[I]t happens also in chemistry as in architecture that “beautiful” edifices, that is symmetrical and simple, are also the most sturdy.
—Primo Levi, 1919–1987, Italian chemist and writer
From The Periodic Table (1984)
Every chemical substance, whether natural or artificial, falls into one of two major categories, according to the spatial characteristic of its form. The distinction is between those substances that have a plane of symmetry and those that do not. The former belong to the mineral, the latter to the living world.… I could not indicate the existence of a more profound separation between the products born under the influence of life and all others.
—Louis Pasteur, 1822–1895, French chemist and microbiologist
From Oeuvres de Pasteur (1922–1939), Vol. 1 (1922)
When Geoff started analyzing leaf waxes, he hadn’t consciously been looking for compounds in living organisms that are likely to survive in sediments, attacking his Derbyshire question from its other end, as is now a standard ploy in bio-marker studies. But when he returned from the Canaries in 1961, it began to dawn on him that this was, in fact, what he had done. It also became apparent that he was not alone in his curiosity about the fate of organic compounds in things like rocks and tars. In fact, he had some very good company.
Two prominent Nobel chemists gave talks in Glasgow that year, both posing fundamental questions about the stuff of life. And both, Geoff found, shared his maverick interest in the organic chemistry of rocks and oils. Sir Robert Robinson sought to resolve the apparent paradox of petroleum, which appeared to have formed from the buried detritus of plants and algae that had been subjected to high pressures and temperatures, and yet also contained organic compounds that were quite different from any known in plants and algae. And Melvin Calvin, who had discovered how CO2 is converted to organic molecules in photosynthesis, talked about the origin of life. Experiments done in the past decade had given new meaning to such questions, by showing that simple organic chemicals could form spontaneously under conditions similar to those that were likely to have prevailed on the early, prebiotic earth. The mystery of life’s origin suddenly seemed approachable, and discussions had shifted from the purely theoretical and philosophical toward the empirical, and from the paleontological to the chemical. On the contemporary earth, life has something of a monopoly on making organic compounds. But if amino acids and sugars, the basic building blocks of living things, were produced by other than living things on the early earth, before the advent of life, then one could imagine the world before photosynthesis, before bacterial chemosynthesis. One could imagine some form of chemical evolution that predated Darwin’s biological evolution and launched the simplest, most primitive of bacteria. And one could look to the oldest rocks for evidence.
Calvin immediately embraced Geoff’s idea of using the new gas chromatographic separation techniques to facilitate the hunt, and he invited Geoff to spend a sabbatical year working in his laboratories in Berkeley. At a time when the natural sciences were growing and dividing into ever more disparate new disciplines, Calvin had gathered scientists from all camps—biologists, biochemists, organic chemists, and physicists—into a specially designed laboratory building that was a model of interdisciplinary collaboration. Geoff says it was a wonderfully creative environment when he was there in 1963. The building was round and set up like a doughnut, with the labs in a ring around a meeting area in the middle, and no doors, so that everyone always knew what everyone else was doing. “The only problem,” he says, “was that the well-equipped chemistry lab Calvin promised me was completely empty. There wasn’t even a bloody test tube!” Once he got over the shock, however, starting out with a blank slate turned out to be not such a bad thing. It so happened that a local company had started producing commercial GCs, so he acquired the most up-to-date instrument. And a student from someone else’s project heard about what he was doing—or supposed to be doing—and showed up one day to help. Ted Belsky turned out to be indispensable to the whole undertaking. He’d served a stint as a mortuary technician in the U.S. Navy and was very practical, the sort who got things done: he begged and borrowed equipment from around the Berkeley campus, and Geoff soon found himself with a fully furnished lab.
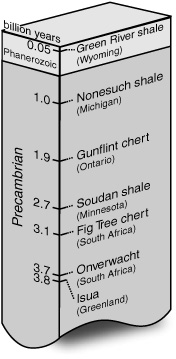
Calvin had acquired samples from some of the oldest known sedimentary rock formations, and he was impatient for Geoff to start analyzing them. So far, a clear history of life as told by fossils went back only about 600 million years, to the advent of multicellular organisms a little before the beginning of the Cambrian period. The earlier strata bore so few legible clues to history that paleontologists had delegated them to geologic prehistory and referred to the entire expanse as the Precambrian, undivided by the eras, periods, and epochs that they meticulously assigned based on the fossil assemblages in more recent strata. Only in the past decade had radiometric dating techniques revealed that this prehistory corresponded to seven-eighths of the earth’s existence, and it was just in the past few years that geologists and paleontologists had begun finding indirect, circumstantial evidence that life may have been present for most of it.
The earth’s oldest minerals had been dated at about 4.5 billion years, and a sedimentary formation in Greenland was dated as 3.8 billion years old. Other sedimentary rocks in Africa, the northeastern United States, and Canada ranged between 1 and 3.5 billion years old. All of them contained organic matter, and many had laminated, mushroom-shaped “stromatolite” structures that looked very much like some that grow in shallow waters on the contemporary earth. In modern stromatolites, the distinctive laminated layers of mud and calcium carbonate are formed by photosynthetic uptake of CO2 and excretion of a sticky substance by cyanobacteria. Were these ancient stromatolite structures formed by similar, oxygen-producing photosynthetic bacteria?
Ancient rocks, 1960s

The rocks Calvin wanted to analyze spanned the period from 1 to 3.7 billion years ago. Paleontologists had just developed new microscope techniques that allowed them to detect the fossils of microorganisms, and there were recent and very tentative reports of fossil bacteria in some of these rocks: tiny marks that appeared to be coated with residual organic matter and bore a close resemblance to the shapes of contemporary bacteria. In younger rocks, the cell wall and detailed structure of the bacteria might actually be apparent with an electron microscope, but in these ancient Precambrian rocks it was unclear whether the marks were the remains of organisms, or simply serendipitous marks left in the matrix of the rock by water, or gases, or mineral crystallization.
If there had been microorganisms, then perhaps they had left molecular remains. Maybe the rocks contained recognizable remnants of biological molecules, or clues of early life-forms with different biochemical systems. Or maybe they contained traces of prebiotic organic chemicals like those formed during experiments that simulated conditions on the early earth. But how could one recognize primordial biological molecules if they were radically different from contemporary ones? For that matter, how could one distinguish a biological from an abiotic or prebiotic organic molecule?
