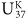 index.
index.Cross-references to other glossary entries are shown in italic.
Acid See proton, carboxylic acid.
Acritarch Microfossil of uncertain biological origin, composed of insoluble, acid-resistant organic material in the form of a single cell or cyst.
Aerobic Metabolic processes (e.g., respiration) that make use of O2 and take place under oxic environmental conditions. (See anaerobic.)
Alcohol A molecule containing one or more hydroxyl groups (–OH). Examples include methanol, ethanol, phytol, cholesterol, and bacteriohopanetetrol.
Alkane Synonym for saturated hydrocarbon, that is, a chemical compound that contains only carbon and hydrogen, and has no double bonds or aromatic units. Alkanes can be straight chains of CH2 groups (n-alkanes), contain one or more rings (cyclic hydrocarbons), or they can have one or more alkyl side groups (branched alkanes).
Alkenone A common name for dialkyl ketones with at least one double bond. In geochemistry, the term usually refers specifically to a group of long-chain unsaturated ketones with 37–39 carbon atoms and 2–4 double bonds, which are biosynthesized by coccolithophore algae and used to determine past sea surface temperatures via the 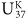 index.
index.
Alkyl An alkane unit that is attached to any sort of molecule; the alkyl group is composed only of carbon and hydrogen, but the rest of the molecule can have any elemental or structural composition.
Amino acid A chemical compound with a carboxyl (−COOH) and an amino (−NH2) group. Organisms make α-amino acids, where the amino and carboxyl groups are attached to the same carbon atom. Peptides and proteins are composed of many of these linked together by amide bonds (−CO–NH–). All but one of the amino acids used in proteins are chiral molecules, and they are present almost exclusively in the L configuration (see stereoisomers).
Anaerobic A metabolic process that does not make use of oxygen and takes place in an anoxic environment.
Anammox An acronym for anaerobic ammonium oxidation, a microbial process wherein ammonia (NH3) reacts with nitrite (NO2−) to produce molecular nitrogen (N2).
ANME Anaerobic methanotrophic archaea. These microorganisms participate in the anaerobic oxidation of methane and incorporate carbon from the methane. At least three phylogenetically distinct groups of ANME have been identified; these appear to be related to several genera of methanogens. ANME are often found living in close association with sulfate-reducing bacteria, sometimes in consortia where methane is oxidized and sulfate reduced. Distinctive lipids include those listed for methanogens, but can be distinguished by their 13C-depleted isotope signatures. (See methanotroph.)
Anoxic, anoxia The absence of O2 in a body of water or in sediments. Anoxic conditions develop in lakes, ocean basins, or sediments when large amounts of organic matter are introduced, because aerobic microorganisms consume the organic matter and use up all the oxygen, which is not replenished quickly enough. In natural waters, oxygen can be incorporated from the air by waves near the surface, or it can be replenished by deep currents. In sediments, the pore size and permeability determine whether oxygen can be replenished from overlying oxic water. Organic matter is better preserved under anoxic than oxic conditions. Cretaceous black shales and Mediterranean sapropels were deposited under anoxic conditions.
Anoxygenic phototroph Photosynthetic bacteria that do not use water as an electron donor or produce molecular oxygen. Typically, they use sulfide or other reduced sulfur compounds, molecular hydrogen, or a variety of small organic molecules as electron donors. There are four known phylogenetic groups of anoxygenic phototrophs: the green sulfur bacteria, filamentous anoxygenic phototrophs (Chloroflexus), purple sulfur bacteria, and heliobacteria.
Archaea Comprises one of the three domains in the phylogenetic tree of life. Originally called Archaebacteria, it was identified as a separate division of prokaryotes by Carl Woese and George E. Fox in 1977. One of their distinguishing features is that their cell membranes are composed of ether-linked lipids based on the phytane structure (isoprenoid glycerol ethers). Initially believed to occur only in extreme environments, archaea are now known to be widely distributed, in all kinds of habitats. Those discovered to date fall into three main phylogenetic groups, the Korarchaea, Crenarchaea, and Euryarchaea.
