Citric acid, antioxidant effects in health
Sushil Kumar Singha, Rahul Kaldateb, Arti Bishtc
aDBT NECAB, Assam Agricultural University, Jorhat, Assam, India
bDepartment of Agrilcultural Biotechnology, Assam Agricultural University, Jorhat, Assam, India
cG.B. Pant National Institute of Himalayan Environment and Sustainable Development, Kosi-Katarmal, Almora, Uttarakhand, India
4.5.1 Introduction
The citric acid (C6H8O7) is also known as tricarboxylic acid (TCA) metabolite, synthesized by condensation of acetate with oxaloacetate in the cellular oxidative metabolism of mitochondria. Its nomenclature originally comes from the Latin word citrus, a plant with phenotypically similar fruit and taste (Max et al., 2010). It is naturally acidic in taste and acts as a good preservative. It can also easily manufacture and converted into a soluble form. It is widely used as an acidifier, flavoring agent, chelating agent, and utilized for the stability of the fruit (Apelblat., 2014; Vandenberghe et al., 1999; Hotha et al., 2014). The annual production of citric acid is exciding million tons every year. In 2007, the total production of citric acid was 1.6 million tones around the world, with a 3.5 – 4.0% annual rise in demand and expenditure (Anastassiadis et al., 2008). In the last 20 years, the availability of citric acid has been increased globally, from 0.5 to 2 million tons (Ciriminna et al., 2017).
In a human mitochondrial cell, citric acid is synthesized in-between the metabolic pathways of TCA cycle where pyruvate manufactured through glycolysis is breakdown into CO2, 4H+, and NADH, provides energy to the cell by utilizing 4H+ by electron transport chain. It is naturally available in tissues of many plants, animals, and physiological fluids. It can be originated from natural sources like citrus fruits or industrial production (e.g., chemical reaction and microbial fermentation). Citrus fruits (lemons, oranges, tomatoes, beets, etc.) contain a higher concentration of citric acid than other fruits, and hence, they are grouped into acid fruit. It is most concentrated in lemons and limes (Muller et al., 1996), lemons contain 4.0%–8.0%, black currants 1.5%–3%, grapefruits 1.2%–2.1%, oranges, tangerines, red currents, raspberries, and strawberries carry a range between 0.6% and 1.3% of the dry weight basis (Apelblat, 2014).
The world production of citric acid by industry-based microbial fermentation technology is rapidly enhanced with the help of various molds, yeasts, and bacteria. With the advancement in metagenomics technologies, numerous different species of microorganisms have been identified and studied in the last few years. Swain et al., 2011 enlisted these microorganisms capable of producing citric acid in which 26 native strains of bacteria, mainly belonging to Aspergillus and Penicillium genus, 14 strains of yeasts and three strains of bacteria are mentioned. Genetically modified microorganisms are developed in such a way that commercially enhances the production of citric acid. Moreover, Aspergillus niger was mostly used in industry for large scale production of citric acid due to its superior production yield as compared to other microorganisms (Pau et al., 2015).
Nowadays, the production of citric acid is carried out by solid-state fermentation, utilizing agricultural residues like sugarcane and cassava bagasse, and food-producing solid wastes, such as grape and apple pomace with a range of 22%–155% yield (Swain et al., 2011). In 1880, Grimaux and Adam, firstly, chemically produced citric acid by purely chemical reactions using glycerol as a starting material.
4.5.2 Chemistry
The IUPAC nomenclature of citric acid is 2-hydroxy-1, 2, 3-tricarboxylic acid with a chemical formula of C6H8O7 and structural formula as represented in Fig. 4.5.1 (PubChem Identifier: CID 311, URL: https://pubchem.ncbi.nlm.nih.gov/compound/Citric-acid).
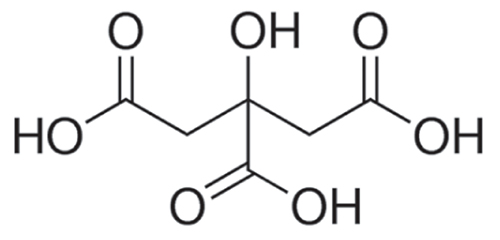
In 1934, the anhydrous crystalline structure of citric acid was first explained by Bennett and Yuill with the help of X-ray diffraction (Bennett and Yuill, 1935). Citric acid is synthesized as a critical metabolic output in the mitochondrial TCA cycle. Citric acid monohydrate has 210.14 g/mol concentration (NCBI, PubChem Database), which contains three similar COOH- functional groups at different positions contributing 3.1, 4.7, and 6.4 values of pKa (Papagianni, 2007). It is odorless, colorless, slightly hygroscopic, and readily dissolvable in water (62.07% at 25°C) (Dalman, 1937). The absence of asymmetric carbon atoms in its structure makes them optically inactive.