There was a conscious naiveté to the assumptions that had to be made as Calvin, Geoff, and the small team they assembled tried to track life backward through geologic time, an unavoidably circular logic. They were trying to find out when and how biochemical pathways had changed over time and, ultimately, how they had come into existence. And yet, of necessity, they were looking for molecules they could recognize, molecules that resembled those they knew from contemporary life-forms. The assumption here was that even as life-forms evolved and changed dramatically, the materials used in building them had remained constant over hundreds of millions of years. Such an assumption mirrored nineteenth-century geology’s premise that the geological processes now at work within the earth have operated with general uniformity over immensely long periods of time: it was Charles Lyell’s Uniformitarianism applied to biochemistry. Geology has now outgrown Lyell’s brand of all-encompassing Uniformitarianism, but it was doctrinaire in the 1960s when Geoff and Calvin started dabbling in geology. Its basic tenet—that the present is the key to the past—was crucial in understanding the geological record, and has proven valid in many, if not most, instances. To what degree it would hold true in the case of biochemical pathways remained to be seen, and the chemists adopted it not as doctrine or even hypothesis, but as a provisional supposition, which they questioned at every turn.
Microfossils in the Gunflint chert 1960s microphotographs
Though Calvin wanted Geoff to leap right in and start analyzing the Precambrian rocks, Geoff was skeptical. For one thing, the rocks had been sitting on the shelf for years, and Calvin kept going in and picking them up to look at them. They were likely loaded with contaminants from his hands and the environment. And even if they could cut off the outside layers and get a pristine sample, Geoff balked at beginning their hunt with rocks about which so little was known, where the organic matter was so sparse and difficult to detect, and the possibility of contamination with organic compounds from later deposits so high. Had the sediments formed in a lake or marsh, near the coast, or in the deep sea? How deeply had they been buried, how much had they been heated within the earth, and how long? It seemed to him that all of these factors would have had an effect on the structures of any organic compounds they found, and on the degree to which they might or might not resemble their biological precursors. How could they recognize clues to past life-forms in such ancient rocks, when they didn’t even know what they looked like in younger rocks? Which of life’s molecules survived the first onslaught of bacterial breakdown in the sediments, and how were they transformed? For that matter, obtaining and analyzing an extract from a rock was not quite the same as dipping leaves in chloroform—shouldn’t he practice a little before starting in on Calvin’s precious Precambrian collection?
Geoff was still getting set up and considering other options when a geologist he’d been talking to in the earth science department came dashing into his half-furnished lab, excitedly waving a single sheet of paper. It was just a one-paragraph abstract from a recent conference, but it was, as his colleague surmised, exactly what Geoff was looking for: a couple of scientists at the U.S. Bureau of Mines had tentatively identified two hydrocarbons in a sample of 55-million-year-old shale from the Green River Basin in the western United States. Geologists had been looking for oil in the area since the 1920s, so quite a lot was known about the basin’s geologic history, which was apparently relatively simple. Much of the Great Plains region was covered with shallow freshwater lakes during the Eocene epoch, and the rock that the Bureau of Mines scientists studied was a shale made up of compressed, solidified sediments that had formed in these lakes. The shale contained large numbers of fossils and a relatively high content of organic matter, and it was old enough that sediment bacteria would long since have eaten or transformed the most reactive organic compounds, leaving only those that were stable on a geological timescale of tens of millions of years. But it had never been deeply buried and subjected to the high pressures or temperatures that were likely to completely destroy most organic molecules. Most promising of all, the two compounds identified, though transformed by bacteria and time, retained certain structural idiosyncrasies that linked them firmly to source molecules in living organisms, and were just the sort of molecules Geoff would need if he were going to track life back into Calvin’s ancient Precambrian rocks.
In his initial experiments, Geoff used the Green River shale to develop dependable extraction and separation procedures, confirming and extending the results of the Bureau of Mines scientists. The organic matter in the rock was the chemical debris from a wide range of organisms and contained a much more complex mixture of compounds, in more minute quantities, than anything he’d ever analyzed—and most of that was bound up in an insoluble, unextractable, and impossible-to-analyze organic matrix. You could grind up the rock, extract it with every imaginable solvent, even dissolve the mineral in acid, and end up with everything in solution except its organic matter, some 90% of which remained like a bad joke in the bottom of the flask, a fine brown or black powder that to this day defies chemists’ attempts to fully characterize it. Geologists had given it the Greek name “kerogen” because it produced a waxlike oil when heated. No one knew quite what it contained or how it had formed. It appeared to be made of all sorts of organic molecules glued together in an apparently random manner by bonds so strong that it took excessive heat or a strong oxidizing agent to break them. Of the 35% organic matter in Geoff’s Green River shale sample, some 95% was insoluble kerogen. He did what any good organic chemist would do, and focused his attention on the 5% that he could get into solution: after slicing off the rock’s outer surface to remove contaminants, he ground it to a powder, heated it in solvent, and then threw away the insoluble residue. The result was a surprisingly yellow-green solution that he figured must be full of pigments, probably from algae that had lived in the lakes.
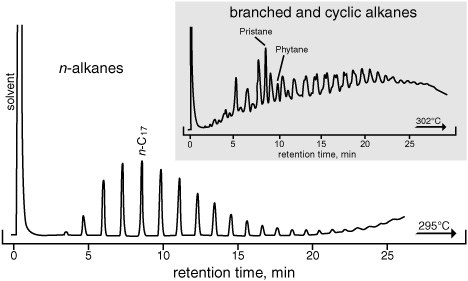
Gratifying as it was to actually be able to see pigments left in a rock 55 million years ago, for the moment Geoff concentrated on the alkanes, which were easier to analyze and, with their complete lack of double bonds and functional groups, most likely to have persisted in older rocks. After all, Calvin’s rocks were almost 50 times as old as the Green River shale! He poured the extract onto an alumina column, which retained all the pigments and other relatively polar compounds, and collected the first fraction to wash through. This contained only the colorless alkanes, but it was still a much more complicated mixture than anything he’d analyzed in the leaf waxes, so he split it into two fractions by pouring it into a molecular sieve, a mineral that catches the slim n-alkanes in its pores and separates them from all the alkanes that have branches or bulky rings. If he then ran each of these simplified mixtures on the GC, he could separate many of their individual compounds.
