In AD 1054, a star in the constellation of Taurus (named by the Greeks for its apparent likeness to a bull14) flared dramatically in brightness; so much that it could even be seen during the day, when the Sun is bright enough to outshine all other stars. Chinese astronomers referred to these brightened stars as ‘kèxīng’ (客星) – ‘guest stars’ – and meticulously recorded their appearance. They noted that the guest star of 1054 was visible for another 642 nights in the night sky (around twenty-one months!), before fading away entirely.
Today, almost a thousand years later, if you were to take a telescope and look at that very same position in the sky in the constellation of Taurus, you would see something dramatically different from a star: you would see a nebula. A maelstrom of gas and dust lit from the centre by the glowing embers of a star too faint to see. This is the leftovers of a dead star, one that ran out of hydrogen fuel and as it desperately tried to prevent the inevitable, outshone every other star in the sky for those short few months, before leaving behind a shadow of what it once was. This ghostly scene is known as the Crab Nebula, and it is a milestone in the history of humanity’s knowledge of the death of stars, and our realisation of the existence of black holes.

The Crab Nebula; the remnant of supernova SN 1054.
While the Crab Nebula is not one of the brightest objects in visible light that we can see with our eyes, it is one of the brightest emitters of incredibly high-energy light known as gamma rays. Light comes in all different shapes and flavours, which are determined by the amount of energy the light wave has. In visible light (also known as optical light), the different colours that we can see with our eyes are due to different wavelengths of light. Blue colours are more energetic – there are more waves that arrive each second – whereas red colours are less energetic, with fewer waves arriving each second. The number of waves that arrive each second is measured as the frequency, or alternatively you can measure it as the distance between the peaks of the waves, known as the wavelength.
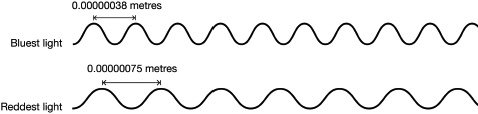
The different wavelengths of red and blue light.
Our eyes can only detect colours that have waves separated by just 0.00000038 metres for blue light and 0.00000075 metres for red light (a frequency range of 790–400 trillion waves per second). ‘White’ light, like that from a torch or the Sun itself, is a mixture of all the colours; as demonstrated beautifully by a rainbow. As light from the Sun passes through drops of water in the air, it is split into its component colours for us all to marvel at. What’s wonderful to think about when you see a rainbow is that you’re barely even seeing the full picture. There are colours beyond the red at the top of the rainbow and the blue at the bottom; but they’re colours that our eyes can’t see. The Sun doesn’t just emit visible light, it emits light of all wavelengths, from the least energetic light, the laziest, with huge, kilometres-long separations between wave peaks, to the most energetic light, with tiny separations between wave peaks of just the width of an atom.
We roughly categorise the different wavelengths of light into different types. From the largest wavelength to the smallest, these are: radio waves; microwaves; infrared; visible; ultraviolet; X-rays; and gamma rays. This spread of the different wavelengths of light is the true full spectrum of light; the entire rainbow, of which we only get the tiniest glimpse. Despite not being able to see these wavelengths of light, this hasn’t stopped us from exploiting them for our gain. From using radio waves to communicate, microwaves to cook our food, infrared light in our TV remotes, ultraviolet for killing bacteria, X-rays for getting a glimpse inside our bodies, and gamma rays in radiotherapy to fight cancer.
However, the more energetic the light, the more dangerous it becomes to life here on Earth. Thankfully, the Earth’s atmosphere filters out the majority of the wavelengths of light produced by the Sun. The highest-energy ultraviolet light is absorbed by oxygen atoms in our atmosphere, producing the ozone layer. Similarly, oxygen and nitrogen atoms absorb all the X-rays and gamma rays, and moisture in the atmosphere absorbs microwaves. The only light that makes it to the ground is visible light, some ultraviolet (which can burn our skin – sunburn) and harmless radio waves. The Sun is 10 million times brighter in visible light compared to radio waves, so it’s no surprise that human eyes evolved to see the bright kind of sunlight which actually makes it to the ground. Perhaps on another planet, with a different type of atmosphere, our eyes would be able to detect a completely different part of the spectrum of light, with brand new colours that we don’t even have a hope of visualising.
