Carotenoids (Xanthophylls and Carotenes)
Koula Doukania,b, Ammar S.M. Sellesc, Hasna Bouhennia, Meriem Chafaaa, Leila Soudania
aFaculty of Nature and Life Sciences, University of Tiaret, Algeria
bLaboratory of Sciences and Technics of Animal Production, University of Abdelhamid Ibn Badis, Mostaganem, Algeria
cInstitute of Veterinary Sciences, University of Tiaret, Algeria
4.4.1 Carotenoids
Carotenoids are one of the most frequent groups of natural colors. They are produced by plants and some species of archaea and fungi as well as photosynthetic bacteria and algae. However, animals must obtain them through diet. The red pea aphid (Acyrthosiphon pisum) and spider mite (Tetranychus urticae) are the only animals that make carotenoids from fungi via gene transfer. There are over 1100 known carotenoids which have been identified and characterized and are responsible for the red, orange, and yellow colors (Yabuzaki, 2017).
4.4.2 Chemical composition
Xanthophylls and carotenes are two types of carotenoids that vary only in their oxygen content. The base structure of carotenoids is identical, consisting of eight isoprene molecules. Isoprene molecules have 5 carbons each, making a total of 40 carbons. Tetraterpenoids are a group of carotenoids that all have the same structure (Rao and Rao, 2007).
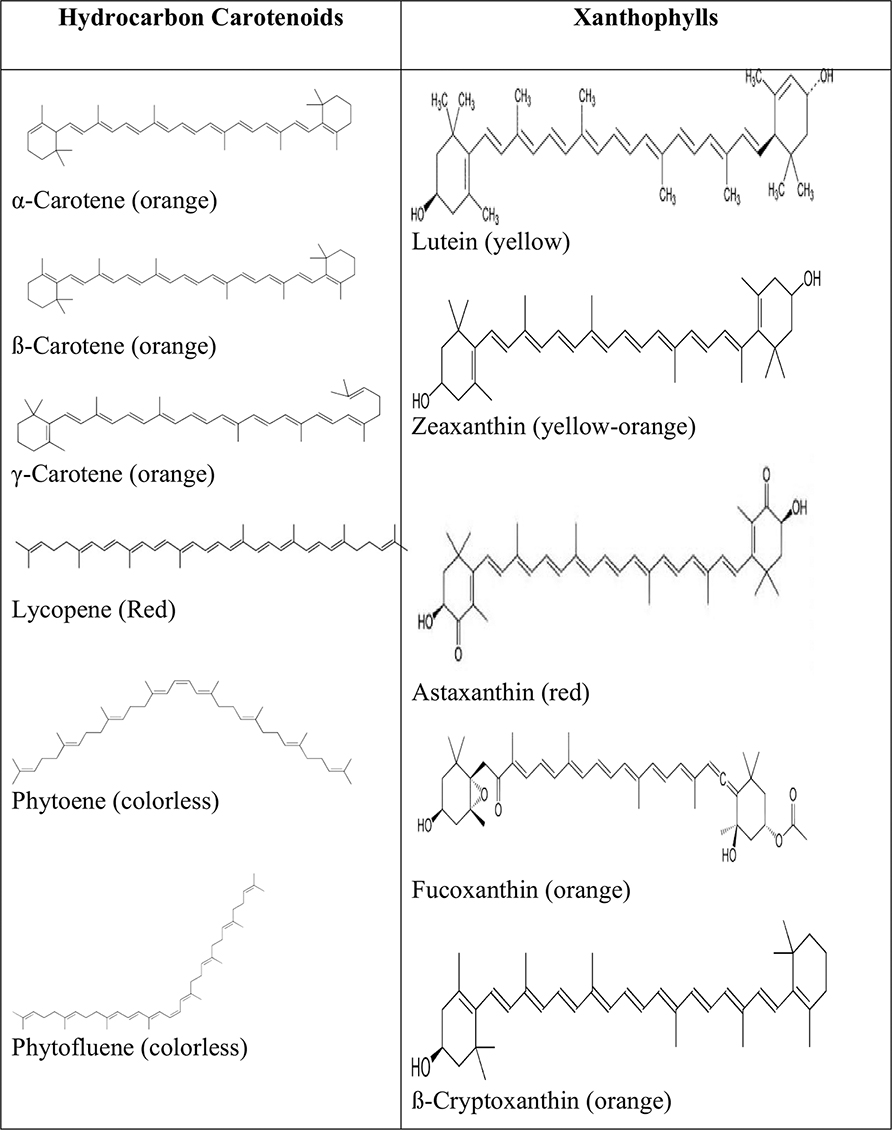
Carotenoids are classified into two groups based on their chemical composition (Fig. 4.4.1): (1) carotenes, which are composed of only carbon and hydrogen molecules, for example, α-carotene, β-carotene, γ-carotene, lycopene, phytoene, and phytofluene and (2) xanthophylls, which contain oxygen functionality at the cyclic end groups in the form of methoxy, hydroxy, keto, carboxy, and epoxy positions. Examples of these compounds are zeaxanthin, lutein, β-cryptoxanthin, astaxanthin, fucoxanthin, spirilloxanthin, echinenone, and antheraxanthin (Goodwin, 1980).
Because of the several conjugated double bonds in the polyene backbone, many geometric isomeric forms are possible. The most common isomer found in nature is the linear all-trans isomer, and many physicochemical and biological properties of these compounds are due to their electron-rich polyene backbone (Britton, 1995).
Carotenoids are generated in plants through a complex process that combines C5-isoprenoid units which are biosynthesized into C20 molecules (geranylgeranyl pyrophosphate GGPP) by the geranylgeranyl pyrophosphate synthase; however, carotenoids with 40 carbon atoms are the most common, which is the product of phytoene synthase mediated condensation of two molecules of 20 carbon atoms (Liang et al., 2006).
Carotenoids are naturally synthesized by all photosynthetic and non-photosynthetic species, including bacteria, fungi and archaea, which have a complex carotenogenic metabolism (Britton et al., 2004). They are categorized as C30, C40, C45, and C50 carotenoids based on the number of carbons in their structure, but only the C40 carotenoids are present in nature in greater abundance and, as a result, are more studied in the literature. Furthermore, species such as eukaryotes, archaea, and bacteria biosynthesize C40 carotenoids, and their chemical structures are made up of a variety of terminal classes. C30 and C50 carotenoids, on the other hand, are generated by archaea and bacteria and contain only 6 and 10 isoprenoid units, respectively. On the contrary, only bacteria are capable of producing C45 carotenoids, which are made up of nine isoprenoid units (Yabuzaki, 2017).
The acyclic C40 isoprenoid (tetraterpene) lycopene is the source of all C40 carotenoids. Lycopene is made up of eight C5 isoprene units in total. Initially, four C5 units combine to form geranylgeranyl diphosphate, a C20 intermediate. The C40 intermediate phytoene, a more saturated precursor of lycopene, is formed when two C20 precursors merge head-to-head. By stepwise enzymatic dehydrations, the other double bonds are introduced in phytoene. Various modifications, such as cyclizations, oxidative functionalizations, rearrangements, and oxidative degradations, are used to produce other carotenoids from lycopene (Zechmeister, 1962).
Carotenoids geometric isomers, as well as carotenoid epoxides, can be found in all-trans and cis forms. While the all-trans isomer is the most common type of carotenoid, the cis isomers are only found in trace amounts. The amount of cis carotenoid isomers could be increased by heating and thermal processing. Due to carotenoid deficiency, the depletion of carotenoids in fruits and vegetables is a major concern. Controlling carotenoid geometry isomer degradation and improving the efficiency of dietary carotenoids should be prioritized. Color variations in carotenoids during geometric isomerization, thermal processing, and even fruit ripening have all been studied extensively. However, the chemical and kinetic mechanisms of color changes during carotenoid degradation and isomerization are still unknown. More research into the isomerization of carotenoids in relation to the colorful pigments found in the biodiversity of fruits and vegetables is required in the future (Khoo et al., 2011).
4.4.3 Sources of carotenoids
Vegetables and fruits are the most major sources of carotenoids in the human diet (Elvira-Torales et al., 2019). Yellow-orange fruits and vegetables, as well as dark green, leafy vegetables, are high in carotenoids (Britton and Khachik, 2009). Animal-derived foods, such as dairy products, eggs, and some fish and seafood, can also contain large amounts of carotenoids (Mezzomo and Ferreira, 2016).
Carotenoids are found in a wide range of fruits and vegetables, and their composition and content are influenced by a number of factors, including variety, genotype, season, geographical location/climatic conditions, soil, maturation stage, type of processing, and storage conditions (Yahia and Ornelas-Paz, 2010; Alonso, 2017).
In addition, food cooking and processing techniques and methods of cultivation differ widely across the world and can have a significant impact on the stability and hence the quality of carotenoids (Maiani et al., 2009).
4.4.3.1 Carotenes
- Beta carotene is abundantly found in foods that has the highest provitamin A activity. It is mostly found in yellow-orange and dark green fruits and vegetables (Gul et al., 2015) with large amounts in buriti (Mauritia vinifera Mart.), camu-camu (Myrciaria dubia), tucumã (Astrocaryum aculeatum), bocaiuva (Acrocomia mokayayba Barb. Rodr.), some varieties of pumpkin, acerola, mango, carrot, spinach, squash, papaya, sweet potatoes, apricots, nuts, rose hip fruits carrot noodles, and oil palm (Mezzomo and Ferreira, 2016).
- Lycopene is a red color that exists naturally only in the tissues of vegetables and algae. It is deficient in provitamin A activity and is responsible for the red to pink colors in fruits and vegetables, such as tomatoes, red grapefruit, watermelon, guava, and papaya (Kong et al., 2010). Tomatoes and their derivatives, such as soups, juices, sauces, and ketchup are commonly the most commonly cited sources of lycopene. However, even greater concentration in cherry, guava and guava products, similar concentrations in watermelon and Thai papaya, and smaller amounts in Solo and Formosa papaya can be found (Mezzomo and Ferreira, 2016).
- Phytoene (PT) and phytofluene (PTF) are carotenes found in a wide variety of fruits and vegetables, for example, in tomatoes, carrots, and light orange apricots (Meléndez-Martínez et al., 2015).
4.4.3.2 Xanthophylls
- Lutein and zeaxanthin are found in green and dark green leafy vegetables, such as parsley, spinach, broccoli, and Brussels sprouts (Mezzomo and Ferreira, 2016). Egg yolk is a highly bioavailable source of zeaxanthin and lutein (Handelman et al., 1999).
- Lutein is a non provitamin A that is distributed in a wide variety of vegetables, such as kale, some varieties of pumpkin, acerola, spinach, and winter squash, caja (Spondias lutea) and camu-camu (Myrciaria dubia) (Mezzomo and Ferreira, 2016) and fruits, such as mango, papaya, peaches, plums, and oranges (Elvira-Torales et al., 2019). It is also produced by some microalgae, such as Chlorella sorokiniana MB-1, Chlorella vulgaris, and Scenedesmus obliquus CNW-N (Mezzomo and Ferreira, 2016).
- Zeaxanthin is found in high amounts in pequi (Caryocar villosum) and even the native marine microalgae Chlorella saccharophila (Mezzomo and Ferreira, 2016).
- Astaxanthin is a characteristic marine carotenoid in crustaceans (lobster, crab, and shrimp). Also, high amounts of this pigment are produced by the microalgae Chlorella vulgaris, Phaffia rhodozyma, and Haematococcus pluvialis (Ambati et al., 2014).
