CHAPTER 17
Sleep and Sleep-Wake Disorders
Sudha Tallavajhula, M.D.
Joshua J. Rodgers, M.D.
Jeremy D. Slater, M.D.
What is sleep? For most, one could paraphrase Supreme Court Justice Potter Stewart—“I know it when I see it” (although he was referring to obscenity). Sleep is characterized by typical changes in posture, reduced motor activity, and a threshold for response to external stimuli that increases progressively as sleep deepens (Datta 2010). Sleep was historically viewed as a largely passive state, a mechanism for the brain to go “off-line.” We now know that the brain functions in and transitions between three physiologically distinct states, namely, wakefulness, non–rapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep. The nuclei, networks, and neurotransmitters that generate and regulate the transitions between these states are also integral to regulation of homeostasis, sensorimotor function, emotion, behavior, and cognition (Dyken et al. 2012). The process of sleep fundamentally affects the brain in nearly every possibly way, from the regulation of genes to the plasticity of widespread networks (Abel et al. 2013), and the quality and quantity of sleep is a biomarker of the functional state of the brain and health in general (Luyster et al. 2012). Thus, there is a reciprocal relationship between sleep and the general medical, neurological, and psychological aspects of health—you cannot have one without the other—and disturbances in one system commonly cause disturbances in the other, either directly or indirectly.
Because sleep is so fundamental to health, the presence of sleep disruption or disorder represents “low-hanging fruit”—a therapeutic target for which the appropriate intervention, most often behavioral and noninvasive, can have a beneficial impact on comorbid medical, neurological, or psychiatric disorders and improve overall morbidity, mortality, and quality of life (Bloom et al. 2009; Dyken et al. 2012; Schutte-Rodin et al. 2008; Watson and Viola-Saltzman 2013). In this chapter, we review the physiological aspects of sleep and the pathophysiology, diagnosis, and treatment of common sleep-wake disorders, with an emphasis on the neuropsychiatric aspects of sleep. The reader is also referred to the resources available from the American Academy of Sleep Medicine (AASM), particularly the clinical practice guidelines for the individual categories of sleep disorders (https://aasm.org/clinical-resources/practice-standards/practice-guidelines/).
Sleep Physiology
The timing of the sleep-wake cycle results mainly from the interaction of a homeostatic drive (process S)—originating in the basal forebrain and promoting sleep in a near-linear fashion with time spent awake—and a circadian drive (process C)—originating in the suprachiasmatic nuclei (SCN) and promoting wakefulness in an oscillatory fashion with a period of about 24 hours. The state of NREM sleep is divided into N1 (characterized by theta frequencies), N2 (characterized by the presence of sleep spindles and K-complexes), and N3 stages, where N3 represents slow-wave sleep (SWS), replacing the old nomenclature of stages 3 and 4. Periods of NREM sleep alternate with periods of REM sleep (characterized by beta frequencies) in a healthy human in roughly 60- to 90-minute cycles and in a ratio of 3–4:1(Dijk and Lockley 2002).
Circadian Clock
The biological basis of the circadian clock and the rhythm it produces rests in genetic feedback loops of relatively fixed timing; clock genes code for clock proteins that are enzymatically transformed into transcription factors that can repress or activate the expression of those of other clock proteins (Lück et al. 2014). The resultant biological clock is resynchronized to the day/night cycle by a variety of environmental cues termed zeitgebers. The predominant zeitgeber is light itself (Duffy and Wright 2005), but temperature, social interactions, exercise, and the timing of meals may exert influence as well. The entrainment effect of light is mediated through photosensitive retinal ganglion cells that project directly to the SCN via the retinothalamic tract and affect abrupt changes in clock protein degradation rates and gene expression. SCN inputs from the thalamus and midbrain raphe allow for nonphotic entrainment to occur (Toh 2008). Conditions that impact entrainment of the circadian clock, either from external factors (shift work, living too near either pole) or internal factors (blindness, glutamate antagonists—any process interfering with SCN inputs), can result in sleep disruptions and somatic complaints. As clock genes are present in virtually every tissue type in the body (Yamazaki et al. 2000), circadian disturbances can trigger far-reaching and complex effects (Duguay and Cermakian 2009).
The SCN projects to other hypothalamic nuclei and the pineal gland that secretes melatonin to regulate temperature, hormone fluctuations, and other bodily functions. Melatonin acts as a signal for “biological night” for the rest of the body (Arendt and Skene 2005).
Wake
The waking state is primarily driven by the ascending reticular activating system (ARAS), a collection of neuronal circuits originating in the pontine reticular formation (Schwartz and Roth 2008). Other areas, including the locus coeruleus, dorsal raphe, median raphe, and hypothalamus, are commonly included in the ARAS. The ARAS projections have two major branches, the first of which consists of primarily cholinergic neurons originating in the pedunculopontine and laterodorsal tegmental nuclei and projects through synaptic relays in the rostral intralaminar and thalamic nuclei to the cerebral cortex. The source neurons are most active during wake and REM sleep and least active during NREM sleep. The second branch of the ARAS projects to the lateral hypothalamus, basal forebrain, and cerebral cortex. This branch carries noradrenergic inputs from the locus coeruleus, serotonergic inputs from the dorsal and median raphe nuclei, dopaminergic input from the ventral periaqueductal gray, and histaminergic input from the tuberomammillary nucleus (TMN). The monoaminergic inputs are most active during wake, are less active during NREM sleep, and go silent during REM sleep. Other cortical afferents originating in the basal forebrain (cholinergic and γ-aminobutyric acid [GABA]–ergic) follow the same firing pattern as the cholinergic inputs to the primary ARAS branch, active in wake and REM sleep (Table 17–1).
Neurotransmitter |
Nuclei |
Target |
Wake |
NREM |
REM |
Monoaminesa |
PAG, TMN, LC, D/MR |
Diffuse cortex; TMN→VLPO nucleus |
++ |
+ |
− |
Acetylcholine |
PPT, LDT |
Diffuse cortex |
++ |
− |
++ |
Orexin |
Lateral hypothalamus |
Throughout CNS; ARAS; cortex |
++ |
− |
++ |
GABA, galanin |
VLPO nucleus |
TMN; lateral hypothalamus |
− |
++ |
+ |
eVLPO nucleus |
Spinal interneuronsb and basal forebrain |
− |
− |
++ |
Note. Relatively more active (++), less active (+), and inactive (–) firing.
Abbreviations: ARAS=ascending reticular activating system; CNS=central nervous system; D/MR=dorsal and median raphe; eVLPO=extended region of the ventrolateral preoptic; GABA=γ-aminobutyric acid; LC=locus coeruleus; PAG=periaqueductal gray; LDT=laterodorsal tegmental; PPT=pedunculopontine; TMN=tuberomammillary nucleus; VLPO=ventrolateral preoptic.
aDopamine, histamine, epinephrine, norepinephrine, serotonin.
bIndirect target; see text for details.
An additional set of cortical afferents originates in the lateral hypothalamus from orexin-producing cells.1 The afferents from orexin-producing cells in the lateral hypothalamus follow the same firing pattern as the cholinergic wake pathways (Sakurai 2007). These orexin-producing neurons project widely throughout the central nervous system (CNS), with particularly dense excitatory connections throughout the ARAS. During wakefulness, the orexin-producing neurons appear to trigger increased activation of the ARAS monoaminergic neurons, while the latter provide feedback inhibition to the orexin-producing neurons. The result is stabilization of the waking state. The orexin-producing neurons receive direct inputs from the limbic system, providing a pathway for emotional states to trigger increased alertness. The SCN projects to the nearby subparaventricular zone, which, in turn, projects to the dorsomedial hypothalamic (DMH) nucleus. The DMH nucleus has GABAergic projections to orexin-producing neurons, providing a pathway for circadian influence over this system.