As astronomers, though, we are no longer limited by the puny sensitivity of the human eye. We have ‘evolved’ a step further, developing detectors sensitive to different types of light. The problem is Earth’s pesky atmosphere which, while protecting life from harmful radiation, also scuppers any detection of X-rays coming from the vastness of space. So we strap our X-ray detectors to telescopes and launch them up into orbit around Earth, beyond the atmosphere that blocks our view. With these telescopes we’ve been able to open our eyes to see the tiny pinpricks of light dotting the infrared, X-ray, and gamma-ray sky that for so long remained hidden to us. Including light from the Crab Nebula, which might have outshone the Sun in visible light back in 1054, but now outshines the Sun and nearly everything else in the sky in gamma rays.
It’s the different colours and types of light we receive from stars that let us work out how hot they are, what type they are and what will happen to them when they die. There are some stars, like Betelgeuse in the constellation of Orion, that appear slightly reddish in colour; you can even see this with the naked eye when the sky is dark, but it’s even more obvious if you snap a picture (even a ten-second ‘night mode’ shot on most smartphones will reveal this if you can’t quite make it out with your eye). Similarly, there are some stars, like Sirius, which appear blueish in colour.
So, using the light from stars, astronomers decided to do what all good scientists do and classify them. With a system. In the same way biologists have their classification of the animal kingdom and chemists have their periodic table, astronomers have their star classification system. This was made possible by Fraunhofer’s invention of the spectrograph – splitting the light from a star into its rainbow reveals the gaps where light is missing; the hidden fingerprints revealing what that star was made of. Because, as Fraunhofer had pointed out, not all stars had the same pattern of missing colours as the Sun.
It was this observation that allowed Italian astronomer Angelo Sechhi to sort stars for the first time into three broad categories. In 1863, Secchi began recording the spectrum of light from different stars, just like Fraunhofer first did with the Sun, amassing over 4,000 of them to analyse. He realised that although the patterns of the missing colours varied slightly from star to star, they could be roughly sorted into three categories, which he called I, II and III using Roman numerals (he also ended up adding two more rarer classes to his scheme, IV in 1868 and V in 1877). The Sun is a type-II star according to Secchi, which means it has a lot of missing colours. We now know these missing colours correspond to the Sun having lots of heavier elements like carbon, magnesium, calcium and iron absorbing light at those colours – we call these metal lines: any element heavier than hydrogen is classed as a ‘metal’ by astronomers, much to all chemist’s chagrin.
Secchi wasn’t the only one interested in classifying stars based on their light. In the 1880s, American astronomer and director of the Harvard College Observatory, Edward Pickering, also turned his attention to classifying stars. Pickering amassed over 10,000 star spectra to analyse, but he didn’t do it alone. Pickering had help from the ‘Harvard computers’. The word computer today refers to a machine, but in Pickering’s day computers were people: ‘one who computes’. Teams of people were hired to do repetitive, tedious jobs and incredibly complex mathematical calculations. These computers were more often than not women, who would make discoveries in the data they had been given to process or glean insight that had previously been missed.15 At the Harvard College Observatory men would do the manual labour of moving the telescope and taking the images or spectra on large photographic plates, then the women would do the tedious, repetitive cataloguing of the brightness or spectrum of a star. By today’s definitions, the men were doing the astronomy, the women doing the astrophysics.
Harvard computer Williamina Fleming did the bulk of the classification of Pickering’s 10,000-star spectrum (discovering ten new ‘guest stars’ in the process), and together Pickering and Fleming revised Sechhi’s system to have more specific classes. They split his five broad classes (I–V) into sub-classes using the letters A–Q, giving seventeen different types of stars in total. As you went through the alphabet the amount of absorption by hydrogen decreased. The work was published in 1890 and was known as the Draper Catalogue of Stellar Spectra, having been funded by Mary Anna Palmer Draper, the widow of American doctor and keen amateur astronomer Henry Draper.