Undaria pinnatifida (Wakame), Laminaria japonica (Ma-Kombu), Phaeodactylum tricornutum, and Cylindrotheca closterium are examples of macroalgae and microalgae that contain also a marine carotenoid called fucoxanthin (Zhang et al., 2015a).
β-Cryptoxanthin is a less well- known carotenoids. It is found in carrots, peppers, tangerines, pumpkins, peaches, oranges, and in tropical fruits, such as papaya (Elvira-Torales et al., 2019).
Britton and Khachik (2009) suggested a classification system for carotenoids in fruits and vegetables based on the following levels:
- Low (0–0.1 mg/100 g FW)
- Moderate (0.1–0.5 mg/100 g FW)
- High (0.5–2 mg/100 g FW)
- Very high (>2 mg/100 g FW)
- Table 4.4.1 shows examples of foods having high, high-very high levels of a certain carotenoid.
Table 4.4.1
| High content | High-very high content |
|---|---|
| ß-carotene | |
| Brussels sprouts (Brassica oleracea
[Gemmiera]) Karat banana (Musa troglodytarum) Peach (Prunus persica) Pepper (red, orange, green) (Capsicum annuum) West Indian cherry (Malpighia glabra) |
Apricot (Prunus ameniaca)
Broccoli (Brassica oleracea) Buriti (Mauritia vinifera) Carrot (Daucus carota) Gac oil (Momordica cochinchinnensis) Kale (Brassica oleracea (Acephala)) Mango (Mangifera indica) Red palm oil (Elaesis guineensis) Spinach (Spinacia oleracea) Sweet potato (Ipomoea batatas) Tomato (Lycopersicum sculentum), high-beta |
| ß-cryptoxanthin | |
| Permisimmon (Diospyros kaki)
Pitanga (Eugenia uniflora) |
|
| Lutein | |
| Broccoli (Brassica oleracea [Italica])
Green leafy vegetables Pepper (yellow, green) (Capsicum annuum) |
|
| Zeaxanthin | |
| Buriti (Mauritia vinifera)
Chinese wolfberry (Lycium chinensis) Pepper (orange, red) (Capsicum annuum) |
|
| Lycopene | |
| Carrot (red) (Daucus carota)
Guava (Psidium guajava) Tomato (Lycopersicon esculentum) Water melon (Citrullus lanatus) |
|
4.4.4 Carotenoids accumulation and bioavailability
Plant physiological, genetic, and biochemical characteristics, as well as environmental growth factors, including light, temperature, and fertility, tend to influence carotenoids accumulation in plant tissues (Kurilich et al., 1999; Kopsell et al., 2003). There have been reports of significant differences in carotenoids accumulation among different vegetable crop species (Klein and Perry, 1982; Kimura and Rodriguez-Amaya, 2003). Carrot, corn, kale, lettuce, potato (Solanum tuberosum subsp. tuberosum), pepper, and soybean all have substantial genetic variation within species (Glycine max). Vegetable improvement programs can benefit from genetic variation in carotenoids concentrations within species (Nicolle et al., 2004).
Environmental growing conditions have an effect on the accumulation of carotenoids in plant foods. Carotenoids levels have been shown to rise and fall in response to environmental changes, with varying results for different plant species. Plant carotenoid accumulation is affected by changes in rising air temperature, irradiance level, irradiance photoperiod, and nutritional fertility (Lefsrud et al., 2005; Lefsrud et al., 2006a; Lefsrud et al., 2006b). Increased carotenoids concentrations are also associated with increased coloration in vegetable and fruit tissues as they mature (Russo and Howard, 2002). Carotenoids concentrations in leaf tissues rise with maturity but fall as the plant ages. Manipulation of cultural growing conditions and harvest time will thus influence the concentrations of carotenoids in fruit and vegetable crops (Gross, 1991).
The species and structures of carotenoids present in food, the composition and release of carotenoids from the food matrix, ingested quantities and absorption in the intestinal tract, transportation inside the lipoprotein fractions, the nutritional status of the ingesting host, as well as biochemical conversions and tissue-specific depositions; all affect the bioavailability of carotenoids from plant foods (Faulks and Southon, 2005). Carotenes are fully lipophilic molecules found in plant membranes’ hydrophobic cores. Xanthophylls, on the other hand, are often hydrophobic molecules with polar groups on opposite ends of a non-polar carbon skeleton. Since carotenoids are lipophilic, any biotic or abiotic activity that exposes them to possible oxidation, degradation, or isomerization would have an effect on their biochemistry and bioavailability (Gruszecki, 1999).
The release of carotenoids from the food matrix is the first step in their bioavailability. Carotenoids chemistry shifts as a result of food processing practices such as thermal processing, mincing, or liquefying, most likely due to isomerization or oxidation reactions (Kopas-Lane and Warthesen, 1995). However, by minimizing possible enzymatic oxidation, freezing or low-temperature storage normally preserves carotenoids concentrations. Processing activities normally increase bioavailability by increasing the release of bound carotenoids from the food matrix; however, in some food crops, thermal degradation of carotenoids chemistry can reduce bioavailability (Rodriguez-Amaya, 1999).
Carotenoids are absorbed passively in humans and follow digestive processes similar to lipids. Carotenoids that are bound to proteins or membranes must first be released from tissues and dissolved in a hydrophobic domain (oils, fats or bulk lipid emulsions). They must be moved to bulk lipids or intestinal micelles in the digesta because they are hydrophobic (Faulks and Southon, 2005). The presence of dietary fat in the small intestine, which promotes the release of emulsifying bile acids by the gallbladder, is needed for carotenoids absorption (Zaripheh and Erdman, 2002). Recent research has found that when carotenoids are consumed with dietary lipids, their absorption increases (Brown et al., 2004). Carotenoids are absorbed into chylomicrons after being released into the enterocyte, and then delivered to the bloodstream and, finally, the liver. Until eventual tissue specific deposition, carotenoids compounds can stay in the liver or be transferred to low-density or high-density lipoproteins (Brown et al., 2004).
In dietary trials or cohort studies, carotenoids bioavailability is normally measured in blood serum after ingestion. The relatively simple study measures changes in serum carotenoids levels over time after consumption of whole foods or supplements. The following are several caveats to consider when interpreting serum carotenoids bioavailability: (1) serum responses to single oral doses of carotenoids are greatly variable, (2) carotenoids levels measured in serum indicate an equilibrium between intestinal absorption, breakdowns, tissue uptake, and tissue release, and (3) serum already contains high levels of endogenous carotenoids (i.e. -carotene, -carotene, lycopene, and lutein) (Yeum and Russell, 2002). In vitro Caco-2 human intestinal cell lines have also been used in recent studies to determine carotenoid bioavailability (Chitchumroonchokchai et al., 2004).
4.4.5 Beneficial and detrimental effects of carotenoids on health
4.4.5.1 Beneficial effects
A diet containing carotenoids is essential for normal behavior and good health of humans and animals (Nisar et al., 2015). The activities of carotenoids as well as provitamin A and antioxidants (reduction of oxidative stress by capture of free radicals) increase interest toward these compounds. However, plants are rich sources of carotenoids (Rao and Agarwal, 1999).
4.4.5.1.1 Provitamin A activity
In humans, carotenoids regulate a number of genes related to developmental and physiological processes. Moreover, they are involved in the visual cycle as precursors of vitamin A related retinoids, such as retinol, retinal, and retinoic acid (Bonet et al., 2015). Divided into provitamin A and non provitamin compound (Olson and Krinsky, 1995). Three carotenoids classes (β-carotene, α-carotene, and β-cryptoxanthin) possess this provitamin A activity (Cazzonelli et al., 2010). They help prevent vitamin A deficiency (Stahl and Sies, 2005).
A toxic effect of vitamin A can be seen with diets rich in it. While, an excess of dietary β-carotene is not, therefore plants with a high β-carotene level constitute a safe and effective means as being a source of vitamin A (Cazzonelli et al., 2010), these β-carotenes are cleaved to form two retinals (vitamin A aldehyde) in the human organism by β-carotene 15,15′-dioxygenase (Von Lintig and Vogt, 2000; Wyss et al., 2000).
4.4.5.1.2 Antioxidant activity
Several epidemiological and clinical studies have examined the antioxidant capacity of carotenoids to prevent formation of various ROS in pathological conditions, such as cancer, inflammation, retinal degeneration and neurodegeneration (Cho et al., 2018). Likewise, these antioxidant properties are due to the various molecules of carotenoids such as lutein and zeaxanthin, ketocarotenoids and which form part of the beneficial human dietary components of the man (Jyonouchi et al., 1995; Zhu et al., 2008).
Antioxidant activity is probably due to trapping of singlet oxygen (1O2) and peroxyl radicals. In addition, carotenoids inhibit radicals generation and singlet oxygen by effectively deactivating the electronically excited sensitizing molecules (Truscott, 1990; Young and Lowe, 2001).
However, Miller et al. (1996) show that the presence of carbonyl and hydroxyl groups, in the terminal rings, as well as the number of conjugated double bonds increases the relative capacities of carotenoids to scavenge the ABTS .+ radical cation.
Nevertheless, Edge et al. (1997) considered carotenoids as the most powerful natural scavengers of singlet oxygen, with a rapid extinction rate (10 10 M −1s −1).
Moreover, El-Agamey et al. (2004) and Milani et al. (2017) report the three stages by which carotenoids scavenge radicals:
First step: an oxidation-reduction reaction involving a transfer of electrons:
CAR + ROO → CAR++ ROO–, this electron acceptance is favored by the presence of conjugated double bonds which makes it possible to neutralize free radicals (Rutz et al., 2016; Milani et al., 2017).
Second step: hydrogen abstraction: CAR + ROO– → CAR + ROOH
Third step: addition:CAR + ROO → ROOCAR (El-Agamey et al., 2004; Milani et al., 2017).
Besides, cooperative synergistic effect of vitamins E, C and β-carotene can trap reactive nitrogen species and inhibit lipid peroxidation (Bfhm et al., 1998; Stahl and Sies, 2005; Milani et al., 2017).
Wang et al. (2000) concluded that antioxidants might be a novel strategy for treating H. pylori infection in humans. This conculsion was made on the basis of the in vivo study carried out on Balbe / cA mice infected with H. pylori and treated with algal meal rich in astaxanthin (0.4, 2, and 4 g/kg of body weight, with the astaxanthin content at 10, 50, and 100 mg/kg respectively). The results of this study showed significantly lower colonization rates, significantly decreased lipid peroxidation and lower inflammation scores than animals not treated or treated with a control meal.
El-Akabawy and El-Sherif (2019) reported that zeaxanthin exerts potent anti-inflammatory and antioxidant effects by lowering myeloperoxidase and malondialdehyde and increasing the enzymatic activity of superoxide dismutase and catalase as well as glutathione levels. Additionnelly, zeaxanthin suppressed levels of tumor necrosis factor alpha, interferon-gamma, interleukin-6, interleukin-1 beta, and nuclear transcription factor kappa B, and inhibited the expression of nitric oxide synthase and cyclooxygenase-2.
