By this, the Earth itself, which lyes so neer us, under our feet, shews quite a new thing to us, and in every little particle of its matter, we now behold almost as great a variety of creatures as we were able before to reckon up on the whole Universe itself.
— Robert Hooke, 1635–1703, British natural philosopher, referring to his view of life through one of the first microscopes
From Micrographia: Or Some Physiological Descriptions of Minute Bodies Made by Magnifying Glasses (1667)
With the most rational philosophers an increase in their knowledge is always attended by an increased conviction of their ignorance.
—Georg Christoph Lichtenberg, 1742–1799, German natural philosopher
From Aphorisms (1990 translation)
If I could do it all over again and relive my vision in the twenty-first century, I would be a microbial ecologist. Ten billion bacteria live in a gram of ordinary soil… almost none of which are known in science. Into that world I’d go with the aid of modern microscopy and molecular analysis.
—Edward Osborne Wilson, 1929–, American biologist
From Naturalist (1994)
In the half century since paleontologists began finding putative microfossils in Precambrian sedimentary rocks, it has become apparent that not only is most of life’s history absent from the visible fossil record, but huge sectors of extant life remain to be discovered. Until the early 1980s, the only way to identify and study species of microbes—many if not most of which are morphologically indistinct from each other—was by growing them in the laboratory, isolating the separate colonies of organisms that developed as they reproduced, and then noting differences and similarities in what they consumed and produced. But the capacity to read the information in microbial genes that was developed in the 1980s and 1990s opened an entirely new world for study—much as the invention of the microscope had in the eighteenth century—and laid bare the unnerving fact that the vast majority of microbes on the planet had been boycotting the microbiologists’ carefully prepared cultures.
Microbiologists had spent almost a century painstakingly cultivating, isolating, and classifying microorganisms, and yet they had failed to identify the most abundant microbes in natural waters, sediments, and soils. Indeed, it now appears that the hundreds of thousands of microbes named and maintained in the world’s bacteria zoos, or “culture collections,” invaluable as they are, comprise but a tiny and somewhat random sampling of the microbial world. These microbial cultures provide the only means by which biologists can directly manipulate and study the biochemistry and physiology of this huge sector of life in the laboratory—but they are distinguished more by their ability to prosper under laboratory conditions than by their importance in natural ecosystems. In the past few years, application of new techniques from molecular biology has resulted in the discovery of thousands of strange new types of microorganisms, and there is the implication of countless more: in the twenty-first century we find ourselves unexpectedly gathered at the threshold of a new world, looking not to Mars or Jupiter or to some distant galaxy, but gazing awestruck at the mud beneath our feet and the water in our seas.
The two paradigm-challenging discoveries that would bring us to that threshold were both made in 1977, but had nothing to do with each other. One was a spectacular discovery made by oceanographers exploring the seafloor of the eastern Pacific Ocean in a tiny, three-person research submarine. It immediately galvanized the wider scientific community and, for the first time, brought microbial ecosystems that had nothing to do with disease or food into the limelight. The other discovery was made by a lone molecular biologist poring over tables of gene analyses in a small laboratory in Illinois, and several years would pass before microbiologists and biologists recognized its profundity.
On the seafloor just east of the Galapagos Islands, at one of the mid-oceanic ridges that separate tectonic plates, oceanographers discovered incredible regions of hydrothermal activity, places where geysers of hot water spouted from every crack and the seafloor was festooned with a veritable fantasia of strange mineral formations. This was more or less what oceanographers, geologists, and geochemists expected, if, as all evidence indicated, the ridge was a center of seafloor spreading, with hot magma rising from deep within the earth to form new oceanic crust. Any seawater that percolated through fissures in the rocks would be heated, producing hydrothermal activity. The superheated water would dissolve chemicals in the rocks, and as the water spouted back into the icy deep sea, these would precipitate and form minerals. The scientists in their submersible could actually see the shimmering hot water from the vents turning milky white or pale blue as it cooled and the minerals precipitated. Further exploration revealed places where the geysers were literally black with the fine particles of metal sulfides that precipitated as the hydrothermal fluids cooled. What no one had expected, however, what shocked oceanographers, geologists, and most of all, biologists, was the cornucopia of life they found swarming around the hydrothermal vents.
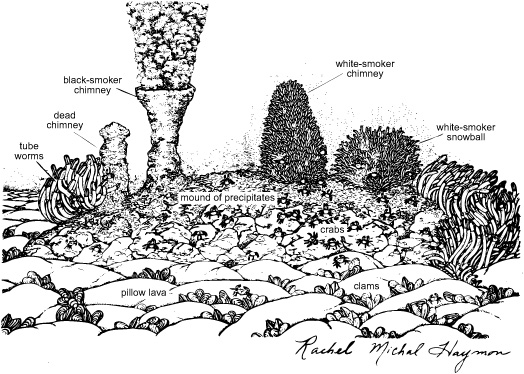
Biologists had long written off the deep sea as a cold, lightless desert, occupied only by the few scavengers that could survive on the meager supply of organic matter from the surface, or by a lone fish that could navigate large distances in search of sustenance. But these hydrothermal oases were surrounded by lush communities of giant mussels, dinner-plate–sized clams and crabs, anemones, pale pink fish, and dense stands of bizarre red-capped tubeworms, all thriving in absolute darkness beneath 2,500 meters of water. Further exploration in the research submersibles revealed that these hydrothermal vents, with their lush ecosystems populated with previously unknown species of invertebrates, were scattered all along the mid-ocean ridges. But what was sustaining them? Where did the energy for all that life come from, if not sunlight and photosynthesis in the surface waters? There couldn’t possibly be enough bits of organic matter raining down from the surface to feed all those mussels and clams and worms. What could they eat? For that matter, how did they eat? The strange tubeworms were just hollow tubes with no mouths, stomachs, or anuses!
Samples of the hot water coming out of the vents were swarming with bacteria; indeed, every hard surface in the vicinity of the vents was caked with thick mats of white or pink or yellow microbes. These, it seemed, were the primary producers for the entire fantastical ecosystem, a resourceful collection of minute autotrophic bacteria that had the wherewithal to harness chemical energy by oxidizing the sulfide in the hydrothermal waters. Many of the mobile vent animals seemed to graze on the microbial mats, like so many deer in a meadow. But what about the immobile, gutless tubeworms and mussels? Geochemists compared the carbon isotope content of tissue from the mussels and tubeworms around the vents with that of mussels from other areas, and found that they were wildly diff erent: the vent organisms clearly did not feed on detritus from the surface, which would have borne the isotopic signature of photosynthetic organisms. In 1980, Colleen Cavanaugh, a young microbiologist at Harvard, proposed that the tubeworms, which obviously couldn’t graze, must have sulfide-oxidizing bacteria living inside their tubes. Cavanaugh was still a graduate student at the time, and it took some doing for her to obtain samples from the strange creatures— but when she did, she found a whole factory of sulfide bacteria living in symbiotic bliss within their tubes. It turns out that the feathery red plume at the end of the tube is full of hemoglobin and serves as a sort of gill, absorbing dissolved compounds from the hydrothermal vent fluid and providing the bacteria with oxygen and with a ready supply of the sulfide they need for energy. The bacteria can then fix carbon and manufacture sugars for their host worm’s consumption. Similar symbiotic relationships were found in many other vent animals, such as mussels, clams, and shrimps, which can actively mine the metal sulfides from the rocks to fuel their bacterial factories. More than 300 new species of invertebrates have been discovered at deep sea hydrothermal vents since 1977, but it’s the incredibly productive chemoautotrophic bacteria at the base of their food chain that has really transformed thinking about life, shifting the focus of biology from plants and animals to microbial life and its interaction with the mineral world.
A hydrothermal vent landscape (1980, East Pacific Rise)

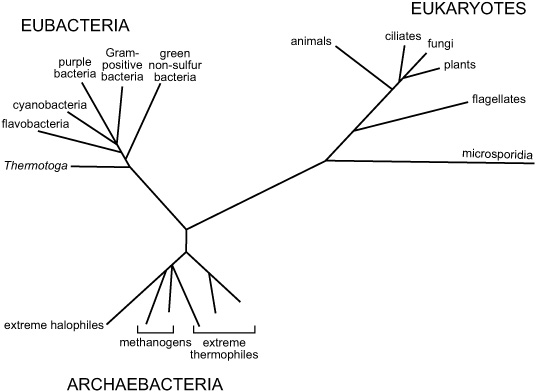
Biologists were still puzzling over the enigma of so much life in the deep sea, and buzzing with excitement about the first new species of invertebrates, when Carl Woese published a paper announcing that the supposedly fundamental dichotomy of life—undiff erentiated unicellular organisms called prokaryotes or, at the time, bacteria, versus compartmentalized cells with membrane-enclosed genetic material, the eukaryotes—was not, in fact, a dichotomy. Woese had been trying to determine the evolutionary relationships among microorganisms and develop a universal system of taxonomic classification that reflected those relationships, something that had eluded biologists for more than a century. Rather than basing classification on scant morphological variations or metabolic diff erences that might or might not be discerned in bacterial cultures, he turned to the ultimate form of chemical taxonomy: diff erences between organisms’ most essential molecules, their genes. By painstakingly comparing sequences of nucleotides in ribosomal RNA molecules from hundreds of species of bacteria, Woese identified the particular nucleotide sequences that were common to all, thus defining them as bacteria … and then he came across a handful of species that were missing those sequences. They were all simple undiff erentiated cells that looked and acted like bacteria, but according to their genes they were as diff erent from bacteria as they were from lions or pine trees. What then? He assigned them a group of their own and soon found more species that fit right in, including all the known species of methane-producing organisms, as well as an odd assortment of extremists that thrived in bizarre environments where it seemed impossible that anything at all could live: halophiles living in extremely saline brines, thermophiles thriving in the near-boiling water of hot springs, and thermoaciophiles, who like their hot water spiked with acid. Woese named his new group archaebacteria, and would eventually propose a new taxonomy based on a universal phylogenetic tree that better reflected evolutionary relationships between three fundamental domains of life: Eubacteria or simply Bacteria, Archaebacteria or Archaea, and Eukarya.
