Chapter 9
Magnets:
How the H*ck Do They Work?
I open the refrigerator to start breakfast, careful not to dislodge the many works of art held to the door with magnets …
The force between two magnets, or between a magnet and a piece of metal, is among the most captivating examples of fundamental physics—for young and old. One of the most enduringly popular toys at my kids’ day care is a set of plastic tiles in simple shapes that snap together thanks to magnets in the edges; almost every day, these are built into elaborate new structures. The local science museum gets a lot of mileage out of a giant horseshoe magnet and several handfuls of steel washers, and adults are as likely as kids to be found trying to see how long a chain of washers they can stick to one of the poles.
In fact, magnets are a great gateway drug to a career in physics. Einstein recalled being captivated by a compass as a child, his wonder at the invisible force that always pulled the needle back to the north launching a lifetime of speculation about the forces of nature. Most physicists I know have childhood memories of, for example, trying to get a small magnet to levitate over a collection of larger ones.1 Even as adults, the fascination remains, and magnetic desk toys are a common feature of faculty offices in physics departments everywhere.
As familiar as they are, the working of magnets is also famously difficult to explain. A frequently shared interview clip from the 1980s shows renowned physicist Richard Feynman declaring flatly that “I really can’t do a good job, any job, of explaining magnetic force in terms of something else you’re more familiar with, because I don’t understand it in terms of anything else that you’re more familiar with.”2 A less highbrow example is the 2009 song “Miracles,” in which the rapping-clown group Insane Clown Posse triggered a thousand unsuccessful attempts to explain magnets with the line “F*cking magnets, how do they work?”
It may seem strange that a phenomenon so common that we use it to hold stick-figure drawings to kitchen appliances is so hard to describe in nontechnical language, but the physics is, in fact, extremely complicated, and depends on subtle details of the microscopic structure of particular materials. And, of course—as you probably guessed—it ultimately traces back to the quantum: the permanent magnets we use to hold pieces of paper up for display would be impossible without electron spin and the Pauli exclusion principle.
Navigating Magnetism
When people ask “How do magnets work?” they are really asking two separate but related questions. A permanent magnet is a macroscopic chunk of material that produces a magnetic field in its vicinity, and one way of interpreting the question is in reference to the general behavior of these magnetic fields. This is, from the standpoint of physics, the easier of the two questions to tackle. The nature of magnetic fields has been understood since the mid-1800s, when Maxwell wrote down his equations showing how currents and changing electric fields create magnetic fields, and vice versa.
Unfortunately, while Maxwell’s equations offer a straightforward way to understand how magnetic fields are created by moving charged particles around, they don’t answer the other question about permanent magnets, namely why those specific chunks of otherwise inert material spontaneously generate magnetic fields in the first place. After all, there don’t seem to be any currents flowing in a hunk of naturally occurring magnetite, and yet this mineral produces a significant magnetic field. The tendency of certain minerals to attract metals has been known since at least the sixth century bce, recorded in Greece and India and China, and it’s been put to practical use since at least the eleventh century ce, by which time the Chinese were using magnetic compasses for navigation. Despite that long history, though, the origin of the magnetic properties of these minerals remained a mystery into the twentieth century.
The existence of permanent magnets defies easy explanation because it involves physics on many levels. Physics on the scale of atoms is obviously involved, because naturally occurring magnetic materials all contain iron, and only a few other elements are clearly magnetic. Atomic-scale physics is not the whole story, though: many materials containing large amounts of iron are not magnetic, including many steel alloys, so the crystal structure of the material must also play a role. And, of course, everything is ultimately pieced together from fundamental particles, so magnetic behavior must have roots in the behavior of individual protons and electrons.
The most useful application of magnets, the constancy of a compass’s direction, also highlights the other issue that adds complexity to the problem of magnetism: magnetic interactions are fundamentally more complicated than the electrostatic attraction or repulsion between charged particles. The electric charge of a particle is a single value, and if you know the charge, you immediately know the force on that particle due to an electric field. The energy of two interacting charges depends on the sign and size of their individual charges, the distance between them, and nothing else.
