Men do generally too much slight and pass over without regard these Records of Antiquity which nature have left as Monuments and hieroglyphick characters of preceding Transactions in the like duration or transactions of the Body of the Earth.
—Robert Hooke, 1635–1703, British natural philosopher, sometimes referred to as “London’s Leonardo”
From The Posthumous Works of Dr. Robert Hooke (1688)
The philosophical study of nature rises above the requirements of mere delineation, and does not consist in the sterile accumulation of isolated facts. The active and inquiring spirit of man may therefore be occasionally permitted to escape from the present into the domain of the past, to conjecture that which cannot yet be clearly determined, and thus to revel amid the ancient and ever-recurring myths of geology.
—Alexander von Humboldt, 1769–1859, German naturalist and explorer
From Views of Nature (1850 translation)
Carl Woese’s drive for a unified system of biological classification didn’t just open the microbial world to exploration: it reshuffled the entire taxonomic system and revolutionized the way that biologists study evolution, reigniting interest in pre-animal evolution. Studies of evolution from the mid-nineteenth through most of the twentieth century relied on the comparison of forms in living and fossil organisms and were limited to the complex multicellular organisms that developed over the past 550 million years. In other words, much was known about the evolution of animals and land plants that left distinctive hard fossils, and very little was known about the unicellular algae and microorganisms that occupied the seas for most of the earth’s history. Woese’s Tree of Life, derived from nucleic acid sequences in ribosomal RNA, has revealed ancestral relationships that form and function don’t even hint at, allowing biologists to look beyond the rise of multicellular life and link it with less differentiated, more primal forms—which was precisely Woese’s intention. But evolution is a history, not just a family tree of relationships. If the information stored in the genes of extant organisms is to provide true insight into that history, it needs to be anchored in time, linked to extinct organisms and to past environments. Ultimately, we must look to the record in the rocks and sediments, just as paleontologists and biologists have been doing for the past two centuries.
In Darwin’s time, that record comprised rocks from the past 550 million years, a span of time that geologists now call the Phanerozoic eon, based on Greek words meaning visible or evident life. The eon began with the rocks of the Cambrian period, in which nineteenth- and early-twentieth-century paleontologists discovered a fabulous assortment of fossils—traces of trilobites, anemones, shrimp, and other multicellular animals that were completely missing from any of the earlier strata. Thousands of new animals and plants, including representatives of almost all contemporary groups, as well as hundreds of now-extinct ones, appeared so suddenly between 542 and 530 million years ago that paleontologists refer to the phenomenon as the Cambrian “explosion.” Darwin speculated that the progenitors of these animals had been evolving for vast periods of time prior to the explosion and that their remains were still hidden somewhere in the earth’s rocks, yet to be discovered. “We should not forget,” he noted in The Origin of Species, “that only a small portion of the world is known.”
Even in the twenty-first century, it’s humbling to consider how dependent our knowledge is on luck and chance—on fortuitous geologic events and the chance of their discovery. Trillions of cubic kilometers of rock strata lie buried and hidden from view unless an oil company decides to drill a core to hunt for oil or a miner excavates a quarry, an engineer cuts a road… or an earthquake creates a rift or a river erodes a canyon. The famous Burgess shale fossil assemblage in the Canadian Rockies owes its preservation to a massive primordial mudslide that buried a complete marine ecosystem in the peak of its Cambrian glory, and its bizarre fossil creatures were discovered by chance when a group of adventuresome nineteenth-century geologists were returning home from their explorations and had to move a stray boulder out of their donkeys’ path. Our understanding of the earliest land plants owes in large part to a volcanic eruption or similar disturbance that covered an ancient moor with a thick layer of dust that formed the chert that some farmer used for his boundary wall 400 million years later—unusual stones that a doctor cum geologist happened to notice as he was out walking near the Scottish village of Rhynie at the beginning of the twentieth century.
