Antioxidants and cardiovascular diseases
Ankita Mandal*, Uday Hossain*, Parames C. Sil
Division of Molecular Medicine, Bose Institute, Kolkata, India
*Both the authors contributed equally to the work.
5.2.1 Introduction
Cardiovascular diseases (CVD) are multifactorial abnormalities including atherosclerosis, coronary heart disease, cardiac ischemia, hypertension, cardiomyopathies, cardiac hypertrophy, and heart failure. Despite of different therapeutic approaches to combat CVD and its related disorders, it accounts for almost quarter of all deaths worldwide and according to World Health Organization (WHO), CVDs are the leading cause of mortality globally (Mendis et al., 2011). Various cardiovascular diseases cause deaths of around 17.6 million people per year worldwide, which is predicted to be 25 million (approx.) by 2020 (Dahlöf, 2010). The predominant cardiac disorders namely cardiac ischemia and strokes accounts >80% of all death caused due to CVD. According to a survey conducted by the Global Burden of Disease (2010), about 24.8% of all deaths in India are caused due to CVD (Kassebaum et al., 2017). Moreover, the age standard death due to CVD is 272 people per 100,000 populations in India, which is higher than average death worldwide (i.e., 235 people per 100,000 populations) (Prabhakaran et al., 2016).
On the basis of existing evidences and ongoing investigations, it is becoming clear that oxidative stress is the leading cause of different cardiovascular diseases. Excessive production of reactive oxygen species (ROS) occurs in various conditions such as diabetes, obesity, metabolic diseases, excessive smoking, etc., can lead to oxidative stress-induced cardiovascular abnormalities (Katagiri et al., 2007). Sources of ROS include mitochondrial electron transport chain, nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase, lipoxygenases, xanthine oxidase, uncoupling of endothelial NO synthase (eNOS), etc. (Lakshmi et al., 2009). Oxidation of low-density lipoprotein (ox-LDL), overload, and desensitization of Ca2+ and uncoupling of eNOS are the major deleterious effects of oxidative stress that results in endothelium dysfunction, atherosclerosis, hypertension, ischemic heart disorders, and subsequently other cardiovascular abnormalities.
In regard of the role of oxidative stress in the onset and progression of various cardiovascular diseases, antioxidants are the most efficient and beneficial therapeutic agents for amelioration of cardiovascular disorders. For last two decades, association of CVD and antioxidant has been intensively investigated in cell culture, animal models as well as in clinical studies. In this book chapter, we focused on the most important natural or dietary antioxidants, namely, vitamins and polyphenolic compounds that have been demonstrated to significantly inhibit oxidative stress-mediated cellular damages which results in cardiovascular disorders. The mechanism of actions of these antioxidants in relation to its role in CVD has also been described with in vitro, in vivo, and clinical trials evidences.
5.2.2 Oxidative stress and its role in cardiovascular disease: a brief idea
Oxidative stress can be broadly defined as a condition in which the balance between ROS and antioxidants in the cell is disturbed (Sinha et al., 2013). Immoderate generation of ROS, exceeding the neutralizing capacity of antioxidant defense system, has been suggested to be the cause of oxidation of biological macromolecules, such as lipids, proteins, DNA, and carbohydrates. Currently, these circumstances are regarded as oxidative stress (Cai and Harrison, 2000). The main source of ROS generation is mitochondrial electron transport chain and in addition to that various enzymes including, (eNOS), cyclooxygenase, (NADPH) oxidase, lipooxygenase, glucose oxidase, xanthine oxidase, lypoxidases (Dahlöf), and myeloperoxidases (MPO) leads to the production of reactive oxygen species (Higashi et al., 2009; Cervantes Gracia et al., 2017). ROS can be of two classes: free radicals, such as superoxide anion (O2−), hydrogen peroxide (H2O2), hydroxyl radical (Podrez et al., 2000), nitric oxide (NO), and peroxynitrite (ONOO−); and nonradical derivatives, such as hydrogen peroxide (H2O2), hypochlorous acid (HOCl), peroxynitrite (ONOO−), and singlet oxygen (1O2) (Sinha et al., 2013; Higashi et al., 2009; Dhalla et al., 2000). Unpaired electrons of free radicals make them more reactive than nonradicals but can oxidize biomacromolecules and to the state of cellular oxidative stress (Fig. 5.2.1).
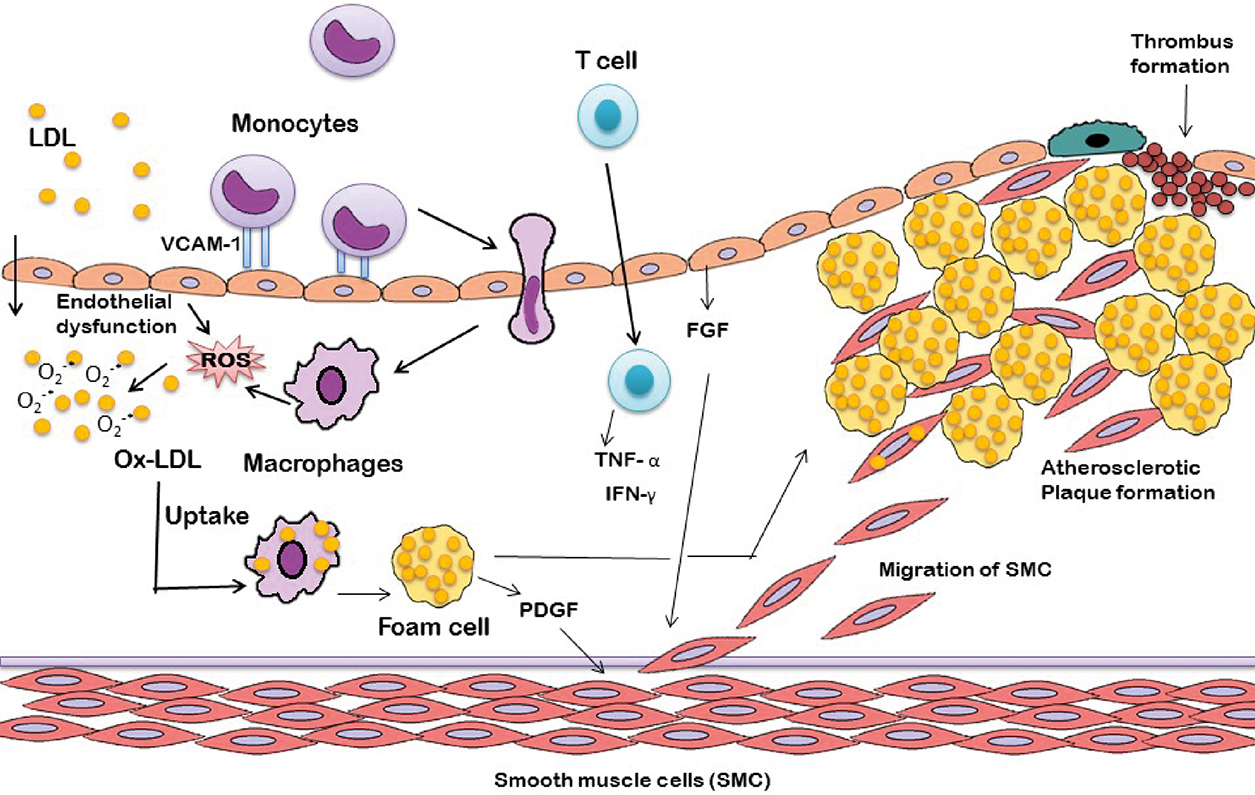
Collective evidences suggested that acute and chronic over production of ROS is essential in the onset of cardiovascular diseases including ischemic heart disease, atherosclerosis, cardiomyopathies, congestive heart failure, hypertension, and cardiac hypertrophy. Complications related to atherosclerosis are the leading causes of most of the cardiovascular disease. Atherosclerosis is characterized by the accumulation of lipids, foam cell formation, hardening, and narrowing of arteries (Cervantes Gracia et al., 2017; Raggi, 2016). Oxidation of low-density lipoprotein (LDL) by reactive oxidation species leads to the initiation of ROS-induced atherosclerosis (Ross, 1999). Oxidized LDL (Ox-LDL) induces vascular cell adhesion molecule (VCAM) expression on endothelial cells resulting in recruitment of monocytes into the intima, which matures and proliferates to form macrophages (Zwaka et al., 2001). These macrophages engulf ox-LDL with the help of CD36 (ox-LDL receptor) and transforms into foam cells (Podrez et al., 2000). Chemokines secreted by endothelial cells and macrophages recruits T cells, which in turn secrete proinflammatory cytokines (Tumor necrosis factor-α, TNF-α and Interferon-γ, IFN-γ) and leads to amplification of inflammation in blood vessels (Pirillo et al., 2013). In addition, fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) secreted from endothelium and foam cells respectively stimulates migration and proliferation of smooth muscle cells, which can also contribute in oxidized lipid accumulation (Raggi, 2016). All together, these events result in the formation atherosclerotic plaque characterized by lump of necrotic foam cells surrounded by smooth muscle cells and a fibrous cap of collagen. The adverse consequence of these events is the reduction of blood supply to the heart, leading to myocardial ischemia and hypertension (Dhalla et al., 1999).
Endothelium dysfunction (Dhalla et al., 1999) is referred to as an early clinical manifestation of atherosclerosis and is the indicator of future cardiovascular pathological events. Superoxide anion (O2*−) leads to oxidative degradation of tetrahydrobiopterin (BH4), an essential cofactor of NO synthase, resulting in uncoupling of eNOS (endothelial NO synthase). As a result superoxide anion (O2*−) is generated instead of NO, leading to reduction of NO bioavailability (Kelly and Fussell, 2017). In addition, O2*− reacts with NO and generates peroxynitrite, leading to the NO loss (Lakshmi et al., 2009). Abnormal endothelial function due to minimal NO availability results in a series of disorders including accumulation of platelets, fibrinolytic imbalance, thrombogenesis, and atheroma formation (Anderson et al., 1995). As a consequence, endothelium-dependent vasodilation is prohibited and ultimately leads to various cardiovascular disorders like hypertension.
Ample amount of ROS generation is associated with myocardial ischemia/reperfusion. ROS can directly impose myocardial cell injury or it effect myocardium via inflammatory consequences. The critical phenomenon related to ROS-induced myocardial injury is protein and lipid peroxidation in myocardial cells, which can cause damage to sarcolemma and contractile proteins (such as troponin, myosin, and α-tropomyosin). As a result of these, overload and desensitization of Ca2+ occur in the region of ischemia/reperfusion of cardiac myocytes, ultimately lead to reduced contraction ability of myocardium and heart failure (Lefer and Granger, 2000).