Under some circumstances carotenoids may act as cellular antioxidants. For example, β-carotene can suppress the upregulation of hem oxygenase-1 gene expression provoked by UVA exposure in human dermal fibroblasts (FEK4) in a dose-dependent manner (Trekli et al., 2003; Elliott, 2005). This is consistent with a direct antioxidant (singlet oxygen quenching) effect as is the observation that UVA exposure caused the depletion of cellular β-carotene and the accumulation of apocarotenal. However, the activation of retinoid signaling via retinoic acid receptors RARs and RXRs could not be ruled out as a possible alternative mechanism. Curiously, the authors of this study also reported that at the lowest level used (0.2 AM) the presence of β-carotene actually augmented UVA induced hem oxygenase-1 induction. Furthermore, others have reported that β-carotene can act as a pro-oxidant augmenting the induction of hem oxygenase-1 in human skin fibroblasts (HFP-1) (Obermuller-Jevic et al., 1999; Elliott, 2005).
Several enzymatic and nonenzymatic antioxidants are involved in the body’s antioxidant defense system (Sies, 1993; Stahl and Sies, 2005). Carotenoids protect cells against oxidative stress by activation of endogenous antioxidant enzymes and reduction of DNA damage, helps protect cells against oxidative stress (Cho et al., 2018).
While, several studies have shown that high concentrations carotenoid increase DNA damage (Woods et al., 1999; Palozza, 2005). These same authors reported that β-carotene increase H2O2-induced oxidative DNA damage has been reported in HepG2 cells (Woods et al., 1999; Palozza, 2005), whereas, Lowe et al. (1999) have observed that a failure of β-carotene and other carotenoids, such as lycopene, protect human cells against free-radical-induced DNA damage.
Another study noted oxidation of β-carotene resulted in a greater increase in DNA oxidation in human Hs68 fibroblasts (Yeh and Hu, 2001).
4.4.5.1.3 Carotenoids effect on immune function
Carotenoids have a stimulating effect on the immune system mediated by their vitamin A metabolism and the subsequent mediation of the RAR/RXR response pathways. However, even carotenoids without provitamin A, such as lutein, canthaxantine, and lycopene have marked effects on the immune system (Chew and Park, 2004; Hughes, 2001; Rühl, 2007).
Several studies have reported the stimulatory effects of β-carotene on immune function. Many clinical trials with various doses of β-carotene have been used (from 15 mg/day to 180 mg/day, administered over periods ranging from 14 days to one year). These studies reported an increase in the helper T lymphocytes number (CD4 +) or CD4 +: CD8 + T cells (T suppressor/cytotoxic cells) and in lymphocytes percentage of expressing the activation markers, interleukin (IL) 2 receptor, and transferrin receptor (Alexander et al., 1985; Watson et al., 1991; Murata et al., 1994). In the same context, Fryburg et al. (1995) suggests that β-carotene appears an immuno-enhancing agent in the management of HIV infections by potentiating the increase in CD4 + cells.
Likewise, dietary carotenoids have a stimulating effect on the immune system in inflammatory diseases or human immunodeficiency (Austin et al., 2006). This stimulation results in an increase in proliferation of lymphocytes induced by mitogens, an increase in myeloperoxidase and phagocytic activity, an increased antibody response and an increase in cytochrome oxidase and peroxidase activities in macrophages due to an increase in respiratory burst, which leads to stimulation of blood neutrophil killing activity (Chew and Park, 2004; Elliott, 2005). Moreover, the administration of β-carotene in patients with acquired immunodeficiency syndrome at 60 mg /day for four weeks leads to a slight increase in the number of CD4 + cells (Fryburg et al., 1995; Hughes, 1999).
Additionally, Chew and Park (2004) reported a stimulating effect of β-carotene on the growth of the thymus gland and a strong increase in the number of thymic small lymphocytes. Likewise, in vivo studies show the stimulatory effect of β-carotene on lymphocytic blastogenesis in rats, pigs, and cattle. Also, oral β-carotene supplementation in adult humans has a stimulatory effect by increasing the number of T helper and T inducing lymphocytes.
Kang and Kim (2017) report that among carotenoids astaxanthin is the best immune stimulator by stimulating production of antibodies directed against T-dependent antigens and the activity of T helper cells. These authors found that the T cell response of mice infected with H. pylori was different in the astaxanthin-treated group with a predominant T-helper (Th) 2 cell-like response and IL-4 release.
4.4.5.1.4 Carotenoids effect on gastrointestinal tract
People with inflammatory bowel disease commonly suffer from vitamin A deficiency (Bousvaros et al., 1998). The study by Głąbska et al. (2019) on 56 individuals with ulcerative colitis in remission with gastrointestinal symptoms (daily number of bowel movements, and the presence of painful tenesmus, flatulence, and constipation) allowed reduction of the incidence of constipation to be observed following consumption of higher doses of lutein and zeaxanthin (varying from 289.0–13221.3 μg and 432.7–1309.0 μg, respectively). However, higher doses of the retinoids, such as lycopene, lutein, and zeaxanthin in individuals with ulcerative colitis in remission lead to a decrease in fecal blood, mucus, and pus but not abdominal pain. While, higher carotene intake in individuals with ulcerative colitis in remission may contribute to higher incidence of fecal mucus (Głąbska et al., 2016).
Wang et al. (2000) found that astaxanthin-rich algae meal posses an inhibitory effect on H. pylori growth in vitro. Likewise, El-Akabawy and El-Sherif (2019) reported that zeaxanthin can be used for the treatment of ulcerative colitis induced in rats from day 15 by transrectal administration of 3% acetic acid and pretreated with zeaxanthin (50 mg/kg/day) orally for 14 days as it causes a significant reduction in disease activity index, wet weight of the colon, ulcer area, macroscopic scores, and histological changes.
4.4.5.1.5 Carotenoids effect on neurological system
Antineuroinflammatory effects of carotenoids have been highlighted (Cho et al., 2018). Several studies have shown the suppression of inflammation murine retinal cells by lutein (Sasaki et al., 2009; Li et al., 2012). Additionally, lutein in the presence of a variety of oxidative stressors suppresses activation of the nuclear factor-κB (NF-κB) pathway causing reduction lipid peroxidation and release pro-inflammatory cytokines (Kim et al., 2012; Liu et al., 2017). Likewise, in brain the inhibition of NF-κB activity and related expression of pro-inflammatory cytokines (Sachdeva and Chopra, 2015) that can contribute to the suppression of Aβ formation (Katayama et al., 2011) and improvement of memory retention (Prakash and Kumar, 2014; Sachdeva and Chopra, 2015).
Furthermore, Lee et al. (2012); and Marcotorchino et al. (2012) reported that lycopene reduces proinflammatory cytokine and chemokine expression in macrophages. In addition, lycopene increases the permeability of the blood-brain barrier. Whereas, Parkinson’s disease and vascular dementia have been observed with significantly low lycopene levels. Also, lycopene can confer protection against amyotrophic lateral sclerosis (ALS) in humans (Rao and Rao, 2007).
Moreover, Nam et al. (2010) report that crocin and crocetin, by sitmulating lipopolysaccharides, interferon γ, and β-amyloids (Aβ) in microglial cells, suppress the production of proinflammatory cytokines and nitric oxide. Though, crocin was shown to be beneficial in both Alzheimer’s disease (Ghahghaei et al., 2013; Asadi et al., 2015) and Parkinson disease (Zhang et al., 2015b; Rao et al., 2016).
In addition, Zhou et al. (2015) and Zhou et al. (2017) found that astaxanthin reduced hippocampal and retinal inflammation in diabetic rats induced by streptozotocin. Also, it attenuated cognitive deficits, retinal oxidative stress, and depression. Furthermore, astaxanthin has been shown to protect neurons in various neurodegenerative diseases, including Alzheimer’s disease (Chang et al., 2010; Lobos et al., 2016), Parkinson disease (Ikeda et al., 2008; Ye et al., 2013), and amyotrophic lateral sclerosis (Isonaka et al., 2011).
As well, Lin et al. (2016) reported that the consumption of fucoxanthin can inhibit acetylcholinesterase and improve the expression of neurotrophic factors derived from the brain, hence these advantages in the treatment of Alzheimer’s disease.
4.4.5.1.6 Anticarcinogenic effects
Several studies have reported the beneficial effects of carotenoids in the treatment of various cancers (Milani et al., 2017). Acting themselves or their metabolites, carotenoids influence the expression of certain genes, inhibit regulatory enzymes, which probably enables preventive properties of cancer (Stahl and Sies, 2005). In addition, a correlation between a high intake of carotenoids in the diet and the reduced risk of breast, cervical, ovarian and colorectal cancers have been mentioned in epidemiological studies (Milani et al., 2017).
Furthermore, several mechanisms are involved in cancer chemoprevention by dietary carotenoids. These have effects on gap junctional intercellular communication, on the growth factor signaling, on cell cycle progression, on differentiation-related proteins, on retinoid-like receptors, on the antioxidant response element, on nuclear receptors, on the AP-1 transcriptional complex, on the Wnt/β-catenin pathway and on inflammatory cytokines. Moreover, carotenoids can stimulate the proliferation of B- and T-lymphocytes, the activity of macrophages and cytotoxic T-cells, effector T-cell function, and the production of cytokines (Milani et al., 2017).
Giovannucci (2002) reported that increased blood levels of lycopene were associated with a decreased risk of prostate cancer. These high lycopene levels are due to consumption of tomatoes and tomato products. Based on the results of previous studies, Kucuk et al. (2001) and van Chen et al. (2001) suggested that supplementation with lycopene or diet rich in lycopene may slow the growth of prostate cancer.
Furthermore, Wang et al. (2015) report, “Neither dietary β-carotene intake nor its blood levels was associated with reduced prostate cancer risk. Dietary α-carotene intake and lycopene consumption (both dietary intake and its blood levels) were all associated with reduced risk of prostate cancer. However, neither blood α-carotene levels nor blood lycopene levels could reduce the risk of advanced prostate cancer”. These authors concluded, “α-carotene and lycopene, but not β-carotene, were inversely associated with the risk of prostate cancer. However, both α-carotene and lycopene could not lower the risk of advanced prostate cancer”.
β-cryptoxanthin acts as a chemopreventive agent against lung cancer. It negatively regulates the α7 / PI3K signaling pathway of nicotinic neuronal acetylcholine receptors (Iskandar et al., 2016). Similarly, this molecule enhances the action of a chemotherapeutic agent, oxaliplatin, in the treatment of colon cancer (San Millan et al., 2015). Likewise, a diet with high levels of lycopene and β-cryptoxanthin could protect against aggressive prostate cancer (Antwi et al., 2016).
Astaxanthin by reactivating the expression of Nrf2 and Nrf2-target genes through epigenetic modification and chromatin remodeling possess a beneficial health effects against the formation and tumor progression of prostate cancer (Yang et al., 2016). Likewise, astaxanthin by inhibiting ERK1/2 activity exerts antitumorigenic and anti-inflammatory effects on human lung cancer cell lines (Liao et al., 2016). Additionally, high concentrations of astaxanthin may suppress mammary carcinoma (Yuri et al., 2016).
In vitro studies indicate that fucoxanthin stimulates apoptosis and decreases proliferation and migration in glioma cancer cell lines U87 and U251 through Akt/mTOR and p38 pathway inhibition (Liu et al., 2016; Merhan, 2017).