There is no magnetic analogue to a single electric charge, however—you never find a magnetic north pole alone without a matching south pole—so magnetic forces depend not only on a simple charge but also on a direction. As anyone who has played with bar magnets knows, the force between two magnets gets stronger or weaker, and even changes from attractive to repulsive, depending on which direction the north pole of each is pointing. To find the energy of a pair of magnets, you need to know not only their strength and separation, but also the angle between their north poles.
This dependence on orientation adds some additional overhead when trying to determine the behavior of particles with magnetic character. Like the electric field, the magnetic field comes with an associated direction, but determining its effect on a magnetic particle placed in the field also requires keeping track of a direction associated with the particle. That extra information also adds to the bookkeeping required to calculate the properties of a large collection of magnets, and opens the possibility of entirely new collective phenomena. A large collection of magnets all pointing in the same direction is a very different thing than a collection where each magnet has its north pole in the opposite direction from its neighbor’s.
We can cut through some of the complexity involved in the multiple scales of magnetism by using the same fundamental principle that explains so much of physics: no matter what scale we look at, any physical system is always trying to find the lowest energy state possible. Finding that minimum energy involves balancing the energy costs of all the different interactions that a given object—whether it’s a fundamental particle, an atom, or a small chunk of mineral—has with the rest of the universe. Keeping that balancing act in mind provides a simple and reliable guide to navigating the complexity of permanent magnets, like a compass needle always pointing the way north.
In general, the energy of a magnetic object, at whatever scale, will be lowest when its north pole is pointing in the same direction as the magnetic field at its position, and highest when it points in the opposite direction. This is what makes a compass work: currents in the core of the earth generate a magnetic field on a grand scale, so that every point on the surface of the planet sits in a small magnetic field pointing in a particular direction. A compass needle is a small, light, permanent magnet that’s able to freely rotate about its center to minimize its energy, which happens when the north pole of the magnet is pointed toward the North Pole of the planet, more or less. We designate the poles of magnets as “north” or “south” depending upon which geographic direction they point to when allowed to rotate freely. By convention, though, the field surrounding a magnetic object points outward in the region of the magnet’s north pole and inward in the region of its south pole, with the field lines in between forming closed loops, like those traced by the familiar demonstration of scattering iron filings over a bar magnet. This north-to-south direction of magnetic fields means that what we call the earth’s “North Pole” actually corresponds to the south pole of a typical magnet.3
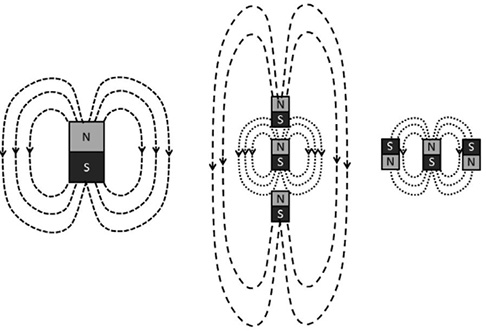
The magnetic field lines for a single magnet, and the lowest-energy configurations for groups of multiple magnets. End-to-end magnets with their north poles aligned generate a larger collective magnetic field, shown by the larger loops, while the fields of side-by-side magnets cancel each other out.
Aligning individual magnets with the field produced by other nearby objects not only changes the energy of the magnets, but also how their individual fields add up to produce the field around the group. If the magnets are positioned end to end, the lowest-energy arrangement will have all the north poles pointing in the same direction; in this case, their individual magnetic fields add to make a stronger effective magnet. On the other hand, magnets placed side by side will prefer their north poles to be in opposite directions, in which case their individual fields will largely cancel out, making a weaker effective magnet.
A three-dimensional material made up of smaller particles with magnetic character will necessarily have some of those particles placed side by side, which is why the vast majority of materials are nonmagnetic. Even strongly magnetic atoms like iron and chromium end up in nonmagnetic forms when combined into minerals or alloys, because the lowest-energy way for those magnetic atoms to arrange themselves in molecules and crystals has the north poles of neighboring atoms pointing in opposite directions.