Analysis of hydrocarbons in ancient shales
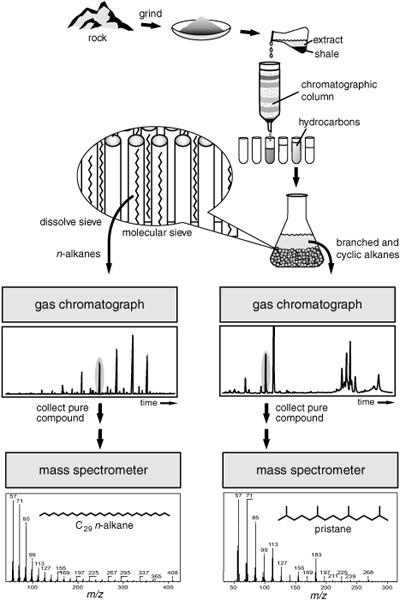
Once he had the compounds separated, he needed to figure out what they were. This was no problem for the n-alkanes, which he could identify from their positions in the gas chromatogram. The branched and cyclic alkanes were more difficult, however, as there were myriad structural possibilities, and Geoff wasn’t sure what to expect. Even when he could synthesize a structure that matched up with an unknown peak in the chromatogram from the shale extract, he couldn’t be positive they were the same, as different alkanes could easily exhibit similar behavior in the GC column, and their peaks might well overlap. Luckily, Al Burlingame, a young analytical chemist who worked with Calvin, had set up an instrument that showed great promise as a tool for determining the molecular structures of organic compounds, a state-of-the-art mass spectrometer that required only a trace of sample. When Geoff’s separated compounds came out the end of the GC column with the gas, they cooled and condensed into a few drops of liquid. If he stood there patiently with a little vial, he could collect the purified compounds and then give them to Burlingame to analyze on the mass spectrometer.
Identifying pristane in the 50-million-year-old Green River shale
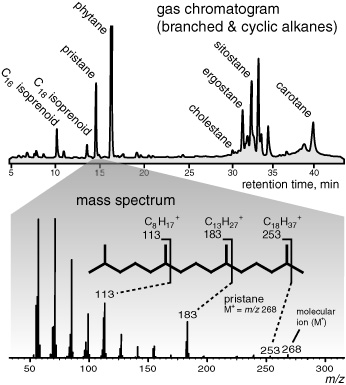
The mass spectrometer is a complex instrument based on a rather juvenile impulse: the best way to see how something is put together is to break it apart. A high-energy electron beam breaks the molecules into charged fragments, or ions, that are then sorted and registered by a detector according to their masses. If the mass of a fragment is known, then the atomic weights of the elements—12 mass units for carbon, 1 for hydrogen, 16 for oxygen, and so forth—can be used to figure out how many carbon, hydrogen, oxygen, or other atoms it has. Some of the molecules in a sample will only be nicked by the electron beam, which just knocks off an electron and produces a molecular ion, so you can calculate the molecular weight of the intact molecule. Others will be hit more directly and break at the structure’s weak spots, yielding large quantities of the fragment that corresponds to the most stable part of the molecule—the base ion—and lesser quantities of various other fragments. It’s like dropping a tray full of wineglasses: a few will escape unscathed, some will shatter completely, but most will break into three pieces—the round cup, the stem, and the thick-glassed base. In a few cases the stem may break in half, or the round part may shatter, but the thick-glassed base will usually stay intact. By examining all these fragments one can, in principle, figure out what an intact wineglass looked like, even if one has never seen an intact wineglass. A branched alkane, for example, usually breaks next to the branches. The mass spectra of two such alkanes in the Green River shale exhibited a very distinctive pattern of fragmentation and showed that they were both open chains of carbon atoms with branches composed of a single-carbon methyl group at every fifth carbon atom, and a total of 19 or 20 carbon atoms—and confirmed the structures of the compounds pristane, with 19 carbon atoms, and phytane, with 20, that the Bureau of Mines scientists had proposed.
It just so happened that the distinctive five-carbon pattern that made the mass spectra of these two compounds so readily recognizable also linked them firmly to biological parent compounds and made them particularly promising as indicators of life in the ancient rocks that Geoff was setting out to analyze. The carbon skeletons of the family of compounds with this pattern, known collectively as isoprenoids, are constructed by stringing these five-carbon branched segments together like beads, joining them head to tail into longer chains and rings, which form the basis for a wide variety of lipid molecules found in all forms of plant, animal, and bacterial life.
It’s a universal method of construction, and it results in a recognizable and idiosyncratic architectural style that is built into the most enduring part of the molecule—its carbon skeleton—and has a very good chance of surviving the ravages of decay, time, pressure, and even heat.
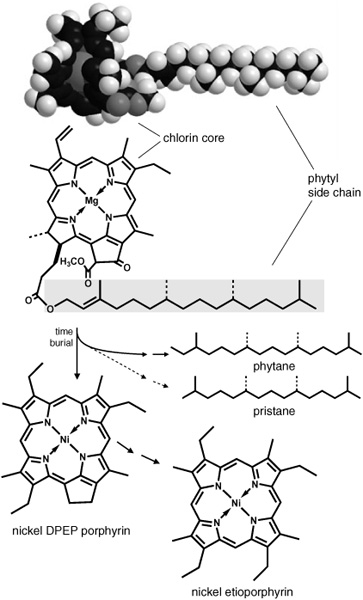
The two isoprenoids that were of such interest in the Green River shale didn’t look like anything Geoff had identified in the leaf waxes, but they were, nonetheless, quite familiar: they looked for all the world like the lopped-off tail of the chlorophyll molecule. In fact, it was the pared down version of chlorophyll’s other piece, its recalcitrant heart, that Alfred Treibs had identified in coal and in oils from even older shales back in the 1930s. Treibs had noticed a certain familial resemblance between the blood-red pigment molecules he extracted from the oil and the chlorin structure at the heart of the green chlorophyll molecule, and had proposed a chemically probable pathway to get from one to the other.