4.4.5.1.7 Carotenoids effect on skin
β-Carotenes have anti-inflammatory properties and have the ability to prevent the formation of reactive oxygen species protects the skin from the harmful effects of UV light, the formation of erythema, premature aging of the skin, the development of photodermatitis and skin cancer (Stahl and Sies, 2007; Cazzonelli, 2011; Merhan, 2017). Moreover, several studies in humans have shown that carotenoids levels in plasma and skin decrease upon UV irradiation; lycopene is lost preferentially as compared to other carotenoids (Ribaya-Mercado et al., 1995).
However, a protective effect on human skin of dehydrated molecules and accumulated phytoene and phytofluene was due to its UV absorption properties and antioxidant and anti-inflammatory activities (Aust et al., 2005; Hsu et al., 2012; Merhan, 2017).
Several studies have investigated the protective effect of supplementation with β-carotene alone at 24 mg/day or associated with supplementation with vitamin E or other carotenoids (lutein and lycopene), for erythema caused by UV or induced by illumination with a solar simulator. The results showed a decrease and an attenuation of the intensity of the erythema (Stahl et al., 2000; Heinrich et al., 2003; Stahl and Sies, 2005).
4.4.5.1.8 Carotenoids and cardiovascular diseases
Many studies have indicated the protective effect of carotenoids against cardiovascular diseases (Stahl and Sies, 2005; Li and van Eck, 2007; Lu and Li, 2008). Likewise, several clinical trials have shown that carotenoids can reduce the risk of developing cardiovascular disease via several mechanisms such as: (1) lowering blood pressure, (2) reducing proinflammatory cytokines, (3) decreasing markers inflammation (e.g., reactive protein C), and (4) improving the sensitivity of the liver, muscle, and fatty tissue to insulin. In addition, it can modulate the expression of specific genes involved in cell metabolism (Gammone et al., 2015; Milani et al., 2017).
4.4.5.1.9 Eye diseases
Between 1987 and 1998, a number of studies indicated that dietary carotenoids (lutein, zeaxanthin, lycopene, carotene, and carotene) from fruits and vegetables could help prevent eye diseases (Sommerburg, 1998). Given the especially high concentrations and exclusive presence of both xanthophylls in some ocular tissues, lutein, and zeaxanthin can play a role in visual health, demonstrating their contributions to vision enhancement or delaying the onset of ocular diseases (Alves-Rodrigues and Shao, 2004). Lutein has been shown to play a central role in reducing the incidence of eye diseases, such as AMD, cataract, and retinitis pigmentosa (Aleman et al., 2001). It is impractical to specifically measure the effect of eye lutein concentration on the occurrence of ocular diseases in living subjects due to the fragile nature of the eye and the intrusive nature of techniques for assessing and quantifying metabolic products in the retina and lens. Direct measurement of the actual concentration of lutein and zeaxanthin in the macular pigment of donor eyes with and without AMD was published in a key study. This study concluded that control subjects with the highest levels of lutein are 82% less likely to produce AMD than those with the lowest levels of xanthophylls after analyzing 56 retinas from AMD and control subjects (Bone et al., 2001). Observational studies are appropriate for demonstrating relations between nutrient supplementation and tissue concentrations of a particular compound with disease risk, but fall short of establishing a clear cause-and-effect relationship between nutrient intake and a specific benefit (Alves-Rodrigues and Shao, 2004).
4.4.5.2 Detrimental effects
We use the term detrimental to refer to both direct and indirect effects (Zahavi and Zahavi, 1997). The theory of direct toxicity is intriguing because, while the toxicity of carotenoids has yet to be thoroughly assessed, certain other chemicals, such as tannins and alkaloids, are considered to be harmful to animals. Toxicity may be especially problematic for herbivorous species, whose diets are typically rich in carotenoids. Detoxifying the components of plant metabolism may be energy intensive for herbivores (Bendich, 1993; Olson, 1993). Furthermore, carotenoids may be poisonous to any organism that consumes them, based on the degree and type of toxicity. This is true regardless of the quantity consumed. The vast majority of human observational studies indicate that carotenoids can minimize the risk of some chronic diseases (Bendich, 1993; Olson, 1993). However, experimental carotenoids supplementation has not always been shown to be successful, and in one review, supplementation in smokers was linked to an increased risk of lung cancer (Mayne, 1996), smoking is an uncommon animal behavior, and associations between disease incidence and carotenoids in smokers cannot actually be extrapolated to other species or non smokers. Despite the evidence to the contrary, Zahavi and Zahavi (1997) proposed that carotenoids may contribute to the disintegration of cell membranes when transported into cells. More research is required to establish carotenoids possible harmful effects.
4.4.6 Toxicity of carotenoids
The enhancement of the immune response observed in animal models, which may be attributed to the synthesis of tumor specific antigens, is one of the main effects of carotenoids that can be linked to cancer prevention (International Agency for Research on Cancer, 1998). Furthermore, carotenoids have been shown to influence cytochrome P450 metabolism, to inhibit arachidonic acid metabolism, chromosome destruction and instability, to influence apoptosis, and to affect a variety of other biological processes (Mathews-Roth, 1993).
For several years, patients with erythropoietic protoporphyria have been treated with doses of 20–180 mg/day ß-carotene with no signs of toxicity or abnormally elevated serum vitamin A concentrations (Mathews-Roth, 1993). Then, Sies and Krinsky (1995) discovered that low dietary intake and plasma concentrations of carotenoids are often linked to an increased risk of cervical dysplasia, cardiovascular disease, lung cancer, cortical cataract, and AMD.
Some studies have shown that people who consume more fruits and vegetables rich with carotenoids and have elevated levels of serum ß-carotene have a reduced risk of cardiovascular disease and cancer. Although no clinical experiments of ß-carotene as a single agent has indicated a decrease in cancer risk at any particular site; contrarily, the risk of lung cancer among smokers and asbestos employees who received elevated doses of ß-carotene supplements (concentrations that were 10–15 times higher than usual in blood) was higher (Bohlke et al., 1999). According to Olson (1994), carotenoids are non toxic when consumed in foods, however, a very high dose of oxocarotenoid and canthaxanthin used for medicinal reasons can induce retinopathy.
Increased consumption of vegetables and fruits rich with carotenoids has generally been related to a lower risk of lung cancer in observational trials, whether prospective or retrospective. Furthermore, in prospective trials, elevated levels of ß carotene in blood were linked to a lower risk of lung cancer (Hennekens et al., 1996). Also, a high dose of ß carotene (in supplement form, 20–30 mg/day) is contraindicated for smokers due to the high risk of lung and stomach cancer, according to a study conducted by a group of researchers in the United States, who tested the effects of a mixture of 30 mg/day ß carotene and 25.000 IU/day retinol vitamin A in more than 18.000 smokers (men and women), former smokers, or others who have been exposed to asbestos on the jobsite. In groups of smokers who took more than 20 cigarettes/day and consume elevated doses of ß carotene supplements (5 to 10 times the recommended dose), the prevalence of lung cancer was higher (16%) after 6 years in the ATBC participants and (28%) after 4 years in the CARET participants (Omenn et al., 1996a).
Furthermore, other studies written about 1990 summarized the literature on diet and lung cancer conducted over the previous 25 years. The consensus was that increased consumption of carotenoids from vegetables and fruits decreased the risk of lung cancer in clinical trials of diet and lung cancer, whether prospective or retrospective. While in prospective trials, elevated levels of ß-carotene in blood were reliably linked to a lower risk of lung cancer. The most basic reason for the epidemiology was that ß-carotene was defensive, despite the fact that other carotenoids or other substances from vegetables and fruits, as well as related dietary habits, had not been thoroughly investigated. Human chemoprevention studies conducted in the last decade have shown that ß-carotene raises the prevalence of lung cancer and death in human smokers. As well, excessive use of certain carotenoids can also result in carotenemia, a reversible skin yellowing (Steinmetz and Potter, 1996). As a result, recent data from human testing suggests that supplementing with ß-carotene (20 mg or more per day) is not recommended for heavy smokers (Hennekens et al., 1996).
Wang and his colleagues at Tufts University attempted to understand the negative effects of high dose ß-carotene supplementation on a molecular degree. They affirmed that a high dose of ß-carotene disrupts retinoid signalling in lung cells, resulting in changes in the expression levels of genes included in tumorigenesis (Liu et al., 2000).
Other researchers looked at the impact of ß-carotene supplements (50 mg/day) on cancer incidence in over 22.000 male doctors in the United States, with 11% of them currently smoking, supplementing of ß-carotene for longer than 12 years was not related to an elevated risk of lung cancer (Scientific Committee on Food on the safety of use of beta carotene from all dietary sources, 2000).
More recently, the Women’s Health Study (WHS) found no elevated lung cancer risk in 40.000 women, including 13% smokers, who received 50 mg ß-carotene a day over two years and two years of follow-up (Lee et al., 1999).
According to Rao and Rao (2007), women with higher circulating levels of ɑ-carotene, ß-carotene, lutein + zeaxanthin, lycopene, and total carotenoids could have a lower risk of breast cancer.
Carotenoids, such as lutein, are adequate to preserve wellness when consumed as part of a well-balanced diet. However, supplementation is needed, in cases of chronic disease or insufficient carotenoids absorption (Ravikrishnan et al., 2011).
According to some reports, lutein intake has no effect on cytochrome P450 enzyme function, implying that lutein may not affect the metabolism of endogenous or exogenous molecules (Zheng et al., 2013). Also, in an animal study, mice deficient in carotene oxygenase 2 developed pathologic carotenoid aggregation as well as mitochondrial dysfunction and oxidative stress (Amengual et al., 2011). This result suggested that, under some cases, a high carotenoid consumption could cause toxicity. Lutein supplementation also raised the incidence of crystalline maculopathy in elderly people (Satia et al., 2009).
While previous research has shown a positive interaction between lutein and the risk of some diseases, the EFSA survey concluded that the data collected were inadequate to prove a negative outcome (European Food Safety Authority (EFSA), 2008).
4.4.7 In-vitro evidence, animal studies, and clinical studies of carotenoids
Total carotenoid concentrations and overall mortality rate in older adults have been studied in several observational trials (Akbaraly et al., 2009; Ray et al., 2006) as well as adults of a variety of ages (Shardell et al., 2011). The majority of studies indicate inverse correlations, while a separate pattern of interaction by gender has been proposed (Ray et al., 2006). Overall mortality for individual plasma carotenoids has been studied in a smaller number of experiments (Bates et al., 2011), but it’s too early to make conclusion about the association for individual carotenoids.
CVD is the leading cause of death worldwide, and diet plays a critical part in its progression (Perk et al., 2012). The evidence for dietary factors and CVD risk was classified as high for vegetables and was rated as moderate for fruit and dietary ß-carotene (Mente et al., 2009). Despite the fact that a recent meta-analysis affirmed the relation between FV intake and CVD risk (Wang et al., 2014). A variety of retrospective trials have been performed in relation to overall or human carotenoid intake or status and CVD outcomes (Goyal et al., 2013; Sesso et al., 2005).
Any detected inconsistencies may be attributed to demographic variability and carotene consumption ratios, as well as the degree of correction for possible confounders within individual studies and the subsequent probability of residual confounding. Most experiments have used a single carotenoid status measurement, and it’s likely that participant’s carotenoid status improved during follow-up, causing certain participant’s long-term carotenoid status to be misclassified, affecting the reported correlations (Aune et al., 2012).