Sleep and Flip-Flop Switches
A subgroup of GABAergic and galaninergic neurons projects to the monoaminergic neuron systems, especially the TMN, as well as the lateral hypothalamus (Schwartz and Roth 2008). The TMN, in turn, projects histaminergic input to the VLPO nucleus. During sleep, increased firing by the VLPO neurons suppresses monoaminergic cell firing, resulting in self-disinhibition and a further increase in VLPO nucleus output. The VLPO nucleus output also suppresses hypocretin output, further dampening the ARAS and promoting sleep. With increased monoaminergic firing during wakefulness, the VLPO nucleus is in turn inhibited, with resultant disinhibition of the monoaminergic and hypocretin systems. The bidirectional feedback loop creates a “flip-flop” switch between sleep and wake, allowing for relative stability for each state once it is reached but working against any intermediate state. The SCN (via the DMH nucleus) also connects to the VLPO nucleus, allowing for another avenue of circadian influence. The relative complexity of the various sleep and wake regulatory centers allows for maintenance of critical physiologic cycles, but also permits flexible adaptation to external influences and changes in the environment.
The extended portion of the VLPO (eVLPO) contains REM-active GABAergic and galaninergic neurons, which project to an apparent “REM-off” site within the ventrolateral periaqueductal gray (vlPAG) (Lu et al. 2006). The vlPAG has dense GABAergic inhibitory projections to the sublaterodorsal (SLD) nucleus within the mesopontine tegmentum, which, in turn, projects similar inhibitory inputs back to the vlPAG. The SLD nucleus contains two populations of glutamatergic neurons, one with descending projections to spinal interneurons that appear to trigger REM atonia and a second with ascending projections to the basal forebrain triggering the cortical desynchronization seen on electroencephalogram (EEG) during REM. The result of the feedback loop between the vlPAG and SLD nucleus appears to be another flip-flop switch, this time supporting the oscillation between REM and NREM sleep.
Table 17–1 lists the summary of overall changes in neurotransmitter systems with respect to state (wake, NREM, and REM). Knowledge of the role of the different systems can sometimes predict the likely adverse effects of commonly used drugs (e.g., the sleepiness triggered by over-the-counter antihistamines) or the impact of pathological states (e.g., hypocretin depletion associated with narcolepsy).
Recent work has revealed that sleep is a dynamic state, specifically dependent on preceding waking activity over specific areas of the cerebral cortex (Clinton et al. 2011; Krueger and Tononi 2011). The accumulation of sleep-regulatory substances, produced as a consequence of the normal metabolic activity of neural networks, appears to trigger the entry of cortical columns into local sleep while awake.
Classification of Sleep-Wake Disorders
There are three major organizing systems used in the classification of sleep-wake disorders, each of which has recently been updated, reflecting the rapid pace of progress in this field. Sleep medicine specialists commonly use the International Classification of Sleep Disorders, now in its third edition (ICSD-3; American Academy of Sleep Medicine 2014), while mental health professionals may refer to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5; American Psychiatric Association 2013), and practitioners of all types refer to the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM; National Center for Health Statistics 2017) for billing codes. A commonality between these systems, and a departure from prior editions in the case of DSM, is classification based on symptom clusters and comorbidity rather than on presumptions of specific etiology. In ICSD-3, sleep disorders are classified into one of seven categories: insomnias, sleep-related breathing disorders (SRBDs), central disorders of hypersomnolence, circadian rhythm sleep-wake disorders (CRDs), parasomnias, sleep-related movement disorders, and other sleep disorders. ICSD-3 also includes appendixes of sleep-related medical or neurological disorders and substance-induced sleep disorders with an ICD-10 coding guide. DSM-5 generally parallels the ICSD-3 classification scheme but does not differentiate the various disorders of hypersomnolence, nor does it include as thorough a classification in regard to the parasomnias or sleep-related movement disorders. Unlike DSM-5 or ICSD-3, ICD-10-CM continues to make a distinction between “organic” (G-code) and “inorganic” (F-code) disorders.
Evaluation of the Patient With Sleep Complaints
Some basic principles of the evaluation of patients with sleep complaints are presented here. (For a comprehensive review of this subject, see Reite et al. 2009.)
History
Complaints presented by patients with sleep-wake disorders usually fall into three categories: insomnia, hypersomnolence, and aberrant nocturnal behaviors (Figure 17–1). Some patients may present with overlapping symptoms—for instance, patients with difficulty sleeping at night may present with excessive daytime sleepiness. Careful questioning can usually elucidate the primary problem and guide further evaluation as outlined below. Essential components of the history include review of the patient’s daytime and nighttime symptoms, daily schedule, bedtime behaviors, sleep environment, daytime napping, current medications or changes, and use of alcohol, caffeine, over-the-counter medications or supplements, and illicit drugs. Obtaining a family history also may help guide diagnoses.
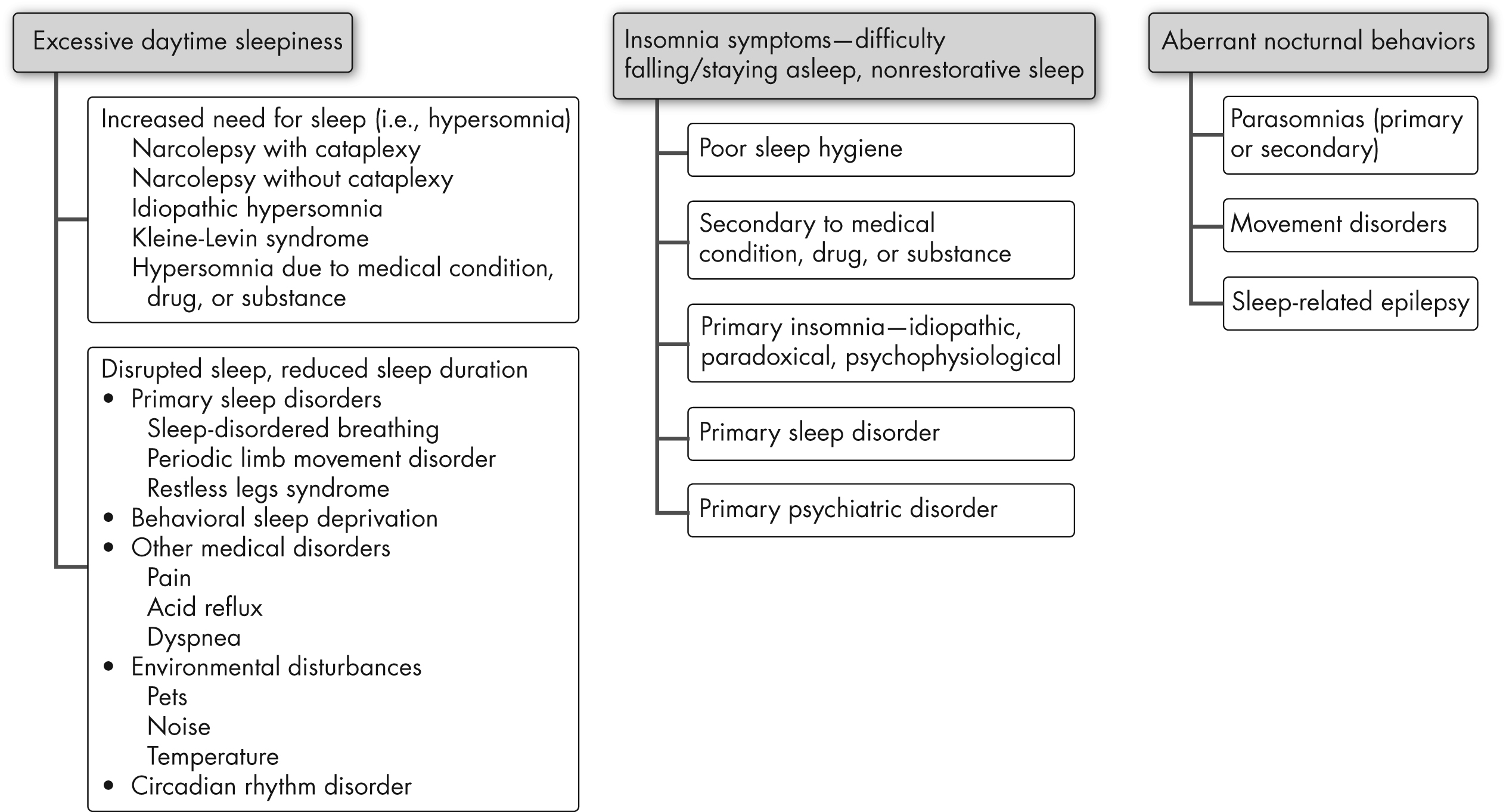
FIGURE 17–1. Sleep complaints and differential diagnosis.