5.2.3 Antioxidants and cardiovascular diseases
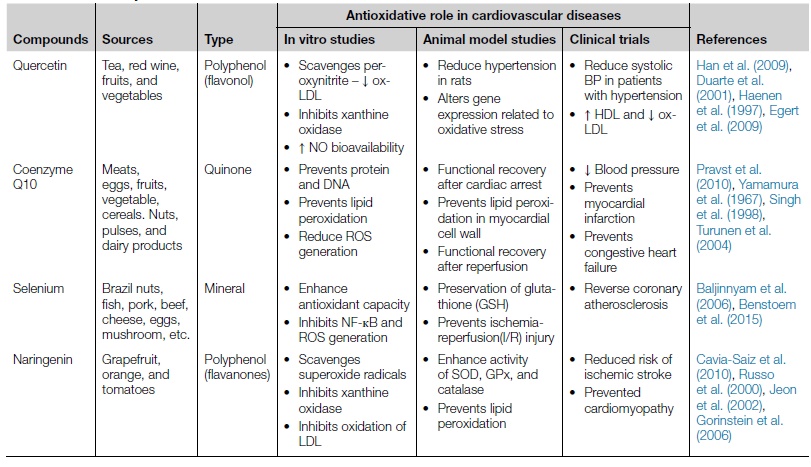
Antioxidants are the substances that possess ROS neutralizing properties and can prevent or slow down the deleterious effect of oxidative stress in various pathophysiological conditions. Antioxidant defense system of human body comprises proteins, enzymatic, and nonenzymatic antioxidants that continuously combat with ROS and neutralize them before they become deleterious to health. The major antioxidant enzymes that act as intracellular armor are superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) that collectively reduce hydrogen peroxide/superoxide to H2O (Goszcz et al., 2015; Nojiri et al., 2004). Nonenzymatic antioxidants include vitamins (A, C, and E), uric acid, bilirubin, ubiquinol-10, carotenoids, α-tocopherol as well as polyphenolic flavonoids compounds and minerals (Nojiri et al., 2004; Halliwell, 2007a). Other antioxidants are proteins such as transferrin, ceruloplasmin, hemopexin, haptoglobin, and albumin (Halliwell, 1988). In addition, some therapeutic agents like quercetin, resveratrol, N-acetylcysteineare, epicatechin, and allopurinol are under clinical trials for their antioxidant effects in relation to CVD (Goszcz et al., 2015). If the intrinsic or endogenous antioxidant defense system fails to conquer the over produced ROS in pathophysiological disorders such as diabetes, cardiovascular disease, etc. than dietary and therapeutic antioxidant supplements are provided to get rid of the deleterious effects of oxidative stress. Collective evidences suggested that oxidation of LDL, uncoupled endothelial NO synthase, and Ca2+ overload resulting due to oxidative stress are the major cause for various CVD, so antioxidants can be an inexpensive of preventing onset or progression of cardiovascular diseases (Table 5.2.1). Some of the major dietary and naturally occurring antioxidants in relation to CVD are discussed in this chapter. In addition, the roles of some other antioxidants that have gained attention for its antioxidative role in cardiovascular disorders are included in Table 5.2.2.
Table 5.2.1
| Endogenous antioxidants | ROS neutralizing reactions |
|---|---|
| Superoxide dismutase (SOD) | O2.− + O2.− + 2H+ H2O2 H2O2 |
| Catalase | 2H2O2 2H2O + O2 2H2O + O2 |
| Glutathione (GSH) | H2O2 + 2GSH  GSSG + 2H2O GSSG + 2H2O
GSSG + NADPH + H+ |
Table 5.2.2
| Compounds | Sources | Type | Antioxidative role in cardiovascular diseases | References | ||
|---|---|---|---|---|---|---|
| In vitro studies | Animal model studies | Clinical trials | ||||
| Quercetin | Tea, red wine, fruits, and vegetables | Polyphenol (flavonol) | Han et al. (2009), Duarte et al. (2001), Haenen et al. (1997), Egert et al. (2009) | |||
| Coenzyme Q10 | Meats, eggs, fruits, vegetable, cereals. Nuts, pulses, and dairy products | Quinone | • Functional recovery after cardiac arrest |
Pravst et al. (2010), Yamamura et al. (1967), Singh et al. (1998), Turunen et al. (2004) | ||
| Selenium | Brazil nuts, fish, pork, beef, cheese, eggs, mushroom, etc. | Mineral | Baljinnyam et al. (2006), Benstoem et al. (2015) | |||
| Naringenin | Grapefruit, orange, and tomatoes | Polyphenol (flavanones) | Cavia-Saiz et al. (2010), Russo et al. (2000), Jeon et al. (2002), Gorinstein et al. (2006) |
5.2.3.1 Vitamins and CVD
SOD, GPx, and catalase are some of the antioxidative enzymes present in the body and provide defense against free radicals. Some vitamins, like β-carotene, vitamin C, and vitamin D, cooperate or sometimes work synergistically to protect the body from oxidative stress. In atherosclerosis, modified LDL accumulates in the intima of the arterial wall and induces plaque progression, which results into different cardiovascular events (Finking and Hanke, 1997; Witztum and Steinberg, 1991).
5.2.3.2 Vitamin E
A group of fat-soluble compounds are collectively termed as vitamin E. These compounds show significant antioxidant properties which is beneficial for our health (Niki and Traber, 2012). The fat-soluble property of the vitamin helps them to get stored within the fatty tissues of humans and other animals. The vitamin E group is divided into two groups, tocotrienols and tocopherols, together termed as tocochromanols. Each group shows four forms, alpha(α), beta(β), gamma(ϒ), and delta (δ) (Traber, 2007). Edible vegetable oil is a rich source of vitamin E. Various seed, nuts, green vegetables contain significant amount of alpha-tocopherol.
It has already been established that atherosclerosis is a chronic inflammatory disease (Diaz et al., 1997) and vitamin E has observed to mediate antioxidant as well as anti-inflammatory properties (Devaraj et al., 1996), which extends its protective role against CVD. Vitamin E decreases monocyte recruitment by reducing foam cell formation(Devaraj et al., 1996; Wu et al., 1999), chemokine secretion(Munteanu et al., 2006), and expression of scavenger receptors on macrophages (CD36; Zapolska-Downar et al., 2000). Vitamin E has the ability to reduce expression of adhesion molecule (Wu et al., 1999; Keaney Jr. et al., 1999), inhibit proliferation of smooth muscle cell (Keaney et al., 1993), and enhance bioavailability of NO (Murohara et al., 2002), which lowers the progression of atherosclerosis. These above-mentioned effects are partially mediated by nonantioxidant properties of vitamin E. It also inhibits the signaling pathway, mainly protein kinase C, activated by oxidized LDL (Sugiyama et al., 1998). It has been observed that vitamin E prevents activation of NF-κB, induced by oxidized LDL, via suppression of protein kinase C (Li et al., 2000) and inhibits IkB degradation (Goya et al., 2006), which further reduces the CVD-mediated inflammatory response. Vitamin E exerts its anti-atherogenic property by controlling the gene expression. Expression of endothelial NO synthase mRNA is upregulated by vitamin E, therefore NO level is also elevated (Keaney et al., 1996) and protects the endothelium. Vitamin E protects the endothelium against oxidized LDL and reactive oxygen species (Ulrich-Merzenich et al., 2002). It stimulates endothelial cell proliferation and reduces endothelial apoptosis (Kuzuya et al., 1991; Haendeler et al., 1996). These help in prevention of endothelial dysfunction. These effects are possibly facilitated via modulation of apoptosis-related proteins of Bcl-2 family (Uemura et al., 2002), caspase-3 inhibition, phosphatase PP2A activation, and inhibition of mRNA and protein upregulation of angiotensin II receptor (AT1R), which is induced by oxidized LDL (Azzi et al., 1998; Koga et al., 2004; Jialal and Grundy, 1991).
5.2.3.2.1 In vitro and animal studies
Different experiments on animals, in vitro and in vivo, lead us toward this option of using vitamin E, and especially α-tocopherol as a protective against cardiovascular disease. It is known that, inflammation, oxidative stress and endothelial dysfunction play key role in the development of atherosclerotic plaque. In some studies, it has been observed that α-tocopherol can prevent above-mentioned processes. In different experiments it is seen that α-tocopherol can reduce endothelium to monocyte adhesion in primary human monocytes (Devaraj et al., 1996) and cultured monocytes. It has been discovered that vitamin E can prevent expression of different adhesion molecules, like intercellular adhesion molecule (ICAM-1) and VCAM-1, which is induced by oxidized LDL in cultured endothelial cell (Cominacini et al., 1997). Alpha-tocopherol can also prevent proliferation of vascular smooth muscle cell by inhibiting activity of protein kinase C (Tasinato et al., 1995). But the protective roles of vitamin E in in vivo animal models are not well understood properly. α-tocopherol can remarkably reduce circulating C reactive protein (CRP), which is a marker of inflammation, linked with atherosclerosis (Patrick and Uzick, 2001). On the other hand, if we look from antioxidant viewpoint, peroxidation of LDL can be inhibited by supplements of vitamin E, which ultimately slows down the atherosclerotic plaque formation (Reaven et al., 1993). α-tocopherol can also scavenge ROS which results into prevention of peroxidation of LDL (Sagach et al., 2002). In an experiment, diabetic mouse model, which is prone to develop atherosclerosis, is treated with vitamin E supplements. Those animals were observed to show lower macrophage activation and intravascular fat deposition (Otero et al., 2005). But on the contrary, in many studies, vitamin E has failed to reduce occurrence of atherosclerosis.
5.2.3.2.2 Clinical studies and trials
The Heart Outcomes Prevention Evaluation Study (HOPE) and the Cambridge Heart Antioxidant Study (CHAOS) are the two major clinical trials conducted on vitamin E. In CHAOS trial, α-tocopherol has succeeded in lowering the occurrence of nonfatal myocardial infarction in coronary artery disease patients, but failed to reduce the mortality rate in cardiovascular disease (Stephens et al., 1996). But the results of the HOPE trial have been considered as failure (Yusuf et al., 2000). It is still not fully understood, why one trial failed and another one succeeded. Despite the unsatisfactory results, scientists are still planning to conduct further clinical studies on this. US Preventative Task Force still has not recommended not using vitamin E supplementation for CVD primary prevention.
5.2.3.3 Vitamin C
Vitamin C or ascorbic acid is a simple carbohydrate with low-molecular weight with an ene-diol structure. Humans lack L-glucono-ϒ-lactone oxidase enzyme, which is needed to synthesize vitamin C. For this reason, it is not synthesized in human body. Because of this, dietary ascorbate is essential. Citrus fruits and leafy vegetables are rich source of vitamin C.
The independent protective role of vitamin C has not been widely studied in clinical trials. Vitamin C can perform synergistically with other vitamins, which might enhance their beneficial role (Myasnikova, 1947). Vitamin C also has some nonantioxidant properties. Administration of vitamin C has been observed to lower the cholesterol level in the hypercholesterolemic patients (Rössig et al., 2001). Vitamin C suppresses apoptosis of endothelium which is mediated by oxidized LDL and inflammatory cytokines (Rayment et al., 2003). It has been observed that vitamin C promotes endothelial cell proliferation and inhibits growth of vascular smooth muscle (Kuzuya et al., 1991) via the extracellular signal-regulated kinase-signaling pathway. Vitamin C is also able to modulate gene expression. It reduces monocyte adherence to endothelium by downregulating the expression of intercellular adhesion molecule-1 gene (Heller et al., 1999). Studies have shown that vitamin C elevates the synthesis of NO in endothelial cells (Gokce et al., 1999). An in vivo study has shown that vitamin C exerts sustained beneficial effects on endothelial-derived NO-dependent flow-mediated dilation (Siow et al., 1999). Apoptosis of vascular smooth muscle cell can be reduced by vitamin C supplementation, which prevents plaque instability seen in late-stage atherosclerosis. Vitamin C gives protection oxidative changes in LDL induced by different types of oxidative stress including metal-induced oxidative stress (Lehr et al., 1994).
5.2.3.3.1 In vivo studies
In one study, Lehr et al. induced atherosclerosis in Syrian Golden hamsters by cigarette smoke. They have observed that vitamin C inhibits the inflammatory response by stopping leucocyte adhesion and aggregation to the endothelium (Padayatty et al., 2003). Different studies have shown that vitamin C has the ability to stop peroxidation of lipid by preventing LDL oxidation and subsequent ox-LDL uptake (Frei, 1991; Maeda et al., 2000). Maeda et al. have shown in their study that L-glucono-ϒ-lactone oxidase enzyme lacking mice shows extensive vascular damage, which includes disruption of elastic lamina, proliferation of smooth muscle cell, epithelial cell desquamation, when their dietary vitamin C had been removed. This indicates the importance of vitamin C in vascular function and development (Nakata and Maeda, 2002).