Woese’s universal phylogenetic tree, 1987
In 1977, when Woese proposed the division of the prokaryotes—broadly defined as organisms whose cells lack internal membrane-bound compartments—into two distinct taxonomic groups based on their rRNA sequences, many microbiologists in the United States ignored or pooh-poohed the distinction as insignificant. But microbiologists in Germany had already noted that the cell walls of methanogens were diff erent from those of other bacterial cells, and gradually other evidence of fundamental diff erences began to accumulate … including one that caught the immediate attention of the organic geochemists in Strasbourg and Bristol. In 1979, just three years after Michel Rohmer tracked the hopanoids to bacterial lipids, biochemists in Canada and Italy confirmed that the methanogenic, halophilic, and thermophilic microorganisms in Woese’s archaea all had cell membranes with an entirely diff erent structure and lipid makeup than either the eukaryotes or the bacteria. In place of the usual phospholipids, where a glycerol molecule is linked to fatty acids by ester bonds, the archaeal membranes consisted of acyclic isoprenoid chains attached to glycerol by ether linkages. Ether bonds are generally more stable than ester bonds, so at first glance this appeared to be an adaptation to the extreme environments where archaea thrived. But it soon became apparent that the strange lipids were indicative of a more profound and universal difference between the Archaea and the other two domains of life.
One of the most widespread compounds was a diphytanyl glycerol diether with two 20-carbon phytanyl chains, which would come to be known as archaeol. Another common compound was a di-biphytanyl diglycerol tetraether wherein two 40-carbon biphytanyl chains were bound at both ends by ether links to glycerol molecules, so that instead of dangling free, the carbon chains were constrained in a long, narrow rectangular sort of structure. The biphytanyl chains themselves were distinct in that they consisted of two phytane molecules connected by a head-to-head link, instead of the usual head-to-tail isoprenoid link. Stranger still, the biphytanyl chains in the tetraethers of some thermophilic organisms contained one or more five-carbon rings, where the methyl side chain of an isoprenoid group had looped around to form a cyclic structure within the chain. How such compounds were assembled to form a cell membrane was not entirely clear, but there was some evidence that the tetraethers traversed the entire membrane to form a relatively dense, solid monolayer, rather than the fluid phospholipid bilayer of eukaryote and bacterial membranes. Whatever the case, it was the sturdy, idiosyncratic isoprenoid chains that caught the attention of organic geochemists in Bristol and Strasbourg. Acyclic isoprenoids in general seemed to be an archaean specialty. Among the nonpolar lipids found in sediments and crude oils, regular acyclic isoprenoid hydrocarbon chains with 15 to 35 carbon atoms, as well as a couple that had tail-to-tail links in the middle like carotenoid pigments, were found in such quantity that it seemed they must have some significant role to play in cell membranes—but to date there is no direct evidence that hydrocarbons lacking a polar group can, as such, form part of a membrane.
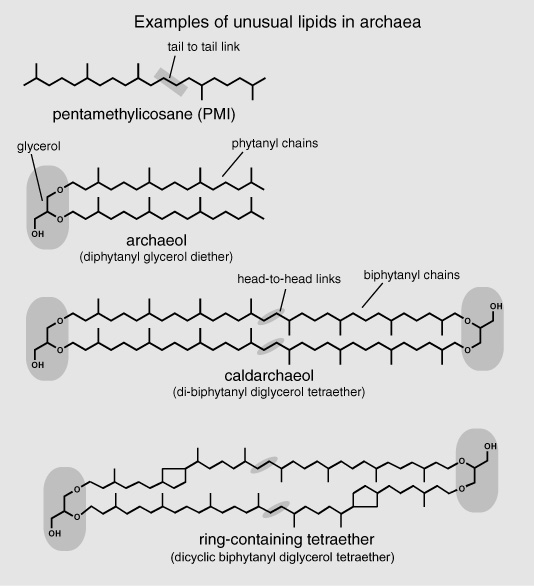
When the archaeal membrane lipids started making news in 1979, there were, as it happened, a number of molecules languishing in Strasbourg’s so-called molecule orphanage that bore a striking resemblance to the weird biphytanyl components of some of the archaeal lipids. They had been discovered in the Messel shale and relegated to anonymity by a visiting German postdoc back in 1972, when Pierre Albrecht and the Strasbourg crew were trying to get a better idea of what, exactly, was in the shale’s kerogen. Walter Michaelis was trying out various chemical treatments to break the bonds in the kerogen and then running the compounds he generated on the GC-MS and figuring out their chemical structures. The reagent boron tribromide, which was strong enough to break all the oxygen bonds, released a preponderance of acyclic isoprenoids, including 40-carbon head-to-head linked biphytanes—some of which had five-carbon ring structures woven in along their chains. Michaelis says that neither he nor anyone else in Strasbourg could find any clues as to the source of these strange compounds, and when his fellowship ended, he left Strasbourg disappointed, assigning them to orphanhood.
In 1979, however, not long after the Italian biochemists who were studying archaeal membranes reported the structures of the biphytanyl diglycerol tetraethers, Moldowan and Seifert at Chevron found a series of ring-containing biphytanes in their petroleum samples. The biochemists had found the ring-containing compounds only in thermophilic organisms, which, the Chevron group postulated, could have lived in the maturing sediments when they reached oil formation temperatures. But what about the compounds Michaelis and Albrecht had found in the immature kerogen of the Messel shale? It had never reached oil formation temperatures, and its plethora of well-preserved animal and plant fossils were ample evidence that the ancient lake itself had not been particularly hot or overly acidic.
Michaelis arranged for a year’s leave from his position at Hamburg University and went back to work with the Strasbourg researchers. This time they worked on sediment extracts and immature oils, rather than kerogen, choosing samples of various ages that had been deposited in low-oxygen or anoxic environments resembling that of the ancient Messel Lake, places like the Cariaco Basin and the Black Sea. They isolated the ethers by thin-layer chromatography, used their chemical treatments to cleave the oxygen bonds—and obtained an entire series of acyclic isoprenoids with a clear structural heritage from the archaeal lipids the microbiologists had isolated. The ether bonds were surprisingly resilient, as the biphytanyl diglycerol tetraethers persisted intact even in immature oils and Cretaceous age sediments.
Identifying methanogen lipids in these sediments was exciting, but not particularly surprising, because the samples came from the sorts of anoxic environments where methanogens thrived. But tetraethers with ring-containing biphytanyl components were also prominent—and, again, none of the environments sampled showed any signs of having ever been hot enough to support thermophilic organisms. In a speculative review of microbial biochemistry published in 1982, Guy Ourisson, still marveling that a bunch of old rocks and mud had led the way to discovery of hopanoids in bacteria and had such an impact on biochemical and evolutionary theories, predicted that these ring-containing biphytanyl tetraethers in the sediments would eventually lead to discovery of new species of methanogenic archaea.
In the Bristol lab, news that some of the acyclic isoprenoid alkanes in ancient sediments might be fossil molecules of anaerobic, methane-producing microorganisms, rather than remnants of chlorophyll or carotenoid pigments, was enough to trigger a reassessment of the scientists’ collection of DSDP sediment samples. If they treated the polar fractions of their extracts to cleave the ethers, would they find the head-to-head linked biphytanyl chains or, perhaps, the distinctive isoprenoid alkanes with the tail-to-tail links? Biologists had known since the nineteenth century that there were microorganisms that produced methane and lived in waterlogged environments where there was lots of organic debris and no oxygen. In many marshy areas, the gas could actually be detected bubbling out into the atmosphere. But only recently, in the late 1970s, when marine chemists began analyzing the dissolved gases in water trapped in the pores of DSDP sediments, had it become apparent that such organisms also lived deep within marine sediments along continental margins, and even deeper beneath the sea floor in the open ocean. Sure enough, when the Bristol group focused on DSDP samples from core sections where methane had been detected, the nonpolar hydrocarbon fractions of the extracts consistently boasted large amounts of two distinctive acyclic isoprenoid alkanes: the 30-carbon squalane, and the 25-carbon pentamethylicosane known as PMI, both with a tail-to-tail link in the middle, and both among lipids of methanogens identified by the microbiologists. Here, finally, was a distinctive series of fossil molecules that seemed to reflect a particular group of microorganisms that could only live in certain environments—anoxic ones. But though PMI, and the diphytanyl glycerol diether known as archaeol, and the simple ringless biphytanyl diglycerol tetraether known as caldarchaeol, with its distinctive head-to-head isoprenoid linkages, were all added to the molecular lexicon in the early 1980s, it would be another 15 years before anyone employed that lexicon in a hypothesis-driven attempt to understand the production—and consumption—of methane in such environments.
Organic geochemists in the 1980s were almost as ignorant of sediment microbial life and its leavings as they were in the 1960s, when Pat Parker first noted that the iso and anteiso fatty acids in his Texas mud had been made by heterotrophic bacteria. They had gained some insight into diagenesis, much of it the result of microbial processes, and marine chemists working with the DSDP and ODP had been making routine measurements of dissolved gases and minerals—microbial waste products and energy sources—in the top few hundred centimeters of marine cores. But direct knowledge of the actual organisms or processes responsible was limited. It was impossible to link the compounds found in the DSDP stamp-collecting endeavors with specific groups of sediment microorganisms, or to disentangle the molecular fossil record of life and the environment in ocean surface waters from that of the sediment microbes. It had taken almost a decade, not to mention a good deal of serendipity, just to trace the hopanoids to a bacterial source—and then most of the specific structural variations in the compounds that Rohmer isolated were in the polar side chain, lost during early diagenesis and of little use in distinguishing bacterial processes that had been prevalent in ancient environments. The few microbiologists who had denounced their discipline’s subservience to medicine and set out to explore the microbial world in nature were still developing tools for their enterprise. Much of their work focused on microbial mats, partly because these were relatively simple ecosystems to study, and partly because they contained enthrallingly exotic organisms that could live in salty brines or near-boiling water and might, potentially, be analogues to ancient life-forms.
