When a distinguished but elderly scientist states that something is possible, he is almost certainly right. When he states that something is impossible, he is very possibly wrong.
—Arthur C. Clarke, 1917–2008, English science fiction writer
From Profiles of the Future (1962)
Anaerobic methanotrophs are not the only ecologically important archaea to surprise microbiologists in the last decade. And their isoprenoid ethers are not the only useful lipids—and certainly not the strangest—to have joined the lexicon of microbial biomarkers. Though much of that lexicon is still too generic to be of much use in understanding geologic history, some of these structures have allowed geochemists to transcend biological complexity and garner clues to past climates and environments.
In the 1990s, when Stefan Schouten first started finding ring-containing biphytanyl ethers in his sediment samples, he was still working on his doctorate at NIOZ. Like everyone else at the time, he assumed that they derived from the lipids of methanogenic archaea and that it was only a matter of time before ring-containing biphytanyl tetraethers would be identified among the lipids of some newly isolated culture of methanogens, as Guy Ourisson had predicted. Schouten was studying oxygen- and sulfur-bound biomarkers, which meant he treated his sediment extracts chemically to cleave the ether and sulfur bonds, and the treatments often turned up biphytanes. But then, he says, he and another student started finding the ring-containing compounds in some really unlikely places, such as the oxic surface layer of marine sediments where neither methanogens nor extreme thermophilic and halophilic archaea were likely to make a home. The only thing they could think of at the time was that the tetraethers had come from methanogens that lived in the oxygen minimum zone, the layer of water beneath the photic zone where heterotrophic bacteria are active, sometimes to the point of using up all of the oxygen.
When Schouten presented these ideas at the 1995 organic geochemistry meeting, Stuart Wakeham immediately piped up with the suggestion that they look for the lipids in the water column—and offered the perfect samples for the enterprise. He had collected particulate matter at diff erent depths in the Black Sea and Cariaco Basin, just the sort of anoxic environments where one might expect to find methanogens in the water column…. But to everyone’s surprise, when they followed through with the analyses, the highest concentrations of biphytanyl compounds weren’t in the samples collected from the anoxic deep water at all, but rather from the plankton and detritus collected in the completely oxic water near the sea surface! Not only that, but the δ13C values of the tetraethers were generally enriched in 13C compared to representative lipids from photosynthetic organisms, whereas methanogen lipids should have been slightly depleted. These biphytanyl ethers apparently came from planktonic archaea that had nothing to do with methane, and certainly nothing to do with extremely hot or acidic water. The isotopic composition and depth distribution of the lipids implied that they might be autotrophic organisms that derived their carbon from dissolved CO2 or bicarbonate—but they clearly weren’t photosynthetic, and what they used for energy was anybody’s guess.
Around this time, Schouten says, he heard about some new species of archaea that marine biologists in California had discovered several years before. What Dave Ward had been doing with cyanobacteria in Yellowstone’s hot springs, Ed DeLong, the leader of the California group, had done with the natural populations of archaea in the ocean. He’d extracted nucleic acids from marine plankton growing in all sorts of decidedly moderate, oxic environments—and found the rRNA sequences of organisms whose closest known relatives lived in hot springs and hydrothermal vents, a subgroup of archaea known as crenarchaea and hitherto comprised exclusively of extreme thermophiles. These new crenarchaea, however, appeared to be mainstream members of the marine plankton, present wherever one looked for them, even in freezing cold Antarctic waters. Were these the producers of the ring-containing biphytanyl compounds that had been puzzling the geochemists? Wakeham, who had also heard about the work in California, teamed up with DeLong to find out.
The planktonic crenarchaea had defied all attempts to grow them in laboratory cultures, but one strain was discovered growing in a sort of natural isolation that made it amenable to study. It lived in symbiotic relationship with a marine sponge that had been collected off the coast of California, and could be maintained in an aquarium, replete with its resident archaea. DeLong’s and Wakeham’s groups analyzed the rRNA, DNA, and lipid content of extracts from the aquarium sponges and compared the results to those from plankton samples they obtained from surface waters in two very diff erent environments—off the coast of Southern California, and in the ocean around Antarctica. The only archaeal genes detected in any of the extracts were those of nonthermophilic crenarchaea, and in each case their presence was correlated with significant amounts of biphytanyl compounds, including ones with two and three rings.
When he saw the results of Wakeham’s and DeLong’s studies, Schouten says, things began to make sense—sort of. The existence of planktonic crenarchaea resolved one paradox—that of finding biphytanyl tetraethers in oxic sediments from cool water environments—and introduced another. Molecular models of archaeal membranes showed that the rings in the biphytanyl components of the tetraethers produced a densely packed and thermally stable membrane that was especially well-suited to extremely high temperatures. But how did these crenarchaea, which apparently had lots of ring-containing tetraethers, get along in the relatively cold ocean? Wouldn’t their membranes be too stiff and impermeable to function? The genetic analyses allowed one to detect new species and place them with respect to known species, but one could only make inferences about their biochemistry, physiology, and biology based on those relationships—and it’s a long haul from an organism that lives at temperatures above 60°C to one that thrives in the frigid waters around Antarctica. DeLong’s surveys of rRNA were beginning to hint that the crenarchaea might hold an important place in marine food chains, but virtually nothing was known about how they lived, what they consumed and produced, or how plentiful they were. Schouten thought that a survey and comparison of the distributions of tetraether structures and isotopic compositions in plankton and sediments from diff erent environments might be revealing—but such a survey was no easy task with the existing techniques. The tetraethers were too big and polar to analyze directly by GC-MS, and treating the extracts to cleave the ether bonds was a tedious multistep process. It took two full days just to analyze one sample, Schouten says, and then one could only determine the amounts and structures of the biphytanyl components, not of the actual tetraethers. The biochemists who had isolated tetraethers from thermophilic archaea had used thin-layer chromatography to separate and purify them, but that was untenable with the complex mixtures and relatively small concentrations in the sediments.