Just as Darwin suspected, the discovery of larger portions of the rock record, combined with advances in microscopic techniques and a better understanding of the mineral and chemical consequences of microbial life, has revealed rocks formed during vast periods of Precambrian time that were teeming with life. Three of the nearly four billion years of earth history that predate the Phanerozoic have now earned division into eons, if not eras and periods: the ill-defined Archean, which includes the first sedimentary rocks and the first putative stromatolites and microfossils, as well as organic carbon that is depleted in 13C compared to carbonates; followed by the long Proterozoic, which extends from 2.5 billion to 542 million years ago and includes evidence of the first continents, the first ice ages, and the first definitive fossils of bacteria and microalgae. Darwin was, however, only partially correct in his expectation. The detailed fossil imprints of a rich variety of relatively large, soft-bodied, apparently multicellular creatures have indeed been discovered in Precambrian rock formations around the world, most notably in Australia’s Ediacaran hills—but only in rocks formed during the 40 million years directly preceding the Cambrian explosion. The fossils are plentiful enough to have earned the time from 620 to 542 million years ago its own name, the Ediacaran period, but they are completely enigmatic with respect to anything that came before or after. In other words, though there is now plenty of evidence to document life’s presence on Earth for billions of years before the Cambrian explosion, we require another echelon of information, beyond the morphological, if we are to understand how, when, and which of the microscopic spheroidal marks and bloblike polymeric cysts that comprise its fossil record gave way to the plethora of complex forms that appeared in the Cambrian.
In fact, the phylogenetic work of the past few decades has shown that a lot of genetic variation and, presumably, evolutionary diversification can occur before it is expressed in morphological traits—not only in simple unicellular microorganisms, but also in complex multicellular plants and animals. Of course, botanists and natural products chemists had been noting this indirectly since the 1960s, when they started using the structures of biochemicals—proteins, or the triterpenoids that Guy Ourisson studied, or Geoff’s distributions of leaf waxes—to distinguish between related groups of plants that morphology failed to clearly differentiate. So it makes sense that fossil lipids, in addition to being the only fossils preserved in many environments, may reflect genetic change that morphology has yet to register, even in highly differentiated, well-characterized, multicellular forms of life such as the flowering plants.
Though biologists and paleontologists have been studying the morphology of flowering plants for almost two centuries and their fossils show up clearly during a relatively well-documented period of earth history—though they are the most abundant plants on the contemporary earth and our agriculture depends on them—we know as little about their early evolution as we do about the unicellular algae that left their amorphous imprints and blobs in Precambrian rocks. Fossils of flowering plants appear in rocks from the mid-Cretaceous period, but paleontologists could find no transitional forms, no morphological characteristics that might indicate when, where, or how the flowering plants diverged from the other groups of seed plants, such as conifers, ginkgoes, and mosses, whose fossils predate them in the rock record. It was, Darwin noted in a letter to botanist James Hooker in 1879, an “abominable mystery,” and the situation wasn’t much improved in the 1990s, when Mike Moldowan left Chevron for an academic post at Stanford and turned his attention to the problem. Even the advent of modern phylogenetic analyses had not shed much light on the origin of the flowering plants: they seemed to have no relatives.
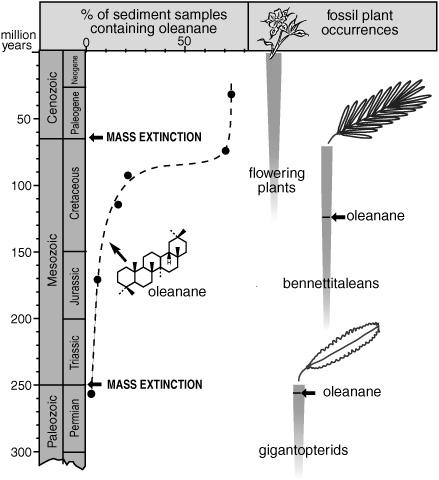
Moldowan’s geochemical approach was based on a phenomenon that he had been seeing in oil and rock samples for years, the chemistry of which Jürgen’s group had recently clarified: the various β-amyrin-type pentacyclic triterpenoids that flowering plants use to ward off insects and other pests are converted to oleanene and oleanane during diagenesis. If, as appeared to be the case, the β-amyrin structure is made by a biosynthetic pathway that is limited to flowering plants, and there are no other sources of oleanane in the sediments; and if one looked for oleanane in ancient marine sediments, rocks, and oils that had formed along continental margins, where runoff and rivers had delivered sediments eroded from large areas of land—then, Moldowan reasoned, one could extend the search for clues to the origins of flowering plants far beyond the paleontologists’ quarries and explorations.