Insomnia
The definition for insomnia requires some form of daytime impairment for diagnosis. Rare individuals that are genetically short sleepers seldom suffer from deficiency in daytime performance. Patients with insomnia may complain about difficulty falling asleep (sleep onset), staying asleep (sleep maintenance), early awakening with difficulty returning to sleep, or any combination of these. Each of these complaints may suggest different types of sleep-wake disorders—for example, patients with restless legs syndrome (RLS) often have sleep onset difficulty but usually do not complain of sleep maintenance problems. Obstructive sleep apnea (OSA) leads to frequent nocturnal awakenings and hence may present as sleep maintenance difficulty. Early morning awakening is often associated with depression but may also result from advanced sleep phase circadian disorder. Inquiring about sleep hygiene—for instance, amount and timing of caffeine intake, use of electronics at bedtime, and inconsistent bedtimes—is also useful. Maladaptive sleep behaviors associated with anxious thoughts concerning sleep point toward psychophysiological insomnia.
Hypersomnolence
Excessive daytime sleepiness is a common complaint reported in 5%–20.6% of the population (Ohayon 2008). In general, daytime sleepiness can result from nocturnal sleep disturbances or from an increased need for sleep. It is critical to make this differentiation to guide rational investigation and management. Nocturnal sleep disturbances could include sleep deprivation or disruption of sleep by clinical sleep disorders. When daytime napping is also nonrestorative, this usually indicates disruption of sleep by clinical sleep disorders. However, patients with primary hypersomnia (increased need for sleep) usually wake up refreshed from naps. Also, associated symptoms like snoring, witnessed apneas, and frequent leg movements at night provide information about clinical sleep disorders.
Aberrant Nocturnal Behaviors
Aberrant nocturnal behaviors constitute a diverse group of pathologies. Particularly in those disorders that occur during REM sleep, the patient may have insight and memory and be able to describe the problem. It is equally important to obtain witnessed accounts. The pattern of aberrant behavior, its timing and duration, patient responsiveness, and patient’s memory for the event are all significant clues. Stereotypic behavior that is short, occurs in clusters, and involves hypermotor manifestations or dystonic posturing suggests nocturnal epilepsy, most commonly frontal lobe epilepsy. REM sleep behavior disorder (RBD) usually presents as dream enactment behavior with vivid recall, often occurring in middle-aged individuals, especially in the latter half of the night. Non-REM parasomnias like sleepwalking and sleep terrors are more often seen early in the night and are less likely to be recalled by the patient.
Examination
In addition to routine physical examination, certain pertinent physical findings can guide in the differential diagnosis of sleep-wake disorders. Height, weight, and body mass index are important to note. Obesity is commonly associated with OSA, narcolepsy, and nocturnal eating disorder. Patients with body mass index greater than 35 kg/m2 are also at risk for obesity-hypoventilation syndrome (Balachandran et al. 2014). Large neck circumference (greater than 16 inches in men and 15 inches in women) is a risk factor for OSA. Craniofacial anatomy, particularly structure of the maxilla and mandible, the hyoid, tongue size, and uvula shape all affect the upper airway resistance to airflow. Retrognathia and micrognathia are risk factors for OSA. Patients with these anomalies are candidates for certain treatment options that are not positive airway pressure (PAP) options, such as the mandibular advancement device. Macroglossia is one of the contributing factors to the development of OSA in patients with Down syndrome. Nasal patency is assessed by having patients breathe through each nostril separately. The oropharynx is commonly assessed by two methods, the Mallampati classification (Mallampati et al. 1985) and the Friedman classification (Friedman et al. 2002). Tonsillar and adenoidal hypertrophy is a common cause of sleep-disordered breathing in young children. Features of heart failure detected during the cardiopulmonary exam—for example, bibasilar crackles and pedal edema—should raise suspicion for central sleep apnea. In addition, chronic pulmonary obstructive disorders may also coexist with OSA.
Careful neurological examination is essential. One of the most fascinating disorders of sleep, RBD is closely related to parkinsonian syndromes. Patients with RBD may present with hyposmia (i.e., impaired sensation of smell) (Miyamoto et al. 2009). Examination of the first cranial nerve is hence important along with the rest of the neurological exam. In patients with clinical features of RLS, examination to detect neuropathy is necessary. Attention should be paid to stigmata of neuromuscular disease, including muscle atrophy, hyporeflexia in lower motor neuron disease, fibrillations, fasciculations, and hyperreflexia in upper motor neuron disease, as well as some characteristic features of individual syndromes. In addition to predisposition to OSA caused by improper muscular tone in the upper airway, these patients are also prone to developing hypoventilation, especially during REM sleep. Obesity resulting from some of the medications used for neurological disorders—for example, valproate for epilepsy and steroids for certain neuromuscular disorders—may also contribute to the development of OSA.
Investigations to Aid in Diagnosis
Use of sleep diaries can help characterize sleep complaints, provide important information about sleep hygiene and circadian disorders of sleep, and help track response to treatment (Schutte-Rodin et al. 2008). Some commonly used questionnaires in sleep medicine history are the Epworth Sleepiness Scale (to quantify daytime sleepiness), the Pittsburgh Sleep Quality Index, the Insomnia Severity Index, the STOP-BANG Questionnaire (a screening tool developed by anesthesiologists to preoperatively assess the risk of SRBDs), and the Morningness-Eveningness Questionnaire (to assess for CRDs).
Actigraphy is a good option to evaluate sleep patterns over prolonged periods of time (Morgenthaler et al. 2007a). This is performed using portable wristwatch-like devices that estimate sleep and wakefulness based on motion. It has been validated in the diagnosis and therapeutic monitoring of insomnia and CRDs.