There happened to be a new instrument in the NIOZ lab around this time that was generally good for separating large polar molecules that couldn’t be vaporized, but it had never been used to analyze anything like the tetraethers. It was an HPLC, like what James Maxwell and Kliti Grice used to analyze plant pigments, except the new instrument in the NIOZ lab had an interface to a mass spectrometer. In 1999, when Sinninghe Damsté got the idea that the machine might be used to separate and determine the structures of intact tetraethers, it was being operated by Ellen Hopmans, a postdoc who was an expert in toxicology and food science and had lots of analytical experience with HPLC. Hopmans knew it was a bit of a long shot—indeed, when she told the analytical chemists at the instrument company that she wanted to analyze dibiphytanyl glycerol tetraethers, they told her in no uncertain terms that the column she wanted to try wouldn’t work and it would all be an exercise in futility. New to the lab, with no experience whatsoever in geochemistry, she was eager to please, and just rash and naive enough to try anyway.
As Schouten tells the story, the reason they happened to have a toxicologist and food scientist in the NIOZ lab at this particular juncture was that he’d gotten the urge to track down his old high school buddies on the Internet and found one of his former fellow nerds working in the food science and technology department at the University of California in Davis. The details may not be relevant to science, but somehow an idle electronic hello from an old school buddy halfway around the world led to the proverbial endless date, and since it was rather difficult for a toxicologist in California and a geochemist in the Netherlands to have much of a marriage, the toxicologist had developed a sudden interest in geochemistry and the NIOZ group had decided it might be able to make use of a toxicologist’s analytical talents.
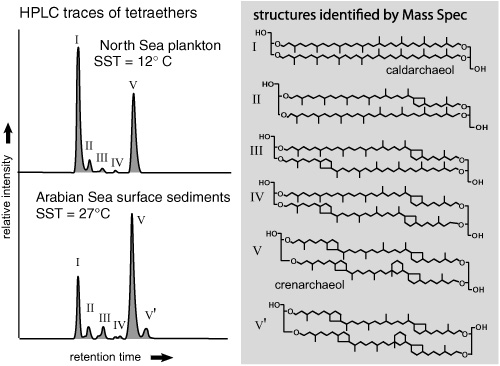
The group obtained or prepared purified tetraethers from two cultures of thermophilic crenarchaea, and though Hopmans says that her first chromatogram would best be described as a “humpogram,” it wasn’t long before she had managed to separate a standard mixture of nine compounds into nine sharp peaks with nine characteristic mass spectra. Now they could detect the entire range of tetraethers, with all of their structural information intact, in one fell swoop—the group immediately began running samples from its extensive collection of sediment samples, from ancient to recent, open ocean to coastal, inland seas, lakes, and peat bogs.
All of the samples contained tetraethers of one sort or another, including many of the same structures that had been isolated from thermophilic archaea. But the tetraether structures present in the sediments were far more diverse than those in the membranes of the thermophilic crenarchaea: their distributions varied across diff erent environments, and it was soon apparent that they came from large and diverse populations of archaea, adapted to all sorts of environments— from oxic to anoxic, surface waters to sediment, freezing to near-boiling—and deriving their carbon and energy from a variety of diff erent sources. This was when Richard Pancost was at NIOZ as a postdoc, finding severely 13C-depleted tetraethers in samples from the Mediterranean mud volcanoes—indicating that at least one of their source organisms was able to oxidize methane. The other thing that became apparent in the group’s onslaught of intact tetraether analyses was that the most common, abundant compound in the sediments had a completely unknown and difficult-to-determine structure.
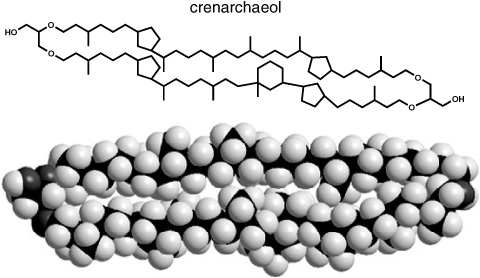
Though the HPLC-MS system provided only basic mass spectral information, most of the unknown tetraether structures were pretty easy to figure out— except for the most prevalent one. Hopmans had to collect peaks from the end of the HPLC column during repeat runs until she had enough of it for other forms of analysis, and then the group went at it with every trick in the organic chemist’s arsenal. Schouten says the structure had them fooled for almost two years, and in the meantime they obtained a sample from DeLong’s sponge crenarchaea and found that the same compound comprised its dominant membrane lipid. More sediment analyses confirmed that it was the core lipid of the planktonic crenarchaea, a compound that the NIOZ group took to be a unique feature, because to date it had never been detected in methanogens or thermophiles and was not among the 13C-depleted tetraethers that seemed to derive from methanotrophs. Finally, in late 2001, Sinninghe Damsté cracked the structure using two-dimensional nuclear magnetic resonance spectroscopy, a favorite tool of natural products chemists that was generally out of bounds for geochemists because of the amount of pure compound required. The main problem in this case, he says, was not isolating the compound, but rather the incredibly complex spectra it yielded and the endless interpretations required to determine the relative positions of each of its atoms. It was distinguished from the other ring-containing tetraethers by a biphytanyl chain that had a six-carbon ring in addition to two of the usual five-carbon rings. Damsté christened the compound “crenarchaeol” and, noting that the six-carbon ring created a sort of bulge in the middle of the tetraether, teamed up with theoretical chemists from the University of Amsterdam to create a molecular model of an archaeal membrane structure with the new compound as a core lipid. The result offered one possible solution to the apparent paradox of cold water crenarchaea: whereas the five-carbon rings made the biphytanyl chains fit together more snugly in a dense structure, addition of crenarchaeol with its bulging six-carbon ring kept them from packing together too closely and would, conceivably, allow the membrane to maintain its fluidity at low temperatures.