The motivations for Geoff’s long-standing interest in microbial mats were not much diff erent, though of course they had a molecular slant. Ever since working on Melvin Calvin’s Precambrian rocks in the 1960s, he had wondered if they might yet find molecular fossil clues to early life in the ancient stromatolites, if only they knew better what to look for—and what better place to find that out than in living stromatolites or, for that matter, microbial mats in general? On a more pragmatic front, microbial mats constituted relatively simple environments where diff erent types of microbes were, presumably, associated with visibly distinct layers that were dominated by a limited variety of organisms. This arrangement made it easier to link the mats’ molecular content with specific groups of photosynthetic or heterotrophic microorganisms, which, in turn, they hoped would help in interpreting the emerging molecular fossil records in marine sediments. But, finally, if one pushes Geoff to explain the logic of his microbial mat studies, he will admit that microbial mats are rather intriguing, slimy-looking things, usually growing in bizarre places where nothing much else will grow, and, as the refrain of 50 years goes, “We just wondered what was in them!” His first real encounter with the slimy things was in 1968, during a teaching stint with Pat Parker in Texas. Over the next decade, he analyzed samples whenever he could find an interested microbiologist to work with, and in the early 1980s he and a group of like-minded geochemists—Jan de Leeuw, Jaap Boon, and Al Burlingame among them—teamed up with a trio of Israeli, German, and Danish microbiologists who were studying the oldest living microbial mat ever discovered, deposited over the past 2,500 years in a shallow tidal lagoon known as Solar Lake, on the Sinai Peninsula.
The Solar Lake mat consisted of more than 70 centimeters of microbial organic matter, in which the geochemists identified a variety of carotenoid pigments, short-chain n- and iso-alkanes and fatty acids, and the mid-chain branched alkanes that appeared to be a cyanobacterial specialty. While they attempted to map the diff erent distributions of compounds in the layers of mat, the microbiologists tried to determine “who” was doing what in each layer—no easy task. This was a molecular-scale world, where a slight chemical gradient in the concentration of a nutrient or gas might be the only salient indication of activity, and the microbiologists had designed tiny electrodes that could be inserted directly into the living mat to measure oxygen concentrations, acidity, and sulfide. The mat layers, they found, were not only less homogeneous than they appeared to be, but also entirely interdependent—the left overs from one layer were the next one’s raw material, and one organism’s waste or refuse was another’s most coveted energy source. Ecology, it seemed, was key. The microbes lived in tight-knit, medieval-style communities whose members were as reliant on each other as weaver and tailor, or winemaker and tippler—isolate them in a laboratory culture, and they were likely to change operations or cease to function altogether.
Microbial mats from around the world

Though most of the compounds the geochemists identified in the Solar Lake mat were either too unspecific or too rapidly transformed by diagenesis to be of much use as biomarkers in the geologic record, their patterns and distributions could, potentially, off er quite a few clues about the living organisms, something that caught the attention of the project’s microbiologists—particularly that of the Dane, Bo Barker Jørgensen, who would collaborate with organic geochemists off and on for the next 20 years. The lipid analyses also impressed a microbiologist who had nothing to do with the Israeli mats at all but was, rather, working at Montana State University and studying microbial mats in Yellowstone National Park. Like the Solar Lake microbiologists, Dave Ward had long suspected that laboratory culture techniques were not telling the whole story when it came to microbial communities, even apparently simple ones like those in the mats he was studying. He says he’d been generally skeptical of laboratory cultures ever since he’d taken a class in aquatic chemistry: the range of temperatures, pH, nutrients, and other chemical constituents in one small sample of water from a local lake were so far removed from the carefully contrived conditions he had to use to get lake bacteria to grow in the laboratory that it was hard to believe his cultured organisms could have played much of a role in the microbial life of a real lake.
When Ward heard about the Solar Lake studies in 1982, he was studying the microbial mats that grow in and around Yellowstone’s hot springs, where the extreme heat and bizarre hydrothermal chemistry presumably limited the range of organisms that could survive. And yet Ward suspected that there was more to the mats than met the eye—that they were far more complex than they appeared under the microscope, and that the few organisms that adapted well to life in a laboratory culture were misleading him. He was thinking about trying to extract the fatty acids from thin slices of mat, to see if the distributions would allow him to diff erentiate and quantify the organisms living in diff erent layers, when he went to a conference at Woods Hole and heard Geoff’s report on the Solar Lake mats. “It was awesome,” Ward says, summing up his original impression, which was that there was a lot more to the molecular composition of the mats—and the information it might provide—than he had realized. He immediately queried Geoff about taking a year’s sabbatical leave in Bristol to learn the methods.
Geoff, of course, was keen on any project that involved something as beautiful and exotic as Yellowstone’s hot springs, not to mention close collaboration with a young and upcoming microbiologist who knew the hot spring mats as well as they could, to date, be known. He and Ward scheduled a sabbatical stay for Ward in Bristol, and a visit to Montana for Geoff —but the summer before the sabbatical, Ward received a call from a well-known molecular biologist who also wanted to visit Yellowstone and, as it turned out, was developing a technique for in situ study of microbes that looked even more promising than lipid biomarkers.
Norman Pace was one of Woese’s most stalwart supporters and a principal contributor to his growing, rRNA-based phylogenetic scheme. Like Ward and the Solar Lake group, Pace and his colleagues were trying to develop ways to characterize populations of microorganisms within their natural environments, and it had occurred to them that they might be able to apply their rRNA techniques directly to the mixed populations in water and sediments. They wanted to collect water samples from the Yellowstone hot springs, filter out the microbes and extract their nucleic acids, isolate and sequence the rRNA, and compare the sequences to those in the phylogenetic database of microbial species. If the sequences belonged to known species of microbes, then they could determine the relative abundances of those microbes in the hot spring microbial community. If they discovered new sequences, they would gain some knowledge of the diversity of organisms in the hot spring environment; and if they could fit those new sequences into the existing phylogenetic tree of evolutionary relationships, they might gain a few clues to the physiology and metabolism of the new organisms. That was the idea, anyway. It was precisely what Ward was looking for: he arranged to spend most of his sabbatical at Pace’s lab in Denver, and feeling a bit chagrined, apologized to Geoff and scaled back his Bristol stay to a few months. That was enough, however, to launch a long series of collaborative projects with organic geochemists—first with Bristol, and then later with NIOZ.
The Yellowstone hot springs were typical of the strange environments that archaea were known to be fond of, and several species had already been isolated by microbiologists. When the Montana-Bristol alliance started work in 1984, both Geoff, in his molecule-collecting fervor, and Ward, who had spent years studying methanogenic archaea, were eager to look for distinctive isoprenoid ether lipids. They started with an alkaline hot spring mat where methanogens were clearly active. Working with students and postdocs from both universities, they identified archaeol and caldarchaeol, both among the lipids in the one species of methanogenic archaea that had been isolated from the mat and, it was beginning to seem, common to most if not all archaea. But they also found the strange ring-containing biphytanyl compounds, which none of the cultures of organisms grown from the mat contained. The same compounds turned up again in an acidic hot spring mat, but again, none of the known mat organisms contained them. It seemed that even these simple, well-studied, contemporary microbial mats harbored organisms that Dave Ward and the various other microbiologists who’d spent years scrutinizing them were completely unaware of.
Much of the interest in the Yellowstone microbial mats focused on their intriguing assortment of primary producers, photosynthetic organisms that were likely to bear a strong resemblance to those that colonized a young, unsettled earth. Years of culture work and microscopic observation had led microbiologists to conclude that the top few millimeters of each mat was dominated by one or two species of photosynthetic organism, depending on the temperature and chemical makeup of the particular hot spring: eukaryotic algae in relatively cool acidic springs, thermophilic cyanobacteria in alkaline hot springs, and thermophilic anoxygenic photosynthetic bacteria—which utilize reduced sulfur compounds and don’t generate oxygen—in sulfidic springs. The lipid analyses indicated that the only mats to contain significant amounts of sterols were those formed by eukaryotic algae, in keeping with observations that, of the three domains of life, only members of the Eukarya made sterols. The productive layers of mats formed by anoxygenic photosynthetic bacteria were characterized by distinct series of C29–C33 alkenes and of C28–C38 wax esters. The mats made by cyanobacteria sported the same set of mid-chain branched 17-carbon alkanes that had been found in the Solar Lake mat. The alkenes and wax esters weren’t of much use to geochemists trying to read the geologic record, because the distributions were quickly lost to diagenesis and would, in any event, be obscured by inputs from eukaryotes in more complex environments. But the mid-chain branched alkanes were distinctive and persistent, and had even been identified among the hydrocarbons in Precambrian rocks and stromatolites—putative evidence for the presence of cyanobacteria on the early earth.
For microbiologists, one of the most important contributions of the Montana-Bristol alliance, and later of the Montana-NIOZ alliance, was in understanding the structure of microbial communities and how they depended on the flow of energy. Just as the Solar Lake studies had shown, the mat layers were not as distinct as they appeared, and organisms could actually move up and down in the mat, following the diurnal to nocturnal shift in chemical gradients created by the photosynthetic organisms. But the distributions and relative amounts of compounds did reflect the distribution of organisms from various links in the food chain— photosynthetic organisms enjoying the sunshine at the top of the mat, aerobic heterotrophic community members performing the first round of decomposition beneath them, and then anaerobic fermentative microbes breaking up the energyrich sugars and carbohydrates, while the sulfate-reducing bacteria and methanogenic archaea mopped up the left overs at the bottom. The lipid distributions also provided clues to the microbes’ respective metabolic roles and the flow of energy in the mat. In an alkaline hot spring mat where primary production was shared by cyanobacteria and anoxygenic photosynthetic bacteria, mid-chain branched alkanes, wax esters, and alkenes, as well as straight-chain C16 and C18 fatty acids, were in greatest abundance. The C15 and C17 iso and anteiso acids typical of aerobic heterotrophs and fermentative bacteria were next in abundance. And the alkyl glycerol ethers associated with sulfate-reducing bacteria, which depend on small two- and three-carbon organic compounds from the heterotrophs and use sulfate instead of oxygen, were present in trace amounts, as were the isoprenoid glycerol ethers typical of methanogens, which compete with sulfate reducers for small molecules from the heterotrophs or use the CO2 they generate.
The lipid work on the Yellowstone mats served as the basis for what is now a useful and actively growing lexicon of microbial lipid biomarkers—in large part because of the addition of the isotopic dimension, on the one hand, and the gene-based techniques that Pace and his colleagues had been developing, on the other. Geoff was at the Montana lab when Ward obtained his first rRNA sequence from one of the surface layers of the mats they had been studying. Ward excitedly read off the new sequence of nucleotides, and Geoff compared it to that from the one species of anoxygenic phototroph that had been isolated from the mat—a distant relative, as it turned out, of the one Ward had just discovered. By 1990, Ward’s survey of the rRNA gene sequences in the mats’ surface organisms made it clear that what had appeared to be virtual microbial monocultures were, in fact, composed of many diff erent, hitherto unknown species. In one of the most thoroughly studied mats, the species of cyanobacteria and anoxygenic phototrophs that had appeared to share exclusive rights to the mat’s primary production were not even the most prevalent primary producers. As Ward had long suspected, the organisms he and his colleagues had isolated in cultures were misleading them about the diversity and ecology of organisms in even these most rudimentary of microbial communities.