Nocturnal, attended polysomnography (PSG) is considered the gold standard test for many sleep-wake disorders. It is a comprehensive assessment of many physiological parameters of sleep, including electroencephalography, chin and limb electromyography, electrooculography, electrocardiography, respiratory effort, respiratory flow, oxygen saturation, and several other parameters like end-tidal capnography. The AASM recommends criteria for the performance and analysis of PSG. PSG is diagnostic in most instances and is usually followed by PAP titration studies if the initial study demonstrates sleep-disordered breathing. This paradigm is now undergoing change in the era of cost-effective medicine. Portable sleep testing (limited channel cardiopulmonary testing) has been developed as a tool of verification of diagnosis for patients who have a high pretest probability of having SRBDs. This is a resource for patients that do not have significant comorbid conditions like congestive heart failure or neuromuscular disease. If confirmed to have OSA by portable sleep testing, these patients may undergo treatment with auto-titrating PAP, without undergoing PSG for PAP titration. AASM criteria have also been proposed for patients who are suitable for portable sleep testing (Collop et al. 2007).
PSG is usually not indicated in the routine evaluation of insomnia. It has some utility in paradoxical insomnia to convince patients of existence of electrographic sleep. It is the test of choice for patients who are suspected of having neurological disorders of sleep and SRBDs in the context of complex medical conditions. The Multiple Sleep Latency Test (MSLT) is an extended form of PSG that is used objectively to quantify daytime sleepiness. The rationale behind the MSLT is that those patients who have uninterrupted sleep at night and continue to demonstrate daytime sleepiness have a centrally mediated increased need for sleep. Further testing with dedicated EEG, cerebrospinal fluid testing, neuroimaging, endocrine and genetic testing, arterial blood gas, pulmonary function testing, dedicated electrocardiogram, echocardiogram, and serum chemistry may be considered on the basis of individual diagnoses.
Common Sleep-Wake Disorders and Their Management
In the following subsections, we describe common sleep disorders, based on ICSD-3, and their management.
Insomnia
Most definitions of insomnia recognize this as a disorder of persistent difficulty with sleep initiation, duration, consolidation, or quality that occurs despite adequate opportunity for sleep and results in daytime impairment in some form. The insomnia organization presented in ICSD-3 represents a consolidation of the multiple chronic insomnia diagnoses in prior editions. This was done both for the sake of simplicity and to be more in line with clinical practice. Currently recognized subtypes of insomnia include chronic insomnia, which requires the patient to have complaints for longer than 3 months, and short-term insomnia, which is of shorter duration as the name implies. Another category of insomnia disorder is reserved for patients with more nonspecific complaints that do not meet full criteria for either chronic insomnia or short-term insomnia. Insomnia resulting from other medical and psychiatric disorders or from the use of drugs or substances is independently classified as such.
The previously delineated clinical and pathophysiological subtypes of insomnia include psychophysiological insomnia—characterized primarily by maladaptive behavior and heightened arousal surrounding sleep—and paradoxical insomnia, also called sleep state misperception—characterized by the patient’s inability to perceive neurophysiologically documented sleep as the sleep state. Childhood insomnia has two main defined categories. Sleep onset association type is consequential to the child’s dependence on specific environmental circumstances for sleep. Limit-setting type is usually seen as resistance to go to bed because of inadequate limit setting by the parent.
The evaluation of insomnia, as outlined in the guidelines published by the AASM (Schutte-Rodin et al. 2008), is based on published evidence emphasizing that insomnia is primarily diagnosed by a clinical evaluation. Supporting tools like questionnaires and technology help characterize insomnia better but continue to be secondary.
The treatment of chronic insomnia involves a multimodality approach. In addition to optimizing the treatment of comorbid sleep disorders and/or medical disorders, the AASM clinical guidelines (Schutte-Rodin et al. 2008) designate cognitive-behavioral therapy for insomnia (CBT-I; described further below) as a first-line treatment of insomnia. This recommendation is based on strong evidence from systematic reviews and meta-analyses that established that CBT-I is effective (70%–80% of patients can be expected to benefit) and durable (effects persist) and, when used as the only treatment, may result in better long-term outcomes than pharmacotherapy alone or in combination with CBT-I. The use of pharmacotherapy should be considered as a short-term, adjunctive aid to cognitive and behavioral therapies (Mitchell et al. 2012).
Pharmacotherapy
A wide array of agents are used in the pharmacological management of insomnia. Table 17–2 provides characteristics of some of the more commonly prescribed agents, although it should be noted that not all of the agents listed are approved by the U.S. Food and Drug Administration (FDA) for the treatment of insomnia. Current FDA-approved treatments include several benzodiazepine receptor agonists (BzRAs), melatonin receptor agonists (ramelteon), and the relatively new orexin (hypocretin) receptor antagonists (suvorexant). Selection of an agent involves consideration of several factors, including the type of insomnia, timing of symptoms (sleep onset vs. sleep maintenance insomnia), comorbid disorders, adverse effects, past treatment experience, contraindications and medication interactions, cost, and patient preference. The recommendation is to begin treatment with a short- or intermediate-acting BzRA or ramelteon. Low-dose sedating antidepressants are used as second-line measures. Physicians should be familiar with the nuances of using these medications—namely, the potential for BzRAs to cause automatic behaviors and depress respiratory drive (the patient must avoid concomitant use of alcohol or other sedative agents; adequate sleep opportunity should be ensured), the potential for daytime drowsiness and impaired psychomotor performance, and the potential for tolerance with some of these agents as well as the dangers of abrupt withdrawal.
Medication class |
Medication |
Half-life (hr) |
Dose (mg) |
Significant adverse effects |
Benzodiazepines (BZDs) |
Temazepam |
8–20 |
15–30 |
Sleep architecture changes. Residual sedation, amnesia, abuse potential, automatic behavior in sleep, falls, dizziness, cognitive effects |
Non-BZD BZD-receptor agonists |
Zaleplon |
1 |
5–20 |
Sleep architecture changes. Residual sedation, amnesia, abuse potential, automatic behavior in sleep, falls, dizziness, cognitive effects; metallic taste (eszopiclone) |
Zolpidem |
1.5–2.4 |
5–10 |
||
Zolpidem CR |
1.6–4.5 |
6.25–12.5 |
||
Eszopiclone |
6 |
1–3 |
||
Melatonin receptor agonists |
Melatonin |
0.6–1 |
0.3–10 |
Drowsiness, dizziness |
Ramelteon |
0.8–2 |
8 |
||
Sedating antidepressants |
Trazodone |
7–15 |
25–150 |
Dizziness, orthostatic hypotension, weight gain, urinary retention; priapism (trazodone) |
Doxepin |
15.3 |
3–6 |
||
Amitriptyline |
1.5–4 |
10–100 |
||
Antihistamines |
Diphenhydramine |
3.4–9.2 |
25–50 |
Sedation, dizziness, orthostatic hypotension, tachycardia, urinary retention |
Doxylamine |
10 |
25–50 |
||
Antipsychotics |
Quetiapine |
7 |
25–200 |
Dry mouth, tachycardia, weight gain, orthostatic hypotension |
Orexin receptor antagonists |
Suvorexant |
12 |
10–20 |
Automatic behavior, cataplexy-like symptoms, sleep paralysis, sleep-related hallucinations |
Cognitive and Behavioral Management Strategies
The use of CBT-I is informed by multifactorial models that, in addition to recognizing the role of precipitating stressors and predisposing trait characteristics of the patient, emphasize the roles of maladaptive cognitions, beliefs, attention to stimuli, coping strategies, and safety behaviors in the process of conditioning insomnia (Perlis et al. 2011; Schutte-Rodin et al. 2008). For example, maladaptive strategies such as spending more time in bed (e.g., going to bed earlier, sleeping in, taking naps) and staying in bed while awake (e.g., tossing and turning attempting to force sleep) result in decreased sleep efficiency and association of the bed with arousal and, thus, can condition insomnia.