The anhydrous citric acid has a density of 1.665 g cm−3 at a temperature of 18°C with a melting point of 156–157°C (Apelblat, 2014) and density of its monohydrate form is 1.542 g cm−3 at 25°C (Laguerie et al., 1976). In 1991, Trask-Morrell and Kottes Andrews reported that its anhydrous form melts at 152–154°C and disintegrate at 228–242°C, which is solid at room temperature. It can occur mainly as the trivalent anion at physiologic pH and smaller concentration extent in the urine.
4.5.3 Bioavailability
The fresh juice of citrus fruit like lemon, orange, and lime supply more citric acid per liter than cane-packed juice of grapefruit, and other extracted juice from the citrus fruit (Penniston et al., 2008). Citric acid helps in increasing the accumulation of Ca, Mg, P, and Zn. In contrast, that of ascorbic acid only ameliorates the concentration of Fe and assist in improving the bioaccumulation of other micronutrients of chicken eggshells (Siddique et al., 2016). The bioaccumulation of calcium and phosphorus upon intake of citric acid in the food were studied by modifying the dietary habits of a rat. This study indicates that taking citric acid supplementation together with a Ca-rich diet enhances the bioavailability of Ca and P in the bone. Continues intake of Ca citrate may, therefore, aid in improving the deposition of Ca in the bone (Lacour et al., 1997). Moreover, it has been reported that the accumulation of citric acid reduces the uptake of both lead and cadmium in the body. It was observed that the accumulation of citric acid to the soil altered the concentration and relative abundance of other forms of acids (Chen et al., 2003). It may reduce the phytoremediation of heavy metal ions lead and cadmium due to the decline of soil pH after the addition of citric acid.
4.5.4 Mechanisms of action
Citric acid acts as an oxygen scavenger and delays unsaturated lipid/free fatty acid oxidation by depletion of essential agents like metals-based oxygen scavenger (Rostamzad et al., 2011). It plays an active role in limiting the degree of lipids degradation due to oxidation of food materials (Gordon, 1990) and chelates photo-oxidative metal ions by combining metal ions with free radicals of carboxyl or hydroxyl groups thereby quench catalytic oxygen (Anon, 1985). The concentration of available dissolved oxygen is a vital factor for lipid oxidation, and reducing it can help to enhance the stability of lipids. Chelate complexes have inhibited oxidation process due to the following regions: (1) formation of unstable complexes, (2) reducing the activity of chelate metals ions, or (3) providing steric hindrance between metals and free triplet and singlet electronic states of oxygen or other constituents of food products (Polumbryk et al., 2013).
Ethylene diamine tetra acetic acid (EDTA) is the well-known chemically synthesized chelating agent that can bind to metal by forming carbonate and amine bonds. This water-soluble EDTA, in combination with lipid-soluble citric acid, can act as an effective antioxidant but not a true antioxidant. The synergistic effect of citric acid is due to its consumption of free radicals when it is associated with a metal chelator like EDTA as Ca-EDTA or Na-EDTA form. This partial antioxidant activity of EDTA basically reduces the oxidation catalyzed by metal, and decrease the amount of free radicals (Phaniendra et al., 2015; Palmer et al., 1997). It catalyzed the production of reactive oxygen species act as a scavenger in the step of propagation while that of chelator at the initial stage (Choe et al., 2009).
4.5.5 Possible proxidant activity
Citric acid was applied to decrease the growth of microorganisms, add sour flavor, and enhance the quality as well as stability of food components in the beverage industry. The aqueous solution of citric acid and other exclusive hydrogen peroxide equalizer is lower down the conversion of H2O2 to H2O and O2 gas. This type of microbial regulator outcome is because of the development of per-organic acids, as shown in the following reaction.
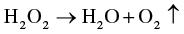
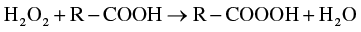
For packaged food products, citric acid, along with other ingredients, can act as an antioxidant preservative and helps in maintaining quality, enhance taste, masking effect, texture improver, color, and freshens of industrially produced packed food. In the case of freshly cut fruit like apple citric acid proved to be the best protective agent that increases the shelf-life of food (Rossle et al., 2009).
The presence of citric acid reduced the pH, and break the lipid membrane of microorganism with the help of H2O2 and other organic acids with free –COOH functional group. Due to membrane disruption property of citric acid, it protects from various foodborne pathogens that can cause severe diseases in human beings, for example, Clostridium botulinum, Salmonella typhimurium, Staphylococcus aureus, Shigella, Listeria monocytogenes, Campylobacter, etc. The treatments of citric acid solution to the raw food before packaging wash out the entire toxic components.
4.5.6 Safety profile or toxicity studies
The safety concern related to citric acid depends on the effect of toxicity at diverse concentrations. The FDA enlisted citric acid in categories of generally recognized as safe (GRAS), direct food additives based on the report of dermal toxicity, which proved to be safe for ingestion. However, FDA makes it compulsory to label as a warning note in the cosmetic product containing AHA, which may increase the skin sensitivity under the sun, causing sunburn (Fiume et al., 2014).