For his part, Schouten says, the ongoing analyses of tetraethers in sediments and plankton had almost gotten boring, because the compounds were so widespread. It seemed they were everywhere, all the time—in plankton at all depths and seasons, recent sediments, ice age sediments, even Cretaceous sediments, from all around the world. The group did another study with Wakeham, examining particulate matter in the Arabian Sea and comparing the concentrations of tetraethers with those of algal steroids and fatty acids. The results indicated that these planktonic crenarchaea could live not only at all depths, but also at very low oxygen levels. They were certainly not photosynthetic, and they weren’t particularly abundant in the zones where organic matter accumulates and heterotrophic bacteria prosper. There was some correlation between the concentration of crenarchaeol and the concentrations of nitrate and nitrite, as if the organisms might be using the former and producing the latter. But the ecology and metabolic strategy of these tiny but ubiquitous organisms were still a mystery when Schouten and Hopmans noticed another pattern, this time in the overall distributions of the tetraethers.
Schouten says that he was standing by the HPLC-MS with Sinninghe Damsté and Hopmans late one aft ernoon, contemplating the chromatograms from the day’s last analyses and getting ready to go home, when he wondered idly why the relative amounts of tetraethers in the two chromatograms were so diff erent. “Oh, it’s probably just temperature,” Hopmans said. She was too new to geochemistry to know about the alkenone  index or to realize the importance of her insight, but Schouten and Sinninghe Damsté turned and stared at each other. Of course.
index or to realize the importance of her insight, but Schouten and Sinninghe Damsté turned and stared at each other. Of course.
One of the samples was from North Sea sediments, and the other from the Arabian Sea. The latter contained more tetraethers with lots of rings in them, and fewer of the ones with zero or one ring: warm water, more rings. This even made some biochemical sense, more so than the alkenones’ much-studied decrease in unsaturation with increasing temperature. There was overwhelming evidence that ring-containing tetraethers were components of cell membranes in the phylogenetically related thermophilic crenarchaea. And experiments with cultures of the thermophiles had shown that they biosynthesized tetraethers with more rings when grown at higher temperatures, in keeping with the observation that the five-membered rings caused the biphytane chains to pack together more closely and make a denser, more protective membrane.
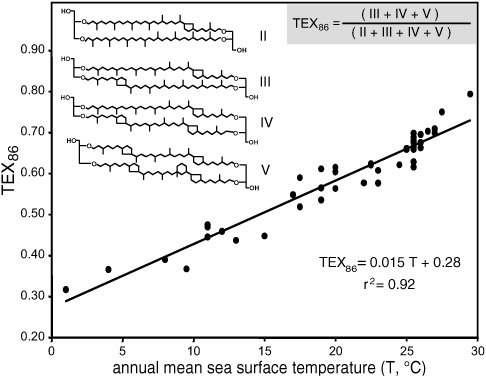
It was a quarter to five in the aft ernoon when the group had this epiphany, and the problem with working at NIOZ—or advantage, if one happens to be a workaholism-prone scientist with a family—is that it is on an island, and the last convenient ferry and train connection to Amsterdam departs at 5:00 P.M. sharp. But the following morning Schouten set to work trying to quantify the relationship between temperature and the relative amounts of the various tetraethers. His first attempts were, Schouten says, a bit discouraging. He used a weighted average of the number of rings in the compounds, which gave a positive correlation with temperature, but the data were too scattered to make a good temperature proxy. Then he tried using the ratio of isomers with more rings to isomers with fewer, and finally, when he left caldarchaeol out of his equation, he got a clean correlation. Precisely why was not entirely clear, but it now appears that caldarchaeol is a core lipid in many groups of archaea, and large contributions from other organisms in the sediments may have been skewing the data. Whatever the case, the correlation between the new index and temperature, based on 40 surface sediment samples from around the world, was worthy of the best proxy. Following suit with the Bristol group’s  , they called their proxy the “Tetraether Index for tetraethers with 86 carbon atoms” and gave it the acronym TEX86, a small tribute to local pride, as the Dutch island where NIOZ is located is called Texel.
, they called their proxy the “Tetraether Index for tetraethers with 86 carbon atoms” and gave it the acronym TEX86, a small tribute to local pride, as the Dutch island where NIOZ is located is called Texel.