Though Geoff was unaware of Treibs’s work when he first turned his attention to rocks, it wasn’t long before he realized that he had been unwittingly following its lead and that a small tribe of chemists was scattered around the world doing likewise, defining a field they called organic geochemistry. Many of them were working for oil companies that forbid them to publish their results, so the organic geochemistry literature was scarce and often not known to organic chemists working in universities and research institutes. But a few key observations did make their way into the wider scientific community during those early years, published either by petroleum geochemists who managed, by sheer force of will, to sidestep company policies, or by geochemists at the enlightened Mobil Oil, which actually encouraged its scientists to publish. Keith Kvenvolden, who worked for Mobil, says that the management had figured out that when its scientists’ work went unpublished, there was no outside scientific scrutiny to verify it.
While Geoff had been in the Canary Islands noting the odd-over-even carbon number preference of long-chain n-alkanes in leaf waxes, a couple of oil company geochemists in Texas had published a paper describing similar patterns in sediments and rocks. The n-alkanes in Geoff’s Green River shale analysis also showed this distribution: a predominance of compounds with an odd number of carbon atoms, with the 27-, 29-, and 31-carbon compounds particularly prominent. But whereas none of the plants Geoff had analyzed contained any n-alkanes fewer than 25 carbon atoms long, the shale contained the 17-carbon n-alkane in almost equal abundance with the long-chain compounds. Had the n-alkanes in the Green River shale come from land plants, washed into the lake from the surrounding watershed? Or from the algae growing in the lake? Or both? What about the 17-carbon n-alkane that was so prevalent? Had that come from the algae? Or, perhaps, from bacteria living in the sediments? Very little was known about the lipid constituents of either algae or bacteria. For that matter, this short-chain n-alkane might not have been synthesized as such by any organism. It might have been a chemical degradation product of some other compound altogether. These questions about the precise sources of the compounds extracted from the shale would remain unanswered for years to come. But there was little doubt that the pattern of alkane abundance and the prevalence of the isoprenoids, pristane and phytane, in the Green River shale constituted a record of life in North America 55 million years ago, even if scientists couldn’t yet decode the details. With the hope that similar patterns might provide a record of life in rocks formed during the long prehistory of geologic time, when fossil-forming organisms were scarce or absent, Geoff turned his attention to analyzing Calvin’s Precambrian rocks.
Geological transformation of chlorophyll a
Copper mining in northern Michigan had provided access to the gray and black shales of the one-billion-year-old Nonesuch Formation, which were so rich in organic matter that drops of oil seeped from their pores. Unlike the Green River shale, which was loaded with fossils of plants and animals, the only physical fossils that had been found in these rocks were submicroscopic bits of organic material in the shape of microbial filaments or spores. Geoff began by extracting the alkanes from the oil, and found that the n-alkane distribution was quite different from what he’d found in plants and what the Texas geochemists had found in contemporary sediments. Like the Green River shale, the Nonesuch contained an abundance of the 17-carbon n-alkane, but in the Nonesuch only n-alkanes with fewer than 20 carbon atoms were present, and these included both odd and even carbon-number homologues, with little preference for the odd. Where did these short-chain n-alkanes come from? Nothing like them was known in organisms, and indeed, they seemed to defy what was known about the biosynthesis of straight-chain compounds. The Texas petroleum geochemists had found similar distributions in extracts of crude oil from much younger rocks. Could such compounds have been generated by some purely chemical, nonbiological means in the earth’s crust?
The one-billion-year-old Nonesuch shale
Gas chromatograms of alkanes, packed column, 1964
Chemists trying to simulate the chemical reactions that might have occurred on an early, lifeless earth had generated hydrocarbons from inorganic chemicals by dissolving iron carbide in hydrochloric acid, or by applying an electrical discharge to frozen methane. But these chemical processes produce thousands of different combinations of carbon and hydrogen, rather than the discrete selection of n-alkanes Geoff found in the Nonesuch shale oil. This alone implied that these hydrocarbons derived from some more discriminating biological reactions. There was, however, another, more direct and informative indication of past life in the one-billion-year-old rocks: the branched alkane fraction of the extract was loaded with pristane and phytane.
As Geoff and his colleagues were nearing completion of the Nonesuch study, Calvin had a visit from Phil Abelson, the editor of Science and director of the Carnegie Institute’s Geophysical Laboratory in Washington, D.C. Abelson was interested in the chemistry of organic compounds on the early earth, and had himself been trying to determine how long the distinctive components of life such as proteins and amino acids could survive when they were trapped in the mineral matrix of a fossil shell. Geoff still remembers showing him a chromatogram of the branched alkanes in the Nonesuch shale, pointing out the pristane and phytane peaks and mumbling that they might be of interest—at which point the rather large, imposing senior scientist suddenly looked up from the chromatogram, fixed him in his gaze, and exclaimed heartily, “You bet!”
Abelson told Geoff to hurry up and submit the Nonesuch study for publication, which turned out to be timely advice: unbeknownst to either of them, Warren Meinschein, a geochemist at Esso Oil who somehow managed to keep a foot in academic science—and publish his results—had been thinking along similar lines and working on the Nonesuch shale with a couple of Harvard paleontologists. Abelson published Meinschein’s paper back to back with Geoff’s in the July 1964 issue of Science. Both reported the identification of fossil organic molecules in the Nonesuch shale—but different ones. Geoff and crew had identified the acyclic isoprenoids phytane and pristane, and Meinschein and his colleagues had found porphyrins, the complex ring structures that Treibs first identified in oil. It was a sensational finding, but they needed more information, more knowledge, before it could be verified, let alone teach them anything about Precambrian life. Were the porphyrins and isoprenoids remnants of chlorophyll—its chlorin heart and phytyl tail—that had served to harvest light and energy for organisms that lived more than a billion years ago? Or had they come from some other source? Longer isoprenoid chains had just been identified in the membranes of certain salt-loving microbes, and phytane and pristane could, conceivably, be fragments of such chains, perhaps formed in the membranes of some yet-undiscovered but ubiquitous bacteria. Here they were, trying to recognize the molecular remnants of ancient life, but they didn’t have a complete inventory of life’s molecules, let alone an understanding of how those molecules are transformed over time in the rocks—the central conundrum of the whole enterprise.