5.2.3.3.2 Clinical trials
As vitamin C is synthesized in huge number of animal species, animal studies of this vitamin is difficult. Few studies have been held to discover the role of vitamin C in vascular health-related factors. It has been observed that proper dose of vitamin C (3 g) helped to improve endothelium-dependent vasodilation of epicardial coronary artery in hypertension patients (Solzbach et al., 1997). Vitamin C has major effect on endothelial dysfunction. Patients with NO-mediated or hyperglycemia-induced vasodilation showed good result with intra-arterial infusion (10 min of 24 mg/min) or oral dose (6 g over 2 days) administration of vitamin C (Levine et al., 1996).
Reduction of arterial stiffness and aggregation platelet in healthy person by oral administration of vitamin C (2 g) has been observed, though the exact mechanism behind this is not known properly (Wilkinson et al., 1999). A study by the European Prospective Investigation into Cancer and Nutrition (EPIC) has shown that vitamin C concentration in plasma has inverse relationship with mortality due to cardiovascular disease (Sargeant et al., 2001).
5.2.3.4 β-Carotene and vitamin A
The carotenoids are brightly colored pigments (red, yellow, and orange) found largely in vegetables and fruits. This compound is also lipid soluble. Synthesis of retinol or vitamin A in gut can occur from β-carotene, before as well as after absorption (Norum and Blomhoff, 1992). Lycopene is the precursor of β-carotene. It is an efficient scavenger of singlet oxygen and a second-line antioxidative defense for LDL compound after vitamin E is utilized (Jessup et al., 1990). Carotenoids as an antioxidant are pretty unpredictable. Studies show that it can act as neutral, pro-, and antioxidant depending on the situation (Princen et al., 1992; Tsuchihashi et al., 1995). Dietary consumption of β-carotene in high amount is linked with lowered mortality in CVD as well as in all-cause (Buijsse et al., 2005), but this effect is limited to elderly persons. Whether β-carotene can prevent LDL oxidation or not, has not been explored yet. A number of studies have been performed to examine LDL oxidation inhibition by β-carotene, but the results are inconclusive.
5.2.3.4.1 In vitro studies
In vitro studies show conflicting results regarding the protective role of β-carotene in CVD. In one study, β-carotene prevents peroxidation of LDL which is mediated by Cu2+ (Romanchik et al., 1995). But in another study of similar kind, β-carotene shows no beneficial effect upon peroxidation of lipid (Dugas et al., 1998). 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase is a rate-limiting enzyme helps in synthesis of cholesterol. In vitro studies show that β-carotene can reduce plasma cholesterol level by inhibiting HMG-CoA reductase. It has been studied that β-carotene and fluvastatin reduce HMG-CoA activity to same extent, which suggests that carotenoids can be used as therapeutic drug. Activity of LDL receptor of macrophage is increased with the help of carotenoids which reduces circulating LDL. From this it might be concluded that carotenoids have protective effect on CVD (Rao, 2002).
5.2.3.4.2 Clinical studies and trials
A protective property of carotenoids against CVD has been proven in clinical studies. In a report of Physicians Health Study, it has been observed that people consuming vegetables containing high carotenoids are less prevalent to heart disease (Liu et al., 2001). Some current studies like the young adult longitudinal trends in antioxidants (YALTA) and coronary artery risk development in young adults (CARDIA) have shown that high concentrations of plasma carotenoid are related with lowered inflammation, endothelial dysfunction, and oxidative stress, three significant features of atherosclerosis (Hozawa et al., 2007). On the other hand, reduced occurrence of atherosclerosis has been observed in Bruneck study with higher plasma β-carotene as well as α-carotene (D’Odorico et al., 2000). Endothelial dysfunction markers, like ICAM-1, as well as inflammation (CRP) are reduced due to more vegetables and fruit consumption containing high carotenoids (Watzl et al., 2005). On the contrary, in the β-carotene and retinol efficacy trial (CARET), inverse relation has been noticed between risk cardiovascular disease and combinatorial supplementation of retinol and β-carotene (Omenn et al., 1996). Strong correlation has been observed between lowered risk of MI and serum β-carotene (Karppi et al., 2011). Similarly, vitamin E has found to be more effective than β-carotene in reducing the oxidation of LDL (Reaven et al., 1993). On the other hand, lycopene, present tomatoes in large amount, the precursor of β-carotene is receiving increasing interest as an antioxidant. EURAMIC study has shown that high concentration of adipose lycopene can correlated with lowered mortality in CVD (Kohlmeier et al., 1997). Epidemiological studies have revealed that supplement- and diet-derived lycopene lowers oxidative stress and inflammation in healthy as well as overweight individuals, which results into reduced CVD occurrence (O’Kennedy et al., 2006).
5.2.4 Polyphenols
Polyphenol is a group of compounds that consists of two or more phenol groups and can be categories into flavonoids, stilbenes, phenolics acids, and lignans (Halliwell, 2007b; Manach et al., 2004). The relative position and number of OH and catechol groups in chemical structures are responsible for their antioxidant property (Quideau et al., 2011). The mechanism of action involves direct interaction with reactive oxygen species, metal ions chelating, upregulation of endogenous antioxidants, etc. (Goszcz et al., 2015). Herein we discussed about the major polyphenols that are investigated in animal studies, in vitro and clinical trials and their role in CVD are mostly understood.
5.2.4.1 Anthocyanins
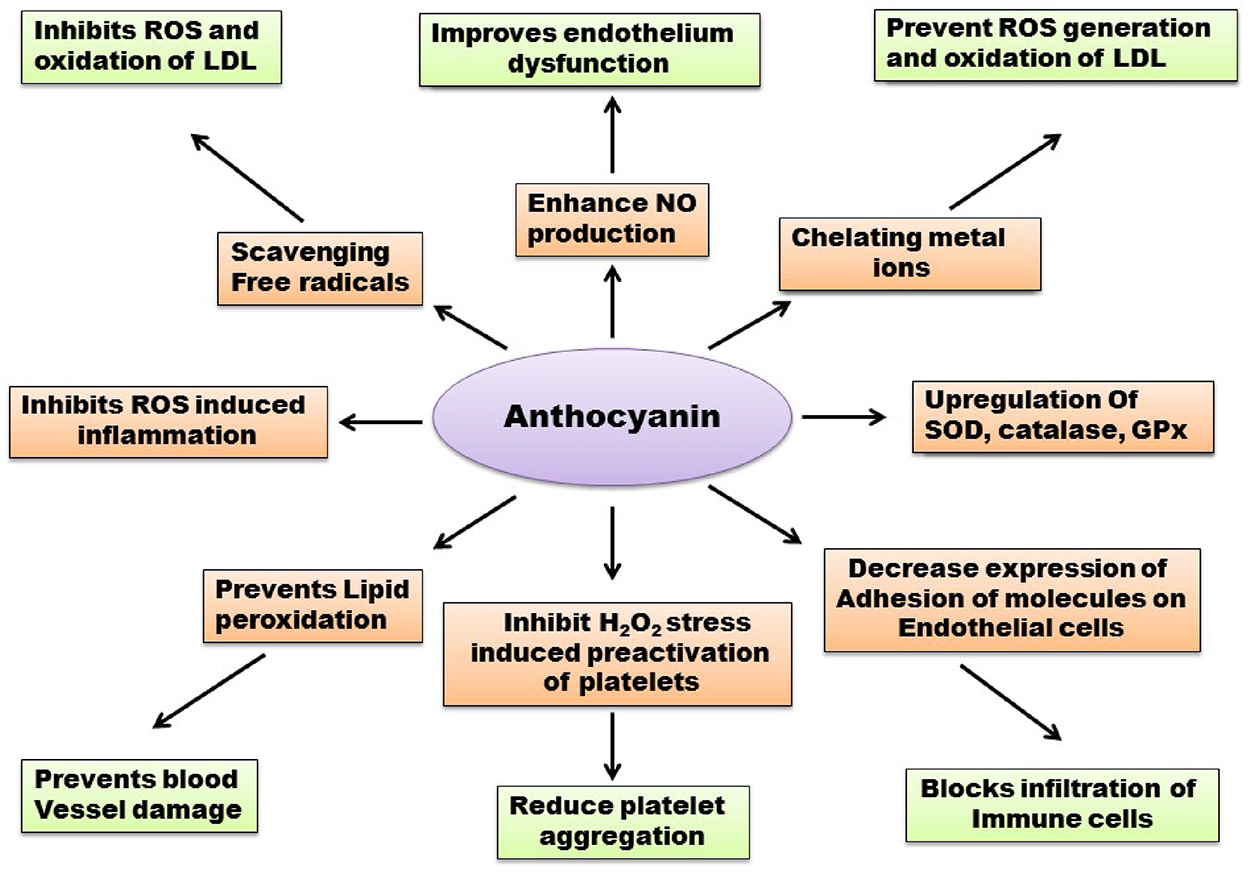
Anthocyanins are group of water-soluble polyphenol flavonoid constituents that occurs abundantly in plant realm forming red–orange to blue–violet pigments of vegetables, fruits, grains, flowers, and other edible parts of plants (Wallace, 2011). Anthocyanin possess various pharmacological properties owing to their chemical structures, most importantly anti-inflammatory and antioxidant activity (Castaneda-Ovando et al., 2009). Collective evidences from several epidemiological studies suggested that regular intake of flavonoid-rich food materials is associated with decrease chance of CVD onset and progression as well as reduced mortality due to cardiac disorders. Regular intake of anthocyanin is estimated to be nine folds higher (approx. 180–215 mg/d) than other dietary flavonoids (approx. 20–25 mg/d) (Hertog et al., 1993). The mechanism of action of anthocyanin includes inhibition of oxidative stress via free radical scavenging, endothelial dysfunction, and inflammation, as well as metal ions chelating. The antioxidant role of anthocyanin regarding CVD has been demonstrated in various in vitro, in vivo (animal models) as well as clinical trials and human epidemiological studies (Fig. 5.2.2).
5.2.4.1.1 In vitro studies
Youdim et al. (2002) isolated four types of anthocyanins from elder berries and incorporated them into plasma, cytoplasm, and lemma of endothelial cells in vitro and examine their role directly. The result of their study indicated that anthocyanins can protect endothelial cells from oxidative stress significantly via direct incorporation into them (Youdim et al., 2002). Anthocyanins and proanthocyanidins, found in red–black rice grains and grape seeds, can scavenge hydroxyl radical and superoxide radicals in vitro (Da Silva et al., 1991; Walter and Marchesan, 2011). Delphinidin, a type of anthocyanin, prevents apoptosis of endothelial cells in vitro in presence of NOS inhibitors, via regulation of NO-signaling pathways and maintaining calcium ion homeostasis (Martin et al., 2003). In another study, it was demonstrated that anthocyanin prevents thrombosis or atherosclerotic plaque formation by inhibiting platelet activation via inhibition of P-selectin expression and thrombin receptor activating peptides (TRAP) and H2O2 stress-induced preactivation of platelets (Rechner and Kroner, 2005). Moreover, anthocyanin from purple grape extract can prevent activation of platelets in both in vitro and in vivo, by reducing ROS generation and enhancement of NO production (Goszcz et al., 2015; Freedman et al., 2001). Viana et al. reported that flavonoids can prevent LDL oxidation by metal chelating mechanism in vitro.