It shows low irritation at a concentration of 10% and mild irritation at 30% concentration in rabbit eyes. Similarly, up to 10% of citric acid in skin cream is safe to use. Still, the concentration above that shows a significant increase in epidermal thickness along with Langerhans cells and glycosaminoglycan (GAG) content. Spermicidal activity determined that a concentration of 0.1% and 1% citric acid show slight and complete immortality of sperm, respectively. However, citric acid does not show any sign of genotoxicity, both in vivo and in vitro (Fiume et al., 2014). It has been reported that oral intake of citric acid is easily absorbed by the gastrointestinal tract and metabolized approximately 2 kg of citric acid a day to provide energy. The physiological level of citric acid in the body is about 25 mg/L (Dzik and Kirkley, 1988). It does not act as a mutagenic, carcinogen, or teratogenic substance in vivo or in vitro. It can be easily filterable from the glomerulus of the kidney. The NOAEL toxicity dose rate is 1200 mg/kg/d. Due to the low toxicity and biodegradable nature of citric acid, it can be considered as safe for the environment.
4.5.7 Beneficial and detrimental effects on health
Citric acid and citrates are extensively used as a food additive for additional protection against oxidation and show no side effects on human health (Lawrence, 1998; Voss, 2002; Lidon et al., 2014; Inetianbor et al., 2015). The composition of citric acid with K-citrate and Na-citrate is utilized for the treatment of kidney diseases such as kidney stones or gout or metabolic acidosis. A skin cream containing citric acid helps to remove skin infection. It can also help in retarding the acidic content of urine. It helps in recovering damaged tissue in the body, protects the brain and liver from oxidative damage, and reduces aging, stress, and acid in the urine due to its antioxidant properties. In our body it is present in every tissue, take part in oxidative metabolism, play a role for reducing lipid peroxidation and inflammation through antioxidant redox homeostasis mechanism of decreasing the level of PMN degranulation and release of IL-1beta, platelet factor-4, elastase and myeloperoxidase in the cell (Gabutti et al., 2004). It can also help in the recovery of CCl-4 induced hepatocellular necrosis injury in rats (Abdel et al., 2009).
The fresh juice of lemon or lime is regarded as the most abundant source of citric acid, and its intake substantiates as useful for treating oxalate stones (Halliwell, 1992; Kang et al., 2007; Haleblian et al., 2008). It can be quickly broken down in the water surface and readily biodegradable to the environment, declared as safe for human consumption (Ciriminna et al., 2017). It is now well known that citric acid, along with their salts, are metal chelators and create an acidic buffer for preservation (Oktar et al., 2001; Kim et al., 2006). Due to its antioxidant properties, citric acid acts as an inert substance in pharmaceutical drug development. It reduced the delivery of drug in vitro, when combining with multiple tablets and delayed the absorption of drug in vivo. It enhances the acidity while adjusting the pH and provides stability of food ingredients, chelate blood Ca ion, and prevents blood clotting. Due to its unique property of pH adjustment, chelation, masking agent, preservative, antioxidant and dissolution, citric acid has widely been utilized in the food and pharmaceutical industry. Hence, daily intake of citric acid increases in the body through both in the form of processed food, drugs, and natural sources.
4.5.8 Animal studies and clinical studies
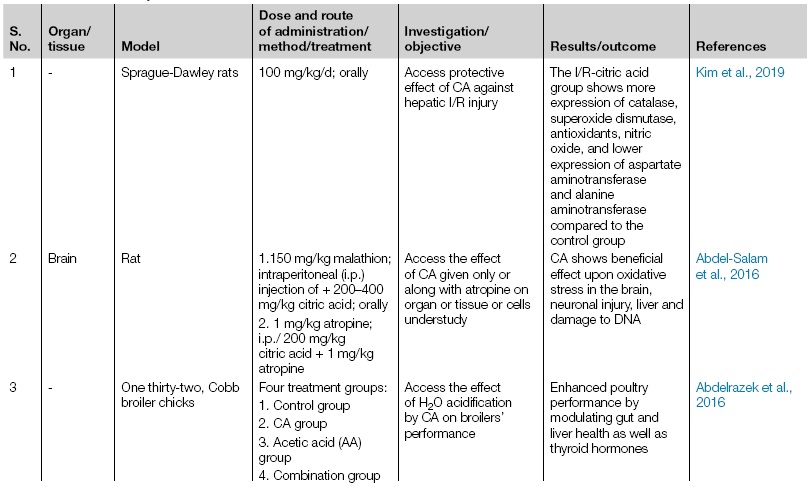
There are various in vivo/in vitro clinical and animal trials are investigated to assess the effectiveness of citric acid, which is summarised with details in Table 4.5.1. Multiple researchers have studies clinical clearance and established safety concerns of citric acid. In support of this in vivo animal studies, Omer et al. (2013) observed the effect of citric acid under endotoxin-induced oxidative damage in the brain and liver tissue of mice. They found that 1–2 g/kg dose of citric acid retard brain lipid-peroxidation and inflammation, liver damage, and DNA-fragmentation upon LPS (lipopolysaccharide) induced oxidative stress. The study of antioxidative activity of citric and ascorbic acids helps in lipid oxidation, was observed in the experiment of frozen Persian sturgeon fillets, which revealed significant differences in biochemical parameters when added with citric acid compared with control. It might be due to oxygen scavenger property, which delays lipid oxidation by reducing oxygen and metals. In different studies, the detailed result was observed by Aubourg et al. (2004) that acetic acid and citric acid have a synergistic outcome on each other. It can also change the fat improperly due to the reduction of lipid oxidation. Hence, ascorbic acid, along with citric acid, demonstrated an active role in avoiding lipid oxidation in freeze stored fillets (Rostamzad et al., 2011).