In 1964, as Calvin and Burlingame continued their analyses of the Precambrian rocks in Berkeley, Geoff returned to Glasgow and began to confront that conundrum. He enlisted the help of the only two Glasgow chemists who had experience with complex mixtures of hydrocarbons, largely from doing medical research aimed at identifying carcinogenic compounds in lubricating oils used by industrial workers and machine operators. One of those chemists, Archie Douglas, got interested in how the carcinogenic hydrocarbons formed to begin with and had already started working with petroleum geochemists. An analytical wizard by all accounts, Douglas was pivotal to assembling a laboratory that was up to the challenges posed by geologic materials of the sort Geoff wanted to study. Within the year, the fledgling organic geochemistry group had acquired two other essential resources—one in the form of a disgruntled graduate student, and the other, a newly minted analytical instrument. James Maxwell tells me that he’d started his doctoral work in an organic synthesis laboratory, but his hands were covered with horrible skin diseases from the chemicals and he was shopping for another project. “I looked like Frankenstein!” he says, 40 years later. He was not particularly interested in rocks when he queried Geoff about working in the organic geochemistry group … but he was easily seduced by the research papers Geoff directed him to read, which included the Nonesuch papers. As for the laboratory’s star instrument, it had been designed by biomedical chemists in Stockholm, but it was precisely what one needed to decipher the chemical structures of unknown compounds in complex mixtures extracted from rocks: a gas chromatograph linked directly to a mass spectrometer, immediately christened with the acronym GC-MS. Now, as the compounds came through the GC column, they were routed one after the other directly into the mass spectrometer for structural analysis. Not only could Geoff’s group now separate the mess of compounds in the mixtures extracted from rocks, it now had a much better chance of identifying what those compounds were. The group could, in principle, expand its horizons beyond phytane, pristane, and the n-alkanes.
When he’d first started his career at Glasgow, Geoff had shied away from natural products chemistry because the field was growing so fast and producing such a huge body of literature. “You had to spend all your time reading and trying to catch up,” he says. But now he found himself not only reading the burgeoning natural products literature, but scanning the works of biochemists, paleontologists, and analytical chemists, even hunting down the elusive petroleum geochemists, in his search for relevant information, which, it seemed, could be tucked away in the most obscure journals. “It was hard to know what I should be reading and who I should be talking to,” he says. “It was bewildering … but exciting.”
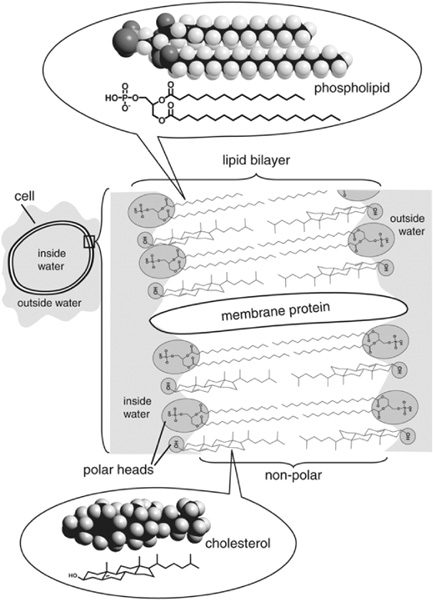
During this period in the mid-1960s, a handful of petroleum geochemists were attempting to follow the geological fate of a class of lipids that, in addition to being the biosynthetic precursors to the n-alkanes, are much more prevalent in living organisms than the n-alkanes: the straight-chain fatty acids. These compounds had been extensively studied for years, partly because of their crucial roles in organisms, partly because of their commercial value for the food and detergent industries, and partly because the carboxyl group at the end of the carbon chain makes them easy for chemists to work with. In organisms, fatty acids are an important means of storing energy, and they are the main ingredient of cell membranes. They come in both saturated and unsaturated versions, the latter with double bonds between some of the carbon atoms; both the chain length and the number and placement of double bonds vary among different groups of organisms. The carboxyl group is bound by an ester linkage to form the phospholipids that make up most cell membranes, and Geoff had found that the leaf waxes contained long-chain fatty acids bound by ester linkages to alcohols. Such ester bonds are easily hydrolyzed and cleaved, however, so as soon as the organism dies and begins to decompose, free fatty acids are released. The carboxyl group at the end of the carbon chain in these free acids makes them more polar and soluble in water, more chemically reactive, and easier for bacteria to consume than the alkanes. And yet some fatty acids appeared to be surprisingly persistent. Petroleum geochemists found them in shales and crude oils, and for a time even postulated that fatty acids play a pivotal role in petroleum formation. Abelson and others found them in the Green River shale, and Kvenvolden found them in even older Cretaceous-age rocks.
Cell membrane
Like the n-alkanes, the fatty acids in the oils and ancient rocks posed a number of enigmas that couldn’t be explained by their known occurrence in organisms. Though the only known biosynthetic pathways for fatty acids produced mostly acids with an even number of carbon atoms, and these were by far dominant in organisms, the distributions of fatty acids in older oils and rocks didn’t seem to reflect this. It appeared, rather, that the even-over-odd carbon number preference in fatty acids was progressively erased, over tens of millions of years, just as was the odd-carbon-number preference in n-alkanes. Kvenvolden and his colleagues at Mobil came up with a plausible, even elegant, chemical reaction scheme that very neatly explained this observation, as well as the general excess of short-chain n-alkanes found in petroleum: fatty acids reacted with each other in the sediments, producing acids with an odd number of carbon atoms and n-alkanes with an even number. Not until years later would it become apparent that the process is not so tidy, that the distributions of n-alkanes and fatty acids in sediments, rocks, and oils are influenced by the two most difficult to analyze aspects of the geologic record: the insoluble kerogen, and the multifarious and ever-elusive microbes.
Back in the 1960s, while Kvenvolden was at Mobil in California trying to figure out where the compounds in oil came from, and Geoff and James Maxwell were in Glasgow beginning analyses of the fatty acids in the Green River shale, Max Blumer at the Woods Hole Oceanographic Institution in Massachusetts was finding some of the mysterious short-chain n-alkanes in samples of phytoplankton collected from the surface waters of the ocean. Blumer was another wayward organic chemist whose early fascination with fossils had diverted him briefly into the petroleum industry, and from there to Woods Hole. In one of the first forays into the organic chemistry of the marine environment, Blumer was attempting to trace the pathways from organic compounds made by marine organisms to the organic compounds in ocean sediments. The n-alkanes made by the phytoplankton apparently remained unchanged as they passed through the food chain, because Blumer found the same distributions in zooplankton and in detritus filtered from the water—even bacteria didn’t seem to be particularly fond of the n-alkanes. This all made chemical sense, as it’s hard to cleave a single carbon–carbon bond when it’s part of a straight chain, with no functional groups or other weak links. And yet, when Blumer and his students analyzed extracts of recently deposited ocean sediments, they found significantly different distributions of n-alkanes and concluded that they must have had some other source or sources—but what?