Despite the relatively stable relation between increased carotene intake and status and the risk of CVD, carotene intake has been shown to have little effect on cardiovascular disease (CVD) or lung cancer in randomized controlled trials (RCTs) (Voutilainen et al., 2006). According to meta-analyses, carotene supplements have been linked to an increased risk of death, especially in smokers (Bjelakovic et al., 2013), as a result, the data from retrospective trials and RCTs on the impact on CVD results has been inconsistent. The RCTs’ design has been criticized (Riccioni et al., 2012, Stanner et al., 2004). Supplements were used in the trials and doses were within the pharmacological spectrum, the observed findings may have been influenced by genetic differences in supplementation response, the wellbeing of study subjects was also taken into consideration, participants were normally at high risk of CVD and present a considerable degree of atherosclerosis, as a result, the chance of the intervention minimizing risk is decreased. Furthermore, it has been hypothesized that supplementation would only help those with low nutritional status in RCTs (Goyal et al., 2013).
Indeed, results were found in those with originally lower nutrient status in the only two carotene-containing, as conclusion, trials demonstrate a beneficial influence of supplementation (Blot et al., 1993, Hercberg et al., 2004). Bjelakovic et al. (2013) studies found that at larger doses, vitamins have little benefit or have a negative effect, however, as a result of these carotene trial results, health care organizations have advised against taking carotene supplements for the treatment of CVD or cancer. To better evaluate the impact of carotenoids on CVD risk, RCTs that consider the health and the nutritional status of participants entering trials are needed. Despite the scholarly interest in doing such research, it is unlikely that further supplementation trials will be performed.
Depending on the cancer site, the evidence that increased FV intake was associated with reduced cancer risk was graded as “probable” or “limited-suggestive” as reported by the World Cancer Research Fund (2007). More recent findings have also shown that there is a poor association (Boffetta et al., 2010). Although it’s still possible that one kind of FV, or a certain compound within certain FV, is linked to a lower risk of cancer, or that FV in general is protective at certain cancer sites, and those who consume very little FV may still benefit from raising their intake (Key, 2011).
The WCRF reported the evidence for carotenoid containing foods at various locations in terms of carotenoids and cancer risk. It was classified as probable that foods containing carotenoids protected against cancer in the mouth, pharynx, and larynx, as well as lung cancer, whereas, this association is improbable for prostate cancer and nonmelanoma skin cancer. On the other hand, foods rich in lycopene are thought to protect against prostate cancer (Bjelakovic et al., 2013).
A number of other studies, including of lycopene supplementation, have looked into the correlation between lycopene and prostate cancer risk. Meta-analyses found no impact of lycopene supplementation on benign prostate hyperplasia (BPH) or prostate cancer danger, while a meta-analysis of two studies found a decrease in prostate specific antigen levels in men diagnosed with prostate cancer who obtained lycopene. Given the small number of RCTs and their variable quality, the researchers found that it is currently impossible to endorse or deny the use of lycopene for the prevention or treatment of BPH or prostate cancer (Ilic and Misso, 2012).
Another topic that has gotten a lot of attention since the WCRF study was released in 2007 is the relation between carotenoid intake and status and breast cancer risk. A meta-analysis found a relation between higher levels of carotene, cryptoxanthin, lutein + zeaxanthin, lycopene, and total carotenoids in the blood and a lower risk of breast cancer (Eliassen et al., 2012), whereas another meta-analysis supports this relation for carotenoid status, it also indicates that blood carotenoids concentrations are more closely correlated with reduced breast cancer risk than carotenoid consumption as measured by a dietary questionnaire (Aune et al., 2012).
According to epidemiological research, there is a positive correlation between higher dietary intake and tissue carotenoids concentrations and a lower risk of chronic diseases (Johnson, 2002; Agarwal and Rao, 2000), ß-carotene, and lycopene have been linked to a lower incidence of coronary disease and some tumors, while lutein and zeaxanthin have been linked to ocular disorders (Ribaya-Mercado and Blumberg, 2004). Carotenoids’ antioxidant properties have been proposed as the primary mechanism by which they achieve their beneficial effects. Carotenoids can also exert their effects by other pathways such as cell growth control, gap junction connectivity, modulators of phase I and II drug metabolizing enzymes, modulating gene expression, and immune response (Astrog, 1997; Jewell and O’Brien, 1999; Bertram, 1999).
Carotenoids, including ɑ- and ß-carotene, as well as ß-cryptoxanthin, have the additional benefit of being able to be converted to vitamin A, which has a role in disease growth and prevention. Carotenoids, including ß-carotene and lycopene have been shown to have antioxidant effects in various in vitro, animal, and human studies. ß-carotene suppressed the upregulation of heme oxygenase-1 gene expression in human dermal fibroblasts (FEK4) exposed to UVA in a dose-dependent manner (Elliott, 2005). Worth noting that ß-carotene has been documented to behave as a pro-oxidant in some circumstances. UVA-induced heme oxygenase-1 induction was enhanced by ß-carotene at a concentration of 0.2 mM, suggesting a pro-oxidant function (Obermuller-Jevic et al., 1999). In another study, 10 mM ß-carotene increased the generation of reactive oxygen species (ROS) and the levels of cellular oxidized glutathione in leukemia and colon adenocarcinoma cell lines in vitro (Palozza et al., 2003). In rats, the pro-oxidant role of ß-carotene was also shown, with increased phase I enzyme production in the liver, kidney, and intestine, as well as increased oxidative stress (Paolini et al., 2001). Human experiments confirm also the pro-oxidant properties of ß-carotene. The alpha-tocopherol ß-carotene (ATBC) trial found that supplementing ß-carotene at pharmacological levels improved lung cancer incidence in smokers (Alpha Tocopherol beta Carotene Cancer Prevention Study Group, 1994). The ß-carotene and retinol efficiency trial (CARET) found elevated CVD mortality in a population of users, former smokers, and asbestos exposed people (Omenn et al., 1996b). These findings point to a potential biphasic reaction of ß-carotene, which may encourage wellbeing when consumed in small quantities but may have negative consequences when consumed in larger amounts. While scientists are still debating the validity of the results of human trials and the specifics of research designs, questions about ß pro-oxidant carotene’s properties which could increase the risk of lung cancer and CVD in smoking, have prompted a moratorium on further intervention studies using ß-carotene. ß-carotene has been used as a “gold standard” model to research the association between oxidative stress and chronic diseases for a long time. The subject of study has now moved to lycopene, a different carotenoid antioxidant (Ma et al., 2018).
Conclusion
Today, scientific research has demonstrated a range of carotenoids health benefits in food. These antioxidant, immune stimulating, pro-inflammatory, and provitamin A activities have enabled it to have beneficial effects on several organs. These compounds are recognized, as safe and side effects are very rare and generally mild when they do occur, which allows their are use in many treatments. However, their overall effects are variable depending on the total food intake of carotenoids. Further research must be carried out to establish adequate doses used in the various pathologies studied as well as a clarification of the synergistic or antagonistic effect of these compounds with the other antioxidants contained in foods to better understand their therapeutic effects.
Abbreviations
GGPP Geranyl geranyl pyrophosphate
FW Fresh weight
ROS Reactive oxygen species
UVA Ultraviolet A
RARs Retinoic acid receptors
RXRs Retinoid X receptors
ALS Amyotrophic lateral sclerosis
DNA Deoxyribonucleic acid
Aβ β-amyloid
Nrf2 Nuclear factor erythroid-2-related factor 2
ERK Extracellular signal-regulated kinases
CVD Cardiovascular disease
AMD Age-related macular degeneration
NF-ΚB Nuclear factor-Kappa B
α7 / PI3K Phosphatidyl inositol 3-kinase
Akt /mTOR Protein kinase B/mammalian target of rapamycin
FV Fruits and vegetables
RCTs Randomized controlled trials
WCRF World Cancer Research Fund
BPH Benign prostatic hyperplasia
FEK4 Normal human dermal fibroblasts
ATBC Alpha-tocopherol beta-carotene
CARET Carotene and Retinol Efficiency Trial
References
Agarwal, S., Rao, AV., 2000. Carotenoids and chronic diseases. Drug Metab. Drug Interact. 17 (1–4), 189–210.
Akbaraly, T.N., Favier, A., Berr, C., 2009. Total plasma carotenoids and mortality in the elderly: results of the epidemiology of vascular ageing (EVA) study. Br. J. Nutr. 101 (1), 86–92.
Aleman, T.S., Duncan, J.L., Bieber, M.L., De Castro, E., Marks, D.A., Gardner, L.M., Steinberg, J.D., Cideciyan, A.V., Maguire, M.G., Jacobson, S.G., 2001. Macular pigment and lutein supplementation in retinitis pig-mentosa and usher syndrome. Invest. Ophthalmol. Vis. Sci. 42(8), 1873–1881.
Alexander, M., Newmark, H., Miller, G., 1985. Oral beta-carotene can increase the number of OKT4 positive cells in human blood. Immunol. Lett. 9 (4), 221–224.
Alonso, B. 2017. Carotenoids: content in foods, in diet and bioavailability. Sci. News Lett. 2, 1–9.
Alpha-Tocopherol beta Carotene Cancer Prevention Study Group. 1994. The effects of vitamin E and beta-carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 330, 1029–1035.
Alves-Rodrigues, A., Shao, A., 2004. The science behind lutein. Toxicol. Lett. 150 (1), 57–83.
Ambati, R.R., Phang Siew, M., Sarada, R., Ravishankar Gokare, A. 2014. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications. Mar Drugs. 12, 128–152.
Amengual, J., Lobo, G.P., Golczak, M., Li, H.N., Klimova, T., Hoppel, C.L., Wyss, A., Palczewski, K., vonLintig, J. 2011. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 25, 948–959.
Antwi, S.O., Steck, S.E., Su, L.J., Hebert, J.R., Zhang, H., Craft, N.E., 2016. Carotenoid intake and adipose tissue carotenoid levels in relation to prostate cancer aggressiveness among African-American and European-American men in the North Carolina-Louisiana prostate cancer project (PCaP). Prostate 76 (12), 1053–1066.
Asadi, F., Jamshidi, A.H., Khodagholi, F., Yans, A., Azimi, L., Faizi, M., Vali, L., Abdollahi, M., Ghahremani, M.H., Sharifzadeh, M., 2015. Reversal effects of crocin on amyloid β-induced memory deficit: modification of autophagy or apoptosis markers. Pharmacol. Biochem. Behav. 139 (Pt A), 47–58.
Astrog, P., 1997. Food carotenoids and cancer prevention: an overview of current research. Trends Food Sci. Technol. 8, 406–413.
Aune, D., Chan, D.S., Vieira, A.R., 2012. Dietary compared with blood concentrations of carotenoids and breast cancer risk: a systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 96, 356–373.
Aust, O., Stahl, W., Sies, H., Tronnier, H., Heinrich, U., 2005. Supplementation with tomato-based products increases lycopene, phytofluene, and phytoene levels in human serum and protects against UV-light-induced erythema. Inter. J. Vitam. Nutr. Res. 75 (1), 54–60.
Austin, J., Singhal, N., Voigt, R., Smaill, F., Gill, M.J., Walmsley, S., Salit, I., Gilmour, J., Schlech, W.F., Choudhri., S., Rachlis, A., Cohen, J., Trottier, S., Toma, E., Phillips, P., Ford, P.M., Woods, R., Singer, J., Zarowny, D.P., Cameron, D.W., 2006. A community randomized controlled clinical trial of mixed carotenoids and micronutrient supplementation of patients with acquired immunodeficiency syndrome. Eur. J. Clin. Nutr. 60, 1266–1276.