The incidence of oleanane in the large group of samples that Moldowan and his colleagues analyzed was in rough accord with the fossil evidence, generally increasing from the late Cretaceous onward. The additional chemical information invites speculation about why the flowering plants diversified when they did: is this expansion of triterpenoid-producing flowering plants—presumably representative of the success of organisms with the β-amyrin biosynthetic capability— linked to the evolution, diversification, and expansion of insect pests? We can, to date, only speculate. The presence of oleanane in early Cretaceous and older rocks and fossils has, however, finally revealed a connection between flowering plants and older forms of seed plants—a connection based not on common morphological characteristics or on genetic information in extant species, but on a common biosynthetic pathway for a particular group of secondary metabolites. Oleanane was detected in rocks that formed during the Permian period, some 100 million years before unequivocal fossils of flowering plants or their pollen begin to show up in the rocks. In an attempt to pin down its source, Moldowan’s group analyzed samples that contained recognizable fossil leaves or fragments, and even managed to extract the lipids from some of the fossils themselves. The results were provocative. Oleanane was absent from most of the fossil conifers and seed ferns the researchers analyzed, just as it and its precursor triterpenoids are absent from living representatives of these groups of seed plants. An extinct group of cycad-like seed plants were an exception: fossils of three species of bennettitaleans, found in rocks from the early Cretaceous period, contained significant amounts of oleanane. More intriguing still, oleanane was present in an even older group of extinct fossil plants, the gigantopterids.
About 65 million years ago, at the end of the Cretaceous period, a giant asteroid apparently crashed into Mexico, frying a good part of North America and wreaking havoc with the earth’s climate system. The extent to which the asteroid was responsible is still a matter of hypothesis, but massive die-outs and extinctions of entire groups of Mesozoic era organisms, including dinosaurs, are apparent in the narrow strata of rocks and sediments deposited at this time. According to the fossil record, the bennettitaleans were among the victims of this holocaust, but unless the ability to make β-amyrin-type pentacyclic triterpenoids evolved more than once, some bennettitalean offshoot that paleontologists haven’t found must have survived, eventually giving rise to the flowering plants. And, given the presence of oleanane in gigantopterid fossils from the Permian period, it may not have been the first time that the oleanoid producers slipped by: the fossil record indicates that the gigantopterids disappeared during an even more catastrophic mass extinction of species at the beginning of the Triassic, some 251 million years ago.
Geologic records of oleanane in ancient sediments and fossil plants

Botanists have found various reasons beyond oleanane to believe that the bennettitaleans and gigantopterids were related to the group of plants that eventually took over the continental landscape and won the hearts, not to mention the stomachs, of its human inhabitants—in which case the flowering plants may indeed be the sole survivors of a larger group of seed plants that diverted onto its own evolutionary pathway during the Permian period. But another possible explanation for the oleanane data is that the ability to produce β-amyrin evolved more than once in different, unrelated groups of plants at various times in the earth’s history. John Volkman has become increasingly interested in the evolution of biosynthetic pathways in recent years, inspired by the writings of Guy Ourisson and Michel Rohmer, on the one hand, and by the dynamic group of molecular biologists at his institute in Australia on the other. He points out that, like sterol biosynthesis, the biosynthesis of β-amyrin begins with squalene epoxide and is catalyzed by an enzyme that is similar to the enzymes that catalyze sterol biosynthesis, so perhaps chance mutations leading to the ability to make it were not such a rare event. Triterpenoids with the oleanoid skeleton have, in fact, been found in a species of marine fungus, which might lend some support to the hypothesis that this biosynthetic capability evolved repeatedly in different groups of organisms. Or perhaps, Volkman notes, rather than a biochemical déjà vu, the ability to make oleanoids was a common property of early eukaryotic cells that was subsequently lost in most lineages—with the exception of those leading to the flowering plants and, apparently, certain fungi. If this were the case, however, the fossil oleanane trail, which seems to begin with the gigantopterids in the Permian period, might be expected to extend back even beyond the first fossils of land plants, which are about 425 million years old, to some common ancestor among the algae and the early eukaryotes that emerged during the Proterozoic eon.