Aromatic ring A hexagonal, six-carbon ring structure with the equivalent of three conjugated double bonds; in reality, the electrons are not localized in three separate bonds but shared among the six carbon atoms. The term “aromatic” stems from the fact that many naturally occurring compounds containing aromatic rings have distinctive scents. Polycyclic aromatic hydrocarbons (PAHs) have two or more fused aromatic rings and can be quite large; present in petroleum and formed by combustion of organic matter, they are common air and water pollutants.
Asphalt A semisolid or solid form of bitumen with no precise chemical definition, often found at natural oil seeps. In the United States, asphalt is the residue from petroleum distillation used to pave roads. Sometimes referred to as “bitumen” in ancient texts and by archaeologists.
Asphaltenes A high-molecular-weight polar fraction of bitumen. In chemical composition and polarity, asphaltenes are intermediate between resins and kerogen.
Autotroph An organism that produces complex organic compounds from simple inorganic molecules (e.g., CO2 or HCO3-) and an external source of energy, such as sunlight or chemical reactions of inorganic compounds. An autotroph is a primary producer at the base of the food chain. Photosynthetic organisms are the most widespread and productive autotrophs, but chemosynthetic organisms are important in some ecosystems.
Average chain length (ACL) Used to compare carbon number distributions of unbranched compounds. The ACL of odd-carbon-number n-alkanes from 27 to 33 carbon atoms is given by ACL27–33 = (27[C27] + 29[C29] + 31[C31] + 33[C33]) / ([C27] + [C29] + [C31] + [C33]), where [Cx] is the amount of alkane with x number of carbon atoms.
Bacteria One of the three domains in the phylogenetic tree of life (sometimes called Eubacteria). Until archaea were recognized as a separate phylogenetic division, the term “bacteria” was synonymous with prokaryote and included both eubacteria and archaebacteria, so there is still some confusion in the literature.
Benthic Refers to the sediment surface and water at the bottom of a lake or ocean, used to describe organisms that live there.
Bicarbonate Formed when atmospheric carbon dioxide is dissolved in water. Reaction with water yields an equilibrium mixture of dissolved carbon dioxide (CO2), bicarbonate (HCO3−), and carbonate (CO32−). Bicarbonate is the preferred carbon source for most aquatic photosynthetic organisms.
Biomarker An organic compound in natural waters, sediments, soils, fossils, crude oils, or coal that can be unambiguously linked to specific precursor molecules made by living organisms (also: biological marker, molecular fossil, fossil molecule, geochemical fossil).
Biomass The total weight of all living biological material in a given area.
Biphytanyl A molecular component comprised of two phytanyl units linked together head-to-head via a covalent carbon–carbon bond. Biphytanyl groups are prominent in the cell membrane lipids of archaea, where their apparent function is to add rigidity across the membrane. In some archaea, there are cyclopentyl or cyclohexyl rings within the biphytanyl units.
Bitumen The part of the organic matter in geological samples that dissolves in organic solvents; also called extractable or soluble organic matter.
Black shale A sedimentary rock that contains exceptionally large amounts of organic matter, which makes it dark colored or black. (See oil shale, sapropel.)
C3 and C4 Two diff erent metabolic pathways for carbon fixation during photosynthesis. Most broad-leafed and temperate land plants use the C3 pathway, whereas most tropical grasses use the C4 pathway. The two pathways produce diff erent amounts of carbon isotope fractionation, such that biomarkers of the two plant groups can be distinguished by their δ13C values.
Cambrian The period in the earth’s history (542–488 million years ago) during which the first calcareous algae and exoskeletal invertebrates (e.g., trilobites, mollusks, ostracods, foraminifera) evolved. The radiation of animal phyla during this time is called the “Cambrian explosion.”
Carbon number In this book, refers to the total number of carbon atoms in a molecule and indicated by a subscript (e.g., C22 indicates a 22-carbon compound). In many publications carbon number is also used to designate a specific position in a molecule as determined by the nomenclature of organic molecules, which unequivocally numbers each carbon atom; in this case no subscript is used (e.g., C-22 refers to the 22nd carbon atom in a molecule’s numbering scheme).