Bates, C.J, Hamer, M., Mishra, G.D., 2011. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of people aged 65 years and over. Br. J. Nutr. 105, 123–132.
Bendich, A., 1993. Biological functions of dietary carotenoids. Ann. New York Acad. Sci. 691 (1), 61–67.
Bertram, J.S., 1999. Carotenoids and gene regulation. Nutr. Rev. 57, 182–91.
Bfhm, F., Edge, R., McGarvey, D.J., Truscott, T.G. 1998. Beta-carotene with vitamins E and C offers synergistic cell protection against NOx, FEBS Lett. 436, 387–389.
Bjelakovic, G., Nikolova, D., Gluud, C., 2013. Antioxidant supplements to prevent mortality. JAMA 310 (11), 1178–1179.
Blot, W.J., Li, J.Y., Taylor, P.R., 1993. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Natl. Cancer Inst. 85, 1483–1492.
Boffetta, P., Couto, E., Wichmann, J., 2010. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 102, 529–37.
Bohlke, K., Spiegelma, D., Trichopoulou, A., Katsouyanni, K., Trichopoulos, D., 1999. Vitamins A, C and E and the risk of breast cancer: results from a case-control study in Greece. Br. J. Cancer. 79, 23–29.
Bone, R.A., Landrum, J.T., Mayne, S.T., Gomez, C.M., Tibor, S.E., Twaroska, E.E., 2001. Macular pigment in donor eyes with and without AMD: a case-control study. Invest. Ophthalmol. Vis. Sci. 42(1), 235–240.
Bonet, M.L., Canas, J.A., Ribot, J., Palou, A., 2015. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch. Biochem. Biophys. 572, 112–125.
Bousvaros, A., Zurakowski, D., Duggan, C., Law, T., Rifai, N., Goldberg, N.E., 1998. Vitamins A and E serum levels in children and young adults with inflammatory bowel disease: Effect of disease activity. J. Pediatr. Gastroenterol. Nutr. 26, 129–135.
Britton, G., Khachik, F. 2009. Carotenoids in food.Carotenoids, Volume 5: Nutrition and Health. Birkhäuser Verlag Basel.
Britton, G., 1995. Structure and properties of carotenoids in relation to function. FASEB J. 15, 1551–1558.
Britton, G., Liaaen-Jensen, S., Pfander, H., 2004. Carotenoids: Handbook. Springer Basel, Switzerland AG.
Brown, M.J., Ferruzzi, M.G., Nguyen, M.L., Cooper, D.A., Eldridge, A.L., Schwartz, S.J., White, W.S. 2004. Carotenoid bioavailability is higherfrom salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am. J. Clin. Nutr. 80, 396–403.
Cazzonelli, C.I., 2011. Carotenoids in nature:insights from plants and beyond. Funct. Plant Biol. 38, 833–847.
Cazzonelli, C.I., Nisar, N., Hussain, D., Carmody, M.E., Pogson, B.J., 2010. Biosynthesis and regulation of carotenoids in plants - micronutrients, vitamins and health benefits. In: Pua, E.C., Davey, M.R. (Eds.), Plant Developmental Biology- Biotechnological Perspectives, vol. 2. Springer Nature Switzerland AG, pp. 117–137.
Chang, C.H., Chen, C.Y., Chiou, J.Y., Peng, R.Y., Peng, C.H., 2010. Astaxanthine secured apoptotic death of PC12 cells induced by β-amyloid peptide 25–35: its molecular action targets. J. Med. Food 13(3), 548–556.
Chen, L., Stacewicz-Sapuntzakis, M., Duncan, C., Sharifi, R., Ghosh, L., van Breemen, R., Ashton, D., Bowen, P.E., 2001. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J. Natl. Cancer Inst. 93, 1872–1879.
Chew, B.P., Park, J.S., 2004. Carotenoid action on the immune response. J. Nutr. 134 (1), 257–261.
Chitchumroonchokchai, C., 2004. Assessment of lutein bioavailability from meals as a supplement using simulated digestion and Caco-2 human intestinal cells. J. Nutr. 134, 2280–2286.
Cho, K.S., Shin, M., Kim, S., Lee, S.B., 2018. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxid. Med. Cell Longev. 2018, 412–458.
Edge, R., McGarvey, D., Truscott, T., 1997. The carotenoids as anti-oxidants. A review. J. Photochem. Photobiol. B 41 (3), 189–200.
El-Agamey, A., Lowe, G.M., McGarvey, D.J., Mortensen, A., Phillip, D.M., Truscott, T.G., 2004. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 430, 37–48.
El-Akabawy, G., El-Sherif, N.M., 2019. Zeaxanthin exerts protective effects on acetic acid-induced colitis in rats via modulation of pro-inflammatory cytokines and oxidative stress. Biomed. Pharmacother. 111, 841–851.
Eliassen, A.H., Hendrickson, S.J., Brinton, LA., 2012. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J. Natl. Cancer Inst. 104, 1905–1916.
Elliott, R., 2005. Mechanisms of genomic and non-genomic actions of carotenoids. Biochim. Biophys. Acta 1740, 147–154.
Elvira-Torales, L.I., García-Alonso, J., Periago-Castón, M.J. 2019. Nutritional importance of carotenoids and their effect on liver health: a review. Antioxidants 8, 229.
European Food Safety Authority (EFSA). 2008. Safety, bioavailability and suitability of lutein for the particularnutritional use by infants and young children—scientific opinion of the panel on dietetic products, nutrition and allergies EFSA J. 823, 1–24.
Faulks, R.M., Southon, M., 2005. Challenges to understanding and measuring carotenoid bioavailability. Biochim. Biophys. Acta Mol. Basis Dis. 1740, 95–100.
Fryburg, D.A., Mark, R.J., Griffith, B.P., Askenase, P.W., Patterson., T.F., 1995. The effect of supplemental beta-carotene on immuno- logical indices in patients with AIDS: a pilot study. Yale J. Biol. Med. 68 (1-2), 19–23.
Gammone, M.A., Riccioni, G., D'Orazio, N., 2015. Carotenoids: potential allies of cardiovascular health?. Food Nutr Res 59, 26762.
Ghahghaei, A., Bathaie, S.Z., Kheirkhah, H., Bahraminejad, E., 2013. The protective effect of crocin on the amyloid fibril formation of Aβ42 peptide in vitro. Cell Mol. Biol. Lett. 18 (3), 328–339.
Giovannucci, E., 2002. A review of epidemiologic studies of tomatoes, lycopene, and prostate cancer. Exp. Biol. Med. 227, 852–859.
Głąbska, D., Guzek, D., Zakrzewska, P., Lech, G., 2019. Intake of Lutein and Zeaxanthin as a possible factor influencing gastrointestinal symptoms in Caucasian individuals with ulcerative colitis in remission Phase. J. Clin. Med. 8(1),77.
Głąbska, D., Guzek, D., Zakrzewska, P., Włodarek, D., Lech, G., 2016. Lycopene, Lutein and Zeaxanthin may reduce faecal blood, mucus and pus but not abdominal pain in individuals with ulcerative colitis. Nutrients 8(10), 613.
Goodwin, T.W., 1980.The biochemistry of the carotenoids, Vol.1 Plants, second ed. Chapman and Hall, New York.
Goyal, A., Terry, M.B., Siegel, A.B., 2013. Serum antioxidant nutrients, vitamin a, and mortality in US Adults. Cancer Epidemiol. Biomarkers Prev. 22(12), 2202–2011.
Gross, J., 1991. Pigments in Vegetables: Chlorophylls and Carotenoids, AVI/Van Nostrand Reinhold. Springer US.
Gruszecki, W.I., 1999. Carotenoids in membranes. In: Frank H.A., Young, A.J., Britton, G., Cogdell, R.J. (Eds.), The Photochemistry of Carotenoids. Advances in Photosynthesis and Respiration, 8, Kluwer Academic Publishers, pp. 363–379.
Gul, K., Tak, A., Singh, A.K., Singh, P., Yousuf, B., Wani, A.A., 2015. Chemistry, encapsulation, and health benefits of β-carotene - A review. Cogent Food Agric. 1 (1), 101869.
Handelman, G.J., Nightingale, Z.D., Lichtenstein, A.H., Schaefer, E.J., Blumberg, J.B. 1999. Lutein and zeaxanthin concentrations in plasma after dietary supplementation with egg yolk. Am. J. Clin. Nutr. 70, 247–51.
Heinrich, U., Gärtner, C., Wiebusch, M., Eichler, O., Sies, H., Tronnier, H., Stahl, W., 2003. Supplementation with beta-carotene or a similar amount of mixed carotenoids protects humans from UV-induced erythema. J. Nutr. 133, 98–101.
Hennekens, CH., Buring, JE., Manson, JE., 1996. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasm's and cardiovascular disease. N. Engl. J. Med. 334, 1145–1149.
Hercberg, S., Galan, P., Preziosi, P., 2004. The SU.VI.MAX Study: A randomized, placebo controlled trial of the health effects of antioxidant vitamins and minerals. Arch. Intern. Med. 164 (21), 2335–2342.
Hsu, B.Y., Pu, Y.S., Inbaraj, B.S., Chen, B.H., 2012. An improved high performance liquid chromatography-diode array detection-mass spectrometry method for determination of carotenoids and their precursorsphytoene and phytofluene in human serum. J. Chromatogr. B 899, 36–45.
Hughes, D., 1999. Effects of carotenoids on human immune function. Proc. Nutr. Soc. 58(3), 713–718.
Hughes, D.A., 2001. Dietary carotenoids and human immune function. Nutrition 17 (10), 823–827.
Ikeda, Y., Tsuji, S., Satoh, A., Ishikura, M., Shirasawa, T., Shimizu, T., 2008. Protective effects of astaxanthin on 6- hydroxydopamine-induced apoptosis in human neuroblas- toma SH-SY5Y cells. J. Neurochem. 107(6), 1730–1740.
Ilic, D., Misso, M., 2012. Lycopene for the prevention and treatment of benign prostatic hyperplasia and prostate cancer: a systematic review. Maturitas 72(4), 269–276.
International Agency for Research on Cancer 1998. IARC Handbooks of Cancer Prevention Vol. 2, Carotenoids. IARC, Lyon, France.
Iskandar, A.R., Miao, B., Li, X., Hu, K.Q., Liu, C., Wang, X.D., 2016. β-cryptoxanthin reduced lung tumor multiplicity and inhibited lung cancer cell motility by down regulating nicotinic acetylcholine receptor α7 signaling. Cancer Prev. Res. 9 (11), 875–886.
Isonaka, R., Hiruma, H., Katakura, T., Kawakami, T., 2011. Inhibition of superoxide dismutase selectively suppresses growth of rat spinal motor neurons: comparison with pho phorylated neurofilament-containing spinal neurons. Brain Res. 1425, 13–19.
Jewell, C., O'Brien, N.M., 1999. Effect of dietary supplementation with carotenoids on xenobiotic metabolizing enzymes in the liver, lung, kidney and small intestine of the rat. Br. J. Nutr. 81, 235–242.
Johnson, E.J., 2002. The role of carotenoids in human health. Nutr. Clin. Care 5(2), 47–49.
Jyonouchi, H., Sun, S., Tomita, Y., Gross, M.D., 1995. Astaxanthin, a carotenoid without vitamin A activity, augments antibody responses in cultures including T-helper cell clones and suboptimal doses of antigen. J. Nutr. 125, 2483–2492.