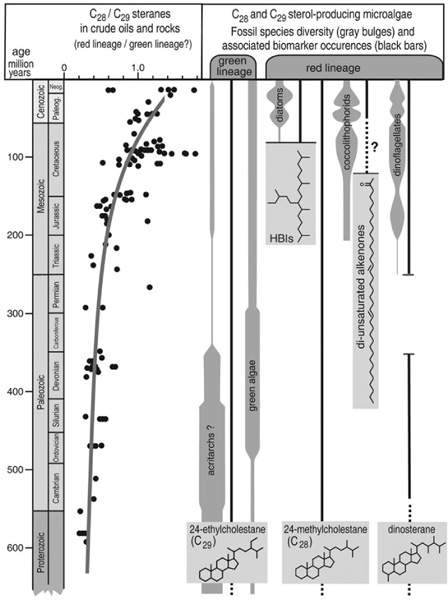
Similarities in cell structure and pigment composition imply that the precursors to land plants may have been members of the green algae, one of the three broad groups of algae—red, brown, and green—that can be distinguished by their different pigment compositions and types of photosynthetic apparatus. Phylogenetic analyses also indicate that the green algae are related to liverworts and mosses, and the oldest fossils of land plants are very similar to contemporary members of these two groups. Jan de Leeuw has speculated that the cross-linked alkyl chains of algaenan may have acted as a sort of plastic coating that provided protection against dehydration for early land-colonizing algae and allowed them to survive dry spells in shallow-water environments or during transport through the air by wind. But, of course, we don’t know when or even which green algae first started producing algaenan, and attempts to distinguish it or similar biological macromolecules in ancient kerogen and use it as a biomarker have, to date, been unsuccessful. On the contemporary earth, green algae are a minority group among marine phytoplankton, generally prominent only in freshwater environments, where they are often the dominant group. But microfossils in rocks formed prior to the Mesozoic era indicate that they may have been more widespread and prevalent in marine environments in times past. Paleontologists are at a loss to apply specific taxonomic assignments to many of these fossils, which are composed of insoluble, acid-resistant organic material in the form of a cell or cyst. They have been lumped under the ignominious heading “acritarch,” meaning “of uncertain origin” in Greek, but many of those found in marine rocks from the Paleozoic era exhibit just enough detail to be broadly identified as deriving from some species of green algae.
The distribution of steranes in ancient oils and rocks indicates that green algae may actually have dominated marine systems throughout the Paleozoic, and that the triumvirate of diatoms, photosynthetic dinoflagellates, and coccolithophores that currently rules ocean surface waters didn’t begin its rise to power until the Mesozoic era. Geochemists have been noting since the 1980s that the steranes in pre-Mesozoic crude oils and source rocks were predominantly C29 compounds, whereas those formed later were predominantly C28 compounds. By the 1990s, Volkman’s and others’ analyses of algal sterols were showing that, like most land plants, green algae typically produce relatively large amounts of stigmasterol and sitosterol, both of which are reduced in the sediments to some stereoisomer of the C29 sterane 24-ethylcholestane. The most abundant sterols in diatoms, dinoflagellates, and coccolithophores—all phylogenetically related to the red lineage of algae—are compounds such as brassicasterol, ergosterol, and 24-methylenecholesterol, which are reduced in the sediments to the C28 sterane 24-methylcholestane. Recently, Roger Summons has been working with the geochemist John Zumberge, whose private consulting company, GeoTech Oils, has assembled a database of results from careful, standardized biomarker analyses of hundreds of well-characterized and dated crude oils and source rocks: here the ratio of C28 to C29 steranes is consistently less than 0.6 from the late Proterozoic eon to the beginning of the Mesozoic era, but increases to more than 1.0 by the end of the Cretaceous period.
Silica frustules, calcium carbonate coccoliths, and elaborate organic cysts document the general expansion and diversification of diatoms, coccolithophores, and dinoflagellates, as well as the fluctuations in the relative status of their populations, since the Cretaceous. Diatoms appear to be the most recent of the three to gain prominence, with the largest increase in diversity having occurred within the last 50 million years. But like the fossil record of land plants and animals, the preservation of all these tiny microfossils is dependent on the whims of nature, in this case on the depth and chemical composition of the ocean water at a given time and place—and, of course, there is only so much one can learn about the evolution of an organism from its mineral encasement or, in the case of dinoflagellates, the cysts formed during its dormant periods. Here again, the molecular fossils tell a longer and potentially enlightening story.