CBT-I includes three core components—sleep hygiene, stimulus control, and sleep restriction therapy—and three adjunctive therapies—cognitive therapy, relaxation training, and phototherapy (Perlis et al. 2011). Depending on the problem and selected goals, the appropriate component or combination can be implemented (Table 17–3). Sleep hygiene is the set of routines and environmental factors that are conducive to circadian alignment and the promotion of consolidated sleep, including keeping consistent routines, avoiding maladaptive use of substances, and not spending excessive time awake in bed. The aim of stimulus control therapy is to pair the stimulus of the intended sleep environment to a sleep response by reducing autonomic arousal and aligning intrinsic homeostatic and circadian processes with the environment.
Goal |
Sleep hygiene and other behavioral interventions |
CBT-I interventions |
Create sleep-conducive environment |
Keep the room dark and quiet (white noise is OK). Use an eye mask and ear plugs. |
Sleep hygiene |
Do not use the TV to fall asleep. Avoid fluid intake in the evening. |
||
Inpatient: minimize staff intrusions, alarms, and bright light at night. |
||
Facilitate circadian rhythm entrainment |
Keep a regular schedule (routine) for bedtime, wake time, eating, and activities. |
Sleep hygiene |
Get bright light in the morning and avoid bright light at night (including TV and computer screens); use a night-light (low light) if needed at night. |
Phototherapy |
|
Increase drive for sleep at night |
Get regular daytime exercise and avoid exercise at night. Consolidate sleep at night and avoid taking naps during the day. |
Stimulus control, sleep restriction, cognitive therapies |
If possible, dose sedating medications at night and stimulating medications in the morning. |
||
Avoid caffeine or other stimulants after noon. |
||
Do not use tobacco. |
||
Do not use alcohol to sleep (it disrupts sleep continuity). |
||
Decondition insomnia |
Use the bed for sleep (no TV, eating, or worrying allowed). |
Stimulus control, sleep restriction, cognitive therapies |
Sleep when tired. |
||
If awake >20 minutes, get up, do something nonstimulating, and return to bed when tired. |
||
Relax, reduce autonomic arousal |
Have a relaxing bedtime routine. Make a list of problems or worries for later. Avoid stressful or anxiety-provoking conversations and media (e.g., books, movies, news) before bed. |
Relaxation training, hypnosis, biofeedback, mindfulness |
Note. CBT-I=cognitive-behavioral therapy for insomnia.
In sleep restriction therapy, homeostatic and circadian forces are leveraged by first establishing a fixed wake time and a fixed sleep opportunity—limited initially to a sleep diary–derived average of time spent sleeping—and then progressively advancing the scheduled bed time while maximizing sleep efficiency. (Because sleep deprivation may precipitate mania or seizures, sleep restriction therapy is contraindicated for patients with these conditions.)
Cognitive therapy consists of psychoeducation and guided identification and restructuring of maladaptive thoughts through learning and practicing specific skills, including paradoxical intention, attention bias, and imagery rehearsal. Relaxation training aims to reduce the physiological arousal associated with insomnia and may include progressive muscle relaxation, diaphragmatic breathing, biofeedback, hypnosis, and mindfulness. A great deal of practice in session and during waking hours is required to learn these skills for them to be readily used and effective at bedtime and not to be quickly abandoned or contribute to the already conditioned insomnia response.
Phototherapy also may be helpful in insomnia or jet lag. This therapy involves the use of bright light in the morning or evening depending on whether there is a phase delay or phase advance component, respectively.
Hypersomnias
The central theme of these disorders is an increased need for sleep. The main subtypes include narcolepsy (type I and type II), idiopathic hypersomnia, Kleine-Levin syndrome, and hypersomnias secondary to medical or psychiatric conditions and medications. The MSLT is the gold standard test for defining daytime sleepiness. The clinical manifestations of narcolepsy include excessive daytime sleepiness as the cardinal symptom, and a mean sleep latency of less than 8 minutes and two or more sleep-onset REM periods during the MSLT are required for the diagnosis of narcolepsy. Although cataplexy (episodic, sudden loss of muscle tone with retained consciousness often triggered by certain emotions, most commonly laughter) is associated with narcolepsy type I, the latter may be diagnosed even in the absence of cataplexy if associated with low serum hypocretin levels. Other symptoms include sleep paralysis, sleep stage transition (hypnagogic and hypnopompic) hallucinations, and disrupted nocturnal sleep. Narcolepsy is also associated with the HLA DQB1*0602 or DRB1*1501 allele (but this is not diagnostic; see Kumar and Sagili 2014). Also associated are obesity, other primary disorders of sleep (e.g., REM sleep behavior disorder), and anxiety disorders.
Stimulant medications like modafinil, methylphenidate, amphetamine, and methamphetamine are used for the treatment of daytime sleepiness due to narcolepsy (Morgenthaler et al. 2007b). Wake-promoting agents such as modafinil or armodafinil have more favorable adverse effect profiles. Sodium oxybate is effective for the treatment of cataplexy and daytime sleepiness and for consolidating sleep in narcolepsy. Tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), and venlafaxine may also be effective for the treatment of cataplexy as well as sleep paralysis and hypnagogic hallucinations. Scheduled naps can also ameliorate daytime sleepiness.
There is less robust evidence for symptomatic treatment in other central hypersomnias (Morgenthaler et al. 2007b). Modafinil has been found to improve daytime sleepiness in patients with idiopathic hypersomnia. Lithium carbonate is also thought to be effective for treatment of recurrent hypersomnia and behavioral symptoms due to Kleine-Levin syndrome. This rare syndrome is characterized by recurrent episodes of severe sleepiness, in association with cognitive, psychiatric, and behavioral disturbances.
Sleep-Related Breathing Disorders
SRBDs include the OSA disorders, central sleep apnea syndrome, sleep-related hypoventilation disorders, and sleep-related hypoxemia disorder. Young et al. (1993) reported prevalence rates for OSA of about 9% in women and 24% in men. Predisposing factors include obesity, craniofacial abnormalities, male gender, and endocrine disorders. In younger children, adenotonsillar hypertrophy is the most common cause of upper airway narrowing. The pathophysiology of OSA involves repetitive, intermittent upper airway obstruction during sleep. As implied, an apnea is characterized by complete obstruction, whereas a hypopnea is a partial obstruction. Typical symptoms of OSA include snoring, witnessed apneas, gasping arousals, and daytime sleepiness. The spectrum of severity of upper airway obstruction ranges from simple snoring to obesity hypoventilation syndrome (associated with hypercapnia). OSA is associated with hypertension, atrial fibrillation, type 2 diabetes, coronary artery disease, and congestive heart failure, as well as mood disorders and pain disorders. Primary options for OSA include PAP (continuous [CPAP], biphasic [BIPAP], or auto-titrating [APAP]) therapy and lifestyle modification such as weight loss through healthy diet and exercise. Although several surgical approaches have been proposed, these usually remain second-line options after PAP therapy. The exception is adenotonsillectomy in the pediatric population. Oral appliances have demonstrated efficacy, particularly in mild, supine positional OSA.