This method of classifying stars was deemed overly complicated by some, especially another Harvard computer called Annie Jump Cannon. In 1890, Harvard College Observatory branched out from studying stars only in the northern hemisphere sky and built an observatory in Arequipa, Peru to get data from (the many more) stars in the southern hemisphere sky. Cannon was tasked with classifying all the stars in the southern sky down to a certain brightness for a revised version of the Draper catalogue. While doing so, she also simplified the classification system, sticking to the alphabetised letters but dropping all but A, B, F, G, K, M and O. She noticed that most stars were a mix of two types, a halfway house between the A and B type, for example. So, instead of seventeen individual types, she added a number between 0 and 9 to specify if the star was between two types, e.g. an A5 star. The Sun is a G2 star in Cannon’s system, Sirius with its blueish colour is an A1 and Betelgeuse with its reddish colour is an M2 star.
Pickering and Cannon first published this system in 1901, but the work didn’t end there. The Draper catalogue was not yet complete, with many more stars in the sky yet to be classified. The full catalogue of 225,300 stars was published in volumes between 1918 and 1924; in working towards it Cannon and her computer colleagues at the observatory were classifying the spectra of over 5,000 stars per month using her system.
So by the early twentieth century, astronomers had a system for sorting stars, but understanding why they could be sorted that way would take that little bit longer. What made the spectrum of stars look different? What made them shine with slightly different colours? Well, during the completion of the Draper catalogue in 1911, Danish chemist and astronomer Ejnar Hertzsprung had worked out the distance to some of the stars listed. With the distance he could work out their actual brightness, rather than the brightness they appear to be from Earth, and noted that the actual brightness was proportional to the amount of light stolen away in the absorption lines (the absorbed wavelength/colour doesn’t disappear completely, but is very faint compared to the overall amount of light received from the star). Hertzsprung plotted this on a graph, showing how the two were correlated. By 1913, American astronomer Henry Russell had collated yet more distance measures to stars, allowing him to calculate more absolute brightnesses and revise Hertzsprung’s diagram to yet again show off the correlation between brightness and absorption-line strength. The brightness obviously had something to do with the amount of absorption in the spectrum of stars, but what was the link?
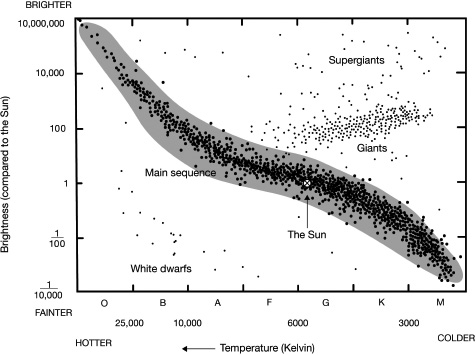
The Hertzsprung–Russell diagram for nearby stars. The ‘main sequence’ of stars is the correlation originally seen by Hertzsprung and Russell and is where normal hydrogen fusing stars are found. The x-axis of temperature is inverted because it was originally plotted as least to most absorption. Like most things in astronomy: if at first it doesn’t make sense, it’s a historical thing.
For that, we come back to the work of Cecilia Payne-Gaposchkin (who, if you remember from the previous chapter, showed in her 1925 PhD thesis that the Sun was made mostly of hydrogen). Edward Pickering’s inclusion of women computers at Harvard College Observatory, allowing them to publish their work under their own name (uncommon at the time), paved the way for many more women in astronomy. After Pickering died in 1919, the new director of the Harvard College Observatory was American astronomer Harlow Shapley. Shapley established a graduate program in astronomy for women at the observatory in conjunction with the nearby Radcliffe College for women.
Payne-Gaposchkin wasn’t hired as a computer, but taken on as a graduate student and subsequently became the first person to be awarded a PhD in astronomy from Radcliffe College at Harvard University.16 What Payne figured out during her PhD was how the classes of stars (A, B, F, G, K, M and O) were related to their temperature. She had read the work of Indian physicist Meghnad Saha, a professor at Allahabad University in Uttar Pradesh, who was studying the behaviour of gases at high temperatures. Saha used the ideas of quantum mechanics, i.e. how tiny particles behave, to work out what happened to atoms at incredibly high temperatures and pressures. He realised that the higher the temperature or pressure, the more ionised a gas became. The more ionised a gas, the more electrons are released from their orbits around the centres of atoms, giving free-roaming negative electrons and positive nuclei. He wrote this all down in a nice neat equation known as the Saha equation.17
Other physicists, such as British astronomer Ralph Fowler, realised the implications of Saha’s work; that this would cause different amounts of absorption in the spectra of stars. Too cold and there wouldn’t be enough energy to boost electrons up to higher orbits, and so there would be less absorption of light by the electrons. Too hot and there would be so much ionisation that there would no longer be any electrons left in orbits around atoms to steal away the light, and so there would again be less absorption of light. There should then be a sweet spot where the most absorption of light happens by the electrons, at that perfect Goldilocks temperature, giving many gaps in the spectrum of a star.