According to their microfossil record, the diatom family has been around for about 130 million years. Fossils of the genera known to produce HBIs aren’t present until about 70 million years ago, but in a massive survey of sediments and oils formed over the past 500 million years, Sinninghe Damsté’s NIOZ group discovered that the HBIs had been around for much longer. According to this biomarker evidence, HBI-producing genera appeared on the scene about 91 million years ago—just after one of the most pronounced Cretaceous oceanic anoxic events, so it seems that the reorganization of marine ecology and nutrient cycles may have paved the way for their rise to dominance. The physiological role of HBIs in contemporary species of diatoms remains a mystery, but the Plymouth group’s investigations of how the organisms make them have raised some interesting questions. The two most prominent groups of HBI-producing marine diatoms, Haslea and Rhizosolenia, use two different biosynthetic mechanisms to produce the isoprene building blocks for their HBIs. Furthermore, phylogenetic analyses of more than a hundred species from all four HBI genera indicate that the Haslea and the Rhizosolenia fall into two very distinct genetic groups. It seems, then, that the ability to make HBIs evolved independently in different groups of organisms during at least two different episodes in earth history, implying that the compounds provided an important evolutionary advantage— but what that advantage was and how and whether it was related to the transformation of marine ecosystems that occurred during the oceanic anoxic event 93 million years ago are still anybody’s guess.
Another group of organisms that may play an important role in contemporary marine ecosystems, but is unrelated to any algal group and left no hard fossils whatsoever to attest its passage, may have made an even more decisive evolutionary leap during another of the Cretaceous anoxic events. The preponderance of 2-methyl hopanoids and relative paucity of algal steroids in black shales deposited during the oceanic anoxic events at 120 and 93 million years implies that nitrogen-fixing cyanobacteria were the primary producers of choice for these stratified, low-oxygen ocean regimes. But all Cretaceous black shales were not, apparently, created equal, despite their similar appearance. Black shales deposited in the newborn Atlantic and shrinking Tethys oceans about 112 million years ago contain an entirely different contingent of organic matter than anything that came before or after. Algal steroids are scarce, as in other black shales, but 2-methyl hopanoids are entirely absent, and the extractable organic matter consists mostly of archaeal tetraethers, with crenarchaeol being the most abundant compound in the polar fraction, and of acyclic isoprenoid alkanes, including pentamethylicosane and a similar compound, tetramethylicosane. Stranger still, the kerogen consists of a macromolecular material composed almost entirely of the latter, laced together by a network of ether linkages. In both the extract and the kerogen, these lipids are significantly enriched in 13C compared to the algal steroids—as is typical for crenarchaeal lipids. Faced with this evidence, the NIOZ group hypothesized that during this particular Cretaceous anoxic event, it was the planktonic crenarchaea, rather than cyanobacteria, that gained an upper hand. Perhaps the event was even a major turning point in crenarchaeal evolution, marking the expansion of cool-water, crenarchaeol-producing planktonic forms.