5.2.4.1.2 In vivo studies
There are evidences supporting the fact that anthocyanins can increase antioxidant capacity in vivo due to its higher radical scavenging activity and adaptive improvement in the activity of catalase and SOD (Chiang et al., 2006). In a study conducted by Karthikeyan et al. (2007), significant improvement of SOD, catalase, GSH, and GPx activity was observed in isoproterenol-induced injected rat model (Karthikeyan et al., 2007; Karthikeyan et al., 2009). In this study, it was also observed that pretreatment with proanthocyanidins leads to significant improvement of activities of the heart mitochondrial enzymes involved in electron transport system. Rodriguez et al. (2013) showed that supplementation of blueberries containing anthocyanin improves endothelial function and diminish blood pressure in rats fed with high-fat diet(HFD; Rodriguez-Mateos et al., 2013). In a study with hypercholesterolemic rabbits, Sozanski et al. demonstrated that a diet of cornelian cherry (100 mg/kg) for 60 days decreases triglycerides, LDL levels, and atherogenic index in blood. It was also evaluated that GSH and PPARα expression levels were elevated while GPx and SOD showed no differences (Sozański et al., 2014).
5.2.4.1.3 Clinical trials
In a study, Mazza et al. found that when adult male human subjects were supplemented with anthocyanin (1.2 g), the serum antioxidant capacity is directly proportional to the serum anthocyanins concentration (Mazza et al., 2002). This event suggests that anthocyanins may play a crucial role in reducing superoxide production by NADPH oxidase because diminishing activity NADPH oxidase may cause increase in serum antioxidant potential (Wallace, 2011). In a study conducted by Alvarez et al. (2014), they showed that a diet of fresh fruits (strawberry) containing anthocyanin for 30 days can lower cholesterol, LDL, and triglycerides level and partially prevent the pathogenesis of CVD (Alvarez-Suarez et al., 2014). Qin et al. (2009) studied the role of 17 purified anthocyanins from blackcurrant and blueberry, in ameliorating CVD abnormalities in 120 dyslipidemic subjects and they observed significant decrease in LDL and (CETP) cholesteryl ester protein and increase in HDL concentration in blood (Qin et al., 2009). In another study, 66 patients with obesity and metabolic syndrome were suggested to have blueberries (containing anthocyanin) in their diet for 8 weeks and after evaluation at the end of this period, it was found that serum level of ox-LDL and MDA were significantly reduced with almost normal blood pressure (Basu et al., 2010). This result suggests a close association of blueberries and improvement of CVD.
5.2.4.2 Catechins
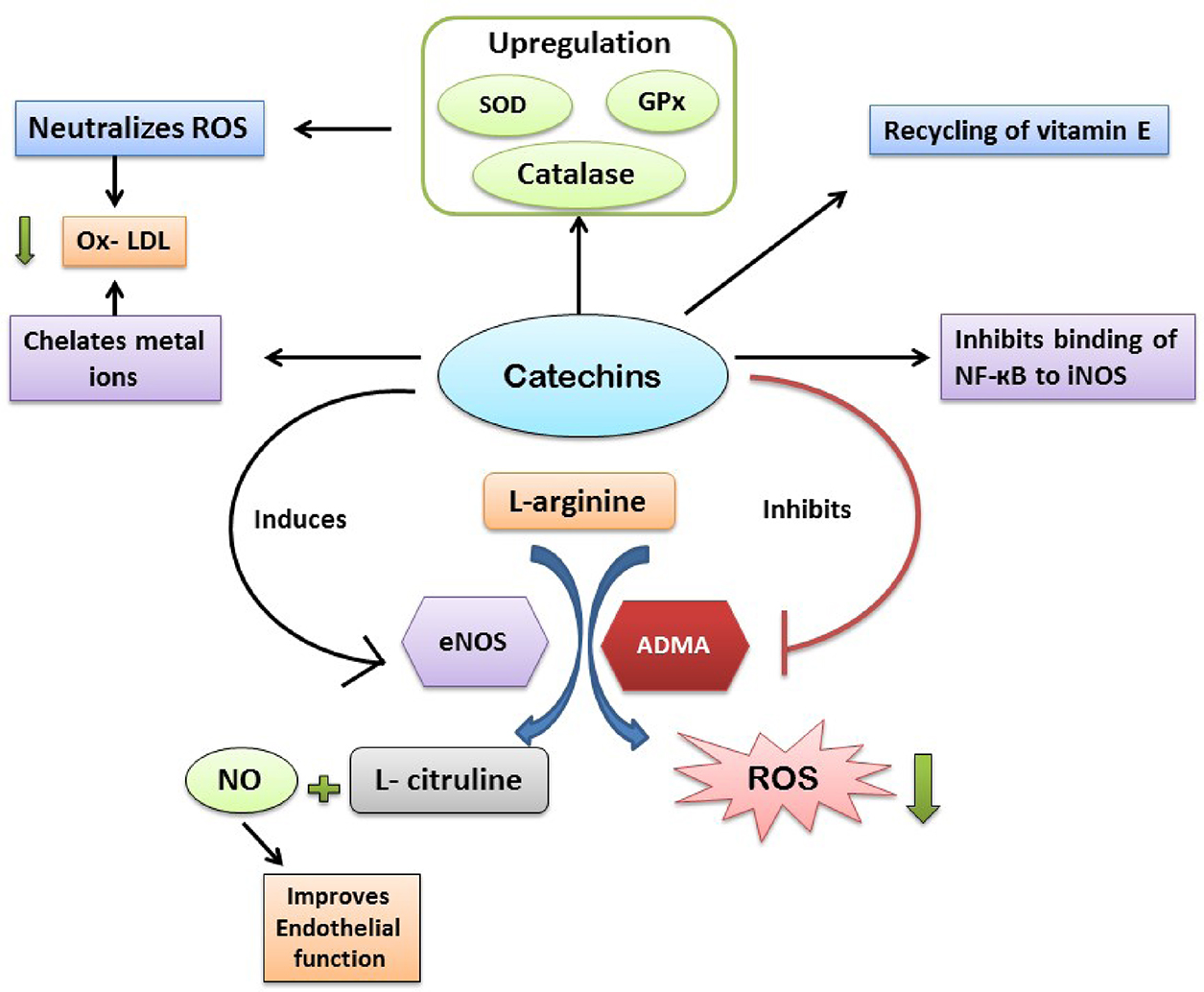
Catechins are the major group of flavanols found in green tea and epidemiological, in vitro and in vivo studies postulated a strong association between cardiovascular conditions and green tea consumption. The most active biologically active catechin in green tea is epigallocatechin-3-gallate (EGCG) and others catechins are gallocatechin and epicatechin (Islam, 2012). Catechins exhibit cardiovascular protective role via various mechanisms such as antihypertensive, antithrombogenic, anti-inflammation, antioxidative, and lipid minimizing effects (Balentine et al., 1997). Catechins exert antioxidant activities by metal ion chelating, free radical scavenging, inactivation of pro-oxidant enzymes activity (iNOS), promoting activity of antioxidant enzymes (SOD, catalase, and GPx) and inactivation of transcription factors (NF-кB) involve in redox reactions (Babu and Liu, 2008). The mechanism behind prevention of oxidative injury by catechin via scavenging reactive oxygen species may include hydrogen bond formation, electron delocalization, molecular rearrangements, and metal ion chelating (Babu and Liu, 2008) (Fig. 5.2.3).
5.2.4.2.1 In vitro studies
There are evidences regarding antioxidant activity of green tea extracts or catechins in in vitro studies, indicating metal ion chelating and superoxide scavenging properties of catechins (Deka and Vita, 2011). In another study, it has been reported that catechins in tea extract can prevent lipid peroxidation (Miura et al., 1994). In an ex vivo study, Pearson et al. (1998) demonstrated that when aortic endothelial cells and human LDL was incubated with green tea extract (5 and 0.08 ppm, respectively), oxidation of LDL was inhibited significantly (Pearson et al., 1998). Chan et al. (1997) conducted a cell culture study and showed that EGCG prevents iNOS expression in LPS-treated macrophages in a dose-dependent manner by inhibiting binding of NF-кB to iNOS and lowering the production of harmful nitric oxide (Chan et al., 1997). In many experimental studies, catechins have been able to improve endothelium dysfunctions but the mechanism behind this role is not fully understood. However in some recent ex vivo studies with aortic tissues of rat and rabbit demonstrated that catechins can induce endothelium-dependent vasorelation via increment in NO bioavailability (Schroeter et al., 2006).
5.2.4.2.2 Animal studies
In a mice model for atherosclerosis, Miura et al. (1994) demonstrated that treatment of EGCG increases apolipoprotein E in circulation and improvement of antioxidant capacity in vascular tissues (Chyu et al., 2004). Several investigations with animal model confirmed that catechin can enhance antioxidant enzymes activity. For instance, when 0.2% catechins are given to mice via drinking water results in significant upregulation of SOD, catalase, and GPx and promote ROS scavenging (Khan et al., 1992). In another study, 2 weeks of green tea consumption by hypertensive rats, catechins enhanced catalase expression in aorta (Babu et al., 2006). Moreover catechins also take part in recycling of vitamin E and supplement the action of glutathione (Zhu et al., 1999).
5.2.4.2.3 Clinical studies
In a recent study, Wang et al. (2011) reported that consumption of one or more cup of green tea per day may prevent the risk of coronary artery disorders development (Wang et al., 2011). In a clinical trial, it has been demonstrated that green tea consumption reversed endothelial dysfunction and significantly reduces 8-iso-prostaglandin-F2α which is an oxidative stress index (Bhardwaj and Khanna, 2013). Consumption of 600 mL green tea (with 5.2 g solid tea) regularly for 4 weeks results in significant reduction of LDL oxidation (Sung et al., 2005). It was reported that regular consumption of 120 ml per day of green tea prevented hypertension development in Chinese population (Deka and Vita, 2011). Long-term ingestion of high-dose of catechins significantly increases bioavailability NO and decreases malondialdehyde (MDA) and chemotactic protein-1 (MCP-1; Oyama et al., 2010). Daily consumption of catechins or green tea improves endothelial function in patients affected with coronary heart disorder (Islam, 2012). In human studies with EDCG (10 μM) reduces oxidation by 68% in dose-dependent manner (Liao et al., 2001).
5.2.4.3 Resveratrol
Resveratrol (3,4’,5-trihydroxystilbene) is a natural compound which is produced by some spermatophytes, like grapevines. It has been observed that resveratrol is the main active compound of phytoalexins and it might have a beneficial role upon human health. Resveratrol is also used in traditional oriental medicine. As skin of grapeberry contains resveratrol, not flesh, red wine has larger amount of resveratrol than that of white wine. Resveratrol shows antioxidant properties. It has been noticed that resveratrol can modulate lipid metabolism, inhibit LDL oxidation, and platelets aggregation. Also, resveratrol, being a phytoestrogen, might play a role in cardiovascular disease prevention. Besides being an antioxidant, resveratrol also contains anticancer and anti-inflammatory properties (Fig. 5.2.4).