Kang, H., Kim, H., 2017. Astaxanthin and β-carotene in Helicobacter pylori-induced gastric inflammation: a mini-review on action mechanisms. J. Cancer Prev. 22(2), 57–61.
Katayama, S., Ogawa, H., Nakamura, S., 2011. Apricot carotenoids possess potent anti-amyloidogenic activity in vitro. J. Agri. Food Chem. 59 (23), 12691–12696.
Key, T.J., 2011. Fruit and vegetables and cancer risk. Br. J. Cancer 104, 6–11.
Khoo, H.E., Prasad, K.N., Kong, K.W., Jiang, Y., Ismail, A. 2011. Carotenoids and their isomers: color pigments in fruits and vegetables. Molecules 16, 1710–1738.
Kim, J.E., Clark, R.M., Park, Y., Lee, J., Fernandez, M.L., 2012. Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet. Nutr. Res. Pract. 6(2), 113–119.
Kimura, M., Rodriguez-Amaya, D.B., 2003. Carotenoid composition of hydroponic leafy vegetables. J. Agric. Food Chem. 51, 2603–2607.
Klein, B.P., Perry, A.K., 1982. Ascorbic acid and vitamin A activity in selected vegetables from different geographic areas of the United States. J. Food Sci. 47, 941–948.
Kong, K., Khoo, H., Prasad, N., K., I., A., C.-.P., T., R., N., F. 2010. Revealing the power of the natural red pigment lycopene. Molecules 15, 959–987.
Kopas-Lane, L.M., Warthesen, J.J. 1995. Carotenoid photostability in raw spinach and carrots during cold storage. J. Food Sci. 60, 773–776.
Kopsell, D.E., Kopsell, D.A., Randle, W.M., Coolong, T.W., Sams, C.E., Celentano, J.C., 2003. Kale carotenoids remain stable while flavor compounds respond to changes in sulfur fertility. J Agric. Food Chem. 51(18), 5319–5325.
Kucuk, O., Sarkar, F.H., Sakr, W., Djuric, Z., Pollak, M.N., Khachik, F., Li, Y.W., Banerjee, M., Grignon, D., Bertram, J.S., Crissman, J.D., Pontes, E.J., Wood Jr, D.P., 2001. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol. Biomark. Prev. 10, 861–868.
Kurilich, A.C., 1999. Carotene, tocopherol, and ascorbate in subspecies of Brassica oleracea. J. Agric. Food Chem. 47, 1576–1581.
Lee, I-M., Cook, NR., Manson, JE., Buring, JE., Hennekens, CH., 1999. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Womens´ Health Study. J. Natl. Cancer. Inst 91, 2102–2109.
Lee, W., Ku, S.K., Bae, J.W., Bae, J.S., 2012. Inhibitory effects of lycopene on HMGB1- mediated pro-inflammatory responses in both cellular and animal models. Food Chem. Toxicol. 50, 1826–1833.
Lefsrud, M.G., Kopsell, D.A., Augé, R.M., 2006. Biomass production and pigment accumulation in kale grown under increasing photoperiods. Hort. Sci. 41(3), 603–606.
Lefsrud, M.G., Kopsell, D.A., Kopsell, D.E., Celentano, J.C., 2006. Irradiance levels affect growth parameters and carotenoid pigments in kale and spinach grown in a controlled environment. Physiol. Plant. 127, 624–631.
Lefsrud, M.G., Kopsell, D.A., Kopsell, D.E., Celentano, J.C., 2005. Air temperature affects biomass and carotenoid pigment accumulation in kale and spinach grown in a controlled environment. Hort. Sci. 40 (7), 2026–2030.
Li, L., van Eck, J., 2007. Metabolic engineering of carotenoid accumulation by creating a metabolic sink. Transgenic Res. 16, 581–585.
Li, S.Y., Fung, F.K., Fu, Z.J., Wong, D., Chan, H.H.L., Lo, A.C.Y., 2012. Anti-inflammatory effects of lutein in retinal ischemic/hypoxic injury: in vivo and in vitro studies. Invest Ophtalmol. Vis. Sci. 53 (10), 5976–5984.
Liang, C., Zhao, F., Wei, W., Wen, Z., Qin, S., 2006. Int. J. Biol. Sci. 2(4), 197–207.
Liao, K.S., Wei, C.L., Chen, J.C., Zheng, H.Y., Chen, W.C., Wu, C.H., 2016. Astaxanthin enhances pemetrexed-induced cytotoxicity by down regulation of thymidylate synthase expression in human lung cancer cells. Regul. Toxicol. Pharmacol. 81, 353–361.
Lin, J., Huang, L., Yu, J., Xiang, S., Wang, J., Zhang, J., 2016. Fucoxanthin, a marine carotenoid, reverses scopolamine-induced cognitive impairments in mice and inhibits acetylcholin- esterase in vitro. Mar. Drugs 14 (4), 67.
Liu, C1., Wang, XD., Bronson, RT., Smith, DE., Krinsky, NI., Russell, RM., 2000. Effects of physiological versus pharmacological beta-carotene supplementation on cell proliferation and histopathological changes in the lungs of cigarette smoke-exposed ferrets. Carcinogenesis 21(12), 2245–53.
Liu, Y., Zheng, J., Zhang, Y., Wang, Z., Yang, Y., Bai, M., Dai, Y., 2016. Fucoxanthin activates apoptosis via inhibition of PI3K/Akt/mTOR pathway and suppresses invasion and migration by restriction of p38-MMP-2/9 pathway in human glioblastoma cells. Neurochem. Res. 41, 2728–2751.
Liu, T., Liu, W.H., Zhao, J.S., Meng, F.Z., Wang, H., 2017. Lutein protects against β-amyloid peptide-induced oxida- tive stress in cerebrovascular endothelial cells through modu- lation of Nrf-2 and NF-κB. Cell Biol. Toxicol. 33 (1), 57–67.
Lobos, P., Bruna, B., Cordova,A., Barattini, P., Galáz, J.L., Adasme, T., Hidalgo, C., Muñoz, P., Paula-Lima, A., 2016. Astaxanthin protects primary hippocampal neurons against noxious effects of Aβ-oligomers. Neural Plast. 34, 567–583.
Lowe, G.M., Booth, L.A., Bilton, R.F., Young, A.J., 1999. Lycopene and β- carotene protect against oxidative damage in HT29 cells at low concentrations but rapidly lose this capacity at higher doses. Free Radic. Res. 30, 141–151.
Lu, S., Li, L., 2008. Carotenoid metabolism: biosynthesis, regulation, and beyond. J. Integr. Plant Biol. 50 (7), 778–785.
Ma, E., Iso, H., Yamagishi, K., Ando, M., Wakai, K., Tamakoshi, A., 2018. Dietary antioxidant micronutrients and all-cause mortality: the Japan collaborative cohort study for evaluation of cancer risk. J. Epidemiol. 28(9), 388–396.
Maiani, G., Periago Castón, M.J., Catasta, G., Toti, E., Cambrodón, I.G., Bysted, A., Böhm, V. 2009. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res 53, S194–S218.
Marcotorchino, J., Romier, B., Gouranton, E., Riollet, C., Gleize, B., Malezet- Desmoulins, C., Landrier, J.F., 2012. Lycopene attenuates LPS-induced TNF-alpha secretion in macrophages and inflammatory markers in adipocytes exposed to macrophage-conditioned media. Mol. Nutr. Food Res. 56, 725–732.
Mathews-Roth, MM., 1993. Carotenoids in erytropoietic protoporphyria and other photosensitivity diseases. Ann. N Y. Acad. Sci. 691, 127–138.
Mayne, S.T., 1996. Beta-carotene, carotenoids, and disease prevention in humans. FASEB J. 10, 690–701.
Meléndez-Martínez, A.J., Mapelli-Brahm, P., Benítez-González, A., Stinco, C.M. 2015. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Arch. Biochem. Biophys 572,188–200.
Mente, A., de Koning, L., Shannon, H.S, Anand, S.S. 2009. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 169(7), 659–669.
Merhan, O., 2017. The biochemistry and antioxidant properties of carotenoids. In: Cvetković, D.J., Nicolić, G.S.(Eds.), Carotenoids, InTechOpen, Rijeka, pp. 51–66.
Mezzomo, N., Ferreira, S.R.S., 2016. Carotenoids functionality, sources, and processing by supercritical technology: a review. J. Chem 3, 164–312.
Milani, A., Basirnejad, M., Shahbazi, S., Bolhassani, A., 2017. Carotenoids: biochemistry, pharmacology and treatment. Br. J. Pharmacol. 174 (11) 1290–1324.
Miller, N.J., Sampson, J., Candeias, L.P., Bramley, P.M., Rice-Evans, C.A., 1996. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 384 (3), 240–242.
Murata, T., Tamai, H., Morinobu, T., Manago, M., Takenaka, H., Hayashi, K., Mino, M., 1994. Effect of long-term administration of beta- carotene on lymphocyte subsets in humans. Am. J. Clin. Nutr. 60 (4), 597–602.
Nam, K.N., Park, Y.M., Jung, H.J., 2010. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur. J. Pharmacol. 648(1–3), 110–116.
Nicolle, C., 2004. Genetic variability influences carotenoid, vitamin, phenolic, and mineral content in white, yellow, purple, orange, and dark-orange carrot cultivars. J. Am. Soc. Hortic. Sci. 129, 523–529.
Nisar, N., Li, L., Lu, S., Khin, N., C., P., J., B., 2015. Carotenoid metabolism in plants. Mol. Plant 8, 68–82.
Obermuller-Jevic, U.C., Francz, P.I., Frank, J., Flaccus, A., Biesalski, HK., 1999. Enhancement of UVA induction of haem oxygenase-1 expression by beta-carotene in human skin fibroblasts. FEBS Lett. 460, 212–216.
Olson, J.A., 1993. Molecular actions of carotenoids. Ann. New York Acad. Sci. 691, 156–166.
Olson, J.A., 1994b.Vitamin A and carotenoids in modern nutrition. Health Dis. 8, 287–307.
Olson, J.A., Krinsky, N.I., 1995. Introduction: the colorful fascinating world of the carotenoids: important physiologic modulators. FASEB J. 9, 1547–1550.
Omenn, G.S., Goodman, G.E., Thornquist, M.D., 1996. Effects of a combination of beta-carotene and vitamin A on lung cancer and cardiovascular disease. N. Eng. J. Med. 334, 1150–1155.
Omenn, GS., Goodman, GE., Thornquist, MD., 1996. Risk factors for lung cancer and for intervention effects in CARET, the Beta-carotene and retinol efficacy Trial. J. Natl. Cancer. Inst 88(21), 1550–1559.
Palozza, P., 2005. Can h-carotene regulate cell growth by a redox mechanism? An answer from cultured cells. Biochim. Biophys. Acta Mol. Basis Dis. 1740, 215–221.
Palozza, P., Serini, S., Torsello, A., 2003. Beta-carotene regulates NF-kappaB DNA-binding activity by a redox mechanisms in human leukemia and colon adenocarcinoma cells. J. Nutr. 133, 381–388.
Paolini, M., Antelli, A., Pozzetti, L., 2001. Induction of cytochrome P450 enzymes and over-generation of oxygen radicals in beta-carotene supplemented rats. Carcinogenesis 22 (9),1483–1495.