Carbon Preference Index (CPI) A numerical means for determining the odd- or even-carbon-number predominance in a homologous series of n-alkanes, n-alcohols, or n-carboxylic acids based on the ratio of the amounts of odd-carbon-number to even-carbon-number compounds. Amounts are obtained by measuring compound peak heights or areas in the gas chromatograms. Initially defined for the long-chain C24 to C34 n-alkanes, it can be similarly used for other carbon number ranges.
Carbonyl The functional group (C=O) that characterizes the ketones.
Carboxylic acid A chemical compound that is characterized by the −COOH functional group. Carboxylic acids that have a straight or monomethyl-branched chain, with or without unsaturation, are also called fatty acids (see proton).
Catagenesis The thermocatalytic transformation, or maturation, of organic matter in sedimentary rocks that occurs at great depths (usually >2 km) and high temperatures (>50°C). Results in “cracking” and the generation of smaller molecules, releasing liquid and gaseous hydrocarbons from the kerogen into the bitumen. Under some geological conditions, these form accumulations of crude oil and natural gas.
Cell membrane (plasma membrane) The envelope around a cell that encloses the cytoplasm and forms a semipermeable barrier between it and the environment. In plant cells and prokaryotes, it is usually surrounded by a cell wall, whereas in animal cells it is the only barrier between the cytoplasm and the outside of the cell. It is composed of glycerol-based phospholipids in which a variety of protein molecules are embedded and act as channels and pumps. Sterols, bacteriohopanoids, or tetraethers may act to strengthen and stiff en the otherwise quite fluid membrane in eukaryotes, bacteria, and archaea, respectively.
Chert A white, dense, hard sedimentary rock composed of microcrystalline quartz (SiO2) from the chemical or biological precipitation of silica. Chemical impurities may give rise to colored analogues.
Chiral Describes a molecule that can exist in two nonsuperimposable mirror image structures, due to the presence of one or more asymmetric carbon atoms, that is, carbon atoms with four diff erent atoms or groups of atoms bound to them. In solution, chiral compounds rotate plane-polarized light in either clockwise or anticlockwise direction (optical activity/rotation). The term is also used to denote the asymmetric centers, or atoms, in a molecule.
Chromatogram The visual result of chromatographic analysis, now also used for the graphic representation of the result, where signal intensity is on the y-axis, and time on the x-axis. For example, a gas chromatogram may show the response of a flame ionization detector as a function of time. The term can also refer to the result of mass spectrometric analysis, where a mass chromatogram may show ion intensity versus time for the sum of a range of ions or for specific selected ions. (See mass spectrometry.)
Ciliates A group of mobile, heterotrophic, unicellular eukaryotes, typified by undulating hairlike extensions that are used for swimming, crawling, attachment, feeding, and sensation. They feed on detritus and bacteria and are common in natural waters. Some ciliates biosynthesize tetrahymanol, a pentacyclic triterpenoid that is diagenetically transformed into the saturated hydrocarbon gammacerane.
Coccolithophores A group of single-celled marine planktonic algae that secrete hubcap-shaped calcium carbonate (calcite) shields called coccoliths. Members of the Haptophyta phylum, they are widespread and abundant in the contemporary ocean. The most prominent contemporary species of coccolithophore, Emiliania huxleyi, produces the long-chain alkenones used by geologists and paleoceanographers to determine past sea surface temperatures (see 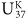 ). Coccoliths comprise a major component of carbonate marine sediments deposited in the past 150 million years.
). Coccoliths comprise a major component of carbonate marine sediments deposited in the past 150 million years.
Column chromatography A technique for separating mixtures of organic compounds into pure compounds or simpler mixtures that involves dissolving them in a mobile phase and passing it through a column filled with some sort of stationary phase. Compounds in the mixture have diff erent affinities for the mobile and stationary phases. They are adsorbed onto the stationary phase and then, as the mobile phase flows through the column, separated and sequentially released and collected. A compound’s residence time in the column is determined by the particular combination of materials used for the stationary and mobile phases, and by its boiling point, molecular size, molecular shape, and polarity. The unembellished term column chromatography refers to use of a vertically positioned glass column filled with silica gel or aluminum oxide, as stationary phase; an organic solvent dripped into the top of the column, as mobile phase; slow percolation through the column under the force of gravity; and separated groups of compounds collected as they drip out the bottom. In a more general sense, gas chromatography and high-performance liquid chromatography are forms of column chromatography.