Making a strong permanent magnet requires finding a way to put particles together so that the minimum energy at every scale—that of fundamental particles, magnetic atoms, and chunks of mineral—comes when the north poles of the individual magnetic components are aligned. This can’t be managed with magnetic interactions alone; it requires an additional interaction that increases the energy of the nonmagnetic state so that the magnetic state is preferred. This is very tricky to arrange, and in the end requires us to factor in not only the electrostatic repulsion between electrons, but also, once again, the Pauli exclusion principle.
Magnetic Electrons
Magnetism begins at the level of fundamental particles, and the intrinsic magnetic character of electrons is the ultimate source of the magnetic field of a permanent magnet. The interaction between pairs of elementary particles also provides a clear illustration of the energy balancing that governs the whole process.
As we saw when we first introduced the notion of Pauli exclusion in Chapter 6, a single electron has “spin,” a purely quantum property that can take on only two possible values. This spin gives the electron a small amount of magnetic character, and in the presence of a magnetic field, the two values of spin produce two states of slightly different energy. These states are traditionally called “up” and “down,” depending on whether the electron’s internal magnet points in the same direction as the local magnetic field or in the opposite direction.
Of course, the magnetic character of the electron doesn’t just give it a preferred direction, it also creates a magnetic field, which affects other nearby particles. A second electron placed in the vicinity of the first will tend to align its spin with this field, giving that second electron a preferred direction that depends on whether it’s end to end or side-by-side with the first. If we considered magnetic interactions only, the electrons would tend to arrange themselves into long chains with neighboring chains having alternating spin, the whole arrangement in the end producing no net magnetic field.
Of course, two electrons in close proximity don’t interact only via their magnetic properties, they also feel electrostatic interactions, and they repel each other very strongly because they have the same negative charge. This repulsion is vastly stronger than the tiny magnetic interaction, so two electrons don’t stick around long enough for the magnetic interaction between their spins to matter. While the pair of electrons can lower their energy by pointing their spins in opposite directions, they can lower the energy much more by moving farther apart, and as a result, they end up separated by enough distance that the tiny magnetic interaction makes no discernible difference.
This magnetic character does have measurable effects, though, when two particles with spin can be induced to hang around each other a little longer. If we take an electron and a positron—the positively-charged antimatter version of an electron—and bring them close together, they can form a short-lived “atom” held together by the attraction between their charges. As in an ordinary atom, the two particles can lower their energy by drawing closer together, but putting them into a smaller volume causes an increase in their kinetic energy, and the balance between these two determines the atom’s optimum size. Their mutual attraction keeps the electron and positron in this “positronium atom” close enough together that their magnetic interaction produces a measurable effect. The lowest energy state for positronium is split into two states depending on the relative alignment of the spins of the electron and positron: when both north poles are in the same direction, the energy is slightly higher, and when they’re in opposite directions, the energy decreases. The “hyperfine splitting” between these states has been measured experimentally: positronium has a spectral line in the microwave region of the spectrum, corresponding to photons with a frequency of about 203 GHz.
This magnetic interaction also comes into play in more ordinary matter. A proton also has a quantum-mechanical spin, and thus produces a magnetic field, so an electron bound with a proton to make a hydrogen atom also has its energy shifted by the magnetic interaction between them, splitting hydrogen’s lowest energy state into two. The energy separation corresponds to a photon with a frequency of 1.4 GHz, in the radio region of the spectrum,4 and light emitted by hydrogen moving between these states is one of the principal tools used by radio astronomers to study distant clouds of hydrogen gas.
The magnetic interaction energy in both of these cases is only a tiny perturbation to the electrostatic interaction—the energy difference between the two hyperfine levels in positronium is about 1/10,000th of the energy difference between the two lowest-energy electron orbitals. This is why the original Bohr model was able to completely neglect magnetic interactions: at the scale of fundamental particles, the electrostatic interaction absolutely dwarfs any magnetic effect. As we move to the scale of multi-electron atoms, though, the situation becomes more complicated, and as the Pauli exclusion principle comes into play, the extreme strength of electrostatic interactions becomes a crucial factor in producing magnetic atoms and minerals.