Tetraether distributions: North Sea vs. Arabian Sea
The possibilities for the new proxy were exciting. The tetraethers were found in significant quantities in sediments dating back to the Cretaceous, in Arctic and Antarctic waters, and in lake sediments—times and places where use of the  temperature proxy was proving problematic. In 1999, an international conference on the alkenones had convened in Woods Hole, examining the
temperature proxy was proving problematic. In 1999, an international conference on the alkenones had convened in Woods Hole, examining the  proxy from all angles, reinforcing its success, highlighting its limitations, and, in the process, providing a recipe book for development and validation of new temperature proxies. Does the calibration hold up under controlled conditions in the laboratory? Is it affected by changes in salinity and growth rate? Are there diff erent strains of crenarchaea with diff erent temperature dependences? For that matter, are the tetraethers used in TEX86 found in other archaea that might confuse the signal in the sediments? Do some of the compounds break down more quickly than others, such that the ratios are affected by diagenesis? And so on.
proxy from all angles, reinforcing its success, highlighting its limitations, and, in the process, providing a recipe book for development and validation of new temperature proxies. Does the calibration hold up under controlled conditions in the laboratory? Is it affected by changes in salinity and growth rate? Are there diff erent strains of crenarchaea with diff erent temperature dependences? For that matter, are the tetraethers used in TEX86 found in other archaea that might confuse the signal in the sediments? Do some of the compounds break down more quickly than others, such that the ratios are affected by diagenesis? And so on.
Calibration of the Tetraether Index (TEX86) in surface sediments, 2002
The NIOZ group had one difficulty the Bristol contingent hadn’t had: whereas Emiliania huxleyi was already happily growing and reproducing in the laboratories of the Marine Biological Association in Plymouth, the crenarchaea had defied all attempts at domestication. Sinninghe Damsté’s lab had recently acquired both a microbiologist and a molecular biologist—another affair of the heart, which NIOZ seems to have had good luck with—but how could the two do experiments without a laboratory culture of the organisms in question? They didn’t necessarily need a pure culture…. Could they just obtain a large sample of water where crenarchaea were growing naturally, put some in a tank where the temperature could be adjusted, and see if they’d keep growing? The very idea was anathema to most microbiologists, and Schouten says everyone he talked to thought such an experiment was preposterous, doomed to failure. But his student Cornelia Wuchter took up the challenge: she used water from the North Sea and a series of simple 20-liter tanks, and then monitored the total concentration of tetraethers to see if and how fast the crenarchaea were growing.
Remarkably, the organisms no one could get to grow in a carefully controlled bacterial medium were off and running within a week. Molecular biologist Marco Coolen analyzed the rRNA and DNA and found that the North Sea water contained a simple mixture of two strains, one of which eventually died out. The “preposterous” improvisation would provide Wuchter with results for an entire doctoral thesis. She could heat the tanks at diff erent temperatures and calibrate TEX86, and she could track the chemistry of the water and learn something about what the crenarchaea were consuming and producing.
The first round of studies with the captive crenarchaea not only verified that the lipid composition within a single strain varied with temperature, but also provided a calibration of TEX86 that was gratifyingly close to that from the sediments…. But if, as DeLong’s rRNA studies and the NIOZ team’s lipid surveys indicated, the crenarchaea thrived throughout the water column, what temperatures would they be recording? It was all very nice that the values of TEX86 in the sediments correlated so well with sea surface temperatures—but why would they, when temperatures at 500 meters depth were some 10°C colder? More studies of water column particulate matter with Wakeham soon made it apparent that this was a question not only of where in the water column the crenarchaea live, but also of how their remains get to the sediments once they’re dead.
Crenarchaea are less than one-fift h the size of coccolithophores, and with no heavy coccolith shields for ballast, they apparently remained suspended in the water—unless they were living in the upper 100 meters or so, where they were likely to be consumed by larger grazers. In this case, some undigested crenarchaea lipids might well end up in zooplankton fecal pellets and sink to the seafloor. What exactly happens to the biomass of such tiny, free-living organisms in the deep water, where zooplankton activity is limited, remains a matter of conjecture. But Wuchter’s work with Wakeham indicated that the dominant portion of the crenarchaeal lipids in the sediments came from organisms living in the upper 100 meters of the water, where predators are most active. This might explain the group’s observations from the Black Sea, and those of Kai Hinrichs from the Santa Barbara Basin, where the lipids of organisms that had presumably lived in the oxic surface water—planktonic crenarchaea in the one case and aerobic methanotrophic bacteria in the other—were found in the sediments, and the 13C-depleted lipids of methanotrophic archaea living in the deep anoxic water were not. And it is the best explanation to date for why TEX86 values in surface sediments at most sites have recorded sea surface temperatures, despite thriving populations of planktonic crenarchaea in deeper waters.
Even as the Dutch group worked through the recipes for calibrating and validating its new paleothermometer, first applications of TEX86 began yielding some intriguing results. In sediment cores from the western Arabian Sea, TEX86-derived temperatures are in good agreement with the  temperatures for most of the current interglacial period, but during the last glacial to interglacial transition, the two proxies tell significantly diff erent stories. Whereas the
temperatures for most of the current interglacial period, but during the last glacial to interglacial transition, the two proxies tell significantly diff erent stories. Whereas the  temperatures are correlated with the δ18O values in the ice of the Greenland ice cores, the TEX86 temperatures record a larger temperature shift and follow the rather distinct trend recorded in the Antarctic ice core. The reasons for this discrepancy are not at all clear, but sea surface temperatures in the region tend to exhibit marked seasonal variations due to upwelling of cool water caused by the seasonal monsoon winds. The Dutch group speculated that whereas TEX86 was registering average annual sea surface temperatures, exceptionally large pulses in coccolithophore productivity during upwelling may have skewed the temperatures registered by
temperatures are correlated with the δ18O values in the ice of the Greenland ice cores, the TEX86 temperatures record a larger temperature shift and follow the rather distinct trend recorded in the Antarctic ice core. The reasons for this discrepancy are not at all clear, but sea surface temperatures in the region tend to exhibit marked seasonal variations due to upwelling of cool water caused by the seasonal monsoon winds. The Dutch group speculated that whereas TEX86 was registering average annual sea surface temperatures, exceptionally large pulses in coccolithophore productivity during upwelling may have skewed the temperatures registered by  toward those of the cool deep upwelled water, thus partially obscuring the glacial to interglacial sea surface warming. This raises the possibility that the two proxies can provide complementary climate information, maybe even a record of how seasonal diff erences varied during the ice ages—but it poses the conundrum that both proxies have been calibrated and correlate well with average annual sea surface temperatures, even in the western Arabian Sea. It also highlights the need for careful interpretation of the temperatures obtained with such proxies, because both can, under some circumstances, be affected by the dynamics of seasonal biological cycles and structure of the water column.