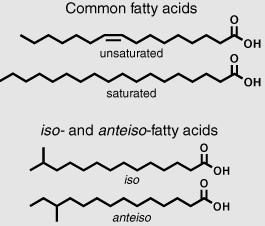
Meanwhile, Pat Parker, a chemist at the Texas Marine Institute, was doing similar sorts of studies in the rich, stinky mud flats and lagoons around the Gulf of Mexico, trying to track the fate of fatty acids in the first stages of their journey from organism to sediment. The laboratory next to Parker’s was occupied by a distinguished microbiologist who was studying the thick mats of photosynthetic bacteria growing in the shallow waters around the institute, and Parker managed to interest him in the enterprise and obtain samples from his cultures of cyanobacteria and algae. Extracts of these organisms contained simple mixtures of both saturated and unsaturated fatty acids, dominated by the 16- and 18-carbon compounds—which was precisely what they found when they analyzed extracts from the surface layer of sediments in the lagoons. The unsaturated acids disappeared quickly, however, and were absent from the deeper layers of mud, presumably because the double bonds made them more accessible to bacterial scavengers in the sediments. No one had ever actually observed such scavengers in sediments, but one assumed they were living in the top layers just as they did in soil or anyplace else where organic detritus accumulated. If something disappeared quickly, it was reasonable enough to blame the invisible bacteria. But the Texas group also found a series of unusual acids that seemed to increase in the sediments and didn’t resemble any of the compounds they’d isolated from the cultures of algae and cyanobacteria. Commonly known as iso- or anteiso-compounds, these were fatty acids with a single methyl group branch on the second or third carbon atom from the end.
A review of the biochemical literature revealed that the iso- and anteiso- acids had been identified among the lipids of heterotrophic bacteria—just the sort of scavengers that might be making a last-ditch effort to recycle organic matter before it was forever buried and turned to stone. Most microbiologists were too busy studying the kind of bacteria that make people sick to get excited about something found in a bucket of mud, but Parker’s distinguished neighbor was intrigued enough to isolate cultures of bacteria from the mud itself. Sure enough, when Parker prepared a solvent extract of these bacteria and ran the fraction with the fatty acids through the GC, he found the same iso and anteiso acids he’d found in the mud itself. On the one hand, it was exciting to find the source of the acids, like finding the treasure in a treasure hunt after only a few clues. On the other hand, it brought home just how complicated it was going to be to read the molecular cipher in the rocks. Not only did you need to think about what the enigmatic sediment bacteria ate —or more precisely, what they disdained to eat and left intact or slightly transformed—but you had to consider what they produced, as well.
Members of Calvin’s lab had also started analyzing extracts of algae and bacteria, looking for the sources of the compounds they were finding in ancient rocks. Not much was actually known about the microscopic inhabitants of natural waters and sediments, but even a limited sampling from the organisms that botanists and microbiologists had managed to isolate and grow in culture provided a potential explanation for some of the hydrocarbons that were being found in sediments and rocks. The long-chain compounds that were so prevalent in land plants, and missing from the Precambrian rocks, were entirely absent from the algae and bacteria analyzed—as might be expected, since organisms that live in water would have no need for long, hydrophobic molecules to protect them from drying out. The 17-carbon n-alkane that was absent from higher plants, but a prominent component of the organic matter in both the Green River and the Precambrian rocks, turned out to be the most abundant straight-chain alkane in the algae and photosynthetic cyanobacteria. All of the bacteria analyzed, including heterotrophs, contained odd-carbon-number short-chain n-alkanes ranging from 11 to 24 carbon atoms, with the 15-carbon compound in relative abundance. In a few cases, though, they also contained the corresponding even-carbon-number compounds, a finding that cast doubt on the reputedly dominant and universal biosynthetic pathway for making straight-chain compounds and caused confusion about the sources of these alkanes in oils and rocks … until someone figured out that these compounds had come not from the organisms under study, but from the plastic hoses used to bubble air into the water while they were growing. The plastic was, of course, made from petroleum, and while it would be many years before anyone figured out how these short-chain n-alkanes were generated in the petroleum, one thing was becoming clear: petroleum hydrocarbons were everywhere in the man-made environment, and they were confounding attempts to understand the presence of biological lipids and their decomposition products—which included many of the same petroleum hydrocarbons—in nature.
One of the main tasks confronting the scattered cadre of organic chemists who were trying to analyze geologic materials in the 1960s was that of developing experimental protocols that eliminated contamination—plastic containers, impure solvents, car exhaust drifting in through an open laboratory window, pollutants in the ocean and lakes. When it came to analyzing Precambrian rocks the problems were even more insidious. Unlike the Nonesuch shale, most of these rocks contained such small quantities of organic compounds that the merest trace of contamination could render results completely meaningless. Indeed, much of the excitement and funding for such work was coming from NASA, which was preparing for its Apollo 11 mission: not only would men be landing on the moon in 1969, but they would be bringing moon rocks back to Earth for chemical analysis. Those analyses would include a search for minute traces of organic compounds, and NASA was offering generous funding for anything that might provide a knowledge base, including much of the Precambrian work and Burlingame’s analytical facilities in Berkeley. One of the most frustrating contamination problems that Geoff had faced with the Precambrian rocks was simply that they’d been sitting around on Earth for so long that they had become contaminated by organic matter that had seeped in from later deposits. Calvin had maintained that the ancient rocks were impervious, until Geoff did a simple experiment: he soaked a representative chunk of rock in water containing long-chain fatty acids that had been prepared for biochemical studies and “labeled” with radioactive carbon atoms. As feared, when he analyzed the rock, he found that the labeled compounds had made their way deep into its middle. The Nonesuch shale had been relatively easy to work with, and he was able to compare the hydrocarbon content of different components and layers of the rock, thus verifying that the compounds identified were indeed indigenous to the one-billion-year-old shale. But verifying that the minuscule amounts of hydrocarbons in older, more altered rocks actually derived from organic matter that was deposited with the sediments that formed the rocks was a difficult, if not impossible, task—one of the reasons Geoff focused the Glasgow efforts on learning about the fate of organic constituents in younger and more genial geologic material. In this younger material, which ranged from 5,000-year-old lake sediments to Geoff’s all-time favorite rock, the 55-million-year-old Green River shale, Maxwell identified a series of acyclic isoprenoid acids—including a substantial amount of pristanic acid, which Blumer also found in his analyses of zooplankton from the marine environment.