Magnetic Atoms
One tempting but wrong idea about the origin of magnetism at the scale of atoms is that it is the result of orbiting electrons behaving like the current in an electromagnet. While this would fit nicely with Maxwell’s equations of classical electromagnetism, it doesn’t fit the evidence. Every atom in the universe consists of electrons orbiting a nucleus, but only a handful of elements in the middle part of the periodic table show significant magnetic character. Magnetism in atoms can’t be solely a result of electron orbits.5
The idea of orbital motion as a source of magnetism was behind the original Stern-Gerlach experiment, discussed back in Chapter 6, in which a beam of silver atoms was split by a special magnet. Unfortunately, as the physicists who grappled with Stern and Gerlach’s results found, that theory didn’t match the behavior of the atoms—differences in orbital motion ought to split the beam into at least three components, where Stern and Gerlach saw only two. Their result helped point toward the existence of an electron property with only two values, namely spin; for our current purposes, it’s also a clear hint that magnetism in atoms is ultimately due to the spin of their electrons.6
Making a magnetic atom, then, is a matter of getting the tiny magnetic fields produced by the electrons inside the atom to add together to make a bigger magnet. This means getting the electron spins pointing in the same direction, so their “north poles” align. This goal faces a major obstacle, though: the fact that the magnetic interaction between electrons favors states where the spins point in opposite directions.
At first glance, the Pauli exclusion principle, which forbids any two electrons from having exactly the same quantum state as determined by the four quantum numbers n, l, m, and s, would seem to make this worse, because it builds in this kind of pairing of electrons. As we saw in Chapter 6, the lowest energy state for the electrons in any particular atom is found by “filling up” the available energy states of the atom (determined by n, l, and m) with at most two electrons each: one spin up (s = +½), the other spin down (s = –½). This natural pairing of spin up and spin down explains why none of the atoms near the edges of the periodic table are strongly magnetic. Those elements have their outermost energy levels nearly or completely filled, with their electrons paired up so their magnetic fields cancel out.
In elements near the middle of the periodic table, though, Pauli exclusion combines with the repulsion between electrons to create a situation where the electron spins want to line up with each other. This has to do with the deeper meaning of the Pauli principle discussed in Chapter 7, as a requirement on the symmetry of a collection of electrons.
An element from the middle few columns of the periodic table will have its outer shell half full of electrons, which seems to give it several options for how to arrange those electrons and their spins. The canonical magnetic element, iron, for example, has six electrons to place in a state with l = 2, which has five distinct sublevels of the same energy but different values of m. There are lots of ways to arrange these electrons, but for the purposes of understanding iron’s magnetic properties, we can focus on only two: one where all six electrons are clustered in just three of the sublevels, and another where the electrons are spread more evenly, with only one sublevel having an electron pair.

Two possible arrangements of electron spins for the half-filled outer shell of iron, one nonmagnetic (top), the other magnetic.
The Pauli exclusion principle dictates that whenever two electrons are paired up in the same sublevel, they will have opposite spins. Both of these states satisfy Pauli exclusion, but the one with all six electrons paired up is nonmagnetic, while the more distributed state has four unpaired electrons pointing in the same direction, giving it strong magnetic character. The energy of all five n, l, and m sublevels is the same in both arrangements, however, so it may seem like there’s no reason one should be any more likely than the other.
That analysis, though, neglects the energy contributed by the repulsive interaction between nearby electrons. This increases as the separation between electrons decreases, and a pair of electrons occupying the same spatial sublevel would be very close together indeed. The repulsion between paired electrons raises the energy of the nonmagnetic state, making the magnetic state with aligned spins the lowest-energy state available.
You might reasonably object that you could make a nonmagnetic state with the electrons distributed over more sublevels, by flipping the spins of two of the unpaired electrons, so that the state has one electron pair, two single spin-up electrons, and two spin-down electrons. But the symmetry aspect of the Pauli exclusion principle takes care of that; how it does so is easiest to understand if we consider only two electrons and two sublevels.