Compound General term for a material with a specific chemical structure. Compound, substance, and molecule are often used interchangeably.
Continental shelf The gently sloping seaward extension of the continent, generally covered with water during interglacial periods like the present, and exposed during glacial periods. Water depth on the continental shelf is less than 200 m, and the sediments typically contain a relatively large amount of organic and mineral matter that derives from the land. Beyond the shelf, the edge of the continent drops off more steeply and water depth increases dramatically.
Copepod A large group of small crustaceans found in the sea and most freshwater habitats. There are both planktonic and benthic species. In the ocean, copepods are the most abundant zooplankton and form a crucial link in the food chain between the primary producers (phytoplankton) and fish.
Covalent bond A chemical bond between atoms in which two atoms share a pair of electrons. Covalent bonds are what holds organic compounds together. They are much stronger than the ionic bonds in inorganic salts, where the anion hosts the electron pair and crystals are formed by strong electrostatic interaction between anions and cations. Other weaker types of bonds include hydrogen bonds and van der Waals interactions.
Crenarchaea A taxonomic grouping of Archaea. Included in the Crenarchaea are hyperthermophiles, which can live in extremely hot environments (over 100°C); autotrophic organisms that are widespread in both the surface and deep waters of the ocean; and heterotrophic organisms that live in sediments and soils. Their cell membranes are composed of isoprenoid glycerol ethers such as caldarchaeol, crenarchaeol, and ring-containing biphytanyl tetraethers. (See TEX86.)
Cretaceous A period in the Mesozoic era of the geologic timescale (144 to 65 million years ago), characterized by a warm “greenhouse” climate and by the final breakup of the earlier supercontinent to form the contemporary continents and the Atlantic Ocean (see seafloor spreading), and known as the last period of the dinosaurs’ reign. (See oceanic anoxic event.)
Crude oil A naturally occurring black or brownish liquid formed from kerogen in deeply buried sediments that have been subjected to high temperatures and pressures. often used interchangeably with the more general term petroleum, which, strictly speaking, includes condensate and gas, as well as crude oil.
Culture A population of bacteria or other microbes grown in an artificial medium in the laboratory, or the process of maintaining them. Cultures may be pure, that is, restricted to one specific organism, or they may be mixed, consisting of more than one species or strain. (See isolate.)
Cyanobacteria A phylum of unicellular and filamentous bacteria that includes all oxygen-producing photosynthetic bacteria. They are important primary producers in the ocean, especially prominent in open-ocean, low-nutrient areas; many groups are able to fix molecular nitrogen, allowing them to thrive when conditions are unfavorable for other photosynthetic organisms, which can only obtain their nitrogen from nitrate and ammonium. Unlike other prokaryotes, cyanobacteria often contain fatty acids with two or more double bonds. Unlike other photosynthetic bacteria, their main form of chlorophyll is the same as that in green algae and higher plants (chlorophyll a). Other distinctive lipids are mono-, di-, and trimethyl-branched alkanes, bacteriohopanoids, and, more specifically, 2β-methyl bacteriohopanoids.
Cyclic hydrocarbon A chemical compound made of carbon and hydrogen and having one or more ring structures.
D amino acid See stereoisomers, amino acid.
Dating techniques Radiometric dating techniques provide the absolute age of a fossil or rock based on the observed abundance of naturally occurring radioactive isotopes and their known decay rates. Diff erent isotopes are useful for diff erent timescales. Radioactive carbon dioxide (14CO2) is present in low concentrations in the atmosphere; it is incorporated into biomass by photosynthesis organisms and then passed on to other organisms in the food chain. With the death of an organism, no new carbon is introduced and radioactive decay of the 14C proceeds, such that the age of a fossil (or of biogenic sediments) can be determined from the relative abundance of 14C remaining in the fossil, the initial concentration in the atmosphere, and the decay rate. Radiocarbon dating is accurate up to about 40,000 years, and can be used to date both organic matter and mineral precipitates. Other radioactive isotopes that are used to date mineral precipitates in sediments and rocks over diff erent timescales, include lead (210Pb or 207Pb), thorium (230Th), various isotopes of uranium (234U, 235U, and 238U), potassium (40K), and rubidium (87Rb). These cover timescales of 100 years to 48.8 billion years. The relative amounts of L and D amino acids in fossils and sediments can also be used to determine absolute ages up to 100,000 years old, based on the rate of isomerization between the L form initially made by the organism and the D form. (See stereoisomers.) Biostratigraphy uses the distributions of microfossils within rock strata to match strata that formed at the same time in diff erent regions, that is, it correlates the strata based on the evolution of the organisms. Fossils used typically comprise pollen, foraminifera, dinoflagellates, radiolaria, and so forth. By comparing the relative ages of strata in one region with those in another where radiometric ages can be determined, an absolute age can be assigned.