Blumer’s studies were showing that the zooplankton that grazed on microscopic algae in the surface waters of the ocean contained phytol, the alcohol produced from cleavage of the phytyl tail of chlorophyll, as well as pristane and pristanic acid, which could easily have derived from the phytol via oxidation and loss of a carboxyl group. All three compounds were present in the extracts of zooplankton and in surface sediments, but phytane, which has the same carbon skeleton as phytol, was absent from both, and Geoff’s group was finding that it only appeared in older deposits, apparently produced by the slow reduction of the hydroxyl group in phytol as the sediments aged. The Green River shale, which had spent 55 million years aging, contained phytane, pristane, and their acid versions, but lacked phytol.
Even with this bread-crumb trail of evidence from chlorophyll to phytol to the series of isoprenoid acids Maxwell was finding, and with plausible chemical mechanisms to produce the corresponding alkanes, pristane and phytane, the origin of the acyclic isoprenoids in ancient rocks and oils was far from certain. Luckily, though they are only slightly more complex than the n-alkanes, these molecules are asymmetric and contain a subtle extra source of information in the way their atoms are oriented in space. The tetrahedron formed by a carbon’s four bonds is symmetrical when any two of the attached atoms or groups are the same, as in the n-alkanes, where every carbon is bound to at least two hydrogens. But if all the atoms or groups attached to a carbon atom are different, as they are at the branch carbons where the methyl groups are attached in the middle of phytane and pristane, then an asymmetric, or chiral, center is created and the same molecule can exist as either of two mirror-image configurations, or stereoisomers. These stereoisomers are identical except that, like a left hand and a right hand, they cannot be superimposed one upon the other. They are, then, chemically indistinguishable until they interact with other asymmetric molecules, at which point they react differently, much as when two people try to shake hands right to left instead of right to right.
The amino acids that make up proteins and enzymes are asymmetric—with a chiral carbon atom bonded to an amino group, an acid group, a carbon chain, and a hydrogen atom—and one of the most crucial, and inexplicable, steps in the origin and evolution of life is the adoption of just one configuration from each of the possible pairs. Accordingly, biochemical reactions, which depend on enzymes, distinguish between otherwise identical pairs of stereoisomers and are very specific about which ones they make and use—unlike abiotic chemical reactions. Nineteenth-century chemists recognized this peculiar “one-handed” aspect of life when they shined polarized light through solutions of plant extracts and found that certain extracts bent the rays to the right or left. Indeed, it was this optical activity and a simple bit of geometric reasoning that led to the realization that the four atoms attached to a carbon must lie at the corners of a tetrahedron and ultimately, if you wanted to understand the molecules of life, you needed to know not just what is bonded to what, but how the molecule’s atoms are oriented in space—its stereochemistry.
The phytyl tail of chlorophyll is made with a single, specific configuration at each of its two chiral carbons—in other words, as only one of four possible versions. Any phytol released by cleaving the tail would presumably have the same configuration at those two carbons, and it was likely that any pristane or phytane that came from phytol would retain it. Maxwell says that he still remembers a 1967 paper from Calvin’s group that noted what a major breakthrough it would be if one could correlate the stereochemistry of the isoprenoids in a geological sample with that of their supposed biological precursor, namely, the phytyl tail of chlorophyll. “I took that paper of Calvin’s literally,” he tells me. At the time, it seemed a sure way of tracking a fragment of isoprenoid skeleton through hundreds of millions of years in the sediments.… It also seemed a near-impossible task. How could they possibly separate and determine the configurations of four chemically identical or nearly identical stereoisomers of phytane or pristane, two configurations at each of the two chiral carbons of interest? Perhaps they’d have a chance with the isoprenoid acids found in younger rocks. Acids allowed you to do things with them in the lab. You could form an ester by tacking an alcohol onto the end, and if you used a single stereoisomer of an alcohol with its own chiral carbon, then the esters formed would be more distinctive than the acids alone and, hopefully, easier to separate. Help with the project came from a strange quarter: the Fisheries Research Board in Halifax, Canada, where a couple of biochemists were trying to measure the amounts of phytanic acid in fish oils. A rare genetic defect in humans can cause a dangerous buildup of this acid in the body, and the biochemists were developing a method of checking for its presence in foodstuffs, trying out a new GC column, which, they discovered quite by accident, separated the acid ester into two peaks.
Phytol, and the stereoisomers of phytanic acid
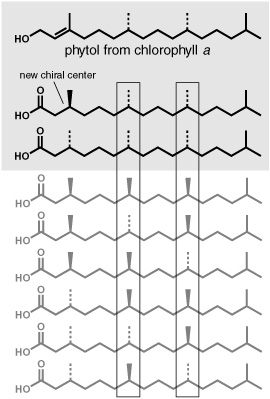
Working with the Canadians, Geoff and Maxwell used phytol from chlorophyll to prepare pristanic and phytanic acids, thus obtaining only the stereoisomers with the same configuration as in chlorophyll—two stereoisomers per acid, due to the creation of a new chiral center in the acids. Sure enough, these were precisely the two stereoisomers—the only two—that they detected among the isoprenoid acids in their extract of Green River shale, the clearest indication yet that the pristane and phytane in the shale came from chlorophy ll.
Did this mean that all of the pristane and phytane they found in geological samples had come from chlorophyll, that there was no other significant source? What of the phytane that had just been reported in a chert from South Africa that was more than three billion years old? Was it possible that they were looking at evidence not only that life was in full swing, but that something as biochemically complex as photosynthesis was already the dominant form of production? Could Maxwell somehow extend his techniques to determine the stereochemistry of isoprenoid alkanes in Precambrian rocks, where the acid forms had long since disappeared?