As discussed in Chapter 7, the Pauli exclusion principle states that the wavefunction for a multi-electron state must be antisymmetric. Because electrons are identical and interchangeable, the measurable properties of the state as a whole cannot change if we swap the labels on two electrons—but the combined wavefunction must change sign after the swap. This antisymmetry requirement applies to the wavefunction as a whole, both the spatial distribution of electrons (determined by n, l, and m) and the distribution of their spins, which means that if one of these is antisymmetric, the other must be symmetric. If both spin and space wavefunctions were antisymmetric, a swap of labels would change the sign twice, putting you right back where you started—in physics as in English, two negatives (awkwardly) make a positive.
Thus, if the two spins point in the same direction, the spin wavefunction is symmetric, and the space wavefunction must be an antisymmetric combination of the two available sublevels. If the spins point in opposite directions, that can be an antisymmetric state,7 in which case the space wavefunction must be symmetric.
We’ve seen that, for a space wavefunction, antisymmetric states exclude the electrons from more space, and that raises their energy slightly, which might make you think that these would be the higher-energy states—and for a single electron, the antisymmetric state is indeed higher energy. The antisymmetric arrangement keeps the electrons farther apart on average, though—you can get a sense of why by thinking about the two-atom states we looked at back in Chapter 7. The excluded region for those wavefunctions is the spot midway between the two atoms, which pushes the two peaks a tiny bit farther apart.
The antisymmetric wavefunctions for electrons in a single multi-electron atom are not split between positions around two nuclei like those in molecular states, but rather are superpositions of different n, l, and m states around a single nucleus. The end result is the same, though: the electrons in an antisymmetric combination of orbitals are a tiny bit farther apart, on average, than those in a symmetric combination. That increase in distance reduces the energy due to their mutual repulsion by more than the energy difference between symmetric and antisymmetric spatial wavefunctions.
Thus, the lowest-energy state available to iron is one in which the outer-shell electrons are distributed among all the available sublevels, with the spins of unpaired electrons aligned. This means that the magnetic fields created by the individual spins add together to produce a larger field, making iron a strongly magnetic atom. The same basic physics is at work in other elements with half-filled outer shells, leading to the cluster of atoms with strong magnetic character in the middle columns of the periodic table.
Magnetic Crystals
Of course, as noted above, just because an atom of a particular element is magnetic doesn’t mean that a solid chunk of that material will be a permanent magnet—if it did, naturally occurring magnets would be everywhere. In fact, some elements that are strongly magnetic at the atomic level (chromium, for example) show almost no magnetic character at all in bulk. The making of a permanent magnet requires not just aligning the spins of electrons within an atom, but aligning the spins of atoms within a crystal.

The process that makes the magnetic arrangement favored in multi-electron atoms. The nonmagnetic arrangement features a symmetric spatial wavefunction and a favorable alignment of spins, both of which lower the energy compared to a state without those effects included (dotted line), but the repulsion between electrons in this state is very strong. In the magnetic arrangement, the antisymmetric spatial wavefunction and the magnetic interaction between spins both slightly increase the energy, but the reduction in the repulsive interaction between electrons is more than enough to compensate.
The phenomenon that makes a magnetic mineral is ultimately the same one that makes a magnetic atom: a combination of Pauli exclusion and repulsive forces that goes by the (somewhat misleading) name “exchange interaction.” The structure of a crystal is determined by the sharing of electrons, which establishes the distance between atoms and their three-dimensional arrangement. This crystal structure then determines the energy bands and band gaps for the electrons in the material, as we saw in Chapter 8, which in turn determines many of their electrical properties.8
When we talked about molecules and solids in previous chapters, we mostly ignored the effect of spin (other than the state-filling effect of Pauli exclusion) and interactions between electrons, but just as they do at the atomic level, these play a key role in magnetism at the level of macroscopic materials. The calculations become much more complicated to carry out, but the mutual repulsion between electrons still increases the energy of states where the electrons are close together. This repulsion tends to be smaller for antisymmetric spatial states, and when electrons are in antisymmetric spatial states, their spins are lined up.