Table 4.5.1
| S. No. | Organ/ tissue | Model | Dose and route of administration/method/treatment | Investigation/objective | Results/outcome | References |
|---|---|---|---|---|---|---|
| 1 | - | Sprague-Dawley rats | 100 mg/kg/d; orally | Access protective effect of CA against hepatic I/R injury | The I/R-citric acid group shows more expression of catalase, superoxide dismutase, antioxidants, nitric oxide, and lower expression of aspartate aminotransferase and alanine aminotransferase compared to the control group | Kim et al., 2019 |
| 2 | Brain | Rat | 1.150 mg/kg malathion; intraperitoneal (i.p.) injection of + 200–400 mg/kg citric acid; orally
2. 1 mg/kg atropine; i.p./ 200 mg/kg citric acid + 1 mg/kg atropine |
Access the effect of CA given only or along with atropine on organ or tissue or cells understudy | CA shows beneficial effect upon oxidative stress in the brain, neuronal injury, liver and damage to DNA | Abdel-Salam et al., 2016 |
| 3 | - | One thirty-two, Cobb broiler chicks | Four treatment groups:
1. Control group 2. CA group 3. Acetic acid (AA) group 4. Combination group |
Access the effect of H2O acidification by CA on broilers’ performance | Enhanced poultry performance by modulating gut and liver health as well as thyroid hormones | Abdelrazek et al., 2016 |
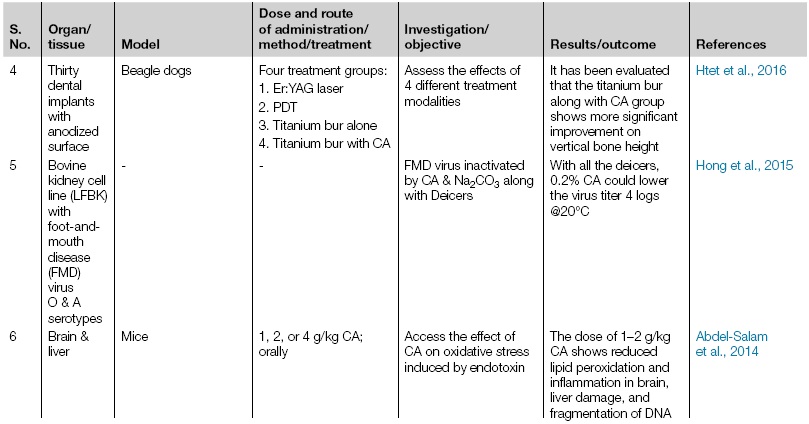
| 4 | Thirty dental implants with anodized surface | Beagle dogs | Four treatment groups:
1. Er:YAG laser 2. PDT 3. Titanium bur alone 4. Titanium bur with CA |
Assess the effects of 4 different treatment modalities | It has been evaluated that the titanium bur along with CA group shows more significant improvement on vertical bone height | Htet et al., 2016 |
| 5 | Bovine kidney cell line (LFBK) with foot-and-mouth disease (FMD) virus O & A serotypes | - | - | FMD virus inactivated by CA & Na2CO3 along with Deicers | With all the deicers, 0.2% CA could lower the virus titer 4 logs @20°C | Hong et al., 2015 |
| 6 | Brain & liver | Mice | 1, 2, or 4 g/kg CA; orally | Access the effect of CA on oxidative stress induced by endotoxin | The dose of 1–2 g/kg CA shows reduced lipid peroxidation and inflammation in brain, liver damage, and fragmentation of DNA | Abdel-Salam et al., 2014 |
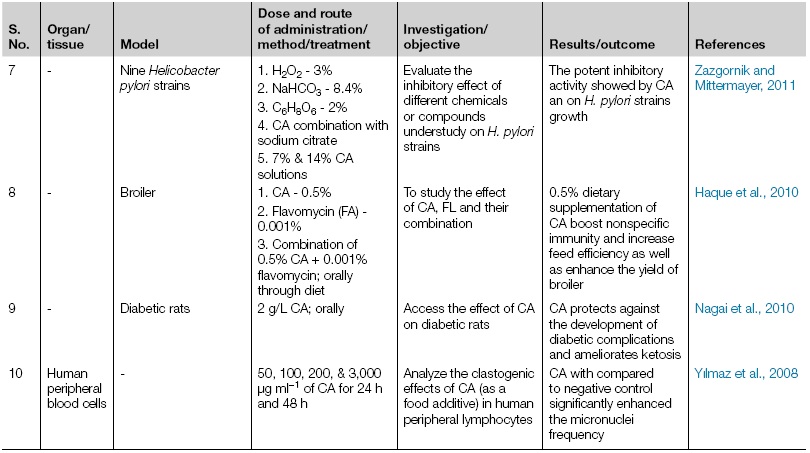
| 7 | - | Nine Helicobacter pylori strains | 1. H2O2 - 3%
2. NaHCO3 - 8.4% 3. C6H8O6 - 2% 4. CA combination with sodium citrate 5. 7% & 14% CA solutions |
Evaluate the inhibitory effect of different chemicals or compounds understudy on H. pylori strains | The potent inhibitory activity showed by CA an on H. pylori strains growth | Zazgornik and Mittermayer, 2011 |
| 8 | - | Broiler | 1. CA - 0.5%
2. Flavomycin (FA) - 0.001% 3. Combination of 0.5% CA + 0.001% flavomycin; orally through diet |
To study the effect of CA, FL and their combination | 0.5% dietary supplementation of CA boost nonspecific immunity and increase feed efficiency as well as enhance the yield of broiler | Haque et al., 2010 |
| 9 | - | Diabetic rats | 2 g/L CA; orally | Access the effect of CA on diabetic rats | CA protects against the development of diabetic complications and ameliorates ketosis | Nagai et al., 2010 |
| 10 | Human peripheral blood cells | - | 50, 100, 200, 3,000 μg ml−1 of CA for 24 h and 48 h | Analyze the clastogenic effects of CA (as a food additive) in human peripheral lymphocytes | CA with compared to negative control significantly enhanced the micronuclei frequency | Yılmaz et al., 2008 |