Phylogenetic analysis does indeed indicate that the marine planktonic crenarchaea diverged relatively recently from their hyperthermophilic antecedents, though just how recently is unclear. Hyperthermophilic crenarchaea have been discovered living within hydrothermal vents, and perhaps more moderate forms developed in the warm outflow waters around the edges of the vents, which were evidently quite plentiful during the mid-Cretaceous. According to Sinninghe Damste’s hypothesis about membrane density, these more moderate thermophiles would have begun synthesizing crenarchaeol, with its six-membered bulge-producing ring … and then what better time for heat-loving, ammonium-using, low-oxygen-tolerant organisms to venture out into the wider world than during an oceanic anoxic event when, according to the TEX86 index and other proxies, surface water temperatures were as high as 35°C? Crenarchaeol or its distinctive biphytanyl component has been found in rocks from the late Jurassic, however, some 50 million years before the mid-Cretaceous expansion. Some researchers think that cold-adapted crenarchaea evolved much earlier, whereas others doubt that crenarchaeol had anything to do with cold adaptation and maintain that it can be made by thermophilic crenarchaea as well. A group of microbiologists and organic geochemists at Harvard and the University of Georgia recently detected crenarchaeol in hot spring microbial mats with temperatures ranging from 40°C to 85°C. They proposed that the ability to biosynthesize crenarchaeol may be more related to metabolic function and the permeability of the membrane to specific compounds than it is to temperature. However, the amount of crenarchaeol in the hottest mats they studied was, in fact, negligible, whereas it comprised a significant fraction of the archaeal tetraethers in the lower temperature ones, and completely dominated them at 40°C—just as one might expect for a lipid component that facilitates membrane function at less extreme temperatures. The presence of crenarchaeol in continental hot spring mats, like its association with heterotrophic organisms in sediments and in soils, indicates that the compound is synthesized by organisms occupying a variety of ecological niches and is not limited to planktonic marine crenarchaea, as initially supposed. Whether it is produced in the hot spring mats by the crenarchaea whose genes the Georgia group found, which are closely related to thermoacidophilic crenarchaea, or by an undetected strain of “cold-adapted” crenarchaea that can survive at higher temperatures, or by some yet-undiscovered evolutionary intermediate is, as of this writing, unclear.
Another story that brings us tantalizingly close to linking the evolution of a particular biochemical trait with a particular set of environmental conditions and juncture in earth history—and is likewise missing an important chapter, or two or three—is that of the alkenone-producing coccolithophores. The incidence of long-chain alkenones and related esters in marine sediments can be loosely correlated with the different forms of coccoliths deposited over the past 50 million years. The most recent of these are, of course, the coccoliths of Emiliania huxleyi and members of the genus Gephyrocapsa. The latter first appear in Pliocene sediments and dominate the coccolith record for about three million years, until Emily gained the upper hand around 75,000 years ago. During the Miocene and Oligocene epochs, the incidence of alkenones correlates roughly with the presence of the now-extinct genus Reticulofenestra, which, based on coccolith morphology, appears to be closely related to Gephyrocapsa. During the Eocene, the record of alkenones becomes sketchier, either because they have been degraded or reduced to alkanes, or because the organisms producing them were scarcer. Prior to the Eocene, the taxonomic association breaks down altogether, as the coccoliths are too morphologically indistinct to classify, and there are only a few reports of alkenones.
Alkenones have, in fact, been identified in Cretaceous sediments as old as 120 million years, but the distribution of chain lengths and saturation patterns is different from the suite of eight made by Emily, most notably in the absence of compounds with more than two double bonds. The triunsaturated compounds are slightly more reactive than their more saturated counterparts and could have simply disappeared from some older sediments—but they are also absent from black shales where much more labile compounds persist, and it seems likely that compounds with more than two double bonds simply didn’t exist yet. The modern-day distribution of homologues, including the triunsaturated 37-carbon alkenones, makes its debut in sediments deposited at high latitudes about 50 million years ago, during the first round of cooling and variable climate regimes that followed the long heat waves of the Cretaceous and early Paleogene periods. Simon Brassell has proposed that this was when the temperature-dependent regulation of unsaturation in alkenone biosynthesis emerged, though whether the algae gained some advantage from storing a larger proportion of unsaturated compounds when the water cooled, or whether this temperature “adaptation” is just the direct effect of temperature on the biosynthetic reactions, is still the question of the day when it comes to alkenones. We remain nearly clueless as to what adaptive benefits these unusual lipids provided for the organisms that started making them to begin with. According to the hard fossil record, phytoplankton diversity, and coccolithophore diversity in particular, was on the rise during the early Cretaceous, implying that competition was also increasing. Trans double bonds make the alkenones difficult for zooplankton to digest and relatively resistant to degradation by sunlight, so perhaps the coccolithophores that invented them were less vulnerable to grazing, or perhaps the alkenones provided a particularly long-lasting energy supply that put them at an advantage when nutrients became depleted in the surface waters.