For the right combination of materials, the iron atoms in a mineral end up separated by just the right distance so that their total energy is lower when the electrons in the crystal fall into antisymmetric space wavefunctions. This means that the spin wavefunctions must be symmetric, with their spins pointed in the same direction and adding together to make a stronger combined magnet.
Getting just the right distance between magnetic atoms depends on subtle details of the chemistry and crystal structure, which is why magnetic minerals are so rare. Even alloys made entirely of magnetic elements can be made nonmagnetic by changing the mix of atoms. A stainless steel alloy consisting of mostly iron with about 15 percent chromium will naturally be magnetic. On the other hand, a different alloy that increases the chromium slightly and adds a bit of nickel (around 8 percent) is nonmagnetic.
This magnetic behavior is also very fragile—the energy shifts involved are generally quite small, and depend again on subtle details of the crystal structure. Some nonmagnetic alloys can even be made magnetic solely by mechanical manipulation: the stainless steel alloy typically used for kitchen appliances is technically nonmagnetic, but the process by which the panels are shaped deforms the crystal structure somewhat, which is why we can use magnets to stick crayon drawings to our “nonmagnetic” stainless steel refrigerators.
When all the various factors involved come together in the right way, the electrons in a particular region will tend to align their spins with those of their nearest neighbors, making a small “magnetic domain” of that piece of the crystal, which acts like a microscopic magnet. Even this is not enough to make a permanent magnet, though. Naturally occurring chunks of metal consist of enormous numbers of little crystals with slightly different orientations, each making a domain with its north pole pointing in a random direction.
If a magnetic material consisting of many little domains pointing in random directions is exposed to a strong magnetic field—say by placing a magnet next to the surface—each of those domains can lower its energy by shifting its electrons around to align with the field. This produces a large number of domains with their south poles pointed at the north pole of the magnet, and it’s responsible for the attractive force between a magnet and a piece of metal. This alignment of domains is only temporary—when the magnetic field is removed, the individual domains return to their original random orientations.
Making a permanent magnet requires rearranging these domains in a more lasting way. This can be done mechanically—if you’re patient, you can turn a steel paper clip into a weak permanent magnet by rubbing it with another magnet—or by heating the material to a high temperature and letting it cool in the presence of a strong magnetic field.9 This results in a material where the electrons in all the individual domains have their spins (more or less) aligned in the same direction, adding together to make a stronger magnet.
Once established, a permanent magnet, as the name suggests, will tend to keep this alignment, even though the crystal structure of the individual domains might favor a different arrangement. While the material’s total energy could be lowered by having the electrons point in the right direction for each domain, the energy would have to increase in the intermediate steps of this process. Again, though, this magnetism is easily disrupted: as a material is heated, the thermal energy added to the motion of the electrons can become large enough to cover the energy increase needed so that electrons will be free to orient their spin however they like—usually in the direction favored by the crystal structure of their particular domain. Magnetic materials thus have a characteristic “Curie temperature” above which their electrons will no longer remain aligned across different domains, and they lose their magnetic character.10
Understanding the physics involved, from electron spins up to crystal domains, has also allowed physicists to engineer magnetic materials that are not found in nature. In particular, since the 1970s, the use of extremely strong magnets based on “rare earth” elements like neodymium has become widespread—they’re found in everything from kids’ toys to magnetic data storage systems. These have made magnetic fasteners in general much more common, and more reliable than they were when I was of an age to make drawings to stick on the refrigerator.
Magnetic Data Storage
While the realignment of domains in a magnetic material placed in a magnetic field is usually temporary, for some materials, applying a sufficiently large field can force a more permanent realignment. Once aligned, these domains will remain in their new orientation after the field is removed, until something else—heating, mechanical manipulation, or a strong enough field in a different direction—disrupts the new arrangement. This persistence of magnetic domains has made these materials an essential part of the data storage industry.
In the early days of computers, many machines used “magnetic core memory,” where bits being used in computation were stored temporarily in small chunks of magnetic material, with the direction of the north pole switched between two values by running a current through a loop of wire around each bit. The magnets in these could be fairly substantial—large enough to create signals picked up by a nearby radio. One of my computer science professors in college told a story about designing a punch-card program that would pointlessly flip bits in the right pattern to play the Sesame Street song “Rubber Duckie” on a radio left next to one of these computers.