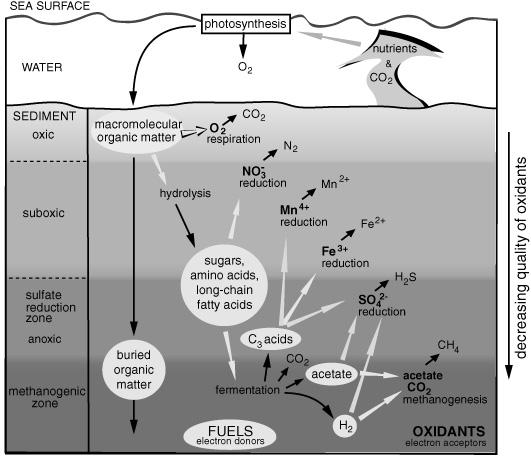
Meanwhile, some headway had been made in identifying microorganisms responsible for the breakdown of organic matter in marine sediments, though understanding was still largely based on what could be deduced from the chemistry of the sediment pore water, namely, changes in concentrations of the dissolved salts and gases that could be used and produced by microbes. These studies had identified a general hierarchy of microbial processes similar to that in the microbial mats, except that primary production and the first steps of decomposition occurred in the surface waters of the ocean and were separated by thousands, rather than thousandths, of meters from the sediment processes. Moving downward into the sediments, the hierarchy of processes was characterized by the changing availability of oxidants available to burn the various microbial “fuels”—electron acceptors and electron donors, respectively. When all the oxygen has disappeared, the next best oxidant, nitrate, was used, and then manganese, ferric iron, sulfate, and, finally, the fermentative bacteria could use components of the organic matter to oxidize other components, and the methanogens could even use CO2 as an oxidant. It was the combination of available oxidants and fuels that called the shots for microbial activity, and as the best oxidants disappeared, the amount of energy available decreased and the fuels that could be used became more limited. Again, it was soon apparent that one organism’s waste was another’s fuel, and in some cases, what was an oxidant for one process could be a fuel for another.
Microbial breakdown of organic matter in marine sediments
Even before microbiologists had a good idea of precisely what organisms were doing and how, the importance of sediment microbial ecosystems in regulating the chemistry and biology of the ocean was apparent. One of the big discoveries of the DSDP and ODP in the 1970s and 1980s was that huge amounts of methane were being produced in the anoxic sediments just below the zone of sulfate reduction, particularly in areas with a high input of organic matter such as along the continental margins. Geologists had long known that there were large stores of the ultimate, one-carbon hydrocarbon trapped deep in the rocks, but they’d assumed it came mostly from petroleum source rocks that had been buried and heated so extensively that all the carbon–carbon bonds had cracked in some portion of the bitumen. Methane that was clearly associated with petroleum deposits and clearly thermogenic had δ13C values around –40 per mil. Most of the methane in marine sediments, however, turned out to be decidedly more depleted in 13C, with δ13C values between –90 and –60 per mil. The studies of isotopic fractionation by methanogens that John Hayes and others did in the 1980s showed that methane generated by methanogens was about 60 per mil more depleted in 13C than the CO2 or small acids they consumed—both of which are generated by decomposition of organic matter in the sediments and already somewhat depleted in 13C relative to the CO2 in the surface waters. So methane with a δ13C value that was more negative than –60 was pretty likely to have come from microorganisms, and not from the thermal cracking of oil.
The chemists, geologists, and oceanographers of the DSDP and ODP explorations were more interested in what happened to all the methane being generated in the sediments than they were in the details of the organisms that produced it. Their measurements indicated that the dissolved methane accumulated until the sediment pore water became saturated, and then free gas began to accumulate, as well. As the sediment was buried and the pores contracted, the methane was squeezed out and migrated slowly upward in the sediments. In some areas along continental margins, large deposits of methane had accumulated beneath the surface from organic-matter–rich sediments that were deposited millions of years ago; sometimes dissolved methane and bubbles of gas could be detected rising from the seafloor, forced to the surface by compaction, or by seawater circulating through cracks created by tectonic activity. If conditions were right in these areas, methane could also be found immobilized as a strange icelike solid in the top few hundred meters of sediment. This methane hydrate, where molecules of methane were trapped in a cage of water molecules, had long been known to chemists as a curious chemical phase—ice that could catch fire!—which formed under very specific conditions of gas concentration, temperature, and pressure. But the explorations of the 1970s and 1980s revealed that the requisite combinations of high methane production, low temperatures, and high, but not too high, pressure attain in the sediments of many of the world’s oceans: along continental margins, near places where tectonic plates converge, in polar seas, and in some shallow basins, sediments are loaded with flammable ice, sometimes embedded up to 500 meters deep, sometimes exposed on the sediment surface, and occasionally piled up in great mounds.
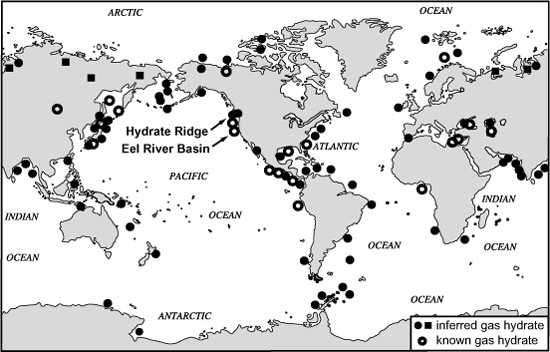
The sheer magnitude of this cache of potentially accessible natural gas was a surprise to geologists and petroleum companies alike. In a 1995 review, Keith Kvenvolden, who had left NASA for a job with the U.S. Geological Survey in the mid-1970s and spent years exploring for and studying methane hydrates, estimated that they contained twice the amount of carbon and energy potential of all known reserves of fossil fuels. But the thing that had geochemists and microbiologists puzzled for more than two decades was that most of the methane they detected rising through the sediment pores neither froze into methane hydrate nor seeped into the sea, but rather disappeared altogether. Something, it seemed, was eating a good portion of the methane produced by the methanogens.
Microbiologists had isolated a number of species of methane-consuming bacteria from soils and freshwater lakes, places where methane generated by methanogens in anoxic sediments seeps up into the oxic sediments or water. But none of these methane munchers, the so-called methanotrophs, could function without oxygen, and the methane in the marine sediments disappeared well within the anoxic sediments, far from any trace of oxygen. Furthermore, microbiologists contended that an organism that consumes methane in the absence of oxygen was absolutely inconceivable, that the process simply couldn’t generate enough energy to be biochemically viable—that it was, in short, impossible. But impossible or not, the geochemists said, such an organism must exist, because methane was disappearing en masse, and there was simply no place else for it to go. In the mid-1980s, their studies showed in no uncertain terms that this disappearing act occurred when the methane reached the band of suboxic and anoxic sediments where sulfate-reducing bacteria live. When Danish microbiologists finally honed in on the methane munchers in organic-matter–rich sediments from the Kattegat Strait, between Denmark and Sweden, they suggested that these organisms were somehow dependent on the sulfate reducers—but the organisms themselves eluded detection, and exactly how they accomplished their impossible task remained a mystery.
In the meantime, Hayes and his colleagues were finding anomalous depletions in the 13C content of the organic matter in ancient rocks—well beyond diff erences of a few per mil associated with changes in CO2 concentrations or phytoplankton productivity—which suggested that there were places and times in the earth’s history when the highly depleted methane generated by methanogens was being consumed by methanotrophic organisms of some sort and channeled back into the food chain. The first compound-specific isotope analyses in the Messel shale had revealed hopanoids and unidentified isoprenoids with δ13C values as low as –60 per mil, which had to include a sizable contribution from methanotrophic organisms of some sort. A similar study of the Green River shale that Roger Summons and one of Hayes’s winter migrant workers did in Australia turned up hopanoids that were even more severely depleted in 13C—including one that was not only isotopically but structurally distinct, with a δ13C of –85 per mil and a methyl group attached at the number 3 position of the A ring. Rohmer had found this structural quirk in the hopanoids of only two of the hundred strains of bacteria he’d analyzed—and both of them were methanotrophs. Presumably, such organisms had lived in the oxic surface waters of the ancient Green River and Messel lakes, consuming methane that bubbled up from the sediments or deep water, much as they did in the modern lakes where they’d been discovered.
Hayes figured that if, as appeared to be the case, there were methane-munching organisms that lived in anoxic sediments, then they would also leave behind lipids that were severely depleted in 13C and, with any luck, had chemical structures that provided clues to the identity of mystery microbes—though what sort of structures and what sort of clues those might be, he had no idea. He wrote up what he says was a shamelessly vague proposal for a collaboration and sent it to Bo Barker Jørgensen, one of the authors of the Kattegat Strait study. But Jørgensen didn’t need much convincing. He obtained another Kattegat Strait core, and one of Hayes’s students did an exhaustive survey of the lipids and their isotopic signatures in sediments from each zone of microbial action that the Danes defined. In his 1992 thesis, Liangqiao Bian notes that only one compound showed unequivocal signs of having been made by any sort of methane-consuming organism. It was present only in the narrow strip of anoxic sediment where Jørgensen detected methane oxidation, and it was even more radically depleted in 13C than the methane in the sediments—but it wasn’t a hopanoid like any of those found in aerobic methanotrophs. It was, rather, a branched alkane that was inseparable from phytane on the GC column and might have eluded detection altogether, if not for the isotope measurements and the carefully defined zones of microbial action in the core. In Bian’s chromatograms, a subtle change in the shape of the phytane peak for sediments from the zone of methane oxidation and an accordant shift in the δ13C from –30 to –80 per mil hinted at the presence of a hidden compound.