δ13C A measure of the relative amounts of the two stable isotopes of carbon in a substance. δ values are generally defined as δ (per mil) = (Rsample/Rstandard – 1)1,000, where R is the ratio of the rare isotope to the abundant one, in this case 13C/12C. Diff erences in isotope ratios are small, measured in parts per thousand (per mil or ‰). The original reference standard was a sample of fossils from an extinct organism called a belemnite collected decades ago near the Pee Dee River in South Carolina. This Pee Dee belemnite (PDB) has long since been used up and replaced by a new standard defined by the International Atomic Energy Agency in Vienna, the so-called Vienna PDB. The δ13C of carbonate mineral deposits reflect the δ13C of the bicarbonate in the water where they formed and vary depending on where and when the deposits formed. Because the 12C isotope in carbon dioxide is preferentially incorporated during photosynthesis, biological organic matter is always isotopically lighter than carbonates, with distinctly negative δ13C values. The δ13C values of an organism’s biomass depends on the type of organism and on the δ13C of its specific carbon source. (See fractionation, C3 and C4.)
δ18O A measure of the relative amounts of the two most abundant stable isotopes of oxygen in a substance, determined as described for δ13C and using the same VPDB reference standard or water defined as “standard mean ocean water” (SMOW) and its updated version, Vienna standard mean ocean water (VSMOW). δ18O values of carbonate foraminifera shells can be used as a climate proxy, because the 18O composition of the oxygen atoms in the bicarbonate and carbonate dissolved in the water reflects the 18O composition of the oxygen atoms in the water, and this varies with climate. The formation of ice sheets on the continents leaves the ocean water enriched in 18O during glacial times, on the one hand, and, on the other, the foraminifera shells have a preference for 18O that increases at cold temperatures. Both effects are recorded in the foraminifera shells, which show the oscillating ice ages and warm periods of the past few million years.
Derivative A compound that has the same basic carbon structure as its “parent” compound, but with diff erent groups or functional groups attached.
Diagenesis The transformation of organic matter in sediments by low-temperature chemical reactions and microbial activity. At temperatures above 50°C, for instance, after burial at greater depth, diagenesis is followed by catagenesis. (Inorganic geochemists use the term diagenesis diff erently.)
Diasterane A product of diagenesis of sterols, produced by structural rearrangement of the intermediate sterenes, specifically by migration of the methyl groups from the upper to the lower face of the ring system (from C-10 and C-13 to C-5 and C-14, respectively). The reaction is catalyzed by acidic sediment components like clay minerals, so diasteranes are generally absent from pure carbonates and evaporites. Diasteranes are not formed by biological processes. Also called “rearranged steranes.”
Diatoms A large, diverse, widespread group of mostly unicellular green alga. Diatoms can be found in the surface waters of seas and lakes, or growing on the surface of sediments or soils. The cell’s ornate skeletal structure (frustule) is made of silica and exhibits a large variety of spectacular forms. Diatom fossils are used in biostratigraphy (see dating techniques). Distinctive lipids include highly branched isoprenoids (HBIs), long-chain alkanediols, brassicasterol (24-methylcholesta-5,22-dien-3β-ol), and fucosterol (24-methylcholesta-5,24(28)-dien-3β-ol).
Dinoflagellates A large, diverse group of unicellular eukaryotes that includes the second largest group of algae in contemporary oceans, as well as many freshwater species and nonphotosynthetic forms. Dinoflagellate cells are poorly preserved as fossils, but many species have a life cycle that includes a “resting phase” when they form cysts that have a more resistant cell wall and are well preserved in ancient sediments. A distinctive lipid common to most members of the group is dinosterol.