It was Celia Payne-Gaposchkin who took these ideas further and demonstrated that Annie Jump Cannon’s classification system could be ordered as O-B-A-F-G-K-M from the hottest to the coolest, with the most absorption happening in A stars at that Goldilocks temperature of not too cold and not too hot. Having realised that the amount of absorption was due to temperature, and not the amount of any particular element, she showed that the Sun actually contained a million times more hydrogen than anything else. Her work was published in 1925, but she was dissuaded from making such a bold claim by her thesis examiner Henry Russell, since it went against the thinking of the time that the Earth and the Sun were made of a similar amount and mix of elements. In 1929, Russell, independently, with a different method, determined that the Sun was mostly made of hydrogen, and although he credits Payne-Gaposchkin’s earlier work, the credit for the discovery is often mistakenly attributed to him.
Thanks to Payne-Gaposchkin’s insight, we now understand how stars shine, the correlation of brightness with the absorption strength and the classification of stars. It’s a simple classification system that is still taught to budding astronomers the world over with the handy mnemonic: ‘Oh Be A Fine Guy/Girl Kiss Me’. It is known as the Harvard Classification Scheme, rather than the perhaps more fitting Cannon Classification Scheme, with many students learning about the scheme but not the women behind it.
So, because the absorption strength in a star’s spectrum is determined by the temperature of the star, the fundamental relation is that the temperature of a star is correlated with its absolute brightness, something that’s now known as the Hertzsprung–Russell diagram. The hotter the star, the more light and, crucially, the more energetic the light will be that shines out. The temperature of the Sun, on average, is 5,778K (kelvin),18 meaning that it emits the most light at a wavelength of around 500 nm (nanometres, or 0.0000005 m), which is greenish in colour. There’s a similar amount of red and blue light being emitted, close enough for it all to mix together to give white light, which is why the Sun doesn’t actually look green. Betelgeuse, with its reddish colour, is cooler at 3,600K, and Sirius with its blueish colour is hotter at 9,940K.
But again, why are brightness and temperature correlated in stars? The last piece of the puzzle in understanding stars is their mass. Edward Pickering, while driving the cataloguing of all those stars at Harvard College Observatory, was himself studying binary stars; pairs of stars that orbit each other. This allowed him to work out how heavy stars of different spectral types were. The heaviest are the O stars, and the lightest are the M stars. Essentially, the more massive the star, the brighter and hotter it will be.
This makes sense if we think about stars, like Lord Kelvin did, as a constant balance between the crush of gravity inwards and energy from nuclear fusion pushing outwards. The most massive stars will exert the greatest crush of gravity inwards, heating up the inside of the star to much larger temperatures than in smaller stars. To resist that bigger force of gravity pushing inwards, more massive stars need a bigger force pushing outwards: they need to burn more fuel each second so they don’t collapse under their own gravity. This is why they’re brighter – they’re fighting harder all the time against their own much stronger gravity. So much so that even though more massive stars are made of far more hydrogen than our Sun, the rate at which they have to fuse that hydrogen means that they live much shorter lives. An O star can be ninety times heavier than the Sun, but live for only a million years (10,000 times less than the Sun’s 10 billion years). Bigger stars live fast and die young.
During their lifetime of happily fusing hydrogen into helium, stars are found on what’s called the ‘main sequence’ of the Hertzsprung–Russell diagram: that main correlation of brightness and temperature. But when stars start to run low on hydrogen fuel, they begin to stray off this correlation, cooling down and changing to a redder colour but somehow staying at the same brightness. They do this by swelling up to a huge size, and we class these stars as ‘giants’ (or perhaps even ‘supergiants’ if they’re particularly large). If you find a big cluster of stars that have all formed at the same time, you can tell how old they are since the brightest O stars will already have died and will be missing from the Hertzsprung–Russell diagram, and there’ll be a point where the main sequence switches back on itself to give a large number of giant stars.