Methane oxidation and carbon-13 depleted crocetane in Kattegat Strait sediments
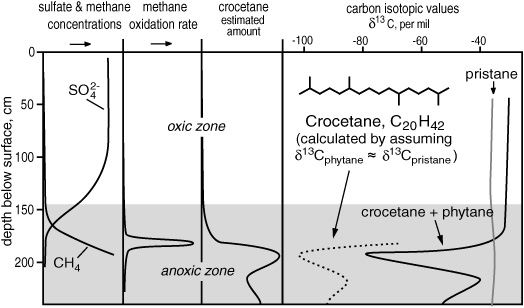
Once they noted its presence, it was possible to obtain a mass spectrum and determine the structure as that of crocetane, a 20-carbon acyclic isoprenoid like phytane, but with a tail-to-tail link in the middle, or like PMI but minus a five-carbon isoprene unit, depending on how one looked at it. Crocetane had never been isolated from any organism, but it could well have been present, even abundant, in sediments and oils and gone undetected, hidden by the omnipresent phytane. When the overlapping peaks were accounted for, the crocetane had a δ13C that ranged from –60 to –100 per mil: it clearly hailed primarily from the organisms responsible for oxidizing methane in the anoxic sediments and was, in fact, the first real physical evidence of their existence. But what were they? Bian wrote a fine master’s thesis in 1992 but immediately abandoned geochemistry for the pharmaceutical industry, and Hayes left the problem of anaerobic oxidation of methane to the microbiologists to figure out—or so he thought.
Meanwhile, Roger Summons had been inspired by the results of the Green River study, among other things, to form another cross-Pacific alliance, this time with Linda Jahnke, a microbiologist who was growing cultures of methanotrophic bacteria and cyanobacteria at NASA’s Ames Research Center in California. Summons had been noting for some time that extended hopanes with an extra methyl group on the A-ring were found only in certain types of ancient rocks and oils, and the distinct methanotrophic isotope signature of the 3-methyl hopanes in the Green River shale fueled his mounting suspicion that the hopanoids in sediments and ancient rocks might harbor more information than they were given credit for. Lipid analyses of Jahnke’s bacterial cultures revealed that many cyanobacteria make extended hopanoids with a methyl group on the number 2 carbon of the A-ring, and the methanotrophs contain large quantities of extended hopanoids with the distinctive 3-methyl ring structure, as well as exceptionally large amounts of the common 30-carbon hopanoids diplopterol and diploptene.
Neither of these embellishments to the hopanoid ring structure had been found in other types of bacteria—and both clearly survived diagenesis and were preserved for posterity in ancient rocks and oils. Studies of isotope fractionation by the methanotrophic bacteria indicated that the hopanoids were as much as 30 per mil more depleted in 13C than the methane the bacteria consumed—if the bacteria lived in marine sediments, their lipids might be expected to have δ13C values of –90 to –120 per mil and be easy enough to spot. Further investigations of isotope fractionation in the cultures, however, would show that all methanotrophic bacteria were not created equal: the two known families employed two diff erent carbon fixation pathways, and the amount of fractionation thus diff ered significantly between them. Like carbon fractionation by CO2-consuming algae, fractionation by methanotrophs also depends on methane concentration. And the δ13C values of the methane itself can be quite variable, with values of –30 per mil and even higher reported for thermogenic methane in a few areas. Extensive uptake by methanotrophs can also leave the residual methane that seeps into surface sediments or water bodies substantially enriched in 13C. Under certain conditions, then, at low concentrations of relatively heavy methane, the δ13C values of hopanoids from certain species of methanotroph could conceivably be as high as –35 per mil, making them hard to distinguish from the hopanoids of other bacteria. As John Hayes is fond of saying, “There are no magic numbers.” Still, there was a reasonably good chance that an active population of methanotrophs in the sediments or water of a marine basin would have left a distinct hopanoid footprint in the sediments, much like that observed in the Green River shale— 3-methyl hopanoids that were over 50 per mil more depleted than the biomarkers from photosynthetic algae—and that was something both Hayes and Summons were keen on tracking.
In the early 1990s, as Summons and Jahnke were beginning their studies of methanotrophic bacteria—and as Freeman, Jasper, and Pagani were correlating alkenone εP values with CO2 concentrations, Kvenvolden was tallying the distributions of methane hydrates, and Hayes was puzzling over isotopic anomalies in the kerogen of Precambrian rocks—it was fast becoming apparent that the amount of methane in the atmosphere varied dramatically over the course of earth history and, alongside CO2, played a principal, if not starring, role in determining climate. Analyses of the gases in the Greenland and Antarctic ice cores were showing that the amount of methane in the atmosphere had varied in concert with Milankovitch cycles and with abrupt climate changes over at least the past 220,000 years. And in 1995, a group of geologists in Michigan published a study in which they hypothesized that one of the most abrupt climate changes evident in the geologic record was due to the sudden release of a massive amount of methane from marine sediments.
In Michigan, Gerald Dickens and his colleagues were trying to ascertain what had happened to the earth’s climate 55 million years ago, during the transition from the Paleocene to Eocene epoch—just prior to the long sustained cooling that led from a greenhouse into an icehouse world. δ18O measurements indicated that temperatures rose dramatically, and globally, within a few thousand years, while the δ13C values of foram carbonate dropped to significantly more negative values, implying that a large amount of 13C-depleted carbon had been released into the oceans. Dickens proposed that a slight heat wave had turned into a cataclysmic global climate change when water temperatures in continental shelf areas rose above the limit for methane hydrate stability and massive amounts of free methane gas were suddenly released into the sediments, ocean, and atmosphere by disintegrating hydrates. The hypothesis was compelling, but there was no direct evidence for methane in the atmosphere or ocean at the time, and the authors could foresee no way of obtaining it.
John Hayes was preparing to move his laboratory from Indiana to Woods Hole when the Dickens paper was published, but its methane hydrate hypothesis was still nagging at him nearly two years later, when he was well settled into his new lab, replete with a new coterie of students and postdocs. Wouldn’t such a massive release of methane into the world’s oceans have resulted in a population explosion of methanotrophic organisms? And wouldn’t they have left their mark in the sediments in the form of 13C-depleted hopanoids? Someone should really take a look at the biomarkers in the Paleocene-Eocene sediments and see if there were any signs of methanotrophs. Jürgen’s student Kai Hinrichs, who went to work on a postdoctoral fellowship with Hayes in 1997, says he couldn’t figure out why Hayes kept giving him papers about methane to read when he was supposed to be working on the alkenone CO2 proxy, expanding on John Jasper’s work. Hinrichs had written a proposal to do alkenone εP measurements in Pleistocene sediments from diff erent geographic locations and determine if and how oceanic sources and sinks for CO2 had changed over time. Hayes had been enthusiastic enough about it when they’d talked the year before, but once Hinrichs got to Woods Hole, the project seemed to languish. For his part, Hayes was concerned that the emerging problems of distinguishing CO2 concentration from growth rate in alkenone εP values would hamper the success of his postdoc’s project … but he was also eager to follow up on Dickens’s paper. Whatever the case, Hinrichs says, getting him interested in methane was one of John Hayes’s most valuable contributions to his career—though it wasn’t immediately apparent.
They requested as many Paleocene-Eocene sediment sequences as they could find in the DSDP and ODP core repositories, but, unfortunately, most of them had come from open ocean sites with low productivity and scant organic matter. “They were really boring,” Hinrichs says. “No peaks, nothing to look at.” A few months into the project, however, Hayes went to a meeting in San Francisco and was offered samples that were a little more interesting—not of Paleocene-Eocene sediments, but of contemporary sediments where methane hydrates were decomposing in real time and methane was actually bubbling into the water.
Oceanographers had been finding these methane seeps along the continental margins since the 1980s, and explorations with the early research submersibles had revealed that they supported oases of life similar to those at the deep sea hydrothermal vents. In the fall of 1997, the Monterey Bay Aquarium Research Institute was making preparations to explore one such seep in the Eel River Basin using an innovative new submersible—as Hayes learned when he got to talking with the institute’s director at the American Geophysical Union meeting. This was a site where methane hydrates were right at their threshold of stability, actively releasing methane into the water—a small-scale, real-time version of the global event that Dickens had proposed to explain abrupt climate and ocean chemistry changes at the onset of the Eocene period. When the institute director offered Hayes samples, he jumped at the chance. Hunting for biomarker evidence of such an event in 55-million-year-old sediments was a little like hunting for fingerprints when you weren’t even sure a crime had been committed, let alone what sort of creature the criminal might have been. But here was a chance to examine the scene of a crime-in-progress, so to speak. They could at least see how evidence of a massive release of methane from methane hydrates 55 million years ago ought to look.
There was plenty of organic matter in the Eel River Basin sediments that Hinrichs analyzed, and lots of methane … but no 13C-depleted hopanoids. The only compounds of interest, he says, showed up as two large peaks with δ13C values of –100 and –110 per mil. They eluted from the column where the hopanols should have been, but they weren’t hopanols. He had treated the extract with a reagent that reacts with alcohols to produce derivatives that are more amenable to gas chromatography, and according to the mass spectra of these derivatives the two compounds were glycerol ethers, now rendered nonpolar and volatile enough to get through the GC column. Hinrichs says he called Jürgen in Oldenburg for help in pinning down the structures, and Jürgen directed him to a paper reporting the mass spectrum for archaeol—but then the isotope machine at Woods Hole broke down and he was left with nothing to do except contemplate his two compounds and read about methane. That was when he started thinking seriously about the lack of oxygen in the Eel River Basin sediments and suspecting that his 13C-depleted compounds might have come from anaerobic organisms that were consuming methane in the anoxic sediments, rather than aerobic methanotrophic bacteria at the sediment surface or in the water.
Meanwhile, back in Germany, a graduate student at the University of Kiel was analyzing sediments from a spectacular methane seep system just to the north of the Eel River Basin, a place off the coast of Oregon where one submarine ridge was literally paved in methane hydrate. Though Marcus Elvert had studied organic chemistry, he ended up doing his Ph.D. with Erwin Suess, a marine geologist who had been exploring the Hydrate Ridge region since the 1980s. Like many of the methane seep sites on the continental margins, it was a subduction zone where two tectonic plates converged and the oceanic plate slipped beneath the continental one to be reabsorbed by the mantle. Suess was among the first to observe the methane bubbling up from the seafloor and the fantastic communities of giant clams, tubeworms, crabs, and carnivorous fish that were thriving in the vicinity, on the otherwise barren seafloor. He was also one of the first to realize that the carbon in everything, from the tissues of the mussels to the surrounding carbonate minerals, was severely depleted in 13C. Suess was interested in understanding the geology of the area. He wanted to know how such systems had evolved over time and where on the globe they had existed in the geologic past. And he wanted to know what happened to all the free methane gas that was bubbling out of the sediments—how it was channeled back into the biosphere, and how the 13C-depleted carbonate had formed. Despite the ecosystem’s apparent likeness to a hydrothermal vent ecosystem and the thick mats of sulfide-oxidizing bacteria covering the exposed methane hydrates, this was clearly a place where methane, not sulfide, called the shots. Suess charged the young organic chemist he enlisted in 1996 with the specific task of identifying biomarkers for methanotrophic bacteria, in the hope that they might shed some light on both the biological and geological questions the methane seeps had raised.