Central sleep apnea syndromes are generally caused by a deficiency in the ventilatory drive and are more prevalent in patients with congestive heart failure, stroke, and/or opioid abuse or in premature infants. In some cases, these syndromes may be a result of PAP treatment of OSA. Treatment includes management of the underlying medical disorder with or without PAP.
Parasomnias
Parasomnias are a fascinating group of disorders that are characterized by undesirable behaviors or experiences that occur during sleep and/or sleep-wake transitions; they are classified based on whether they occur in NREM or REM sleep. NREM parasomnias include sleepwalking, confusional arousals, sleep terrors, and sleep-related eating disorder. REM parasomnias comprise RBD, recurrent isolated sleep paralysis, and nightmare disorder. The entire spectrum of parasomnias is much more common in children. These are also often found associated with other primary disorders of sleep (e.g., OSA). Safety concerns and legal hazards should be addressed at the very beginning. The diagnosis in many cases is purely clinical but in some others (e.g., RBD) requires PSG. Multiple studies may be required to capture an event. Effective treatments include benzodiazepines, tricyclic antidepressants, and cognitive and behavioral therapies, but, depending on the clinical situation and the presence or absence of medical comorbidity, treatment may not be necessary and the focus may be on education and reassurance.
Circadian Rhythm Sleep-Wake Disorders
CRDs are characterized by incongruence between the internal circadian rhythm and timings required by the external environment (Morgenthaler et al. 2007c). Clinically, these disorders often manifest as insomnia symptoms. As with the insomnia group, impairment in functioning is requisite to the diagnosis. Sleep logs and actigraphy are central to the diagnosis and evaluation. Measurement of salivary or plasma dim-light melatonin onset and urinary metabolites of melatonin are also used, most often in research. Circadian chronotype can also be assessed using the Morningness-Eveningness Questionnaire. Delayed sleep-wake phase disorder is most often seen among adolescents and young adults. Social and behavioral factors often play an important role in perpetuating the physiological shift toward later sleep times that is seen in this age group. Advanced sleep-wake phase disorder, in contrast, is often seen with advancing age. Irregular sleep-wake rhythm disorder, as the name implies, is characterized by an erratic sleep-wake cycle. Neurodegenerative disorders often predispose to this form of circadian misalignment.
Therapeutic entrainment of circadian rhythms involves behavioral interventions (most critically sleep hygiene), strategic use of zeitgebers (e.g., light therapy), and pharmacotherapy such as melatonin (dosed to approximate dim-light melatonin onset, i.e., approximately 1 mg in the evening, not at bedtime). High-dose melatonin given later in the night will be soporific but can cause a phase delay and insomnia (Arendt and Skene 2005). Chronotherapy involves gradual advancing or delaying of bedtimes as appropriate to counteract the disturbance. Non-24-hour sleep-wake rhythm disorder is most often found in blind individuals. Recently, tasimelteon, a melatonin receptor agonist, has been approved for the treatment of this disorder. Shift work disorder is characterized by impaired sleep and wake at desired times due to a misalignment between the endogenous circadian clock and environmental time induced by the imposed shift work schedule (Wright et al. 2013). Rapid travel across multiple time zones results in a similar condition colloquially referred to as “jet lag.” The circadian clock can typically adapt to such changes faster if aided by strategic timing of zeitgebers (e.g., bright light, melatonin).
Sleep-Related Movement Disorders
The most common sleep-related movement disorders are RLS and periodic limb movement disorder (Hornyak et al. 2006). Although the two disorders are related, RLS is a sensorimotor disorder and a clinical diagnosis, whereas periodic limb movement disorder is diagnosed when PSG reveals periodic leg movements in sleep (PLMS) (>5 PLMS per hour of sleep in children or >15 PLMS per hour of sleep in adults) and there is also clinical evidence of functional impairment from nonrestorative sleep. RLS is characterized by four cardinal criteria: 1) an urge to move the legs, caused by a usually uncomfortable sensation in the legs, which 2) often begins or worsens with rest or inactivity, 3) is at least partially relieved by movement, and 4) often occurs predominantly in the evening or night. Patients with RLS most often complain of sleep-onset insomnia. RLS may occur at any age, occurs more often in women, and may also appear secondary to other conditions like uremia and pregnancy. The prevalence of PLMS has been found to increase with age. Low brain iron content, as reflected by serum ferritin level, has been found in association with both RLS and PLMS. Iron supplementation is recommended if serum ferritin is less than 50 μg/L. Dopaminergic medications, anticonvulsants (e.g., gabapentin), benzodiazepines, and opioids form the major groups of pharmacological treatment (Aurora et al. 2012).
Sleep Disruption in Medical, Neurological, and Psychiatric Disorders
Sleep in Medical Disorders
Sleep disruption is common in the medically ill, with over 90% reporting symptoms of a sleep disorder (National Sleep Foundation 2002). Sleep is especially problematic for hospitalized patients (Young et al. 2008). Such sleep disruptions can lower the pain threshold, worsen cardiorespiratory status, induce insulin resistance and predict the development of metabolic syndrome, induce changes in cellular processing and production of free radicals, increase the risk of cancer, disrupt autonomic tone, and contribute to poor health and impaired functioning in general (Depner et al. 2014; Luyster et al. 2012). The symptoms of a disorder (e.g., fever, hot flashes, pain, heartburn, nocturia, thirst, dyspnea, and dystonia) or the side effects of a drug (e.g., akathisia) can delay sleep onset or disrupt continuity.
Conversely, the physiological associations of normal sleep can exacerbate some disorders. Examples include the skeletal muscle paralysis and marked increase in blood pressure and heart rate associated with REM sleep that can exacerbate pulmonary or cardiovascular and cerebrovascular disorders, respectively. The former may produce arousals and sleep deprivation and all of its sequelae, and the latter may contribute to the increased risk of sudden cardiac death and stroke in early morning hours, when REM is more prevalent (Verrier et al. 1996) and when coagulability is increased because of circadian-neuroendocrine factors (Dyken et al. 2012; Watson and Viola-Saltzman 2013).
Beyond these basic principles and examples, myriad associations exist between sleep and individual medical disorders and have been well reviewed elsewhere (Luyster et al. 2012; Parish 2009; Young et al. 2008). In the following sections, we discuss the associations between sleep and common categories of neurological and psychiatric disorders.
Sleep in Neurological Disorders
Sleep disruption and disorders can result from any focal lesion (e.g., stroke, CNS tumor, demyelination) or diffuse process (e.g., encephalopathy/delirium, neurodevelopmental disorders) that disturbs the function of any of the sleep-wake and circadian centers and networks described earlier in this chapter. The approach to such sleep disruptions can be informed by the pathophysiology of the underlying neurological disorder (Dyken et al. 2012; Watson and Viola-Saltzman 2013). In CNS infections and autoimmune diseases, inflammation (perhaps mediated by IL-1β and TNF-α) generally has a soporific effect, but a variety of sleep disturbances are possible (e.g., Lyme disease causing poor sleep quality and RLS; HIV causing insomnia in proportion to infection progression; and multiple sclerosis causing disturbed sleep and fatigue and an increased incidence of RBD and narcolepsy) (Parish 2009). Sleep and epilepsy are clearly interrelated, because epileptic seizures commonly occur at least partially or exclusively in sleep or may be precipitated by sleep deprivation, and there are elevated rates of comorbid sleep disorders in patients with epilepsy, including OSA, RLS, PLMS, RBD, and NREM parasomnias (Watson and Viola-Saltzman 2013). Migraine, hypnic, and episodic (but not chronic) cluster headaches are each closely associated with REM sleep, may be exacerbated when REM sleep is increased, and have notable time-of-day periodicity, suggesting roles for REM-promoting regions and SCN dysfunction in these disorders. Neuromuscular diseases typically result in hypersomnolence, strongly correlating with functional disability, either directly by affecting the hypothalamus-hypocretin (orexin) system and serotonergic dorsal raphe nuclei (as in myotonic dystrophy) or secondarily from chronic hypercapnia and SRBD (as in amyotrophic lateral sclerosis [ALS], muscular dystrophies, and myasthenia gravis).