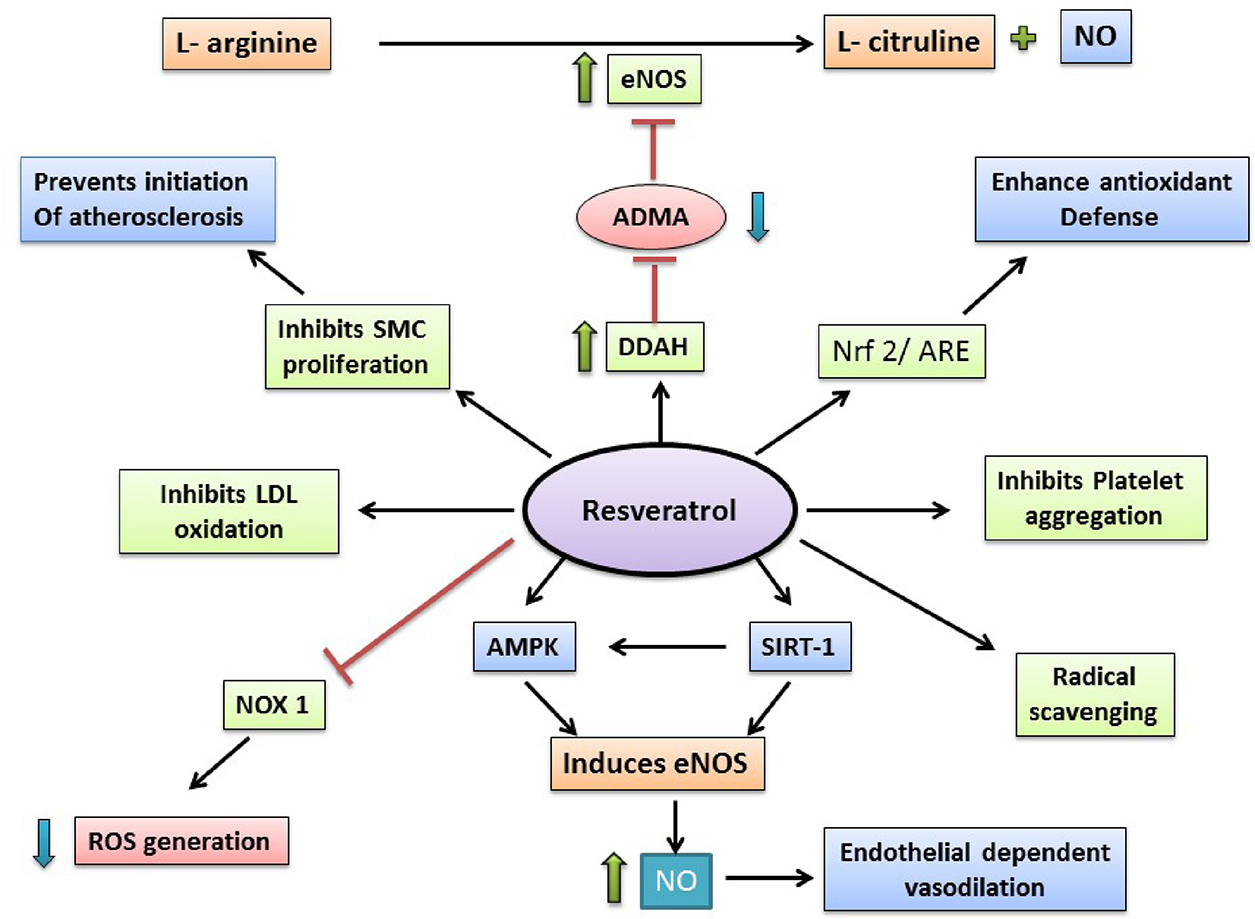
5.2.4.3.1 Antiatherosclerotic effects of RES
The intimal layer of arterial vessel wall gets affected in atherosclerosis. It can be characterized by extracellular lipid deposition, local smooth muscle cell proliferation and migration, and a chronic type inflammation. It results into liminal narrowing, might accompanied with formation of thrombus. All of these eventually lead to clinical proceedings like peripheral arterial disease, coronary artery disease, or stroke (Glass and Witztum, 2001). As lipids, LDLs mainly, are involved in atherosclerosis; improvement of lipid profile might be considered to be beneficial. It is shown in some studies that resveratrol can modulate this lipid profile by reducing LDL-cholesterol level and plasma triglyceride, and elevating the level of HDL-cholesterol (Gocmen et al., 2011). Cho et al. have reported that RES can increase the hypocholesterolemia action of pravastatin via downregulation of 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoA reductase), which is an enzyme takes part in the first step of cholesterol biosynthesis (Cho et al., 2008). Also, RES elevates the expression of LDL-R (LDL receptors) in hepatocytes, which further decreases the LDL-cholesterol level in blood (Yashiro et al., 2012). RES also lowers the oxidation of LDL, which contributes to the antioxidant properties of RES. Migration of smooth muscle cell is inhibited by RES, this is the antiatherogenic property of RES (Lin et al., 2014). RES activates eNOS, antioxidant response element (ARE), SIRT-1 (a class III histone deacetylase), Nrf2 and on the other hand decreases production of TNFα. All these above-mentioned actions of RES lead to activation of endothelium, decrease in endothelial apoptosis, vascular inflammation, which in a way improves the function of endothelium (Haskó et al., 2010). It has been observed that RES can downregulate the expression of different adhesion molecules (ICAM-1, VCAM-1) by inhibiting the activation of NF-κB pathway (Deng et al., 2011). RES can also reduce foam cell formation via inhibition of NADPH oxidase 1 and monocyte chemotactic protein-1 (MCP-1) production (Park et al., 2009). RES exerts its anti-inflammatory action by activating Nrf2 and suppressing the production of proinflammatory cytokines (Hao et al., 2013).
5.2.4.3.2 Antihypertensive effects of RES
Hypertension is one of the main risk factors of CVDs. Several studies have shown that RES has an antihypertensive property. Endothelium-dependent pathways with the involvement of AMPK (an energy metabolism regulator), Nrf2, and SIRT-1 are some of the involved mechanisms by which RES exerts its antihypertensive properties (Zordoky et al., 2015). RES elevates the expression as well as activity of eNOS, which improves the availability of NO, resulting into vasodilation (Leikert et al., 2002). This mechanism is linked with the antioxidant role of RES. RES induces the activation of SIRT-1 which increases expression as well as activity of eNOS (Arunachalam et al., 2010). RES can also induce activation of AMPK, results into increased NO production (Dolinsky et al., 2013). In several studies, it has been also reported that RES perform its antihypertensive role via endothelium-independent mechanism. RES induces activation of AMPK, which inhibits angiotensin II (AngII)-induced phosphorylation of myosin light chain and myosin phosphatase-targeting subunit 1. This results into inhibition of contractility of smooth muscle cell. Also, It has been observed that RES can inhibit contraction of aorta, induced by AngII (Cao et al., 2014).
5.2.4.3.3 In vitro and in vivo study of RES
Many studies have revealed that RES can cure contractile dysfunction and cardiac hypertrophy, one is functional and another one is structural abnormalities linked with hypertension (Chan et al., 2011). Fukuda et al. have shown that RES pretreatment has elevated the expression of inducible and endothelial NOS (iNOS and eNOS, respectively) in rats with ischemic myocardium. Many evidences have shown that the involved mechanism behind the beneficial role of RES against heart failure and cardiac hypertrophy is decrease in oxidative stress. In in vitro study, Tanno et al. have observed that increased level of Mn-superoxide dismutase (SOD2), a mitochondrial antioxidant enzyme, is the reason behind decreased oxidative stress (Tanno et al., 2010). In several in vivo studies, RES has shown significant antihypertensive effect upon different animals in 10–320 mg RES/kg body weight per day (Zordoky et al., 2015).
5.2.4.3.4 Clinical study of RES
High CV risk patients, who are treated with statin for primary prevention, have shown decrease in LDL-cholesterol by 4.5% and decrease in oxidized LDL by 20% with additional RES treatment (350 mg/day RES extract containing 8 mg RES; Timmers et al., 2011). A clinical study was performed on six random group in controlled environment (247 patients), and it showed that higher dose of RES (more than 150 mg/day) is able to lower the blood pressure significantly but lower dose than that has failed to do so (Liu et al., 2015). In order to find out the role of RES regarding secondary prevention, another clinical trial was performed upon patients who already had myocardial infarction. This study was a randomized, placebo-controlled, double-blind 3-month trial, which included 40 Caucasian postinfarction patients (14 women and 26 men). They were treated with either 10 mg/day RES with other additional medication or with placebo. It has been noticed that RES has helped to improve systolic as well as endothelial function, decrease LDL-cholesterol, and lowers aggregation of platelets. From this result the author has derived the conclusion that RES treatment, combined with other standard medication, has a significant beneficial role in lowering the risk of secondary MI in post-MI patients (Magyar et al., 2012).
Conclusion
Oxidative stress plays a critical role in onset and progression antheroclerotic plaque and various other cardiovascular diseases. This leads to the assumption that antioxidant would be an efficient therapeutic agent in this regard. Multiple evidences from in vitro, animal studies, and clinical trials have demonstrated various mechanisms by which antioxidants are capable of regulating oxidative stress and diminish its effects in cardiovascular systems. Among naturally occurring antioxidants, those that belongs to dietary sources have proven to significantly improve the ROS-induced cardiovascular diseases. Vitamins E, C, A, β-carotene, and polyphenols like resveratrol, catechins, anthocyanin, and quercetin are the major dietary antioxidants that have been demonstrated to ameliorate CVD by neutralizing ROS, minimizing lipid peroxidation, upregulation of endogenous antioxidants, reducing expression of adhesion molecules, preventing platelet aggregation, and diminishing oxidation of LDL, proteins, and DNA. In spite of these multiple role of dietary antioxidants in relation to cardiovascular diseases, there are some evidences where they have failed to give significant results. However, if we consider only the positive evidences, dietary antioxidants can be an efficient therapeutic approach for the prevention of cardiovascular diseases. In future more clinical studies should be carried out with these antioxidants in patients with different disorders related to cardiovascular diseases. Investigations must carry on discovering other dietary sources of antioxidants with relevant mechanism of action related to the prevention of ROS-induced atherosclerosis, endothelial dysfunctions, heart failure, and other deleterious CVD.
Abbreviations
ROS reactive oxygen species
CVD cardiovascular diseases
LDL low-density lipoprotein
HDL high-density lipoprotein
eNOS endothelium nitric oxide synthase
MPO myeloperoxidases
FGF fibroblast growth factor
PDGF platelet-derived growth factor
SOD superoxide dismutase
GPx glutathione peroxidase
CRP circulating C reactive protein
EPIC European Prospective Investigation into Cancer and Nutrition
HOPE Heart Outcomes Prevention Evaluation study
EURAMIC European Community Multicenter Study on Antioxidants Myocardial infarction and Breast Cancer
TRAP thrombin receptor activating peptides
CETP cholesteryl ester protein
EGCG epigallocatechin-3-gallate
MDA malondialdehyde
MCP-1 chemotactic protein-1
References
Alvarez-Suarez, J.M., Giampieri, F., Tulipani, S., Casoli, T., Di Stefano, G., González-Paramás, A.M., Santos-Buelga, C., Busco, F., Quiles, J.L., Cordero, M.D., 2014. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 25, 289–294.
Anderson, T.J., Uehata, A., Gerhard, M.D., Meredith, I.T., Knab, S., Delagrange, D., Lieberman, E.H., Ganz, P., Creager, M.A., Yeung, A.C., 1995. Close relation of endothelial function in the human coronary and peripheral circulations. J. Am. Coll. Cardiol. 26, 1235–1241.
Arunachalam, G., Yao, H., Sundar, I.K., Caito, S., Rahman, I., 2010. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: role of resveratrol. Biochem. Biophys. Res. Commun. 393, 66–72.
Azzi, A., Aratri, E., Boscoboinik, D., Clément, S., Özer, N.K., Ricciarelli, R., Spycher, S., 1998. Molecular basis of alpha-tocopherol control of smooth muscle cell proliferation. Biofactors 7, 3–14.