Just as the hydrothermal vent organisms relied on symbiotic sulfide-oxidizing bacteria, at least some of the animals in the methane seep ecosystem clearly relied on symbiotic methanotrophic bacteria. Colleen Cavanaugh, who had discovered the tubeworms’ symbiotic sulfide-oxidizing bacteria, found that the gills of mussels from a methane-seep community contained rRNA genes related to those of methanotrophic bacteria, and Summons and Jahnke had found relatively large amounts of 13C-depleted hopanoids and diploptene in the gills of the same organisms. But the ecosystem’s dependence on methane extended far beyond these symbiotic relationships. The fluxes of methane and sulfate in the Hydrate Ridge sediments indicated that, in addition to the methane bubbling and seeping out of the sediments, a lot of methane was being consumed by some mystery organism deep in the sediments—which, like the Eel River Basin sediments, were anoxic almost to the sediment–water interface—and there was, at the same time, an inordinately active population of sulfate-reducing bacteria that produced an excess of sulfide … which, presumably, fed the extensive mats of sulfide-oxidizing bacteria that covered the sediment surface.
Suess was a marine geologist who did most of his research at sea and had little in the way of laboratory facilities, so he sent Elvert to use the instruments in Michael Whiticar’s lab in Canada, which included a new isotope-ratio-monitoring mass spectrometer. Whiticar himself had been an early student of Suess’s and had been studying the production and consumption of methane since the 1980s. Unlike Hinrichs, Elvert was specifically looking for anaerobic methanotrophs before he even extracted his first Hydrate Ridge sample. Like Hinrichs, however, he started out looking for hopanoids. None of the anaerobic bacteria that Rohmer had examined contained hopanoids, and he and Ourisson had postulated that anaerobes were incapable of making them. There was, however, no specific requirement for oxygen in the biosynthesis of bacteriohopanetetrol, and Elvert thought the methane-munching culprits might be hopanoid-producing methanotrophic bacteria akin to the ones found in mussel gills, aerobic bacteria that had somehow adapted to anoxic conditions in the sediments. He wrote to Roger Summons to request samples from Jahnke’s bacterial cultures—but not long after the cultures arrived, he analyzed his first Hydrate Ridge samples and realized that the hopanoids weren’t going to help him. There simply weren’t any. What there was, was a huge phytane peak—except the mass spectra showed clearly that it wasn’t phytane. It was almost pure crocetane.
Elvert had to wait his turn to use the much-coveted isotope machine in Whiticar’s lab, so weeks went by before he could determine the isotope content of his crocetane. But he knew he was onto something, because Whiticar had told him about a conference poster he’d seen of Bian’s unpublished Kattegat Strait study, which reported 13C-depleted crocetane in the methane-oxidizing zone of the sediments. Elvert changed strategies, turning his attention from hopanoids to crocetane. The Hydrate Ridge sediments were loaded with it, and when he finally got a turn at the isotope machine, he found it had a δ13C of –107 per mil—a sure sign that it was made by methane-consuming organisms. But what were they? This was the question Bian had been unable to answer in the Kattegat Strait study. Elvert, however, had a couple of extra clues. The Hydrate Ridge sediments contained two other isoprenoid alkanes that were equally depleted in 13C: PMI, and an unsaturated counterpart.
Elvert says he read everything he could get his hands on, trying to make sense of his data. Except for Bian’s work, crocetane was unheard of in organisms or sediments. But PMI was apparently synthesized by methanogenic archaea, and the Bristol researchers had found it in their assay of methane-rich marine sediments. Since then, microbiologists had managed to culture methanogens from marine sediments, and the NIOZ group had just reported finding various unsaturated versions of PMI in one of them. But what was going on in the methane seep sediments? Elvert’s PMI was too radically depleted in 13C to have been made by a methanogen. It had to come from something that was eating methane. But how could an organism be eating its own waste product? It was like a human suddenly inhaling CO2 and exhaling oxygen. Finally, he came across a paper by a group of microbial ecologists who had posited just that and offered an explanation: they proposed that the consumption of methane required a consortium of microbes working together, rather than a single organism, and that this consortium consisted of methanogens working in reverse with sulfate reducers to help them along. In other words, the methanogens could oxidize methane back to CO2 and hydrogen, if the sulfate-reducing bacteria immediately sucked up the hydrogen, eff ectively pulling the reaction in an otherwise energetically unfavorable direction.
Elvert was excited—the hypothesis explained what geochemists and marine chemists had been observing for years, the close association between methane oxidation and sulfate reduction that the Danes had demonstrated in the Kattegat Strait sediments. Now he had direct physical evidence to support it: lipids from methanogens that were eating methane. It seemed quite a coup, especially for a graduate student doing organic chemistry in a geology department! As he was preparing his paper for publication, Elvert says, he e-mailed Summons in Australia and asked what he thought of the idea. Summons didn’t take much convincing. “Yes,” he replied, immediately, “I think you’re right.” And then he added the crushing news that Elvert’s discovery was not, as he’d thought, entirely original. The reason Summons was so readily convinced was that he’d just been to a conference where he’d spoken to two scientists who had also found 13C-depleted methanogen lipids in methane seep sediments and had come to similar conclusions. One was Kai Hinrichs at Woods Hole. And the other was Volker Thiel, a postdoc working just down the road, in Walter Michaelis’s Hamburg lab.
By the time their three papers appeared in 1999, the three scientists had started to swap information, much as the Bristol and Dutch groups had done with the alkenones, or Strasbourg and Bristol with the extended hopanoids decades earlier—though in this case, there was a definite sense that the three young researchers were racing for first publication of their discovery. The Nature and Science reviewers, however, weren’t convinced by Thiel’s evidence, or by Elvert’s, and the two had to resubmit to other journals, whereas Hinrichs’s particular assembly of data was finally so compelling that it skated past the Nature editors and appeared promptly—with the result that the race for publication was rendered irrelevant and the first paper completed was the last to appear. Their evidence, in any case, was more complementary than redundant. And, as it turned out, parallel work had been going on under Jaap Sinninghe Damsté’s tutelage in the NIOZ lab, bolstering the case.
Thiel hadn’t been studying an active methane seep at all, but rather a limestone outcrop that showed signs of having formed in a shallow basin where methane seeps were active during the Miocene epoch. Unlike Hinrichs’s and Elvert’s contemporary methane seep sediments, his samples contained significant amounts of a severely 13C-depleted hopanoid. It appeared to be a diagenetic product of diploptene, with the double bond shifted two carbons over. Noting the preponderance of diplopterol and diploptene in the methanotrophic bacteria that Summons and Jahnke had been studying and the general lack of hopanoids in anaerobic bacteria, Thiel speculated that it came from aerobic methanotrophs that had lived in the oxic waters above the seep. But his samples also contained crocetane, PMI, and an unidentified isoprenoid ether lipid that were even more depleted in 13C than the hopanoid, with δ13C values of –105 to –115 per mil. Like Elvert and Hinrichs, Thiel concluded that these compounds had been synthesized by some hitherto unknown methane-consuming archaea. He also found a series of 13C-depleted alcohols that must have come from organisms that had utilized a methane-derived carbon source—though not, apparently, from the methanotrophic archaea that made the isoprenoids. They had δ13C values around –88 per mil, dramatically depleted compared to the rest of the organic matter, but decidedly more positive than the isoprenoid lipids from the putative methanotrophic archaea. Their carbon skeletons were the C14 to C18 iso, anteiso, and mid-chain branched ones typical of many heterotrophic bacterial lipids. Fatty acids with these structures had been found to be particularly plentiful in anaerobic sulfate-reducing bacteria, and noting the extensive evidence linking methane oxidation with sulfate reduction in anoxic sediments, Thiel speculated that these compounds derived from sulfate-reducing bacteria that coexisted with the methanotrophic archaea and somehow utilized carbon from their decaying biomass.
Hinrichs says that it wasn’t until he figured out the structure of the second isoprenoid ether in his Eel River Basin samples that he realized he was seeing geochemical evidence of a biochemical phenomenon that neither he nor Hayes had considered. The compound Jürgen had helped him identify was archaeol, the simple glycerol diphytanyl ether that was common to most archaea. This second compound, though similar, was not so common and took him longer to figure out—partly because he didn’t realize that the standard derivatizing reagent he’d used, which was supposed to react with the alcohol groups and make the molecule more volatile and amenable to GC analysis, had reacted with only one of the molecule’s two alcohol groups. The mass spectrum was difficult to decipher, and Hinrichs actually misinterpreted its fragmentation pattern in his 1999 paper— but he deduced the correct compound, and that was enough to put him on the right geochemical and biochemical track. It was hydroxyarchaeol, with an OH group attached to the second phytanyl chain, a compound that had been found only in methanogenic archaea and was, along with various isomers, a major component only in the order Methanosarcinales. A bit of library research revealed that Methanosarcinales included the most versatile of the known methanogens, organisms that could consume almost any small molecule available from CO2 to acetate, methanol, and … methane? If there was a methanogen that could reverse its chemistry to consume its own waste product, it would surely be related to the Methanosarcinales. Hinrichs, of course, was reading all the same literature as Elvert and Thiel … but he worried that his lipid evidence for such an organism was circumstantial at best. after all, gene-based explorations of microbes in natural habitats had been revealing a plethora of hitherto unknown archaea, thriving in places where no one would have expected archaea to thrive. None of these organisms had ever been isolated in cultures and had their lipids analyzed. How, then, could one argue that archaeol, PMI, crocetane, or even hydroxyarchaeol came from methanogens and not from some unknown, completely unrelated group of archaea? Eff orts to isolate and grow the anaerobic methanotrophs in culture had failed, and the genetic techniques were of little help because one didn’t know what processes were associated with the genes identified—until the microbiologists and geochemists started combining genetic techniques with lipid measurements and compound-specific isotope measurements.