toward those of the cool deep upwelled water, thus partially obscuring the glacial to interglacial sea surface warming. This raises the possibility that the two proxies can provide complementary climate information, maybe even a record of how seasonal diff erences varied during the ice ages—but it poses the conundrum that both proxies have been calibrated and correlate well with average annual sea surface temperatures, even in the western Arabian Sea. It also highlights the need for careful interpretation of the temperatures obtained with such proxies, because both can, under some circumstances, be affected by the dynamics of seasonal biological cycles and structure of the water column.
The new proxy is also proving useful for estimating the temperature history in regions or time periods where δ18O and  measurements are not possible. Planktonic crenarchaea are not as ubiquitous in lakes as they are in the marine environment, but they do seem to be abundant in the largest lakes, and unlike
measurements are not possible. Planktonic crenarchaea are not as ubiquitous in lakes as they are in the marine environment, but they do seem to be abundant in the largest lakes, and unlike  measurements in lakes, calibrations of TEX86 in these environments fit smoothly into the calibration from marine sediments. In a sediment core from southeast Africa’s Lake Malawi, temperatures derived from TEX86 provided new insights into glacial to interglacial temperature changes in Africa, raising hopes that similar studies might fill in missing information about how temperatures on the continents were affected by changes in the earth’s climate over the past few hundred thousand years.
measurements in lakes, calibrations of TEX86 in these environments fit smoothly into the calibration from marine sediments. In a sediment core from southeast Africa’s Lake Malawi, temperatures derived from TEX86 provided new insights into glacial to interglacial temperature changes in Africa, raising hopes that similar studies might fill in missing information about how temperatures on the continents were affected by changes in the earth’s climate over the past few hundred thousand years.
The calibration of TEX86 for temperatures above 27°C is incomplete and appears to deviate somewhat from that at lower temperatures, but with this uncertainty in mind, the tetraethers in sediments from Cretaceous black shales indicate that sea surface temperatures were between 33°C and 36°C during the early to mid Cretaceous, consistent with the upper estimates from 18O measurements— and warmer than anywhere in the contemporary ocean. More provocative is an analysis of tetraethers in Arctic Ocean sediments from the late Cretaceous, which provides one of the first direct estimates of polar sea surface temperatures during the period. The TEX86-derived value of 15°C adds to evidence that the planet was entirely free of ice, on the one hand, but it also implies a steeper pole-to-tropics temperature gradient than previously hypothesized. A recent study of Arctic Ocean sediments from the relatively short, putatively methane-induced heat wave at the end of the Paleocene indicates that sea surface temperatures near the North Pole at this time were even higher, ranging from 18°C to 23°C. Of course, these TEX86-determined temperatures rely rather heavily on the principle of biochemical Uniformitarianism, based as they are on the assumption that lipid biosynthesis in microbes living tens of millions of years ago—for which there is no record but the tetraethers themselves—had the same temperature dependence as it does in contemporary crenarchaea.
Whether or not TEX86 will hold up to scrutiny as well as the  has, and precisely what caveats should accompany its interpretation, remains to be seen. With so much of the microbial world yet to be discovered, the specificity of bio-markers like the ring-containing tetraethers is likely to remain in question for some time. Wuchter’s and Coolen’s combined genetic and lipid analyses of plankton indicate that other planktonic archaea in the surface waters of the ocean do not make them, and tetraethers produced by methanotrophic archaea can usually be distinguished by their pronounced negative δ13C values. But explorations with genetic probes have identified a number of crenarchaeal strains living in soils, and the NIOZ group recently discovered that ring-containing tetraethers, including crenarchaeol, are common components of the organic matter in soils and may complicate the use of TEX86 in areas where there have been relatively large inputs of material from rivers and continental runoff. And Kai Hinrichs’s Bremen group recently spearheaded a big multidisciplinary study of ODP sediments from off the coast of Peru that revealed a whole range of apparently heterotrophic crenarchaea living buried deep in marine sediments and producing crenarchaeol and other ring-containing tetraethers with δ13C values similar to those of lipids from planktonic crenarchaea. Schouten is guessing that the contribution of lipids from these sediment organisms is quantitatively minimal compared to inputs from the autotrophic organisms in surface waters, and thus has little eff ect on TEX86 values and the use of tetraethers to gauge the productivity of planktonic crenarchaea, but Hinrichs is not so sure this is the case.