Among the phytoplankton that may have been competing with the coccolithophores in the oceans of the early Cretaceous, the dinoflagellates appear to have the longest and most puzzling history. Their organic cysts, replete with telltale details and ornamentation that allow paleontologists to classify them, are indisputably present in sediments dating back to the beginning of the Triassic period and reflect a phenomenal degree of evolutionary innovation and diversification during the Jurassic and Cretaceous. Summons and his cohorts found that an increase in the relative abundance of dinosterane is correlated with the incidence of cysts in rocks and sediments, and Moldowan’s group obtained a similar result from a more extensive analysis of triaromatic dinosteroids— presumably diagenetic end members of dinosterol—in mature sediments and oils. The appearance of the dinoflagellates at the beginning of the Triassic is preceded by a mass extinction of invertebrates and land plants that was so pronounced it almost seems like retribution for the earlier excesses of the Cambrian—a severe retrenchment that cleared the earth of more than 75% of its fossil-producing species, including the trilobites that had reigned in the ocean for hundreds of millions of years. Few of the phytoplankton that lived in pre-Triassic times left hard fossils, and little is known about what happened at the base of the marine food chain during the extinction. The Bristol group—now under Richard Evershed’s leadership, with Richard Pancost among its permanent members—recently analyzed rocks spanning this period and found sudden peaks in the abundance of 2-methyl hopanoids immediately following the two stages of invertebrate extinction indicated by the fossils. The cyanobacteria, it seems, were just waiting in the wings to take immediate, though transitory, advantage of the lack of grazing pressure and competition, much as they apparently did during some of the Cretaceous oceanic anoxic events. Kliti Grice, now leader of her own research group at Australia’s Curtin University of Technology, has found isorenieratane and related compounds in rocks from this same period, indicating that the oceans were similarly oxygen depleted. Both the fossil and the biomarker records suggest that the dinoflagellates rapidly evolved and diversified in the wake of the Permian-Triassic holocaust, and there is no sign, either fossil or biomarker, of their existence during the 100 million years prior.…And yet the story is not, apparently, so simple: there are hints in the geological record that this might have been a repeat performance for the dinoflagellates, either a comeback after a long period of reclusive inactivity, or a reinvention.
Evolution and diversification of marine algae: microfossils and molecular fossils
Though there are no signs of dinoflagellates in rocks formed during the Permian and Carboniferous periods, acritarchs that bear a marginal resemblance to dinoflagellate cysts are frequently encountered in rocks that formed before 350 million years ago, and independent studies from both Summons’s and Moldowan’s labs have found relatively large amounts of dinosterane and triaromatic dinosteroids in the rocks associated with them. Did these come from early dinoflagellates or their ancestors? And did they simply recede into obscurity 350 million years ago, eking out a living in some ecological microniche where they survived through the great apocalypse at the end of the Permian—lying low, so to speak, until they could expand into an empty world where their every evolutionary innovation would be accommodated? Many dinosterol-producing dinoflagellates are heterotrophic and would have been less productive than their photosynthetic relatives—perhaps their fossils and biomarkers are simply not plentiful enough for detection. Or perhaps the early dinosterol-producing, cystforming organisms had nothing to do with dinoflagellates at all and went extinct 350 million years ago—perhaps the dinoflagellates did evolve from scratch after the Permian-Triassic mass extinction, and simply reinvented some of the same characteristics, including dinosterol…. But there is another clue, this time from the phylogenetic tree of life: the “primitive” morphology and genetic makeup of contemporary photosynthetic dinoflagellates imply that they diverged onto their own idiosyncratic evolutionary pathway early in the evolution of the eukaryotic cell structure, indeed, not long after the divergence of the red lineage of algae…. And when was that?
The oldest occurrence of fossils that can be confidently identified as red algae have been found in a rock formation in Canada and are about 1.2 billion years old. The consensus among paleontologists is that acritarchs found in Australian shales that formed between 1.49 and 1.43 billion years ago are definitely the remains of some sort of eukaryotic algae. And most paleontologists agree that microfossils found in 1.9- to 1.8-billion-year-old rocks correspond to organisms that boasted a eukaryotic cell structure. But once again, the molecular fossils may have a much longer story to tell, a story that is directly tied to the accumulation of free oxygen on the early earth and the evolution of oxygenic photosynthetic bacteria—and perhaps, counterintuitively, methanogenic archaea—among other things.