Swelling up to these giant sizes is a star’s way of delaying the inevitable. For example, when the Sun starts to run out of fuel in 5 billion years or so it will take a very winding path through the Hertzsprung–Russell diagram, swelling up to a red giant and then eventually down to the white dwarf section (at high temperature but low brightness), after losing its outer layers to space. But why does it do this? What are stars doing when they swell to delay the inevitable?
Once astronomers had finally put all the pieces together in 1929, and worked out that the process fuelling the Sun and all the stars in the sky was hydrogen fusion into helium, the work could truly begin to understand how this actually happened. How do you physically get four hydrogen atoms to come together and fuse to make helium? It was in 1939 that German-American nuclear physicist Hans Bethe worked out the way that fusion was actually happening in stars.19 George Gamow (who worked out the maths for the probability that two hydrogen atoms could overcome the repulsion between them, and realised it was small but not zero) had previously suggested a chain reaction of hydrogen atoms merging; first with two fusing to produce heavy hydrogen, also known as deuterium. Deuterium has one proton in its nucleus, like normal hydrogen, plus a neutron, making it slightly heavier.20 It’s the number of protons that determine what element an atom is; the number of neutrons just determine how heavy the atom is. Normally, atoms will have an equal number of neutrons and protons (except hydrogen, which has no neutrons normally)21, we call these atoms with a different number of neutrons than normal, like deuterium, isotopes. In the chain reaction, the heavy hydrogen then fuses with another hydrogen atom to make light helium (helium-3), which then finally fuses with another hydrogen atom to make helium.
But Bethe wasn’t convinced by this proton chain reaction; what about the heavier elements, like carbon, that we knew were also part of the Sun and stars? How did these get made and how did they influence the nuclear reactions going on in stars? Bethe realised that the presence of carbon could actually act as a catalyst for nuclear reactions, at least when stars were hot enough. Stars could cycle through combining hydrogen with carbon, nitrogen and oxygen to finally make some helium in the end. The cycle goes like this:
(i) carbon fuses with hydrogen (#1) to make light nitrogen
(ii) light nitrogen decays into heavy carbon
(iii) heavy carbon fuses with hydrogen (#2) to make nitrogen
(iv) nitrogen fuses with hydrogen (#3) to make light oxygen
(v) light oxygen decays into heavy nitrogen
(vi) heavy nitrogen fuses with a hydrogen atom (#4) and splits to give carbon plus a helium atom
In this cycle, we started with carbon and ended with carbon, used four hydrogen atoms along the way and made some helium. It’s known as the CNO-cycle (the carbon-nitrogen-oxygen cycle).
Bethe calculated that at hotter temperatures, this process is much more efficient than the proton-proton chain reaction; it’s much more likely to get hydrogen to fuse with either carbon or nitrogen than with itself. Bethe published his work in 1940, and in 1967 he won the Nobel Prize in Physics – he’d cracked exactly how the stars were powered.22 But what it didn’t answer was how did carbon, nitrogen and oxygen get made in the first place? Hydrogen is the simplest element, with just one proton in its nucleus, a basic building block of the Universe. Hydrogen is the most abundant element in the Universe, so there must be some other process converting it to things even heavier than helium.
Bethe never considered this problem of heavy element creation, and it would be a few more years – 1946 – until British astronomer Fred Hoyle did. Hoyle was a lecturer at St John’s College at the University of Cambridge, and his ideas on heavy element production helped make him a household name23 and eventually led to him becoming the first ever director of the Institute of Theoretical Astronomy in Cambridge. Hoyle suggested that when stars run out of fuel to burn and they no longer have any energy pushing outwards against the crush of gravity inwards, they start to collapse under gravity. This crushing of matter down would increase the temperature inside the star to millions of degrees, causing the hydrogen and helium nuclei created in normal fusion to fuse together to make all the elements across the periodic table in roughly equal abundances.