Similarly, Abdelrazek et al. (2016), experimented with studying the effect of citric acid and acetic acid in water acidification on broilers performance concerning thyroid hormones levels. The investigation reveals the further accumulation of citric acid to broilers water, boost broilers’ development by controlling the health of gut mucosal, liver, and thyroid hormones (T3 and T4) after observing lipid summary. Citric acid shows a substantial effect on balancing internal body homeostasis. Nagoba et al. (2011) used citric acid for controlling the growth of microorganisms related to chronic wound infection in animals. There were 38 cases of this infection which was not reacting to the conventional treatment. They are divided into two groups. In group-I, 3%, and in group-II, 5% of citric acid solutions were applied to check its efficiency. They found that the application of citric acid provides faster recovery of wounds in all 38 animals achieving a 100% success rate. Moreover, the use of citric acid was substantiated to be an effective and economical method for the successful recovery of continuously infected wounds in animals.
Significant emphasis has also been given to the safety of citric acid in animals. In this regard, Bonting et al. (1956) identify the result of citric acid in combination with phosphoric acid for its prolonged intake in rats. It was found that a concentration of 1.20% citric acid shows no harmful effect on developmental stages in rats. Moreover, multiple studies display enormous consumption of citric acid composition of beverages and fruits prove to be supportive in treating kidney stones (Seltzer et al., 1996; Penniston et al., 2008; Gul and Monga, 2014). However, it is interesting to note that citric acid displays potential antimicrobial effects on the growth of Helicobacter pylori strains associated with peptic ulcers in human beings (Zazgornik and Mittermayer, 2011). It is now well known that citric acid is used as a sole antimicrobial agent for successful treatment of lepromatous ulcers and chronic oral ulcers as compared to other therapies or treatments (Nagoba et al., 2012; Jamadar et al., 2019). In the case of a patient with anogenital warts, treatment with a composition of 9% citric acid along with 6% sodium nitrite shows promising effects (Ormerod et al., 2015). In another therapeutic approach for the treatment of a 6-year-old girl with propionic acidaemia, an acute life-threatening metabolic disorder, citric acid shows a positive effect in curing the diseases (Siekmeyer et al., 2013). Many methods have been explored for the treatment of wrinkles in which treatment of citric acid was proved to be a better solution or remedy and got patented for this method with U.S. patent No. 5,470,880 in the United States (Yu and Van Scoott, 1995).
Conclusion
The present study indicates that citric acid is a weak acid found in citrus fruit, mostly in lemons and limes. It has been used as a natural preservative and takes part in foods and soft drinks and has an acidic sour taste. The study of clinical and preclinical trail demonstrated the involvement of antioxidative activity in citric acid against several disorders including metabolic, neurological, and cardiological, among others.