Babu, P.V., Liu, D., 2008. Green tea catechins and cardiovascular health: an update. Curr. Med. Chem. 15, 1840–1850.
Babu, P.V., Sabitha, K.E., Shyamaladevi, C.S., 2006. Therapeutic effect of green tea extract on oxidative stress in aorta and heart of streptozotocin diabetic rats. Chem. Biol. Interact. 162, 114–120.
Balentine, D.A., Wiseman, S.A., Bouwens, L.C., 1997. The chemistry of tea flavonoids. Crit. Rev. Food Sci. Nutr. 37, 693–704.
Baljinnyam, E., Hasebe, N., Morihira, M., Sumitomo, K., Matsusaka, T., Fujino, T., Fukuzawa, J., Ushikubi, F., Kikuchi, K., 2006. Oral pretreatment with ebselen enhances heat shock protein 72 expression and reduces myocardial infarct size. Hypertens. Res. 29, 905–913.
Basu, A., Du, M., Leyva, M.J., Sanchez, K., Betts, N.M., Wu, M., Aston, C.E., Lyons, T.J., 2010. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 140, 1582–1587.
Benstoem, C., Goetzenich, A., Kraemer, S., Borosch, S., Manzanares, W., Hardy, G., Stoppe, C., 2015. Selenium and its supplementation in cardiovascular disease–what do we know? Nutrients 7, 3094–3118.
Bhardwaj, P., Khanna, D., 2013. Green tea catechins: defensive role in cardiovascular disorders. Chin. J. Nat. Med. 11, 345–353.
Buijsse, B., Feskens, E., Schlettwien-Gsell, D., 2005. for the SENECA investigators: plasma carotene and α-tocopherol in relation to 10-y all-cause and cause-specific mortality in European elderly: the Survey in Europe on Nutrition and the Elderly, a Concerted Action (SENECA). Am. J. Clin. Nutr. 82, 879–886.
Cai, H., Harrison, D.G., 2000. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 87, 840–844.
Cao, X., Luo, T., Luo, X., Tang, Z., 2014. Resveratrol prevents AngII-induced hypertension via AMPK activation and RhoA/ROCK suppression in mice. Hypertens. Res. 37, 803–810.
Castaneda-Ovando, A., de Pacheco-Hernández, L.M., Páez-Hernández, M.E., Rodríguez, J.A., Galán-Vidal, C.A., 2009. Chemical studies of anthocyanins: a review. Food Chem. 113, 859–871.
Cavia-Saiz, M., Busto, M.D., Pilar-Izquierdo, M.C., Ortega, N., Perez-Mateos, M., Muniz, P., 2010. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J. Sci. Food Agric. 90, 1238–1244.
Cervantes Gracia, K., Llanas-Cornejo, D., Husi, H., 2017. CVD and oxidative stress. J. Clin. Med. 6, 22.
Chan, M.M., Fong, D., Ho, C.T., Huang, H.I., 1997. Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea. Biochem. Pharmacol. 54, 1281–1286.
Chan, V., Fenning, A., Iyer, A., Hoey, A., Brown, L., 2011. Resveratrol improves cardiovascular function in DOCA-salt hypertensive rats. Curr. Pharm. Biotechnol. 12, 429–436.
Chiang, A.N., Wu, H.L., Yeh, H.I., Chu, C.S., Lin, H.C., Lee, W.C., 2006. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids 41, 797–803.
Cho, I.J., Ahn, J.Y., Kim, S., Choi, M.S., Ha, T.Y., 2008. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem. Biophys. Res. Commun. 367, 190–194.
Chyu, K.Y., Babbidge, S.M., Zhao, X., Dandillaya, R., Rietveld, A.G., Yano, J., Dimayuga, P., Cercek, B., Shah, P.K., 2004. Differential effects of green tea-derived catechin on developing versus established atherosclerosis in apolipoprotein E-null mice. Circulation 109, 2448–2453.
Cominacini, L., Garbin, U., Pasini, A.F., Davoli, A., Campagnola, M., Contessi, G.B., Pastorino, A.M., Cascio, V.L., 1997. Antioxidants inhibit the expression of intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 induced by oxidized LDL on human umbilical vein endothelial cells. Free Radic. Biol. Med. 22, 117–127.
Dahlöf, B., 2010. Cardiovascular disease risk factors: epidemiology and risk assessment. Am. J. Cardiol. 105, 3A-9A.
Da Silva, J.M.R., Darmon, N., Fernandez, Y., Mitjavila, S., 1991. Oxygen free radical scavenger capacity in aqueous models of different procyanidins from grape seeds. J. Agric. Food Chem. 39, 1549–1552.
Deka, A., Vita, J.A., 2011. Tea and cardiovascular disease. Pharmacol. Res. 64, 136–145.
Deng, Y.H., Alex, D., Huang, H.Q., Wang, N., Yu, N., Wang, Y.T., Leung, G.P., Lee, S.M., 2011. Inhibition of TNF-alpha-mediated endothelial cell-monocyte cell adhesion and adhesion molecules expression by the resveratrol derivative, trans-3,5,4′-trimethoxystilbene. Phytother. Res. 25, 451–457.
Devaraj, S., Li, D., Jialal, I., 1996. The effects of alpha tocopherol supplementation on monocyte function. Decreased lipid oxidation, interleukin 1 beta secretion, and monocyte adhesion to endothelium. J. Clin. Invest. 98, 756–763.
Dhalla, N.S., Golfman, L., Takeda, S., Takeda, N., Nagano, M., 1999. Evidence for the role of oxidative stress in acute ischemic heart disease: a brief review. Can. J. Cardiol. 15, 587–593.
Dhalla, N.S., Temsah, R.M., Netticadan, T., 2000. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 18, 655–673.
Diaz, M.N., Frei, B., Vita, J.A., Keaney Jr., J.F., 1997. Antioxidants and atherosclerotic heart disease. N. Engl. J. Med. 337, 408–416.
D'Odorico, A., Martines, D., Kiechl, S., Egger, G., Oberhollenzer, F., Bonvicini, P., Sturniolo, G.C., Naccarato, R., Willeit, J., 2000. High plasma levels of α-and β-carotene are associated with a lower risk of atherosclerosis: results from the Bruneck study. Atherosclerosis 153, 231–239.
Dolinsky, V.W., Chakrabarti, S., Pereira, T.J., Oka, T., Levasseur, J., Beker, D., Zordoky, B.N., Morton, J.S., Nagendran, J., Lopaschuk, G.D., Davidge, S.T., Dyck, J.R., 2013. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta 1832, 1723–1733.
Duarte, J., Pérez‐Palencia, R., Vargas, F., Angeles Ocete, M., Pérez‐Vizcaino, F., Zarzuelo, A., Tamargo, J., 2001. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. British journal of pharmacology, 133(1), 117–124.
Dugas, T.R., Morel, D.W., Harrison, E.H., 1998. Impact of LDL carotenoid and α-tocopherol content on LDL oxidation by endothelial cells in culture. J. Lipid Res. 39, 999–1007.
Egert, S., Bosy-Westphal, A., Seiberl, J., Kurbitz, C., Settler, U., Plachta-Danielzik, S., Wagner, A.E., Frank, J., Schrezenmeir, J., Rimbach, G., Wolffram, S., Muller, M.J., 2009. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 102, 1065–1074.
Finking, G., Hanke, H., 1997. Nikolaj Nikolajewitsch Anitschkow (1885–1964) established the cholesterol-fed rabbit as a model for atherosclerosis research. Atherosclerosis 135, 1–7.
Freedman, J.E., Parker Iii, C., Li, L., Perlman, J.A., Frei, B., Ivanov, V., Deak, L.R., Iafrati, M.D., Folts, J.D., 2001. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation 103, 2792–2798.
Frei, B., 1991. Ascorbic acid protects lipids in human plasma and low-density lipoprotein against oxidative damage. Am. J. Clin. Nutr. 54, 1113S-1118S.
Glass, C.K., Witztum, J.L., 2001. Atherosclerosis. The road ahead. Cell 104, 503–516.
Gocmen, A.Y., Burgucu, D., Gumuslu, S., 2011. Effect of resveratrol on platelet activation in hypercholesterolemic rats: CD40-CD40L system as a potential target. Appl. Physiol. Nutr. Metab. 36, 323–330.
Gokce, N., Keaney Jr., J.F., Frei, B., Holbrook, M., Olesiak, M., Zachariah, B.J., Leeuwenburgh, C., Heinecke, J.W., Vita, J.A., 1999. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 99, 3234–3240.
Gorinstein, S., Caspi, A., Libman, I., Lerner, H.T., Huang, D., Leontowicz, H., Leontowicz, M., Tashma, Z., Katrich, E., Feng, S., Trakhtenberg, S., 2006. Red grapefruit positively influences serum triglyceride level in patients suffering from coronary atherosclerosis: studies in vitro and in humans. J. Agric. Food Chem. 54, 1887–1892.
Goszcz, K., Deakin, S.J., Duthie, G.G., Stewart, D., Leslie, S.J., Megson, I.L., 2015. Antioxidants in cardiovascular therapy: panacea or false hope? Front. Cardiovasc. Med. 2, 29.
Goya, K., Sumitani, S., Otsuki, M., Xu, X., Yamamoto, H., Kurebayashi, S., Saito, H., Kouhara, H., Kasayama, S., 2006. The thiazolidinedione drug troglitazone up-regulates nitric oxide synthase expression in vascular endothelial cells. J. Diab. Complic. 20, 336–342.
Haendeler, J., Zeiher, A.M., Dimmeler, S., 1996. Vitamin C and E prevent lipopolysaccharide-induced apoptosis in human endothelial cells by modulation of Bcl-2 and Bax. Eur. J. Pharmacol. 317, 407–411.
Haenen, G.R., Paquay, J.B., Korthouwer, R.E., Bast, A., 1997. Peroxynitrite scavenging by flavonoids. Biochem. Biophys. Res. Commun. 236, 591–593.
Halliwell, B., 1988. Albumin—an important extracellular antioxidant? Biochem. Pharmacol. 37, 569–571.
Halliwell, B., 2007a. Biochemistry of Oxidative Stress. Portland Press Limited.
Halliwell, B., 2007b. Dietary polyphenols: good, bad, or indifferent for your health? Cardiovasc. Res. 73, 341–347.
Han, J.J., Hao, J., Kim, C.H., Hong, J.S., Ahn, H.Y., Lee, Y.S., 2009. Quercetin prevents cardiac hypertrophy induced by pressure overload in rats. J. Vet. Med. Sci. 71, 737–743.
Hao, E., Lang, F., Chen, Y., Zhang, H., Cong, X., Shen, X., Su, G., 2013. Resveratrol alleviates endotoxin-induced myocardial toxicity via the Nrf2 transcription factor. PLoS One 8, e69452.
Haskó, G., Pacher, P., 2010. Endothelial Nrf2 activation: a new target for resveratrol? Am. J. Physiol. Heart Circ. Physiol. 299 (1), H10–H12.
Heller, R., Münscher-Paulig, F., Gräbner, R., Till, U., 1999. L-ascorbic acid potentiates nitric oxide synthesis in endothelial cells. J. Biol. Chem. 274, 8254–8260.
Hertog, M.G., Hollman, P.C., Katan, M.B., Kromhout, D., 1993. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands.
Higashi, Y., Noma, K., Yoshizumi, M., Kihara, Y., 2009. Endothelial function and oxidative stress in cardiovascular diseases. Circ. J. 73, 411–418.
Hozawa, A., Jacobs, D.R., Steffes, M.W., Gross, M.D., Steffen, L.M., Lee,D.-H., 2007. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) study. Clin. Chem. 53, 447–455.