The presence of idiopathic RBD is usually a harbinger of neurodegenerative disease, including, most commonly, the synucleinopathies (Watson and Viola-Saltzman 2013). In fact, RBD is a “suggestive” diagnostic feature of dementia with Lewy bodies and is found in about one-quarter of all patients with Parkinson’s disease. The disruption of cholinergic tone, marking the progression of many dementias and other neurodegenerative disorders, is associated with circadian misalignment (e.g., phase delay in Alzheimer’s disease, phase advance in frontotemporal dementia, and sleep-wake reversal in Parkinson’s disease and progressive supranuclear palsy), reduced REM sleep, and the phenomena of “sundowning,” and these disturbances may aggravate other symptoms, be a major source of discouragement, increase caregiver burden, and lead to earlier nursing home placement.
Contrary to popular belief, the need for sleep does not decrease for older adults, and it would be unwise to dismiss a sleep complaint or take lightly sleep changes in the elderly simply as expected age-related decline (Bloom et al. 2009). Because of medical and psychosocial comorbidities, as well as, in some individuals, loss of VLPO neurons, aging is associated with sleep fragmentation and an increased predilection for CRDs, RLS, and other sleep disorders.
Sleep in Psychiatric Disorders
Sleep disruption is a core diagnostic feature of mood disorders, but this relationship is complex (American Psychiatric Association 2013; Sutton 2014). PSG changes, including reduced REM latency, SWS, total sleep time, and sleep efficiency, are observed during both depressive and manic episodes. This is consistent with evidence for hypothalamic dysfunction in mood disorders and the cholinergic-monoaminergic imbalance hypothesis for depression. The latter may also account for the disturbing dreams and nightmares and the early awakenings associated with depression. This balance is delicate, because treatment with antidepressants (especially SSRIs) may incite insomnia, RLS, or somnambulism. While hypersomnia is characteristic of atypical depression, insomnia is found in typical depression and its severity predicts worse outcomes, including higher rates of suicide. A single night of sleep deprivation, deprivation in the later part of the night, or simply acute REM deprivation—perhaps by normalization of the increased metabolic activity seen in the anterior cingulate gyrus—can temporally relieve depression or can incite mania.
Insomnia is so strongly and bidirectional correlated, both temporally and in severity, with anxiety disorders and posttraumatic stress disorder (PTSD) that problems with insomnia are part of the diagnostic features of these disorders and they are thought to share commonalities in their underlying pathologies (Alfano and Mellman 2010). These disorders are associated with hyperarousal, decreased sleep continuity and SWS, and increased REM density, as well as narcolepsy and sleep paralysis. Secondary sleep disruption by the symptoms of the respective disorder is common. Examples include nocturnal attacks in panic disorder, nocturnal rituals diminishing the time for sleep in obsessive-compulsive disorder and precipitating delayed sleep-wake phase disorder, worry about sleep producing a conditioned psychophysiological insomnia, or claustrophobia symptoms in PTSD that limit compliance with PAP treatment (Sutton 2014).
Worsening insomnia is characteristic of the prodromal phase of schizophrenia, and there is evidence for dysfunction of homeostatic and circadian processes as well as sleep spindle production in this disorder (Sutton 2014). When present at any phase, sleep disturbances are known to dramatically increase the already elevated risk for suicide in schizophrenia, perhaps 12-fold or more. Decreased dorsolateral prefrontal cortex activity in schizophrenia, also found in REM sleep, may contribute to the experience of hallucinations.
There may be common gene abnormalities shared by attention-deficit hyperactivity disorder (ADHD) and delayed sleep-wake phase disorder (Sutton 2014). ADHD is also associated with insomnia (generally a side effect of stimulant treatment), RLS, and OSA.
Sleep-Wake Effects of Medications and Other Substances
Many medications and abused substances cross the blood-brain barrier and act on systems regulating wakefulness, homeostatic and circadian drives, and REM/NREM balance (Conroy et al. 2010; Schweitzer 2011). The overall effect on these systems varies by agent, timing of dosing and half-life, patient genetic factors, comorbid conditions, and drug-drug interactions. Through skillful psychopharmacology, medication effects can be harnessed for the benefit of the patient, but failing to pay heed to these effects can lead to harm, patient dissatisfaction, and noncompliance.
In general, medications that interfere with receptor-neurotransmitter systems involved in wakefulness promotion (i.e., Ach, NE, DA, 5-HT, H1 or H2, alpha1), such as most antipsychotics, tricyclic antidepressants, and antiepileptic medications, are more likely to cause sedation, whereas stimulators of these systems, such as procholinergic (e.g., donepezil) or dopaminergic (e.g., L-dopa) agents, if dosed in the evening, may disrupt sleep and cause disturbing dreams and, in the case of L-dopa, hallucinations, agitation, and sleep attacks (see Table 17–1). Taking advantage of this effect, use of donepezil in patients with Alzheimer’s disease and OSA can stimulate respiratory drive and reduce apneic events (Sukys-Claudino et al. 2012). Secondary effects of medications can disrupt sleep-wake dynamics—for example, exacerbation of OSA secondarily by the muscle-relaxing effects of benzodiazepines or by increased neck circumference due to weight gain from atypical antipsychotics, mood stabilizers, and some antidepressants.
The use of substances to induce sleep or promote wakefulness in our modern society is pervasive and can be problematic. Alcohol is commonly used to induce sleep but, unfortunately, results in a net decrease in total sleep time with decreased sleep efficiency in the second half of the night, reduced restorative (SWS) sleep, and early awakening, and alcohol is associated with precipitating or worsening SRBD and PLMS (Conroy et al. 2010). Caffeine, as an adenosine receptor antagonist, is thought to promote wakefulness by interfering with the homeostatic drive for sleep, and both evening use and regular daily use are associated with disrupted sleep and daytime sleepiness (Roehrs and Roth 2008).
Conclusion
Sleep is an indispensable physiological phenomenon with far-reaching implications for the physical and mental well-being of an individual. Evaluating and addressing sleep-wake disorders should be integral to health care delivery in all specialties of medicine. There is robust evidence of improvement in outcomes in many comorbid illnesses when sleep-wake disorders are managed well. Referral to a specialist trained in the management of sleep-wake disorders should also be considered in the overall treatment paradigm.