Domain One of three branches in the tree of life: Bacteria, Archaea, or Eukarya.
Double bond A covalent bond between atoms in which two atoms share not one but two sets of electrons.
DSDP Deep Sea Drilling Project, a U.S.-based international project (1966–1984) designed to answer fundamental questions about the earth by drilling holes into the ocean floor and investigating the recovered sediments, rocks, and fluids. Plate tectonics, climate history, paleoceanography, and marine geology were among the main study themes. The first research ship was a converted oil drill ship called the Glomar Challenger. The DSDP expanded into the Ocean Drilling Project (ODP) and then, in 2003, the Integrated Ocean Drilling Project (IODP).
Eocene A period in the Cenozoic era of the geological timescale (55–34 million years ago). The modern mammal fauna developed during this time of mostly warm climate. The Green River oil shale (Utah, Colorado, Wyoming) and the Messel oil shale (Germany) are two important organic-matter–rich lake deposits of Eocene age.
Equilibrium A state in a reversible chemical reaction when the rate of the forward reaction equals that of the back reaction, so that the amounts of reactants and products cease to change with time even though both reactions are ongoing. The equilibrium ratio of products to reactants varies with temperature and depends on their thermodynamic stabilities.
Equilibrium mixture The composition of a mixture of chemical compounds at the equilibrium state.
Ester A chemical compound formed by the reaction of a carboxylic acid and an alcohol, with elimination of a molecule of water. Ester bonds (R−CO–O–R′) are relatively easily cleaved (hydrolyzed) by the addition of water in the presence of an acid or base catalyst.
Ether A chemical compound characterized by an oxygen atom bound to two carbon atoms, or R–O–R′ where R and R′ are alkyl or other groups. Ethers are less easily cleaved during diagenesis than esters.
Ethyl group A two-carbon alkyl group in chemical structures (−CH2CH3).
Eukarya One of the three domains in the phylogenetic tree of life. (See eukaryote.)
Eukaryote An organism with a complex cell structure and a membrane-bound cell nucleus, which contains the genetic material. Most eukaryotes employ aerobic respiration. Animals, plants, and a number of unclassified unicellular organisms (protists) are Eukaryotes. Their membrane lipids contain glycerol diesters of straight-chain C16 or C18 fatty acids and sterols as rigidifiers. (See prokaryote.)
Euryarchaea A taxonomic grouping within the domain Archaea that comprises extremely halophilic archaea, methanogens, and anaerobic methane-oxidizing archaea (ANME).
Evaporite A sedimentary rock formed by the evaporation of water and precipitation of salt in arid marine areas. Salts are deposited according to their solubilities in water, with calcite (calcium carbonate) and gypsum (calcium sulfate) precipitated first and halite (sodium chloride) relatively late.
Extraction, extract The process of removing organic matter from a geological or biological sample by dissolving it in an organic solvent, and the solution that results.
Fatty acid See carboxylic acid.
Fermentation An anaerobic energy-yielding process employed by a wide variety of bacteria and some fungi, wherein organic molecules are split into two smaller molecules, one oxidized and one reduced relative to the starting material. In natural waters and sediments, these products, usually small organic acids and alcohols, are used as energy and carbon sources by other bacteria. A well-known example of fermentation is the conversion of the sugar hexose by yeast (a fungus) to produce ethanol (reduction) and carbon dioxide (oxidation).
Flowering plants The most widespread group of contemporary land plants, characterized, among other things, by flowers and by seeds that are encapsulated in an ovary, which often ripens into a fruit. The other main group of extant seed plants, the gymnosperms, have naked seeds exposed on the scale of a cone. Gymnosperms, which include conifers like pine or spruce trees, developed in the middle Paleozoic era and were the dominant land plants until the advent of flowering plants in the Cretaceous. Distinctive lipids found in both groups of land plants include long-chain n-alkanes, alcohols, and fatty acids, and 24-ethylcholesterol. Oleanoid-type pentacyclic triterpenoids are specific to flowering plants.