Perk, J., De Backer, G., Gohlke, H., 2012. European association for cardiovascular prevention & rehabilitation. European guidelines on cardiovascular disease prevention in clinical practice. Int. J. Behav. Med. 19, 403–88.
Prakash, A., Kumar, A., 2014. Implicating the role of lycopene in restoration of mitochondrial enzymes and BDNF levels in β-amyloid induced Alzheimer's disease. Eur. J. Pharmacol. 741, 104–111.
Rao, A.V., Agarwal, S., 1999. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: a review. Nutr. Res. 19, 305–323.
Rao, A.V., Rao, L.G., 2007. Carotenoids in human health. Pharmacol. Res. 55, 207–216.
Rao, S.V., Yenisetti, S.C., Rajini, P.S., 2016. Evidence of neuro- protective effects of saffron and crocin in a Drosophila model of Parkinsonism. Neurotoxicology 52, 230–242.
Ravikrishnan, R., Rusia, S., Ilamurugan, G., Salunkhe, U., Deshpande, J., Shankaranarayanan, J., Shankaranarayana, M.L., Soni, M.G., 2011. Safety assessment of lutein and zeaxanthin (Lutemax 2020): subchronic toxicity and mutagenicity studies. Food Chem. Toxicol. 49, 2841–2848.
Ray, A.L., Semba, R.D., Walston,J., 2006. Low serum selenium and total carotenoids predict mortality among older women living in the community: the women's health and aging studies. J. Nutr. 136,172–176.
Ribaya-Mercado, J.D., Blumberg, J.B., 2004. Lutein and zeaxanthin and their potential roles in disease prevention. J. Am. Coll. Nutr. 23(6), 567S–87S.
Ribaya-Mercado, J.D., Garmyn, M., Gilchrest, B.A., Russell, R.M., 1995. Skin lycopene is destroyed preferentially over h-carotene during ultraviolet irradiation in humans. J. Nutr. 125, 1854–1859.
Riccioni, G., D‘Orazio, N., Salvatore, C., Franceschelli, S., Pesce, M., Speranza, L., 2012. Carotenoids and vitamins C and E in the prevention of cardiovascular disease. Int. J. Vitam. Nutr. Res. 82, 15–26.
Rodriguez-Amaya, D.B., 1999. A Guide to Carotenoid Analysis in Foods, ILSI, Washington DC.
Rühl, R. 2007. Effects of dietary retinoids and carotenoids on immune development. Proc. Nutr. Soc. 66(3), 458–469.
Russo, V.M., Howard, L.R., 2002. Carotenoids in pungent and non-pungent peppers at various developmental stages grown in the field and glasshouse. J. Sci. Food Agric. 82, 615–624.
Rutz, J.K., Borges, C.D., Zambiazi, R.C., da Rosa, C.G., da Silva, M.M., 2016. Elaboration of microparticles of carotenoids from natural and synthetic sources for applications in food. Food Chem. 202, 324–333.
Sachdeva, A.K., Chopra, K., 2015. Lycopene abrogates Aβ (1–42)-mediated neuroinflammatory cascade in an experimental model of Alzheimer's disease. J. Nutr. Biochem. 26 (7), 736–744.
San Millan, C.S, Soldevilla, B., Martin, P., Gil-Calderon, B., Compte, M., Perez-Sacristan, B., Donoso, E., Pena, C., Romero, J., Granado-Lorencio, F., et al. 2015. β-Cryptoxanthin synergistically enhances the antitumoral activity of oxaliplatin through ΔNP73 negative regulation in colon cancer. Clin. Cancer Res. 21, 4398–4409.
Sasaki, M., Ozawa, Y., Kurihara, T., 2009. Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Invest. Ophthalmol. Vis. Sci. 50 (3), 1433–1439.
Satia, J.A., Littman, A., Slatore, C.G., Galanko, J.A., 2009. White, E. Long-term use of beta-carotene, retinol, lycopene, and lutein supplements and lung cancer risk: Results from the Vitamins and Lifestyle (VITAL) study. Am. J. Epidemiol 169, 815–828.
Scientific Committee on Food on the safety of use of beta carotene from all dietary sources (Opinion adopted by the SCF on 7 September 2000).
Sesso, H.D., Buring, J.E., Norkus, E.P, Gaziano, J.M., 2005. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in men. Am. J. Clin. Nutr. 81, 990–7.
Shardell, M.D, Alley, D.E., Hicks, G.E., 2011. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: the Third National Health and Nutrition Examination Survey. Nutr. Res. 31, 178–189.
Sies, H., 1993. Strategies of antioxidant defense. Eur. J. Biochem. 215 (2), 213–219.
Sies, H., Krinsky, N.L., 1995. Antioxidant vitamins and b-carotene in disease prevention. Am. J. Clin. Nutr 62, 1299–1540.
Sommerburg, O., 1998. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. Br. J. Ophthalmol. 82, 907–910.
Stahl, W., Heinrich, U., Jungmann, H., Sies, H., Tronnier, H., 2000. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am. J. Clin. Nutr. 71, 795–798.
Stahl, W., Sies, H., 2005. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 1740, 101–107.
Stahl, W., Sies, H., 2007. Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight. Mol. Biotechnol. 37, 26–30.
Stanner, S.A., Hughes, J., Kelly, C.N., Buttriss, J., 2004. A review of the epidemiological evidence for the antioxidant hypothesis. Public Health Nutr. 7, 407–22.
Steinmetz, KA., Potter, JD., 1996. Vegetables, fruit and cancer prevention. J. Am. Diet. Assoc. 96, 1027–1039.
Trekli, M.C., Riss, G., Goralczyk, R., Tyrrell, R.M., 2003. Beta-carotene suppresses UVA-induced HO-1 gene expression in cultured FEK4. Free Radic. Biol. Med. 34, 456–464.
Truscott, T.G., 1990. The photophysics and photochemistry of the carotenoids. J. Photochem. Photobiol. B Biol. 6, 359–371.
Von Lintig, J., Vogt, K., 2000. Molecular identification of an enzyme cleaving b-carotene to retinal. J. Biol. Chem. 275, 11915–20.
Voutilainen, S., Nurmi, T., Mursu, J., Rissanen, T.H., 2006. Carotenoids and cardiovascular health. Am. J. Clin. Nutr. 83, 1265–1271.
Wang, X., Ouyang, Y., Liu, J., 2014. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose–response meta-analysis of prospective cohort studies. BMJ 349, g4490.
Wang, X., Willén, R., Wadström, T., 2000. Astaxanthin-rich algal meal and vitamin C inhibit Helicobacter pylori infection in BALB/cA mice. Antimicrob. Agents Chemother. 44(9), 2452–2457.
Wang, Y., Cui, R., Xiao, Y., Fang, J., Xu, Q., 2015. Effect of carotene and lycopene on the risk of prostate cancer: a systematic review and dose–response meta-analysis of observational studies. PLoS One 10 (9), e0137427.
Watson, R.R., Prabhala, R.H., Plezia, P.M., Alberts, D.S., 1991. Effect of beta-carotene on lymphocyte subpopulations in elderly humans: evidence for a dose-response relationship. Am. J. Clin. Nutr. 53(1), 90–94.
Woods, J.A., Young, A.J., Bilton, R.F., 1999. Carotene enhances hydrogen peroxide-induced DNA damage in human hepatocellular HepG2 cells. FEBS Lett. 449, 255–258.
World Cancer Research Fund American Institute for Cancer Research. 2007. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. AICR, Washington, DC.
Wyss, A., Wirtz, G., Woggon, W., Brugger, R., Wyss, M., Friedlein, A., Bachmann, H., Hunziker, W., 2000. Cloning and expression of beta, beta-carotene 15,150-dioxygenase. Biochem. Biophys. Res. Commun. 271, 334–336.
Yabuzaki, J., 2017. Carotenoids database: structures, chemical fingerprints and distribution among organisms. Database 17, bax004.
Yahia, E.M., Ornelas-Paz, J.J. 2010. Chemistry, stability, and biological actions of carotenoid. Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value, and stability, In: de la Rosa, L.A., Álvarez-Parrilla, E., González-Aguilar, G.A., (Eds.) Wiley-Blackwell Ames, IA, USA, pp. 177–222.
Yang, Y., Fuentes, F., Shu, L., Wang, C., Pung, D., Li, W., 2016. Epigenetic CpG methylation of the promoter and reactivation of the expression of GSTP1 by astaxanthin in human prostate LNCaP Cells. AAPS J. 30, 795–800.
Ye, Q., Zhang, X., Huang, B., Zhu, Y., Chen, X., 2013. Astaxanthin suppresses MPP+-induced oxidative damage in PC12 cells through a Sp1/NR1 signaling pathway. Mar Drugs 11(12), 1019–1034.
Yeh, S., Hu, M., 2001. Induction of oxidative DNA damage in human foreskin fibroblast Hs68 cells by oxidized h-carotene and Lycopene. Free Radic. Res. 35, 203–13.
Yeum, K-J., Russell, R.M., 2002. Carotenoid bioavailability and bioconversion. Annu. Rev. Nutr. 22, 483–504.
Young, A.J., Lowe, G.M., 2001. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 385, 20–27.
Yuri, T., Yoshizawa, K., Emoto, Y., Kinoshita, Y., Yuki, M., Tsubura, A., 2016. Effects of dietary xanthophylls, canthaxanthin and astaxanthin on N-methyl-N-nitrosourea-induced rat mammary carcinogenesis. In Vivo 30 (6), 795–800.
Zahavi, A., Zahavi, A., 1997. The Handicap Principle: A Missing Piece of Darwin's Puzzle, Oxford University Press, UK.
Zaripheh, S., Erdman, JW.Jr. 2002. Factors that influence the bioavailablity of xanthophylls. J. Nutr. 132, 531S-534S.
Zechmeister, L., 1962. Cis-Trans Isomeric Carotenoids, Vitamins A and Aryl-polyenes, Academic Press, New York.
Zhang, B., Liu, C., Wang, Y., Yao, X., Wang, F., Wu, J., King, G.J., Liu, K., 2015a. Disruption of a carotenoid cleavage dioxygenase 4 gene converts flower colour from white to yellow in Brassica species. New Phytol. 206 (4), 1513–1526.
Zhang, G.F., Zhang, Y., Zhao, G., 2015b. Crocin protects PC12 cells against MPP+-induced injury through inhibition of mitochondrial dysfunction and ER stress. Neurochem. Int. 89, 101–110.
Zheng, Y.F., Bae, S.H., Kwon, M.J., Park, J.B., Choi, H.D., Shin, W.G., Bae, S.K., 2013. Inhibitory effects of astaxanthin, b-cryptoxanthin, canthaxanthin, lutein, and zeaxanthin on cytochrome P450 enzyme activities. Food Chem. Toxicol. 59, 78–85.
Zhou, X., Zhang, F., Hu, X., Chen, J., Wen, X., Sun, Y., Liu, Y., Tang, R., Zheng, K., Song, Y., 2015. Inhibition of inflammation by astaxanthin alleviates cognition deficits in diabetic mice. Physiol. Behav. 151, 412–420.
Zhou, X.Y., Zhang, F., Huetal, X.T., 2017. Depression can be prevented by astaxanthin through inhibition of hippocampal inflammation in diabetic mice. Brain Res. 1657, 262–268.
Zhu, C., Naqvi, S., Capell, T., Christou, P., 2008. Metabolic engineering of ketocarotenoid biosynthesis in higher plants. Arch. Biochem. Biophys. 483, 182–190