Islam, M.A., 2012. Cardiovascular effects of green tea catechins: progress and promise. Recent Pat. Cardiovasc. Drug Discov. 7, 88–99.
Jeon, S.M., Bok, S.H., Jang, M.K., Kim, Y.H., Nam, K.T., Jeong, T.S., Park, Y.B., Choi, M.S., 2002. Comparison of antioxidant effects of naringin and probucol in cholesterol-fed rabbits. Clin. Chim. Acta 317, 181–190.
Jessup, W., Rankin, S., De Whalley, C., Hoult, J., Scott, J., Leake, D., 1990. α-Tocopherol consumption during low-density-lipoprotein oxidation. Biochem. J. 265, 399–405.
Jialal, I., Grundy, S.M., 1991. Preservation of the endogenous antioxidants in low density lipoprotein by ascorbate but not probucol during oxidative modification. J. Clin. Invest. 87, 597–601.
Karppi, J., Laukkanen, J.A., Mäkikallio, T.H., Kurl, S., 2011. Low serum lycopene and β-carotene increase risk of acute myocardial infarction in men. Eur. J. Public Health 22, 835–840.
Karthikeyan, K., Bai, B.S., Devaraj, S.N., 2007. Cardioprotective effect of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats. Int. J. Cardiol. 115, 326–333.
Karthikeyan, K., Bai, B.S., Devaraj, S.N., 2009. Efficacy of grape seed proanthocyanidins on cardioprotection during isoproterenol-induced myocardial injury in rats. J. Cardiovasc. Pharmacol. 53, 109–115.
Kassebaum, N., Smith, A., Bernabé, E., Fleming, T., Reynolds, A., Vos, T., Murray, C., Marcenes, W., Collaborators, G.O.H., 2017. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. J. Dent. Res. 96, 380–387.
Katagiri, H., Yamada, T., Oka, Y., 2007. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals. Circ. Res. 101, 27–39.
Keaney, J.F., Gaziano, J.M., Xu, A., Frei, B., Curran-Celentano, J., Shwaery, G.T., LoScalzo, J., Vita, J.A., 1993. Dietary antioxidants preserve endothelium-dependent vessel relaxation in cholesterol-fed rabbits. Proc. Natl. Acad. Sci. 90, 11880–11884.
Keaney, J.F., Guo, Y., Cunningham, D., Shwaery, G.T., Xu, A., Vita, J.A., 1996. Vascular incorporation of alpha-tocopherol prevents endothelial dysfunction due to oxidized LDL by inhibiting protein kinase C stimulation. J. Clin. Invest. 98, 386–394.
Keaney Jr., J.F., Simon, D.I., Freedman, J.E., 1999. Vitamin E and vascular homeostasis: implications for atherosclerosis. FASEB J. 13, 965–975.
Kelly, F.J., Fussell, J.C., 2017. Role of oxidative stress in cardiovascular disease outcomes following exposure to ambient air pollution. Free Radic. Biol. Med. 110, 345–367.
Khan, S.G., Katiyar, S.K., Agarwal, R., Mukhtar, H., 1992. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: possible role in cancer chemoprevention. Cancer Res. 52, 4050–4052.
Koga, T., Kwan, P., Zubik, L., Ameho, C., Smith, D., Meydani, M., 2004. Vitamin E supplementation suppresses macrophage accumulation and endothelial cell expression of adhesion molecules in the aorta of hypercholesterolemic rabbits. Atherosclerosis 176, 265–272.
Kohlmeier, L., Kark, J.D., Gomez-Gracia, E., Martin, B.C., Steck, S.E., Kardinaal, A.F., Ringstad, J., Thamm, M., Masaev, V., Riemersma, R., 1997. Lycopene and myocardial infarction risk in the EURAMIC Study. Am. J. Epidemiol. 146, 618–626.
Kuzuya, M., Naito, M., Funaki, C., Hayashi, T., Yamada, K., Asai, K., Kuzuya, F., 1991. Antioxidants stimulate endothelial cell proliferation in culture. Artery 18, 115–124.
Lakshmi, S.V., Padmaja, G., Kuppusamy, P., Kutala, V.K., 2009. Oxidative stress in cardiovascular disease.
Lefer, D.J., Granger, D.N., 2000. Oxidative stress and cardiac disease. Am. J. Med. 109, 315–323.
Lehr, H.-A., Frei, B., Arfors,K.-E., 1994. Vitamin C prevents cigarette smoke-induced leukocyte aggregation and adhesion to endothelium in vivo. Proc. Natl. Acad. Sci. 91, 7688–7692.
Leikert, J.F., Rathel, T.R., Wohlfart, P., Cheynier, V., Vollmar, A.M., Dirsch, V.M., 2002. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation 106, 1614–1617.
Levine, G.N., Frei, B., Koulouris, S.N., Gerhard, M.D., Keaney Jr., J.F., Vita, J.A., 1996. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 93, 1107–1113.
Li, D., Saldeen, T., Mehta, J.L., 2000. Effects of α-tocopherol on ox-LDL-mediated degradation of IκB and apoptosis in cultured human coronary artery endothelial cells. J. Cardiovasc. Pharmacol. 36, 297–301.
Liao, S., Kao, Y.H., Hiipakka, R.A., 2001. Green tea: biochemical and biological basis for health benefits. Vitam. Horm. 62, 1–94.
Lin, Y.C., Chen, L.H., Varadharajan, T., Tsai, M.J., Chia, Y.C., Yuan, T.C., Sung, P.J., Weng, C.F., 2014. Resveratrol inhibits glucose-induced migration of vascular smooth muscle cells mediated by focal adhesion kinase. Mol. Nutr. Food Res. 58, 1389–1401.
Liu, S., Lee, I.-M., Ajani, U., Cole, S.R., Buring, J.E., Manson, J.E., 2001. Intake of vegetables rich in carotenoids and risk of coronary heart disease in men: the Physicians' Health Study. Int. J. Epidemiol. 30, 130–135.
Liu, Y., Ma, W., Zhang, P., He, S., Huang, D., 2015. Effect of resveratrol on blood pressure: a meta-analysis of randomized controlled trials. Clin. Nutr. 34, 27–34.
Maeda, N., Hagihara, H., Nakata, Y., Hiller, S., Wilder, J., Reddick, R., 2000. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc. Natl. Acad. Sci. 97, 841–846.
Magyar, K., Halmosi, R., Palfi, A., Feher, G., Czopf, L., Fulop, A., Battyany, I., Sumegi, B., Toth, K., Szabados, E., 2012. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clinical hemorheology and microcirculation, 50(3), 179–187.
Manach, C., Scalbert, A., Morand, C., Rémésy, C., Jiménez, L., 2004. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 79, 727–747.
Martin, S., Giannone, G., Andriantsitohaina, R., Carmen Martinez, M., 2003. Delphinidin, an active compound of red wine, inhibits endothelial cell apoptosis via nitric oxide pathway and regulation of calcium homeostasis. Br. J. Pharmacol. 139, 1095–1102.
Mazza, G., Kay, C.D., Cottrell, T., Holub, B.J., 2002. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 50, 7731–7737.
Mendis, S., Puska, P., Norrving, B., Organization, W.H., 2011. Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: World Health Organization.
Miura, S., Watanabe, J., Tomita, T., Sano, M., Tomita, I., 1994. The inhibitory effects of tea polyphenols (flavan-3-ol derivatives) on Cu2+ mediated oxidative modification of low density lipoprotein. Biol. Pharm. Bull. 17, 1567–1572.
Munteanu, A., Taddei, M., Tamburini, I., Bergamini, E., Azzi, A., Zingg,J.-M., 2006. Antagonistic effects of oxidized low density lipoprotein and α-tocopherol on CD36 scavenger receptor expression in monocytes involvement of protein kinase B and peroxisome proliferator-activated receptor-γ. J. Biol. Chem. 281, 6489–6497.
Mazza, G., Kay, C.D., Cottrell, T., Holub, B.J., 2002. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 50, 7731–7737.
Murohara, T., Ikeda, H., Katoh, A., Takajo, Y., Otsuka, Y., Haramaki, N., Imaizumi, T., 2002. Vitamin E inhibits lysophosphatidylcholine-induced endothelial dysfunction and platelet activation. Antioxidants Redox Signal. 4, 791–798.
Myasnikova, I., 1947. Effect of ascorbic acid, nikotinic acid, and thiamine on cholesterolemia. Voenno-Morskoi Med. Akad. Leningrado 8, 140.
Nakata, Y., Maeda, N., 2002. Vulnerable atherosclerotic plaque morphology in apolipoprotein E-deficient mice unable to make ascorbic acid. Circulation 105, 1485–1490.
Niki, E., Traber, M.G., 2012. A history of vitamin E. Ann. Nutr. Metab. 61, 207–212.
Nojiri, S., Daida, H., Inaba, Y., 2004. Antioxidants and cardiovascular disease: still a topic of interest. Environ. Health Prevent. Med. 9, 200–213.
Norum, K.R., Blomhoff, R., 1992. McCollum Award Lecture, 1992: Vitamin A Absorption, Transport, Cellular Uptake, and Storage. Oxford University Press.
O'Kennedy, N., Crosbie, L., Whelan, S., Luther, V., Horgan, G., Broom, J.I., Webb, D.J., Duttaroy, A.K., 2006. Effects of tomato extract on platelet function: a double-blinded crossover study in healthy humans. Am. J. Clin. Nutr. 84, 561–569.
Omenn, G., Goodman, G., Thornquist, M., Barnhart, S., Balmes, J., Cherniack, M., Cullen, M., Glass, A., Keogh, J., Liu, D., 1996. Chemoprevention of lung cancer: the beta-Carotene and Retinol Efficacy Trial (CARET) in high-risk smokers and asbestos-exposed workers. IARC Sci. Publ., 67.
Otero, P., Bonet, B., Herrera, E., Rabano, A., 2005. Development of atherosclerosis in the diabetic BALB/c mice: prevention with vitamin E administration. Atherosclerosis 182, 259–265.
Oyama, J., Maeda, T., Kouzuma, K., Ochiai, R., Tokimitsu, I., Higuchi, Y., Sugano, M., Makino, N., 2010. Green tea catechins improve human forearm endothelial dysfunction and have antiatherosclerotic effects in smokers. Circ. J. 74, 578–588.
Padayatty, S.J., Katz, A., Wang, Y., Eck, P., Kwon, O., Lee, J.-H., Chen, S., Corpe, C., Dutta, A., Dutta, S.K., 2003. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J. Am. Coll. Nutr. 22, 18–35.
Park, D.W., Baek, K., Kim, J.R., Lee, J.J., Ryu, S.H., Chin, B.R., Baek, S.H., 2009. Resveratrol inhibits foam cell formation via NADPH oxidase 1- mediated reactive oxygen species and monocyte chemotactic protein-1. Exp. Mol. Med. 41, 171–179.
Patrick, L., Uzick, M., 2001. Cardiovascular disease: C-reactive protein and the inflammatory disease paradigm: HMG-CoA reductase inhibitors, alpha-tocopherol, red yeast rice, and olive oil polyphenols. A review of the literature. Altern. Med. Rev. 6, 248.
Pearson, D.A., Frankel, E.N., Aeschbach, R., German, J.B., 1998. Inhibition of endothelial cell mediated low-density lipoprotein oxidation by green tea extracts. Journal of Agricultural and Food Chemistry, 46(4), 1445–1449.
Pirillo, A., Norata, G.D., Catapano, A.L., 2013. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013.