On a smaller scale, flexible strips of magnetic material formed the basis for the cassette and VHS tapes that were staples of my teenage years, storing sounds and video in patterns of magnetic domains written onto the tape using electromagnets in the recorder. These patterns were then read out by a small detector picking up the changing magnetic field caused by the tape passing beneath a coil of wire in the player. Tapes could store data for long periods of time, though the materials used would slowly degrade after many playbacks.
In less obsolete technologies, rewritable magnetic domains are also behind the operation of modern hard disks. The basic principle remains the same: an electromagnet in the “write head” changes the orientation of magnetic domains on the disk to store digital information. Meanwhile, the “read head” detects the pattern of magnetic fields on the disk, converting the stored information back into ones and zeroes in working memory. Decades of engineering effort in developing better magnetic materials and high-performance data-writing and -reading systems has pushed these drives to the point where they can store an incredible amount of data. The four-terabyte drive I use to back up my computer at home is about the size of a box of the 5.25” floppy disks used by my first computer; that whole box would’ve held around one millionth of the data of my current backup drive.
This chapter has only skimmed lightly over the extremely complex physics of magnetic materials, a rich and varied field keeping huge numbers of physicists happily occupied. Whether you’re interested in high-density data storage or just displaying crayon drawings on kitchen appliances, though, all of this physics is deeply rooted in quantum mechanics. Every magnet you encounter is ultimately a quantum object, drawing on the intrinsic spin of the electrons within it.
Notes
1 This won’t work with stationary magnets, but if you make the magnet part of a rapidly spinning top you can, in fact, get it to hang in midair. A toy version of this is available under the name “Levitron” and makes a useful physics demonstration.
2 This is a little unfair to Feynman, who was, in fact, making a larger point about the problem of “why” questions in general. It’s regularly used as a disclaimer before attempts to explain the physics of magnetism, though, so it clearly resonates on that level.
3 This is a great quiz question in introductory physics classes. The north magnetic pole is also slightly offset from the north end of the axis about which the earth rotates, so depending on your position, magnetic north deviates slightly from true north; the difference between the two is well-known, however, and marked on good navigational maps.
4 The shift is much smaller in hydrogen than positronium because the magnetic field generated by a proton is much smaller than that of an electron or positron.
5 Somewhat loosely speaking, this is because an electron is as likely to be orbiting clockwise as counterclockwise, and the magnetic contributions from those two possible orbits cancel each other out.
6 The orbital motion of electrons does affect their interaction with magnetic fields, leading to the Zeeman effect, where a single energy state splits into multiple sublevels when an atom is placed in a magnetic field. These sublevels do not create a magnetic field outside the atom that could be used to power a permanent magnet, though.
7 There’s also a symmetric combination of one spin-up and one spin-down electron, which is often grouped together with the both-up and both-down states, collectively referred to as a “triplet” state in contrast to the “singlet” antisymmetric state.
8 This may seem a little circular, with the electron states determining the arrangement of atoms and then the arrangement of atoms determining the electron states. Theoretical calculations of these things usually involve an iterative process: picking a plausible arrangement of atoms, then calculating the electron states, then recalculating the arrangement of atoms to see if the new electron states favor a shift. In nature, this process just happens automatically; it’s much easier to be an atom than a theoretical physicist.
9 Or even a relatively weak one—rocks cooling in the magnetic field of the earth become slightly magnetized as a result. This is one of the decisive bits of evidence for continental drift: on either side of the mid-Atlantic ridge, we see a pattern of “stripes” with alternating magnetization, as the earth’s magnetic poles have reversed direction many times over millions of years. New rocks formed as magma moves up and out through the ridge trace the history of pole shifts and the spreading of the ocean floor.
10 This is named after Pierre Curie, whose original research was in the physics of magnetic materials. As Marie Curie began to work on radioactivity, though, Pierre abandoned magnets to join her in those experiments, which we’ll discuss in the next chapter.