In the mid-1960s, fueled by NASA funding and encouraged by the success of the Nonesuch shale analyses, there was a small flurry of studies on older and older rocks. Paleontologists found what appeared to be the fossils of photosynthetic bacteria and algae, and chemists, cynical about what looked to them like a few odd marks in the rock, nevertheless looked for their own clues, and continued to find them: in the 1.9-billion-year-old chert from the Gunflint Formation in Ontario, the 2.7-billion-year-old Soudan shale from Minnesota, and the 3.1-billion-year-old Fig Tree Formation in South Africa.… Pristane, phytane, and a preponderance of 17-carbon n-alkane all implied that photosynthetic microbes had been present. In the Soudan shale, Meinschein tentatively identified ring compounds that seemed to be related to sterols like cholesterol—known constituents of the cell membranes of more complex eukaryotic organisms. But when did life begin? Were there any rocks old enough to have recorded a time before life imposed its order, a time when the only organic compounds on Earth comprised the random assortment made by abiotic chemical reactions?
Such was the excitement of the time that when Calvin’s group extracted a 3.7-billion-year-old sample from the Onverwacht Formation in South Africa and showed him the first chromatogram, he wondered if he might actually be seeing the transition from abiotic to biotic production. There was a large hump of hydrocarbons, presumably so myriad and various in structure that they couldn’t be separated, and, rising from the hump, a number of tiny, well-defined peaks corresponding to the alkanes they’d been seeing in contemporary sediments and living matter. “This is precisely the kind of spectrum one might expect from a mixture of abiogenic materials (the continuum) and biogenic materials (the superimposed peaks),” Calvin wrote in his 1969 treatise on the chemical evolution of life. After giving a brief inventory of the caveats, he went on to say, “we may indeed have found a period in time, 3700 million years ago, during which the transition between the abiogenically developed organic substrate was being converted by the newly developed autocatalytic replicating chemical systems which give rise to life.”
But Calvin’s excitement was premature, and his speculation remained unsubstantiated. Even as analytical techniques improved, facilitating the search for more complex and informative organic molecules, doubts were growing about the veracity of what they were finding in any of the Precambrian rocks older than the Nonesuch. Thomas Hoering, a colleague of Abelson’s at the Carnegie Institute, had long been intrigued by the signs of past life in Precambrian rocks, but was keenly skeptical of his own and others’ results. He was always looking for unequivocal evidence that the organic compounds they contained were biogenic and did in fact hail from the early years of earth history when the rocks formed—and not from the dust filter in the lab’s heating system or the ink on the newspaper the rocks were wrapped in by the geologists who’d collected them, or most difficult of all, that the compounds hadn’t leached in from later deposits such that the remains of organisms that lived a few million or hundred million years ago were being confused with those of the first organisms to leave their mark on Earth. Geochemists from the era consistently tell me that it was a 1967 paper of Hoering’s that finally deflated their hopes of getting meaningful results from rocks that were more than two billion years old, and indicated that much of what they’d found was, as Keith Kvenvolden puts it, “bogus.”
Kvenvolden himself had detected amino acids in several Precambrian samples and was, at first, excited to see that they matched those used by organisms in their proteins, providing evidence that “complex organisms were in existence more than 3.1 billion years ago,” with an amino acid composition that had “not changed significantly since very early in earth history.” But when he repeated the analysis with a system that could separate the amino acid stereoisomers—an easier task than separating hydrocarbon stereoisomers—he immediately realized that the amino acids he’d found in the Fig Tree Formation couldn’t possibly have been made by organisms 3.1 billion years ago. Abelson had just discovered that amino acids in fossil shells gradually convert from the biological isomer—whose configuration is designated L by chemical convention—to the nonbiological D isomer as the fossils age. The conversion was slow, but not so slow that it couldn’t be studied in the laboratory, and the basic mechanism had already been elucidated: the hydrogen at the chiral carbon was removed as a naked proton, without its electron, leaving a negatively charged planar intermediate. A proton could then reattach to either side of this flat intermediate with equal probability, producing either the L or the D isomer.
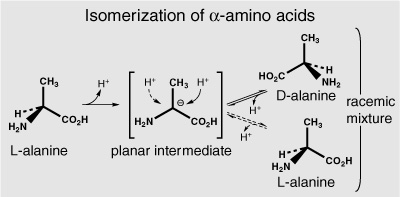
Abelson suggested that one might be able to figure out the precise rate of this process and use the relative amounts of the two isomers to date fossils—but only relatively young ones. The reaction is reversible, with the protons constantly removed and reattached and the molecules inverted and reinverted, until at some point an equilibrium is established where the rate of conversion from D to L balances that from L to D and there is no observable change in the relative amounts of the two isomers. Such an equilibrium mixture, with the two isomers present in near-equal amounts, is obtained after a few tens of millions of years even at low temperatures. If there were amino acids left from organisms that had lived during the Precambrian, billions of years ago, they would surely be present as a 50:50 mixture of L and D isomers, what is known as a racemic mixture of the two mirror images. And yet the amino acids Kvenvolden had extracted from the Fig Tree rock were all in the L configuration that life uses. Clearly, they had leached in from later deposits, and their evidence of primordial life was, indeed, “bogus.”
The chemical record of the origin and early evolution of life that Calvin had hoped to find seemed to have been pushed off the map of the rock record—at least for the time being. “People got frightened and overreacted,” says Maxwell, who spent 1967 as a postdoc in Calvin’s lab. “No one wanted to risk their careers by making bogus discoveries.” Maxwell did in fact develop a method that allowed him to distinguish the stereoisomers of pristane and phytane in geological samples, but he laughs when I ask why he never tried it on the Precambrian rocks. “You want me to be cynical? At that time? Because of the problems of contamination in those rocks, I got frightened off.” Instead he focused his energies on much younger organic-matter–rich oil shales, where the stereochemistry of isoprenoid alkanes would lead him to some unexpectedly practical discoveries—and, along the way, make it clear that knowing the stereochemistry of phytane in the ancient Precambrian rocks would not, in fact, have told him anything about its origin.
By the end of the 1960s, as interest in the Precambrian rocks waned, the small community of organic geochemists that had assembled around their study turned its attention and newly forged analytical methods to the more attainable goal of learning about the molecular remnants of life in younger rocks, sediments, and petroleum … and, at the same time, vied for a chance to analyze some samples that were even more dubious, and more seductive, than the Precambrian rocks. If it was hard to resist the mystique and romance of three-billion-year-old rocks that might contain clues to the origin of life on Earth, it was even harder to resist a hunt for signs of life or its precursors on other planets—if only for the thrill of seeing and handling their rocks.