Podrez, E.A., Febbraio, M., Sheibani, N., Schmitt, D., Silverstein, R.L., Hajjar, D.P., Cohen, P.A., Frazier, W.A., Hoff, H.F., Hazen, S.L., 2000. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J. Clin. Invest. 105, 1095–1108.
Prabhakaran, D., Jeemon, P., Roy, A., 2016. Cardiovascular diseases in India: current epidemiology and future directions. Circulation 133, 1605–1620.
Pravst, I., Zmitek, K., Zmitek, J., 2010. Coenzyme Q10 contents in foods and fortification strategies. Crit. Rev. Food Sci. Nutr. 50, 269–280.
Princen, H., van Poppel, G., Vogelezang, C., Buytenhek, R., Kok, F.J., 1992. Supplementation with vitamin E but not beta-carotene in vivo protects low density lipoprotein from lipid peroxidation in vitro. Effect of cigarette smoking. Arterioscler. Thromb. 12, 554–562.
Qin, Y., Xia, M., Ma, J., Hao, Y., Liu, J., Mou, H., Cao, L., Ling, W., 2009. Anthocyanin supplementation improves serum LDL-and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 90, 485–492.
Quideau, S., Deffieux, D., Douat-Casassus, C., Pouysegu, L., 2011. Plant polyphenols: chemical properties, biological activities and synthesis angew. Chem. Int. Ed. 50, 586–621.
Raggi, P., 2016. Inflammation, depression and atherosclerosis or depression, inflammation and atherosclerosis? Atherosclerosis 251, 542–543.
Rao, A., 2002. Lycopene, tomatoes, and the prevention of coronary heart disease. Exp. Biol. Med. 227, 908–913.
Rayment, S.J., Shaw, J., Woollard, K.J., Lunec, J., Griffiths, H.R., 2003. Vitamin C supplementation in normal subjects reduces constitutive ICAM-1 expression. Biochem. Biophys. Res. Commun. 308, 339–345.
Reaven, P.D., Khouw, A., Beltz, W.F., Parthasarathy, S., Witztum, J.L., 1993. Effect of dietary antioxidant combinations in humans. Protection of LDL by vitamin E but not by beta-carotene. Arterioscler. Thromb. 13, 590–600.
Rechner, A.R., Kroner, C., 2005. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res. 116, 327–334.
Rodriguez-Mateos, A., Ishisaka, A., Mawatari, K., Vidal-Diez, A., Spencer, J.P., Terao, J., 2013. Blueberry intervention improves vascular reactivity and lowers blood pressure in high-fat-, high-cholesterol-fed rats. Br. J. Nutr. 109, 1746–1754.
Romanchik, J.E., Morel, D.W., Harrison, E.H., 1995. Distributions of carotenoids and α-tocopherol among lipoproteins do not change when human plasma is incubated in vitro. J. Nutr. 125, 2610–2617.
Ross, R., 1999. Atherosclerosis—an inflammatory disease. N. Engl. J. Med. 340, 115–126.
Rössig, L., Hoffmann, J., Hugel, B., Mallat, Z., Haase, A., Freyssinet, J.-M., Tedgui, A., Aicher, A., Zeiher, A.M., Dimmeler, S., 2001. Vitamin C inhibits endothelial cell apoptosis in congestive heart failure. Circulation 104, 2182–2187.
Russo, A., Acquaviva, R., Campisi, A., Sorrenti, V., Di Giacomo, C., Virgata, G., Barcellona, M.L., Vanella, A., 2000. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biol. Toxicol. 16, 91–98.
Sagach, V.F., Scrosati, M., Fielding, J., Rossoni, G., Galli, C., Visioli, F., 2002. The water-soluble vitamin E analogue Trolox protects against ischaemia/reperfusion damage in vitro and ex vivo. A comparison with vitamin E. Pharmacol. Res. 45, 435–439.
Sargeant, L., Khaw, K., Bingham, S., Day, N., Luben, R., Oakes, S., Welch, A., Wareham, N., 2001. Fruit and vegetable intake and population glycosylated haemoglobin levels: the EPIC-Norfolk Study. Eur. J. Clin. Nutr. 55, 342.
Schroeter, H., Heiss, C., Balzer, J., Kleinbongard, P., Keen, C.L., Hollenberg, N.K., Sies, H., Kwik-Uribe, C., Schmitz, H.H., Kelm, M., 2006. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. U S A 103, 1024–1029.
Singh, R.B., Wander, G.S., Rastogi, A., Shukla, P.K., Mittal, A., Sharma, J.P., Mehrotra, S.K., Kapoor, R., Chopra, R.K., 1998. Randomized, double-blind placebo-controlled trial of coenzyme Q10 in patients with acute myocardial infarction. Cardiovasc. Drugs Ther. 12, 347–353.
Sinha, K., Das, J., Pal, P.B., Sil, P.C., 2013. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 87, 1157–1180.
Siow, R.C., Richards, J.P., Pedley, K.C., Leake, D.S., Mann, G.E., 1999. Vitamin C protects human vascular smooth muscle cells against apoptosis induced by moderately oxidized LDL containing high levels of lipid hydroperoxides. Arterioscler. Thromb. Vasc. Biol. 19, 2387–2394.
Solzbach, U., Hornig, B., Jeserich, M., Just, H., 1997. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients. Circulation 96, 1513–1519.
Sozański, T., Kucharska, A., Szumny, A., Magdalan, J., Bielska, K., Merwid-Ląd, A., Woźniak, A., Dzimira, S., Piórecki, N., Trocha, M., 2014. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARα activation in hypercholesterolemic rabbits. Phytomedicine 21, 1774–1784.
Stephens, N.G., Parsons, A., Brown, M., Schofield, P., Kelly, F., Cheeseman, K., Mitchinson, M., 1996. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet North Am. Ed. 347, 781–786.
Sugiyama, S., Kugiyama, K., Ogata, N., Doi, H., Ota, Y., Ohgushi, M., Matsumura, T., Oka, H., Yasue, H., 1998. Biphasic regulation of transcription factor nuclear factor-κB activity in human endothelial cells by lysophosphatidylcholine through protein kinase C-mediated pathway. Arterioscler. Thromb. Vasc. Biol. 18, 568–576.
Sung, H., Min, W.K., Lee, W., Chun, S., Park, H., Lee, Y.W., Jang, S., Lee, D.H., 2005. The effects of green tea ingestion over four weeks on atherosclerotic markers. Ann. Clin. Biochem. 42, 292–297.
Tanno, M., Kuno, A., Yano, T., Miura, T., Hisahara, S., Ishikawa, S., Shimamoto, K., Horio, Y., 2010. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J. Biol. Chem. 285, 8375–8382.
Tasinato, A., Boscoboinik, D., Bartoli, G., Maroni, P., Azzi, A., 1995. d-alpha-tocopherol inhibition of vascular smooth muscle cell proliferation occurs at physiological concentrations, correlates with protein kinase C inhibition, and is independent of its antioxidant properties. Proc. Natl. Acad. Sci. 92, 12190–12194.
Timmers, S., Konings, E., Bilet, L., Houtkooper, R.H., van de Weijer, T., Goossens, G.H., Hoeks, J., van der Krieken, S., Ryu, D., Kersten, S., Moonen-Kornips, E., Hesselink, M.K.C., Kunz, I., Schrauwen-Hinderling, V.B., Blaak, E., Auwerx, J., Schrauwen, P., 2011. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 14, 612–622.
Traber, M.G., 2007. Vitamin E regulatory mechanisms. Annu. Rev. Nutr. 27, 347–362.
Tsuchihashi, H., Kigoshi, M., Iwatsuki, M., Niki, E., 1995. Action of β-carotene as an antioxidant against lipid peroxidation. Arch. Biochem. Biophys. 323, 137–147.
Turunen, M., Olsson, J., Dallner, G., 2004. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta 1660, 171–199.
Uemura, M., Manabe, H., Yoshida, N., Fujita, N., Ochiai, J., Matsumoto, N., Takagi, T., Naito, Y., Yoshikawa, T., 2002. α-Tocopherol prevents apoptosis of vascular endothelial cells via a mechanism exceeding that of mere antioxidation. Eur. J. Pharmacol. 456, 29–37.
Ulrich-Merzenich, G., Metzner, C., Schiermeyer, B., Vetter, H., 2002. Vitamin C and vitamin E antagonistically modulate human vascular endothelial and smooth muscle cell DNA synthesis and proliferation. Eur. J. Nutr. 41, 27–34.
Wallace, T.C., 2011. Anthocyanins in cardiovascular disease. Adv. Nutr. 2, 1–7.
Walter, M., Marchesan, E., 2011. Phenolic compounds and antioxidant activity of rice. Braz. Arch. Biol. Technol. 54, 371–377.
Wang, Z.M., Zhou, B., Wang, Y.S., Gong, Q.Y., Wang, Q.M., Yan, J.J., Gao, W., Wang, L.S., 2011. Black and green tea consumption and the risk of coronary artery disease: a meta-analysis. Am. J. Clin. Nutr. 93, 506–515.
Watzl, B., Kulling, S.E., Möseneder, J., Barth, S.W., Bub, A., 2005. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am. J. Clin. Nutr. 82, 1052–1058.
Wilkinson, I., Megson, I., MacCallum, H., Sogo, N., Cockcroft, J., Webb, D., 1999. Oral vitamin C reduces arterial stiffness and platelet aggregation in humans. J. Cardiovasc. Pharmacol. 34, 690–693.
Witztum, J.L., Steinberg, D., 1991. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Invest. 88, 1785–1792.
Wu, D., Koga, T., Martin, K.R., Meydani, M., 1999. Effect of vitamin E on human aortic endothelial cell production of chemokines and adhesion to monocytes. Atherosclerosis 147, 297–307.
Yamamura, Y., Ishiyama, T., Yamagami, T., Morita, Y., Ishio, S., Kashiwamura, S., Terada, A., Tsukamoto, N., Toyama, S., Nakajima, Y., Wada, N., 1967. Clinical use of coenzyme-Q for treatment of cardiovascular diseases. Japanese circulation journal, 31(1), 168.
Yashiro, T., Nanmoku, M., Shimizu, M., Inoue, J., Sato, R., 2012. Resveratrol increases the expression and activity of the low density lipoprotein receptor in hepatocytes by the proteolytic activation of the sterol regulatory element-binding proteins. Atherosclerosis 220, 369–374.
Youdim, K.A., McDonald, J., Kalt, W., Joseph, J.A., 2002. Potential role of dietary flavonoids in reducing microvascular endothelium vulnerability to oxidative and inflammatory insults. J. Nutr. Biochem. 13, 282–288.
Yusuf, S., Dagenais, G., Pogue, J., Bosch, J., Sleight, P., 2000. Vitamin E supplementation and cardiovascular events in high-risk patients. N. Engl. J. Med. 342, 154–160.
Zapolska-Downar, D., Zapolski-Downar, A., Markiewski, M., Ciechanowicz, A., Kaczmarczyk, M., Naruszewicz, M., 2000. Selective inhibition by α-tocopherol of vascular cell adhesion molecule-1 expression in human vascular endothelial cells. Biochem. Biophys. Res. Commun. 274, 609–615.
Zhu, Q.Y., Huang, Y., Tsang, D., Chen, Z.Y., 1999. Regeneration of alpha-tocopherol in human low-density lipoprotein by green tea catechin. J. Agric. Food Chem. 47, 2020–2025.
Zordoky, B.N., Robertson, I.M., Dyck, J.R., 2015. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 1852, 1155–1177.
Zwaka, T.P., Hombach, V., Torzewski, J., 2001. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation 103, 1194–1197.
 2GSH + NADP+
2GSH + NADP+