In various research studies, citric acid has been picked as a possible drug agent for AD. With these studies, we can say that citric acid administration is helpful in the inhibition or reversal of Alzheimer’s disease. Citric acid also increased bioavailability.
Consequently, due to the high national and international demand of the citrus fruit and production of citric acid under the Pharmaceutical sector, these have got much attention and need to be reintroduced for further advanced research.
References
Abdel-Salam, O.M.E., Shaffie, N.M., Sleem, A.A., 2009. Hepatoprotective effects of citric acid and aspartame on carbon tetrachloride-induced hepatic damage in rats. EXCLI J. 8, 41–49.
Abdelrazek, H.M.A., Abuzead, S.M.M., Ali, S.A., El-Genaidy, H.M.A., Abdel-Hafez, S.A., 2016. Effect of citric and acetic acid water acidification on broiler's performance with respect to thyroid hormones levels. Adv. Anim. Vet. Sci. 4(5), 271–278.
Abdel-Salam, O.M., Youness, E.R., Mohammed, N.A., Morsy, S.M.Y., Omara, E.A., Sleem, A.A. 2014. Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice. J. Med. Food 17(5), 588–598.
Abdel-Salam, O.M., Youness, E.R., Mohammed, N.A., Yassen, N.N., Khadrawy, Y.A., El-Toukhy, S.E., Sleem, A.A. 2016. Novel neuroprotective and hepatoprotective effects of citric acid in acute malathion intoxication. Asian Pac. J. Trop. Med. 9(12), 1181–1194.
Anastassiadis, S., Morgunov, IG., Kamzolova, SV., Finogenova, TV., 2008. Citric acid production patent review. Recent Pat. Biotechnol. 2, 107–123.
Anon.,1985. Ullmann's Encyclopedia of Industrial Chemistry, fifth ed. Weinheim, VCH.
Anon.,1985. Ullmann's Encyclopedia of Industrial Chemistry, fifth ed. Weinheim, VCH.
Aubourg, S.P., Perez-Alonso, F., and Gallardo, J.M., 2004. Studies on rancidity inhibition in frozen horse mackerel (Trachurus trachurus) by citric acid and ascorbic acids. Eur. J. Lipid Sci. Technol. 106, 232–240.
Bennett, G.M., Yuill, J.L., 1935. The crystal form of anhydrous citric acid. J. Chem. Soc., 130:130.
Bonting, S.L., Jansen, B.C.P. 1956. The effect of a prolonged intake of phosphoric acid and citric acid in rats. Voeding, 17, 137–148.
Chen, Y.X., Lin, Q., Luo, Y.M., He, Y.F., Zhen, S.J., Yu, Y.L., Tian, G.M. & Wong, M.H. 2003. The role of citric acid on the phytoremediation of heavy metal contaminated soil. Chemosphere 50(6), 807–811.
Choe, E., Min, D.B. 2009. Mechanisms of antioxidants in the oxidation of foods. Compr. Rev. Food Sci. Food Saf. 8(4), 345–358.
Ciriminna, R., Meneguzzo, F., Delisi, R., 2017. Citric acid: emerging applications of key biotechnology industrial product. Chem. Cent. J. 11(1), 22.
Dalman, LH., 1937. The solubility of citric and tartaric acids in water. J. Am. Chem. Soc. 59, 2547–2549.
Dzik, W.H., Kirkley, S.A., 1988. Citrate toxicity during massive blood transfusion. Transfus. Med. Rev. 2, 76–94.
Fiume, M.M., Heldreth, B.A., Bergfeld, W.F., Belsito, D.V., Hill, R.A., Klaassen, C.D., Liebler, D.C., Marks Jr, J.G., Shank, R.C., Slaga, T.J., Snyder, P.W., 2014. Safety assessment of citric acid, inorganic citrate salts, and alkyl citrate esters as used in cosmetics. Int. J. Toxicol. 33(2_suppl), 16S-46S.
Gordon, M.H., 1990. The Mechanism of Antioxidant Action in Vitro. In: Hudson B.J.F. (Ed.), Food antioxidants. Elsevier, New York, pp. 1–18.
Gabutti, L., Nicola, F., Giorgio, M., Mombell, K., Claudio, M., 2004. The favorable effect of regional citrate anticoagulation on interleukin-1beta release is dissociated from both coagulation and complement activation. J. Nephrol. 6 (17), 819–825.
Grimaux, C., Adam, P., 1880. Synthèse de l'acide citrique. C R Acad. Sci. 90, 1252–1255.
Gul, Z., Monga, M., 2014. Medical and dietary therapy for kidney stone prevention. Korean J. Urol. 55(12), 775–779.
Haleblian, GE., Leitao, VA., Pierre, SA., 2008. Assessment of citrate concentrations in citrus fruit-based juices and beverages: implications for management of hypocitraturic nephrolithiasis. J. Endourol. 22, 1359–1366.