has, and precisely what caveats should accompany its interpretation, remains to be seen. With so much of the microbial world yet to be discovered, the specificity of bio-markers like the ring-containing tetraethers is likely to remain in question for some time. Wuchter’s and Coolen’s combined genetic and lipid analyses of plankton indicate that other planktonic archaea in the surface waters of the ocean do not make them, and tetraethers produced by methanotrophic archaea can usually be distinguished by their pronounced negative δ13C values. But explorations with genetic probes have identified a number of crenarchaeal strains living in soils, and the NIOZ group recently discovered that ring-containing tetraethers, including crenarchaeol, are common components of the organic matter in soils and may complicate the use of TEX86 in areas where there have been relatively large inputs of material from rivers and continental runoff. And Kai Hinrichs’s Bremen group recently spearheaded a big multidisciplinary study of ODP sediments from off the coast of Peru that revealed a whole range of apparently heterotrophic crenarchaea living buried deep in marine sediments and producing crenarchaeol and other ring-containing tetraethers with δ13C values similar to those of lipids from planktonic crenarchaea. Schouten is guessing that the contribution of lipids from these sediment organisms is quantitatively minimal compared to inputs from the autotrophic organisms in surface waters, and thus has little eff ect on TEX86 values and the use of tetraethers to gauge the productivity of planktonic crenarchaea, but Hinrichs is not so sure this is the case.
Several studies, including Wuchter’s tank-based experiments, have now confirmed that at least some of the planktonic crenarchaea are in fact autotrophic. Their carbon comes from bicarbonate, which is typically 13C-enriched compared to CO2, accounting for the observation that crenarchaeal lipids are consistently enriched in 13C compared to lipids from photosynthetic organisms or their consumers. And their energy comes from the oxidation of ammonium to nitrite. The discovery of a cosmopolitan marine primary producer that doesn’t require light; is not constrained to nutrient-limited surface waters or to the neighborhood of hydrothermal vents; is adapted to a wide range of climates; converts ammonium from decomposing organic matter in mid-depth waters to nitrite that phytoplankton can more readily use, a process previously thought to be the exclusive domain of a handful of specialized and elusive bacteria; and comprises a major portion of the microbial biomass in the huge expanse of the deep sea raises a lot of questions, to say the least. What does this signify for estimates of primary productivity and the cycles of carbon and nitrogen, both present and past? How did these planktonic crenarchaea aff ect the ocean’s biological pump for CO2—what role did they play in past climates and environments? When did they evolve …? And what other important microbial players have we missed?
Another previously unimagined microbe recently found its way to center stage, thanks in part to the NIOZ group’s discovery of yet another weird lipid molecule—not, initially, in marine plankton or sediments or rocks, but in bacteria from a Dutch wastewater treatment plant. Microbiologists were trying to find a way to get rid of ammonium in the wastewater and had isolated a bacterium that could convert ammonium to nitrogen gas in the anoxic environment of the sewage sludge. Sinninghe Damsté says he got a request from someone at Delft University who had seen one of the NIOZ group’s publications on the archaeal lipids. The microbiologists wanted to know the lipid composition of their bacterium’s cell membrane and wondered if he would do the analysis. It was a routine task that Sinninghe Damsté agreed to do more out of a sense of civic duty than out of scientific curiosity … or so he thought, until he saw the huge unidentified peak in the chromatograms from the extracts.
If the ring-containing biphytanyl tetraethers seemed strange to biochemists and organic geochemists when they first started finding them in the 1970s, the structures that Sinninghe Damsté and his colleagues determined among the lipids of the anaerobic ammonium-oxidizing bacteria were so bizarre it was hard to believe they could possibly exist in nature. Indeed, Sinninghe Damsté says that even the chemists he worked with at the University of Amsterdam couldn’t believe it at first. The ladder-shaped hydrocarbon extensions were made up of fused four-carbon rings, a construction that was so strained it looked like an organic chemist’s joke, something one might try to synthesize on a dare, simply because it looked impossible.
The ladderane lipids made their debut in a poster at the 2002 Gordon Research Conference, and Geoff says he laughed out loud when he saw it. Jan de Leeuw had explicitly led him past the other presentations to show him the poster, which depicted the lipid structures and described their extraction from a wastewater bacterium sporting the unwieldy acronym “anammox,” apparently because they engaged in anaerobic ammonium oxidation. Geoff says he stood there looking at it for several minutes before he decided that his old friend and colleague must be having him on. Ladderane reminded him of his early career, when he considered synthetic organic chemistry a form of modern art in which one tried to outdo one’s cohorts by creating the weirdest shapes. But de Leeuw was indignant at the suggestion that the poster was a prank. “I do not joke about science,” he said. Even then, Geoff wasn’t convinced. Sinninghe Damsté says that on the last night of the conference Geoff took him aside and asked again if the presentation wasn’t, in fact, a joke. For his part, Jürgen refused to believe the structures were correct until he heard that they had been proven by synthesis—which they were in 2004, by one of the world’s foremost synthetic organic chemists.