Hinrichs recalls how Hayes “perked up his ears” when he told him what he’d found in the Eel River Basin sediments and his hypothesis about what it meant— and what he wanted to do next to find out if it was correct. Hayes had expected his postdoc to find 13C-depleted hopanoids in the seep sediments, not hunt down the elusive organisms responsible for the anaerobic oxidation of methane! Hinrichs had been talking to a microbiologist at Woods Hole who’d agreed to extract and analyze the rRNA in sediments from the Eel River Basin and try to identify the organisms responsible for the 13C-depleted hydroxyarchaeol—if Hinrichs could get more samples.
He was in luck: when he called the Monterey Bay Aquarium Research Institute, the director told him that he didn’t need more samples because Ed DeLong, a microbiologist there at the institute, had already extracted and sequenced the RNA from sediments at the same site. When Hinrichs called DeLong and explained his hypothesis about hydroxyarchaeol-producing Methanosarcinales eating methane in the seep sediments, DeLong hadn’t yet analyzed his data. But two days later, he called back, exclaiming into the telephone: “Wow, you’re right!”
The ribosomal RNA sequences indicated the presence of two distinct groups of archaea. None of them matched any of the sequences in the public database that had grown out of Woese’s phylogenetic scheme, but they all fell between or within three orders of methanogens, including Methanosarcinales, and one cluster of sequences formed a distinct group of its own. By this time, the very concept of “species” of microbes had fallen by the wayside, and the Linnaean system of taxonomy had been replaced by phylogenetics: rather than assigning a grandiose Latin scientific name to the new group of putative anaerobic methanotrophs, DeLong and his colleagues celebrated their discovery with the inglorious acronym “ANME 1.” The association between 13C-depleted lipids and phylogenetic group was still circumstantial, but it was enough to convince Hinrichs and his coworkers to submit their paper—and, apparently, to convince the persnickety Nature editors to accept it.
More definitive evidence for a link between the methanotrophic archaea and sulfate-reducing bacteria would come from the NIOZ lab, where Kate Freeman’s student Richard Pancost was working as a postdoc with Sinninghe Damsté. They were analyzing samples from active and extinct “mud volcanoes” in the Mediterranean Sea, places where tectonic pressure causes deeply buried, methane-laden sediments to erupt and flow out over the seafloor. Like Thiel, they found iso and anteiso compounds that were dramatically more depleted than sterols from the eukaryotes or the hopanol typical of heterotrophic bacteria, but less depleted than the acyclic isoprenoid lipids. This time, however, instead of alcohols, the compounds were iso and anteiso fatty acids—the same structures that had been isolated from a number of sulfate-reducing bacteria. Like Elvert, Pancost also found 13C-depleted crocetane and PMI, and their unsaturated versions, and like Hinrichs he found archaeol and hydroxyarchaeol. These compounds were all severely more depleted in 13C than any of the bacterial or eukaryotic lipids in the sediments … but the amount of depletion varied substantially and they appeared to come not from one, but from several species of archaea.
The 1999 papers had sparked a flurry of interest among microbiologists, much of it centered around the Max Planck Institute for Marine Microbiology (MPI) in northern Germany, now headed by Jørgensen. Though ANME continued to elude attempts to isolate it in culture, a group of researchers led by Antje Boetius at the MPI joined an expedition to retrieve fresh samples from the sediments of Hydrate Ridge—and attempted to photograph and count the ANME. They made fragments of RNA molecules that were complementary to the rRNA sequences identified in the Eel River Basin sediments, adding a fluorescent functional group so that they would appear red in the proper light. In what amounted to a needle-in-the-haystack search for the sulfate-reducing bacteria that appeared to be associated with ANME, they also made a set of molecules based on the rRNA of an assortment of sulfate-reducing bacteria, from all sorts of environments. When Boetius mixed these two sets of genetic probes into a Hydrate Ridge sample, they bonded with the complementary rRNA in their target organisms, rendering the archaeal cells red and the sulfate reducers green. The probes for DeLong’s ANME 1 group marked only a scattering of cells. But a probe designed to target another of the Eel River sequences—the one most closely related to Methanosarcinales— produced clumps of red cells that made up more than 90% of the biomass in the Hydrate Ridge sediments. The sulfate reducers were more difficult to find. Boetius says that she had tried 11 of the 12 probes for sulfate-reducing bacteria with no success, when, shortly before midnight and a bit blurry eyed from the long day at the microscope, she looked at her last slide: there were the globs of glowing red spheres, now surrounded by a shell of bright green ones—the long-hypothesized consortium of methanogen-like archaea and sulfate-reducing bacteria. The successful bacterial probe was based on an rRNA sequence that had been detected in polar marine sediments, but the bacteria themselves had never been grown in culture and were just as mysterious as the archaea.
In its Hydrate Ridge samples, the MPI team measured sulfate reduction and methane oxidation rates, and counted cells, and there was every indication that the ANME consortia were responsible for the oxidation of methane. Likewise, the team found all of the lipids that had been identified with the methane-oxidizing archaea—crocetane, PMI, archaeol, and hydroxyarchaeol—with δ13C values as low as –135 per mil, as well as iso- and anteiso-C15 acids with values of –63 and –75 per mil. But could one prove that the 13C-depleted lipids actually came from the organisms in the consortia, that the archaea were, in fact, consuming methane? Hinrichs initiated another collaboration, this time with Christopher House at Pennsylvania State University. House had been working with a technique that allowed him to make carbon isotope measurements on the tip of a microscopic pin, even directly in a single cell. Victoria Orphan, a student of DeLong’s, made fluorescent rRNA probes targeting both of the ANME organisms they had identified, and once the organisms were labeled and could be viewed on a microscope slide, House was able to determine the δ13C of individual ANME cells: here, finally, in 2001, was unequivocal proof that the two groups of methanogen-like archaeal genes did indeed belong to methane-consuming organisms.
Could there be others? Did diff erent organisms thrive in diff erent areas, as the lipid evidence seemed to imply? How did they function, what compounds did the members of the consortium exchange, and how? And the question that had captivated John Hayes and Roger Summons: Could these lipids or their fossil counterparts provide clues to the role methane played in the intertwined history of life, the carbon cycle, and climate over geologic time?
Exactly how the methanotrophic archaea function and how they interact with their bacterial neighbors remains, to date, unclear. Recent work indicates that some methane-oxidizing archaea may contain modified methanogen genes and enzymes, which supports the hypothesis that they reverse the chemistry of methanogenesis. One study of methane seeps in the Gulf of Mexico suggests that some of these organisms can actually switch modes between methanogenesis and the oxidation of methane, depending on environmental conditions. But the microbiologists at the MPI have now disproved the initial hypothesis that the sulfate-reducing bacteria utilize hydrogen produced by the methanotrophs as an energy source. How electrons, energy, and carbon are shuttled between the archaea and their partner bacteria remains unclear. The ANME are clearly using methane as their carbon source, but what do the sulfate reducers use? Why are their lipids so much more depleted in 13C than other heterotrophic organisms in their environment, and yet so much less depleted than the archaeal lipids? In sediments where the anaerobic oxidation of methane does not occur, sulfate reducers use small molecules left over from the decomposition of organic matter by other types of heterotrophic bacteria, and one hypothesis maintained that the sulfate reducers in the consortium derived their carbon from a mixture of this material and the 13C-depleted biomass of the methane-oxidizing archaea. But the MPI scientists found that when they incubated the Hydrate Ridge sediments and added typical organic substrates, the sulfate-reducing bacteria made use of none of them. Another hypothesis is that these particular sulfate-reducing bacteria are autotrophic and derive their 13C-depleted carbon from the CO2 produced by the methanotrophs.
The genes of a number of diff erent bacteria have now been associated with the anaerobic oxidation of methane, and at least three distinct groups of archaea have been identified. Orphan’s extended studies with the fluorescently labeled rRNA probes showed that the tight, hierarchical relationship displayed by the Hydrate Ridge consortia in Boetius’s first attempt is not the only way to go: some consortia are speckled balls where the two types of microorganisms are all mixed up, sometimes groups of methane-oxidizing archaea and sulfate-reducing bacteria live in separate neighboring clusters … and some ANME archaea have even been found living in lonely splendor, keeping their bacterial partners at arm’s distance, so to speak.
The variety of distinct organisms involved in the anaerobic oxidation of methane is reflected by the variety of archaeal and bacterial lipids that have now been associated with the process in diff erent environments. Clearly, a diverse assortment of organisms has, in fact, developed more than one strategy to accomplish the “impossible” task of oxidizing methane in diff erent anoxic environments.
Microbiologists now seem to take such diversity and resourcefulness in stride. “It would be pretty unusual if there were just one bug, or one set of bugs involved,” Mandy Joye told me when I met her at the Hanse Institute in 2003. “If these guys can do it, someone else probably can.” A specialist in microbes that live in extreme environments, Joye was on sabbatical from the University of Georgia, working with Boetius’s group at the MPI. Even a geochemist knows that “bug” is slang for microbe, but it’s not so easy, these days, to know exactly what a microbiologist means by “one” bug. Microbiologists now speak of “strains” and groups of microbes, and it seems that the only way to pin them down to a precise designation of an organism is to listen to the long litany of its evolutionary relatedness—sort of like listening to one’s aging great aunt recount the four generations of divorce, remarriage, illegitimate children, incest, and intercontinental immigration that somehow connect to a second cousin, once removed, who lives in the outer reaches of Mongolia.