Halliwell, B. 1992. Reactive oxygen species and the central nervous system. J. Neurochem. 59(5), 1609–1623.
Haque, M.N., Islam, K.M., Akbar, M.A., Chowdhury, R., Khatun, M., Karim, M.R., Kemppainen, B.W. 2010. Effect of dietary citric acid, flavomycin and their combination on the performance, tibia ash and immune status of broiler. Can. J. Anim. Sci. 90(1), 57–63.
Hong, J.K., Lee, K.N., You, S.H., Kim, S.M., Tark, D., Lee, H.S., Ko, Y.J., Seo, M.G., Park, J.H., Kim, B., 2015. Inactivation of foot-and-mouth disease virus by citric acid and sodium carbonate with deicers. Appl. Environ. Microbiol. 81(21):7610–7614.
Hotha, K.K., Patel, T., Roychowdhury, S., Subramanian, V. 2014. Development of better-quality assay method for the citric acid and sodium citrate in ophthalmic/oral solutions and their application to deformulation studies. Am. J. Anal. Chem. 5(17), 1249.
Htet, M., Madi, M., Zakaria, O., Miyahara, T., Xin, W., Lin, Z., Aoki, K., Kasugai, S., 2016. Decontamination of anodized implant surface with different modalities for peri-implantitis treatment: lasers and mechanical debridement with citric acid. J. Periodontol. 87(8), 953–961.
Inetianbor, J.E., Yakubu, J.M., and Ezeonu, S.C., 2015. Effects of food additives and preservatives on man – a review. Asian J. Sci. Technol. 6(2), 11181135.
Jamadar, N., Nagoba, B., Davane, M., Ahmed, A., Tangsal, A., 2019. Citric acid treatment of oral ulcers refractory to conventional treatment: a case study. J. Wound Care 28(7), 461–463.
Kang, D.E., Sur, R.L., Haleblian, G.E., 2007. Long-term lemonade based dietary manipulation in patients with hypocitraturic nephrolithiasis. J. Urol. 177, 1358–1362.
Kim, S.K., Kang, S.W., Jin, S.A., Ban, J.Y., Hong, S.J., Park, M.S., 2019. Protective effect of citric acid against hepatic ischemia reperfusion injury in sprague-dawley rats. Transplant. Proc. 51(8), 2823–2827.
Kim, S., Lee, K., Park, J., Lee, H., and Hwang, I., 2006. Effect of natural antioxidants on stored freeze dried food product formulated using horse mackerel (Trachurus trachurus). J. Sci. Technol. 41, 90–95.
Lacour, B., Tardivel, S., Drüeke, T., 1997. Stimulation by citric acid of calcium and phosphorus bioavailability in rats fed a calcium-rich diet. Miner. Electrolyte Metab. 23(2), 79–87.
Laguerie, C., Aubry, M., Couderc, JP., 1976. Some physicochemical data on monohydrate citric acid in water: solubility, density, viscosity, diffusivity, pH of standard solution, and refractive index. J. Chem. Eng. Data 21, 85–87.
Lawrence, A., 1998. In: Stephenson, M., Thomas, G. (Eds.), Understanding Food Additives, FAIA – Food Additives and Ingredient Association, University of York, p. 148.
Lidon, F.C., Almeida, A.S., Leitão, A.E., Pinheiro, M.M.N., Macas, B., and Costa, R.., 2014. A synoptic overview of durum wheat production in the Mediterranean region and processing following the European Union requirements. Emirates J. Food Agric. 26(8): 693705.
Max, B., Salgado, JM., Rodríguez, N., Cortés, S., Converti, A., Domínguez, JM., 2010 Biotechnological production of citric acid. Braz. J. Microbiol. 41(4), 862–875.
Muller, M., Irkens-Kiesecker, U., Rubinstein, B., 1996. On the mechanism of hyper acidification in lemon: comparison of the vacuolar H(+)-ATPase activities of fruits and epicotyls. J. Biol. Chem., 271(4), 1916–1924.
Nagai, R., Nagai, M., Shimasaki, S., Baynes, J.W., Fujiwara, Y. 2010. Citric acid inhibits development of cataracts, proteinuria and ketosis in streptozotocin (type 1) diabetic rats. Biochem. Biophys. Res. Commun. 393(1), 118–122.
Nagoba, B.S., Wadher, B.J., Rao, A., Selkar, S.P., Gandhi, R.C., 2012. Treatment of lepromatous ulcers using citric acid as a sole antimicrobial agent. Int. Wound J. 9(5), 553–556.
Nagoba, B.S., Wadher, B.J., and Selkar, S.P., 2011. Citric acid treatment of chronic wounds in animals. Int. J Animal Vet. Adv. 3(1), 26–28.
Oktar, G.L., Sinci, V., Kalaycioğlu, S., Soncul, H., Gökgöz, L., Halit, V., Ersöz, A. 2001. Biochemical and hemodynamic effects of ascorbic acid and alpha-tocopherol in coronary artery surgery. Scand. J. Clin. Lab. Inv. 61(8), 621–629.