Examples of ladderane lipids from anammox bacteria
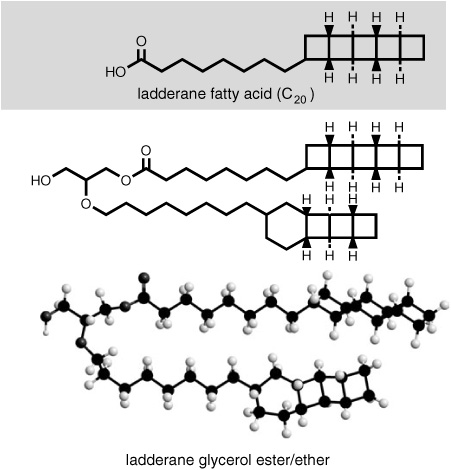
Ladderanes had, in fact, been synthesized by chemists as a novelty and object of theoretical interest. But finding such a thing as a major component in a bacterial membrane was another matter altogether. Sinninghe Damsté, now thoroughly interested in the project, discovered that the ladderane lipids weren’t in the cell membrane, but rather formed part of a dense, relatively impermeable internal membrane. This unusual membrane enclosed the bacterium’s energy-producing reactions and was apparently the solution to one of the enigmas of the anammox, protecting the cell from the indisputably toxic reaction intermediates—which include the common rocket fuel hydrazine—while maintaining the necessary concentration gradients for energy production. The ladderane lipids take a variety of forms: in addition to fatty acids, there are glycerol ethers, esters, alcohols, and dialkyl glycerol diethers, all with ladderane groups attached. Indeed, the diverse anammox lipids even include hopanoids, which were previously considered the exclusive dominion of aerobic bacteria. But for all the novel chemistry and apparatus contained in these tiny organisms, they are, as it turns out, no more a novelty in nature than the anaerobic methanotrophs or the planktonic crenarchaea.
Like methane, ammonium had long been considered biologically inert under anoxic conditions. Likewise, it was oceanographers who first observed that large quantities of the ammonium produced by the decomposition of organic matter disappeared in anoxic waters and sediments, despite the fact that the only bacteria known to oxidize ammonium required oxygen. Unlike methane, ammonium could, in theory, be oxidized by nitrite or nitrate in the absence of oxygen and produce enough energy to be of use to some microorganism. But no such organism could be found, and so the oceanographers’ observations were largely ignored and biologists generally thought they had the marine nitrogen cycle figured out, until the late 1980s when microbiologists in Delft first demonstrated that the process was occurring in an experimental waste-processing plant. The organisms, which they isolated in the mid-1990s, were autotrophic, using CO2 as their carbon source and obtaining their energy by converting ammonium and nitrite—two of the three forms of nitrogen that phytoplankton use as nutrients—to nitrogen gas, which is plentiful in the air and sea but useless to most organisms.
In wastewater facilities and cultures, the anammox bacteria grow exceptionally slowly, and initially microbiologists assumed they wouldn’t make much of a contribution to the cycling of nitrogen in natural ecosystems. But once again, the microbial communities in natural environments exceeded expectations. Explorations with genetic probes have identified several phylogenetically related anammox species in coastal basins and freshwater lakes. Marcel Kuypers, who had been working on his doctoral thesis at NIOZ when the ladderane lipids joined the biomarker lexicon, teamed up with microbiologists at his new post at the Bremen MPI to study the activities of anammox in Europe’s best anoxic laboratory, the Black Sea, where there were high concentrations of ammonium. Together with Sinninghe Damsté and the Delft microbiologists, they used lipid analyses, genetic probes, and water sample incubation experiments and found several of the unusual ladderane lipids and rRNA sequences closely related to those of the cultured anammox, along with nitrite, in a thin layer of water between 90 and 100 meters depth. Ammonium suddenly disappeared at the same depth, though the water was still anoxic. Fluorescent labeling of the genetic probes allowed the group to count anammox cells: even at the exceptionally slow metabolic rates that had been observed for the organism in the laboratory, the anammox in this layer of water were plentiful enough to account for the disappearance of most of the ammonium diffusing up from the deep waters of the Black Sea.
Studies of the oxidation of ammonia by nitrite in oxygen minimum zones in the ocean and in anoxic sediments, now tuned to recognize the anammox process, indicate that anammox may actually be responsible for conversion of up to 50% of the ocean’s nutrient nitrogen to inert nitrogen gas—in other words, for limiting the amounts of ammonium, nitrite, and, indirectly, nitrate that is ultimately available to phytoplankton in the surface waters. Accordingly, these strange organisms, with their rocket-fueled innards, may actually have played a key role in determining the amount and nature of primary productivity in the world’s oceans, particularly at times in the past when oxygen was more limited, such as during the episodes of anoxia and enhanced productivity that produced the Cretaceous period’s ever-enigmatic black shales. One might, naturally, look to the distinctive lipids or, rather, their bizarre ladderane hydrocarbons for evidence—but it seems unlikely that such an incredibly strained structure would be stable for long, and one is hard-put to predict what sort of fossil molecules it might leave behind. Degradation and artificial maturation experiments may provide some clues as to possible fossil structures, and the ladderane lipids do have a distinctive 13C signature that might help, some 45 per mil more depleted than the CO2 the organism consumed. In the meantime, the NIOZ group has hypothesized that the anammox played a pivotal role during two of the Cretaceous oceanic anoxic events, relying on indirect evidence from a more mundane series of microbial biomarkers—the hopanoids produced by organisms at the opposite end of the nitrogen cycle.
Cyanobacteria are among the few known photosynthetic organisms in the ocean that can make use of the abundant nitrogen gas dissolved in the water. They thrive in areas where other phytoplankton are limited by a lack of nutrient nitrogen, and in the contemporary ocean they dominate phytoplankton populations in low-productivity central ocean areas. According to Roger Summons’s and Linda Jahnke’s surveys of bacterial hopanoids, one of the few hopanoid innovations to be incorporated into the sturdy ring structure and preserved for posterity is a methyl group on the second carbon atom of the A-ring, a structure that appeared to be synthesized almost exclusively by species of cyanobacteria. Summons also found that these 2-methyl hopanoids were only prominent in rocks and sediments formed in environments where cyanobacteria were, for one reason or another, prominent. When the NIOZ researchers detected exceptionally large amounts of 2-methyl hopanoids in Cretaceous black shales, they knew they weren’t looking at sediments from an ocean where nutrients were perennially limited and productivity was generally low, as in the contemporary central oceans. Rather, they speculated, something must have specifically depleted the available nitrogen in the photic zone, giving the cyanobacteria a competitive edge, while other key nutrients such as phosphate and iron remained in good supply. In black shales from 93 and 120 million years ago at sites in both the Atlantic and Pacific oceans, the concentration of 2-methyl hopanoids relative to other extended hopanoids increased from less than 2% before shale deposition to more than 20%. The proportion of bacterial hopanoids relative to algal steroids also increased, suggesting that cyanobacteria may have dominated phytoplankton communities in large areas of the world’s oceans during these periods.