Whatever their precise species, strain, or “gene cluster” compositions and metabolic strategies, it is now apparent that anaerobic methane-munching consortia are capable of oxidizing methane at phenomenally high rates and, in some environments, producing a sizable amount of biomass. The MPI group’s incubation experiments showed that both sulfide and bicarbonate are by-products of the consortia activities, which accounts for the extensive mats of sulfide-producing bacteria and precipitates of 13C-depleted carbonates that are so often associated with methane seeps. In the early 1990s, Russian microbiologists did microscopic studies of the microbial mats from the bottom of the Black Sea and posited that they were made up of methane-oxidizing archaea. More recent seafloor explorations of the Black Sea have revealed incredible reefs up to 4 meters high, and analyses with genetic probes show definitively that they are composed of consortia biomass and 13C-depleted calcium carbonate. This is a far cry from the marginal, energetically challenged, biochemically “impossible” organisms once imagined responsible for the disappearance of methane in anoxic marine sediments. According to recent estimates, they consume more than 80% of the methane that might otherwise make its way into the ocean and atmosphere. Aerobic methanotrophic bacteria play a relatively minor role, simply because methane is produced and stored in the deep anoxic sediments and must run the gauntlet of methane-munching archaea before it reaches the oxic surface sediments or water—there just isn’t a lot left over for the aerobic bacteria. Taken together, these populations of methanotrophs act as an efficient control valve on the amount of methane released from the ocean to the atmosphere: despite the huge amount of methane produced and stored in marine sediments, the ocean currently contributes only 3–5% of the global methane flux into the atmosphere. Indeed, most methane now comes from ever-expanding human activities such as rice cultivation, livestock raising, and fossil fuel production, and from the dwindling natural wetlands. But what opens and closes the microbial control valve in the ocean? How would it respond—how did it and how will it respond—to additional inputs of methane from the sediments?
When gas measurements in the ice cores first revealed fluctuations in the methane content of the atmosphere during the last two ice age cycles, climatologists hypothesized that they were linked to a climate feedback eff ect: warming caused increases in tropical rainfall and an extension of wetlands where methanogens were active, and the methane they produced escaped into the atmosphere and caused accelerated warming. But some geologists found this unconvincing, because the fluctuations in methane were closely correlated with episodes of rapid and excessive global warming that didn’t give enough time for wetlands expansion. They suggested that at least some of the fluctuations were caused by the sudden release of methane from “melting” hydrates in the sediments. There was, however, little compelling evidence to support either hypothesis until the spring of 2000, when researchers at the University of California published a study of sediment cores from the Santa Barbara Basin, off the coast of central California.
Geologist James Kennett and his colleagues presented evidence that there had been several sudden, episodic releases of methane into the basin during the past 60,000 years, and that these coincided with brief warm intervals during the same period. They attributed the methane to the sudden breakdown of methane hydrates in the sediments around the shallow edges of the basin and hypothesized that similar events of methane hydrate collapse in continental shelf sediments and shallow basins around the world were responsible for the bursts of methane evident in the ice core gases over the past 200,000 years—less cataclysmic, repetitive versions of the event Gerald Dickens had hypothesized to explain the sudden warming and change in carbonate chemistry at the turn of the Paleocene period 50 million years earlier. Kennett’s hypothesis was controversial, and the evidence—pronounced 13C depletion in the carbonate of planktonic forams in the sediments formed just prior to the warm periods—was ambiguous and limited. As Kvenvolden points out, it was not entirely clear if and how such large stores of methane hydrate could be regenerated rapidly enough to provide for the frequent releases postulated. The implications of the hypothesis were huge. Would the current, human-induced period of global warming trigger a similar collapse of methane hydrates, with a sudden release of methane into the atmosphere and catastrophically accelerated warming?
This time, Hinrichs didn’t need John Hayes to egg him on. To what extent methanotrophs living in the water and sediments of the Santa Barbara Basin would have responded to the hypothesized bursts of methane, and whether their molecular remains would be apparent in the sediments, was still anybody’s guess. But he now had a good idea of what those remains might look like. And, unlike the Paleocene-Eocene sediments he’d tried to analyze, the Santa Barbara Basin sediments were loaded with organic matter. He had done extensive analyses of that organic matter when he was a student with Jürgen, and it was anything but boring.
He chose a site in the middle of the basin, where there was no methane seepage and no methane hydrate at present, and a section of core that spanned a period in the middle of the last ice age, when, according to Kennett’s hypothesis, there had been several releases of methane from the breakdown of methane hydrates in the sediments around the periphery of the basin. In most of the section, molecular fossils of both aerobic and anaerobic methanotrophs were absent. But in the narrow bands of sediment that corresponded to periods of sudden warming, there were pronounced spikes in the concentration of diplopterol, largely absent from the rest of the section, with δ13C values between –55 and –73 per mil. It had to derive from bacteria that were recycling 13C-depleted organic matter and CO2 from methanotrophic organisms, or from the methanotrophic bacteria themselves, and because the bulk organic matter was not significantly depleted in 13C compared to that from primary producers, it probably came from the latter— though the 3-methyl hopanoids often associated with methanotrophic bacteria were absent.
Noting that the only known hopanoid-synthesizing methanotrophs are aerobic bacteria and the sediments in these intervals had clearly been anoxic all the way to the sediment–water interface; that the overall organic matter was not unusually depleted in 13C; and that there were no 13C-depleted carbonate formations like those at methane seeps, Hinrichs concluded that there had been no methane production within the sediments at this site in the middle of the basin— and that the 13C depleted diplopterol was evidence that methane was suddenly released into the water of the basin at some time just prior to these warm spells, in keeping with Kennett’s hypothesis. The 13C-depleted archaeal biomarkers indicative of anaerobic methane munchers were absent throughout most of the sediment section—with one notable exception. In a narrow band of sediments deposited about 44,100 years ago, the δ13C value of archaeol dropped to –55 per mil, and several nonisoprenoid glycerol ethers that have been associated with sulfate-reducing bacteria in methane-oxidizing consortia were present, with δ13C values of –70 to –65 per mil. Hinrichs postulates that the release of methane into the water at this time, about 400 years before the longest of the warm periods, was so massive and fueled so much methanotrophic bacterial activity in the water column that the oxygen in the poorly circulating deep water of the basin became depleted and anaerobic methane munchers flourished briefly at the bottom of the basin.
Biomarkers of methanotrophs in Ice Age sediments from the Santa Barbara Basin: evidence of methane hydrate decomposition?
Though Hinrichs’s results provide some evidence of sudden emissions of methane into the Santa Barbara Basin at times in the recent geologic past, and the breakdown of methane hydrates would seem the most plausible source, the actual extent, timing, frequency, and effects of such events even during the last ice age are far from settled. How much methane was released, and what proportion made it past the microbial control valve into the atmosphere? Did the decomposition of methane hydrate occur in other areas around the world, and to what degree were such events responsible for the fluctuations in atmospheric methane recorded in the ice core records, as Kennett hypothesized? How would this process play out under the current, human-induced warming regime? Answering such questions will require more extensive studies of other basins and continental shelf sediments over longer periods of time. But as Hinrichs found with his Santa Barbara Basin study, interpretation of the biomarker data in such environments is not always straightforward.
In methane seep environments, where methane consumption constitutes the primary means of productivity and is the dominant microbial process, lipids from methanotrophic archaea are prominent constituents of the organic matter and clearly marked for posterity with δ13C values of –130 to –100 per mil. As fossil molecules in ancient sediments and rocks, they document the waxing and waning of methane seeps and mud volcanoes over the course of earth history. But in other environments, where methane is just one of many sources of carbon available to microorganisms, interpretation of biomarker structures and δ13C values requires careful consideration. Freeman’s and Hayes’s early work on the ancient Messel Lake and Green River Basin shales indicate that this is the case even in restricted anoxic basins where methane concentrations are relatively high and methane oxidation is an important microbial process. And recent studies of methane oxidation and methanotroph lipids in the organic matter floating in the Cariaco Basin and Black Sea have raised the concern that even though methane oxidation is occurring in the water column, the organisms responsible may not leave a recognizable signature in the sediments. In the Black Sea, for example, Stu Wakeham and colleagues in the NIOZ group discovered that 13C-depleted bio-markers of methanotrophic archaea in the suspended organic matter in the water either never reached the sediments, or their isotopic signal in the sediments was masked by relatively large quantities of structurally identical biomarkers from other types of archaea. In contemporary environments, the coupling of genetic investigations with lipid analyses and compound-specific isotope measurements provides a powerful means of determining possible sources for the lipids and of characterizing unknown organisms. But nucleic acids generally break down too quickly to be of much use in interpreting the geologic record beyond a few thousand years into the past, and interpretation of isotopic values that fall in the mid-range between those of photosynthetic and methanotrophic products is problematic for all but the most specific of biomarkers.
Does diploptene with a δ13C of –45 per mil indicate that aerobic methanotrophic bacteria were present and their 13C-depleted diploptene mixed with that from other diplopterol-containing bacteria? Or does it mean that the methane concentration was very low? Or that high productivity, rapid burial rate, and restricted water circulation resulted in 13C-depleted carbon dioxide, which produced unusually depleted photosynthetic organic matter—and, accordingly, 13C depletion in diploptene from the heterotrophs that had consumed it? Does archaeol or PMI with a δ13C in the –50 per mil range come from methanotrophic archaea that have consumed thermally generated methane, or does it come from methanogens that have consumed 13C-depleted CO2, or from a mix of the two? Even now, we can hear John Hayes intoning, there are no magic numbers. It takes the compound-specific isotope analysis of an array of fossil molecules to untangle these various influences and gain insight into methane’s role in climate change and earth history: the full spectrum of archaeal biomarkers, biomarkers from representative members of the food chain—phytane or sterols from photosynthetic algae, extended hopanoids for heterotrophic bacteria, and so on—and the isotopic makeup of the total organic matter and carbonate. Even then, interpretation of the microbial fossil record is all too often thwarted by our perennially incomplete understanding of an ecology wherein the “simplest” organisms perform in complex, multifarious ensembles.
The microbial role in controlling the chemistry of the rocks, oceans, and atmosphere has, over the long course of this book’s creation, finally moved to center stage. Over the past few years, the discovery of methane-oxidizing microbial consortia and the meeting of molecular biology and geochemistry that it inspired have had a profound eff ect on the laboratories of organic geochemists, both in the way they operate and in the questions they address. Kai Hinrichs and Marcus Elvert joined forces at the University of Bremen and assembled an organic geochemistry laboratory whose focus is microbial ecology. Their group collaborates closely with the microbiologists and molecular biologists at the MPI, housed on the same campus. At NIOZ, molecular biologists and microbiologists have become a permanent feature in Sinninghe Damsté’s biogeochemistry lab, and certain independent types like Stu Wakeham have spent their sabbaticals learning the genetic techniques themselves. As Geoff found out more than 50 years ago, it’s all very well to work on the premise that “the present is the key to the past,” like geologists did for over a century with their Uniformitarianism— but only if one can understand the present. Now, finally, the combined tools of gene and lipid analyses are providing the means to understand both present and past—just as Geoff and Dave Ward had hoped they would when the techniques first burst on the scene in the late 1980s.