure-Sensitive Adhesives, 2nd ed., Van Nostrand Reinhold, New York, 1987.
14. Skeist, I., Adhesive compositions, p. 482 in Encyclopedia of Polymer Science and Technology, Vol. 1, N. Bikales (ed.), Wiley, New York, 1971.