References
Abel T, Havekes R, Saletin JM, et al: Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol 23(17):R774–R788, 2013 24028961
Alfano CA, Mellman TA: Sleep in anxiety disorders, in Foundations of Psychiatric Sleep Medicine. Edited by Winkelman JW, Plante DT. New York, Cambridge University Press, 2010, pp 286–297
American Academy of Sleep Medicine: International Classification of Sleep Disorders. Darien, IL, American Academy of Sleep Medicine, 2014
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Arlington, VA, American Psychiatric Association, 2013
Arendt J, Skene DJ: Melatonin as a chronobiotic. Sleep Med Rev 9(1):25–39, 2005 15649736
Aurora RN, Kristo DA, Bista SR, et al; American Academy of Sleep Medicine: The treatment of restless legs syndrome and periodic limb movement disorder in adults—an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep 35(8):1039–1062, 2012 22851801
Balachandran JS, Masa JF, Mokhlesi B: Obesity Hypoventilation Syndrome Epidemiology and Diagnosis. Sleep Med Clin 9(3):341–347, 2014 25360072
Bloom HG, Ahmed I, Alessi CA, et al: Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J Am Geriatr Soc 57(5):761–789, 2009 19484833
Clinton JM, Davis CJ, Zielinski MR, et al: Biochemical regulation of sleep and sleep biomarkers. J Clin Sleep Med 7(5)(suppl):S38–S42, 2011 22003330
Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine: Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med 3(7):737–747, 2007 18198809
Conroy DA, Arnedt JT, Brower KJ: Sleep in substance use disorders, in Foundations of Psychiatric Sleep Medicine. Edited by Winkelman JW, Plante DT. New York, Cambridge University Press, 2010, pp 314–329
Datta S: Cellular and chemical neuroscience of mammalian sleep. Sleep Med 11(5):431–440, 2010 20359944
Depner CM, Stothard ER, Wright KP Jr: Metabolic consequences of sleep and circadian disorders. Curr Diab Rep 14(7):507, 2014 24816752
Dijk DJ, Lockley SW: Integration of human sleep-wake regulation and circadian rhythmicity. J Appl Physiol (1985) 92(2):852–862, 2002 11796701
Duffy JF, Wright KP Jr: Entrainment of the human circadian system by light. J Biol Rhythms 20(4):326–338, 2005 16077152
Duguay D, Cermakian N: The crosstalk between physiology and circadian clock proteins. Chronobiol Int 26(8):1479–1513, 2009 20030537
Dyken ME, Afifi AK, Lin-Dyken DC: Sleep-related problems in neurologic diseases. Chest 141(2):528–544, 2012 22315121
Friedman M, Ibrahim H, Bass L: Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg 127(1):13–21, 2002 12161725
Hornyak M, Feige B, Riemann D, et al: Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev 10(3):169–177, 2006 16762807
Krueger JM, Tononi G: Local use-dependent sleep; synthesis of the new paradigm. Curr Top Med Chem 11(19):2490–2492, 2011 21906015
Kumar S, Sagili H: Etiopathogenesis and neurobiology of narcolepsy: a review. J Clin Diagn Res 8(2):190–195, 2014 24701532
Lu J, Sherman D, Devor M, et al: A putative flip-flop switch for control of REM sleep. Nature 441(7093):589–594, 2006 16688184
Lück S, Thurley K, Thaben PF, et al: Rhythmic degradation explains and unifies circadian transcriptome and proteome data. Cell Reports 9(2):741–751, 2014 25373909
Luyster FS, Strollo PJ Jr, Zee PC, et al; Boards of Directors of the American Academy of Sleep Medicine and the Sleep Research Society: Sleep: a health imperative. Sleep 35(6):727–734, 2012 22654183
Mallampati SR, Gatt SP, Gugino LD, et al: A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J 32(4):429–434, 1985 4027773
Mitchell MD, Gehrman P, Perlis M, et al: Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract 13:40, 2012 22631616
Miyamoto T, Miyamoto M, Iwanami M, et al: Odor identification test as an indicator of idiopathic REM sleep behavior disorder. Mov Disord 24(2):268–273, 2009 18972547
Morgenthaler T, Alessi C, Friedman L, et al; Standards of Practice Committee; American Academy of Sleep Medicine: Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 30(4):519–529, 2007a 17520797
Morgenthaler TI, Kapur VK, Brown T, et al; Standards of Practice Committee of the American Academy of Sleep Medicine: Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 30(12):1705–1711, 2007b 18246980
Morgenthaler TI, Lee-Chiong T, Alessi C, et al; Standards of Practice Committee of the American Academy of Sleep Medicine: Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep 30(11):1445–1459, 2007c 18041479
National Center for Health Statistics: International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM), Hyattsville, MD, Centers for Disease Control and Prevention, 2017. Available at: https://www.cdc.gov/nchs/icd/icd10cm.htm. Accessed March 7, 2017.
National Sleep Foundation: 2002 “Sleep in America” poll. April 2002. Available at: http://sleepfoundation.org/sites/default/files/2002SleepInAmericaPoll.pdf. Accessed March 3, 2017.
Ohayon MM: From wakefulness to excessive sleepiness: what we know and still need to know. Sleep Med Rev 12(2):129–141, 2008 18342261
Parish JM: Sleep-related problems in common medical conditions. Chest 135(2):563–572, 2009 19201722
Perlis ML, Aloia M, Kuhn BR: Behavioral Treatments for Sleep Disorders: A Comprehensive Primer of Behavioral Sleep Medicine Interventions. Boston, MA, Academic, 2011
Reite M, Weissberg M, Ruddy JR: Clinical Manual for Evaluation and Treatment of Sleep Disorders. Washington, DC, American Psychiatric Publishing, 2009
Roehrs T, Roth T: Caffeine: sleep and daytime sleepiness. Sleep Med Rev 12(2):153–162, 2008 17950009
Sakurai T: The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci 8(3):171–181, 2007 17299454
Schutte-Rodin S, Broch L, Buysse D, et al: Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 4(5):487–504, 2008 18853708
Schwartz JR, Roth T: Neurophysiology of sleep and wakefulness: basic science and clinical implications. Curr Neuropharmacol 6(4):367–378, 2008 19587857
Schweitzer PK: Drugs that disturb sleep and wakefulness, in Principles and Practice of Sleep Medicine. Edited by Kryger MH, Roth T, Dement WC. Philadelphia, PA, Saunders/Elsevier, 2011, pp 542–561
Sukys-Claudino L, Moraes W, Guilleminault C, et al: Beneficial effect of donepezil on obstructive sleep apnea: a double-blind, placebo-controlled clinical trial. Sleep Med 13(3):290–296, 2012 22281004
Sutton EL: Psychiatric disorders and sleep issues. Med Clin North Am 98(5):1123–1143, 2014 25134876
Toh KL: Basic science review on circadian rhythm biology and circadian sleep disorders. Ann Acad Med Singapore 37(8):662–668, 2008 18797559
Verrier RL, Muller JE, Hobson JA: Sleep, dreams, and sudden death: the case for sleep as an autonomic stress test for the heart. Cardiovasc Res 31(2):181–211, 1996 8730394
Watson NF, Viola-Saltzman M: Sleep and comorbid neurologic disorders. Continuum (Minneap Minn) 19(1 Sleep Disorders):148–169, 2013 23385699
Wright KP Jr, Bogan RK, Wyatt JK: Shift work and the assessment and management of shift work disorder (SWD). Sleep Med Rev 17(1):41–54, 2013 22560640
Yamazaki S, Numano R, Abe M, et al: Resetting central and peripheral circadian oscillators in transgenic rats. Science 288(5466):682–685, 2000 10784453
Young JS, Bourgeois JA, Hilty DM, et al: Sleep in hospitalized medical patients, part 1: factors affecting sleep. J Hosp Med 3(6):473–482, 2008 19084897
Young T, Palta M, Dempsey J, et al: The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328(17):1230–1235, 1993 8464434