Elevated levels of 2-methyl hopanoids in Cretaceous black shales: Did cyanobacteria take over during oceanic anoxic events?
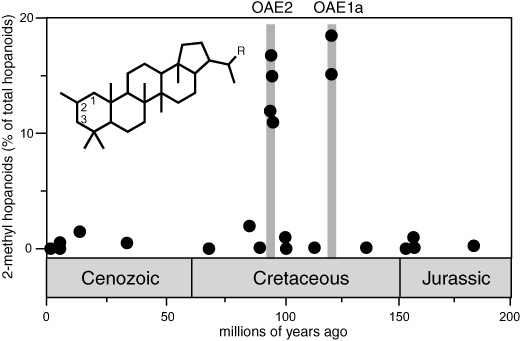
Drawing an analogy to what they’d observed in the Black Sea, the NIOZ group speculated that anammox bacteria could have been active in the deep ocean and even into the photic zone during these periods of ocean anoxia, and that they would have used up the ammonium and nitrite, and indirectly the nitrate, that most phytoplankton require. This would have given the cyanobacteria, with their ability to use the always abundant nitrogen gas, a decided advantage over the coccolithophores, dinoflagellates, and diatoms that generally dominate marine phytoplankton communities. Cyanobacteria not only use the inert nitrogen gas, but also incorporate it into nitrogen-containing organic compounds and ultimately resupply the water with nitrogen in nutrient form, and the NIOZ group suggests that this may have been one of the mechanisms for sustaining the long periods of high primary productivity that produced the black shales.
Surpassing even Guy Ourisson’s enthusiastic prognoses in the 1980s, fossil lipid molecules in sediments and rocks have led to or accompanied the discovery not just of new species, but of energy-harvesting systems that redefine what is “biochemically feasible.” As the anammox bacteria and methanotrophic archaea illustrate, if a reaction is chemically possible and releases some modicum of energy, then it’s quite likely that some microbe somewhere has figured out how to make use of it: off er a free lunch, no matter how humble, and someone, or some few, will find a way to partake.
Recent work verifies that there are microbes living more than half a kilometer beneath the surface of the seafloor, somehow making a home in sediments where the sequence of good oxidants and readily oxidized fuels has long been exhausted and it would seem that not even the most minimal source of energy is available. Microbes have even been found living within the underlying basalt of the oceanic crust and deep within the granite bedrock on the continents. The microbial sector of life is not only vaster and more varied, but also more resourceful and enterprising than even the most forward-thinking microbiologists imagined. Its exploration requires the tools and expertise of chemists, biologists, and geologists, in every combination and permutation. Ironically, organic geochemists, for whom the perfect biomarker is one that survives the ravages of time, now find themselves developing HPLC techniques that allow them to detect the most fragile biological molecules—glycerol ethers, esters, and fatty acids with the hydrophilic phosphate groups still attached—and thus distinguish the lipids of live cells. Used together with fluorescently labeled genetic probes, these bio-markers allow both identification and quantification of the active microbes in a sedimentary environment.
Current hypotheses hold that the organisms in the deep subsurface microbial communities grow in slow motion, so to speak, on a geologic timescale, and that their primary producers derive their energy by exploiting reactions between the reduced minerals in the rocks and the oxidized elements in seawater that circulates through cracks in the igneous rocks beneath the sediments—similar to some of the reactions exploited by hydrothermal vent microorganisms, except at much, much lower temperatures. In some areas, oxidants such as sulfate seem to be introduced from below by seawater that has made its way deep into the basement rocks and is now circulating back toward the surface. Another potential source of energy for these sleepy microbial communities may be the hydrogen generated when water reacts with the reduced metals in the basalt. There is some concern that the presence of live microbes in even the most ancient sediments may hinder interpretation of the molecular fossil record of life and environments in the past, but, so far, it appears that the deep subsurface microorganisms grow so slowly that their lipid biomass is minimal compared to that of the fossil lipids in most rocks.
Exploration of the microbial realm is only just begun, but the repercussions are already sounding in almost every field of the natural sciences. The recognition that microbial photosynthesis is the norm in the surface waters of the ocean, along with the realization that the capture and storage of energy in reduced organic compounds are not confined to the sun-graced surface of the planet but are prevalent in the deep sea and sediments—the two largest habitats on Earth in terms of sheer volume—requires reassessment of how key elements such as carbon and nitrogen cycle through the atmosphere, ocean, and rocks. Organic geochemists are now struggling to understand which and how much of the microbial organic matter formed in the ocean is transported to the sediments, while geologists scramble to understand the movement of nutrient-rich fluids deep within the earth, biologists expand their ideas of what nutrients and energy sources determine primary productivity, climate scientists reconfigure their models for the movement of carbon dioxide and methane into and out of the atmosphere, and paleontologists shift their views of life’s history on the planet— all in an attempt to accommodate the flood of new information from life’s tiniest, most inventive, and most primordial representatives.