The problem with this idea is that those elements then become trapped inside the collapsed star, never to see the light of day. But we know that those elements have to be somehow dispersed across the Universe, to give us the ingredients to cook up the Solar System. So Hoyle revised his theory, thinking about this strange giant phase that stars go through when they run out of hydrogen fuel. It’s only hot enough in the inner core of a star for fusion, so only about 5 per cent of the hydrogen in a star actually gets converted to helium over its lifetime (as Arthur Eddington himself suggested). When a massive star first runs out of hydrogen fuel, the whole thing starts to collapse under gravity, with the outer atmosphere of hydrogen crushing down on the core, now made entirely from helium.
As the star collapses under gravity, the hydrogen closest to the core becomes hot enough to fuse into helium again, and starts heating up the helium core and the hydrogen atmosphere around it. The core contracts some more, getting ever hotter, and the only thing the star can do to balance this out is to swell its outer atmosphere of hydrogen outwards to become really diffuse. It becomes a giant, or if it’s a really massive star, a supergiant (the outer layers of the star cool as they get more diffuse, which is why these giant stars then look red).
Fusion continues in a layer around the core, until the core becomes hot enough to start fusing helium into carbon. Eventually, the hydrogen around the core runs out and the star starts to collapse again, before it becomes hot enough in another layer to kick-start hydrogen fusion again. The previous layer that was fusing is now pure helium, and it starts to fuse that into carbon, while the carbon that was made in the core starts to fuse into oxygen. This process keeps on repeating until you’re left with a star that resembles an onion, with layer on layer of heavier elements made by fusion triggered by ever increasing temperatures as the star tries to prevent its inevitable collapse.
The star will continue this constant fusing of ever-heavier elements in its core until silicon atoms fuse to give iron. Iron is the death sentence for stars. Iron can fuse together to give heavier elements, but you need to put more energy in than you get out, so it can’t be used as a fuel. At this point, when the star contracts again, there’s no extra layer made and there’s no longer any fusion process that can resist the crush of gravity inwards. The lighter elements on the outskirts of the star collapse inwards, briefly exponentially increasing the temperature and producing a huge burst of light that can be seen across galaxies and beyond, before bouncing back off the heavier elements in the core to get flung outwards into space. We call this collapse and rebound a supernova.24
Hoyle published this hypothesis of the onion-like death of stars in 1954, and in 1957 teamed up with three other scientists – American physicist William Fowler, British astronomer Geoffrey Burbidge and British-American astronomer Margaret Burbidge – to write one of the most influential research papers in all of astrophysics: ‘Synthesis of the Elements in Stars’. It’s known as the B2FH paper (from the initials of its authors), and is essentially a review that pulled together all the work that had been done into the production of the heavy elements in fusion (by nuclear physicists), observations of the ratio of the amounts of heavy elements in stars (by astronomers) and Hoyle’s ideas on the onion-death scenario of stars. It identified the nuclear reactions that would occur in each of the layers of the dying star, predicted the amount of each element that would be formed and showed how this matched the amounts measured in astronomical observations of the spectra of stars. It tied up fifty years of research with a nice, neat little bow.
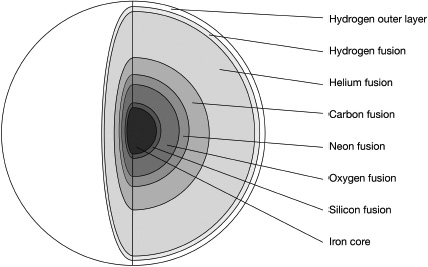
The onion-like structure of a supergiant star nearing the end of its life.
The B2FH paper wasn’t just influential in the field of astrophysics, it captured the attention of the wider public too. If stars are the great forges of the Universe, with all elements made within them and ejected back into the Universe, then that means me, you, the entire Earth even, are all made of ‘stardust’. It sounds very poetic, but my favourite, and I think more accurate, analogy for this process is that these elements are ‘supernova poop’. I realise that ‘we’re all made of supernova poop’ doesn’t quite have the same poetic ring to it, but I like it.
A supernova is what caused the bright ‘guest star’ recorded by Chinese astronomers in 1054 and left behind the ghostly remnants that we see today in the Crab Nebula. But what’s left in the middle of the Crab Nebula, producing all those gamma rays? What happens to the core of a star after the outer layers of a star’s atmosphere have bounced off? What if there’s nothing left to resist the inexorable crush of gravity?
We get a black hole.