Omer, E.A., Said-Al Ahl, H.A.H., El Gendy, A.G., Shaban, Kh.A., Hussein, M.S. 2013. Effect of amino acids application on production, volatile oil and chemical composition of chamomile cultivated in saline soil at Sinai. J. Appl. Sci. Res. 9(4), 3006–3021.
Ormerod, A.D., van Voorst Vader, P.C., Majewski, S., Vanscheidt, W., Benjamin, N., Van der Meijden, W., 2015. Evaluation of the efficacy, safety, and tolerability of 3 dose regimens of topical sodium nitrite with citric acid in patients with anogenital warts: a randomized clinical trial. JAMA Dermatol. 151(8), 854–861.
Palmer, H.J., Paulson, K.E., 1977. Reactive oxygen species and antioxidants in signal transduction and gene expression. Nutr. Rev. 55, 353–361.
Papagianni, M., 2007. Advances in citric acid fermentation by Aspergillus niger: biochemical aspects, membrane transport and modelling. Biotechnol. Adv. 25, 244–263.
Pau, L.S., Kehinde, O.O., Qi, Y.S., Fitri, A.A.Z., John, C.W.L., Tau, C.L., 2015. Overview of citric acid production from Aspergillus niger. Front Life Sci. 8(3), 271–283.
Penniston, K.L., Nakada, S.Y., Holmes, R.P, Assimos, D.G., 2008. Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J. Endourol. 22(3), 567–570.
Phaniendra, Alugoju, Jestadi, Dinesh Babu, Periyasamy, Latha, et al., 2015. Free radicals: properties, sources, targets, and their implication in various diseases. Indian journal of clinical biochemistry 30 (1), 11–26.
Polumbryk, M., Ivanov, S., Polumbryk, O., 2013. Antioxidants in food systems, Mechanism of action. Ukr. J. Food Sci. 1, 15–40.
Rossle, C., Gormley, TR., Butler, F., 2009. Efficacy of Nature seal AS1 browning inhibitor in fresh cut fruit salads applications, with emphasis on apple wedges. J. Hortic. Sci. Biotechnol. 84, 62–67.
Rostamzad, H., Shabanpour, B., Shabani, A., Shahiri, H., 2011. Enhancement of the storage quality of frozen Persian sturgeon fillets by using ascorbic acid. Int. Food Res. J., 18(1 ).
Seltzer, M.A., Low, R.K., McDonald, M., Shami, G.S., and Stoller, M.L., 1996. Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. J. Urol. 156(3), 907–909.
Siddique, S., Firdous, S., Durrani, A.I., Khan, S.J., Saeed, A., 2016. Hesperidin, a citrus flavonoid, increases the bioavailability of micronutrients of Gallus domesticus (chicken) eggshell: in vitro study. Chem. Speciation Bioavailability 28(1-4), 88–94.
Siekmeyer, M., Petzold-Quinque, S., Terpe, F., Beblo, S., Gebhardt, R., Schlensog-Schuster, F., Kiess, W., and Siekmeyer, W., 2013. Citric acid as the last therapeutic approach in an acute life-threatening metabolic decompensation of propionic acidaemia. J. Pediatr. Endocrinol. Metab. 26(5-6), 569–574.
Swain, M.R., Ray, R.C., and Patra, J.K. 2011. Citric acid: microbial production and applications in food and pharmaceutical industries. In: Vargas, D.A., Medina, J.V. (Eds.), Citric Acid. Nova Science Publisher, New York, pp. 97–118.
Trask-Morrell, B.J., Andrews, K., B., A., 1991. Thermoanalytical characteristics of polycarboxylic acids investigated as durable press agents for cotton textiles. J. Appl. Polym. Sci. 42, 511–521.
Vandenberghe, L.P., Soccol, C.R., Pandey, A., Lebeault, J.M., 1999. Microbial production of citric acid. Braz. Arch. Biol. Technol. 42(3), 263–276.
Voss, C., 2002. Veneno no seu prato? Utilidades e Riscos dos Aditivos Alimentares. EDIDECO – Editores para a Defesa do Consumidor Lda, Lisboa.
Yılmaz, S., Ünal, F., Yüzbaşıoğlu, D., Aksoy, H., 2008. Clastogenic effects of food additive citric acid in human peripheral lymphocytes. Cytotechnology 56(2), 137–144.
Yu, J.R., Van Scott, J.E., 1995. Method of using citric acid for the treatment of wrinkles. U.S. Patent No. 5,470,880.
Zazgornik, J., Mittermayer, H., 2011. Citric acid inhibits growth of Helicobacter pylori in vitro: a new strategy for eradication. Wien. Klin. Wochenschr. 123(1-2), 38–40. https://doi.org/10.1007/s00508-010-1524-9