Foraminifera (forams) A large group of unicellular eukaryotes, comprising planktonic and benthic species. Generally heterotrophic, though some species develop a symbiotic relationship with unicellular algae that they have ingested. Most extant forms are marine. Forams are distinguished by their carbonate shells, or tests, which make up a significant component of carbonate marine sediments.
Fractionation 1. Isotope fractionation: the change of isotopic composition during physical, chemical, and biochemical processes, for example, the dissolution of atmospheric carbon dioxide in water, or the photosynthetic transformation of carbon dioxide into organic molecules. 2. The separation of complex mixtures of organic compounds such as those in a rock extract into less complex mixtures, usually by column chromatography.
Functional group A characteristic group of atoms in a molecule that determines its chemical reactions and associates it with a certain type of chemical compound. Some examples are: the hydroxyl group (–OH) in alcohols, the carbonyl group (C=O) in ketones, the carboxyl group (−COOH) in carboxylic acids, or the amino group (−NH2) in amines.
Fungi A group of heterotrophic, generally multicellular eukaryotic organisms characterized by a chitinous cell wall and comprising yeasts, molds, and mushrooms. Distinctive lipids include the 28-carbon sterol ergosterol (24-methylcholesta-5,7,22-trien-3β-ol).
Gas chromatograph (GC) An instrument used for the separation of complex mixtures by gas chromatography. Key components are an injector, a gas chromatographic column, and a detector. Common types of injector for biomarker analyses include split/splitless and on-column; typical columns are 30- to 50-m-long fused silica capillaries (0.2–0.3 mm internal diameter) lined with a stationary phase such as silicone oil; and useful detectors include flame ionization detectors and mass spectrometers.
Gas chromatography A chromatographic method in which the mobile phase is a gas, such as helium or hydrogen, and the stationary phase is a microscopically thin layer of liquid or polymer, which is either coated onto a solid support packing material in the column or on the inside of a narrow capillary tube. As the gas sweeps the molecules in the sample mixture through the column, they are retarded by absorption in the stationary phase. Larger and more polar compounds have a longer residence time in the stationary phase and elute from the column later than smaller and less polar constituents. The temperature of the column can be maintained constant or, when compounds with a wide range of boiling points are analyzed, increased gradually, typically a few degrees a minute. Gas chromatography can be used to identify the components in a simple mixture by comparison with standard mixtures, and a flame ionization detector can be used to quantify amounts of individual components. A mass spectrometer is usually required as a detector for identification of unknown compounds (see column chromatography, GC-MS).
GC-irm-MS Gas chromatography–isotope ratio monitoring–mass spectrometry. A gas chromatograph combined with a combustion interface that burns the separated compounds to CO2 and with a special mass spectrometer that can then measure the relative abundance of isotopes. Used to determine the isotopic composition of individual molecular species.
GC-MS Abbreviation for the combination of gas chromatograph and mass spectrometer.
Genetic probe As used in geochemical applications, a tool for identifying genes in natural waters and sediments. A fragment of DNA or RNA from the phylogenetic group of organisms under study is labeled with a fluorescent chemical group and mixed with the sample, where it binds with the complimentary gene sequence and allows it to be isolated and identified.
Geochemistry Study of the transformation of chemical substances in the geosphere. Subdisciplines are organic geochemistry and inorganic geochemistry; cosmochemistry extends the scope of interest into space.
Geologic timescale An ordered, internationally recognized sequence of time intervals that divide the history of Earth, composed of hierarchical units, from longest to shortest: eons, eras, periods, and epochs.
Geothermal gradient The rate of increase of temperature with depth in the earth. Geothermal heat flow is a physically more precise term that takes into account the diff erent thermal conductivities of the rock and sediment layers. The geothermal gradient can range from 30°C/km in areas of low geothermal heat flow to 80–100°C/km in geothermally hot areas.
Green algae A taxonomic grouping (phylum Chlorophyta) of algae with green chloroplasts that are similar to those found in land plants. Green algae have a wide variety of forms and growth habits: unicellular plankton that grow in lakes and oceans, colonial filaments in pond scum, and leaflike seaweeds that grow along rocky and sandy intertidal areas. Some can live on tree trunks and soil, and others are symbiotic, living together with corals or fungi (lichens). Isomers of 24-ethylcholesterol, often with an additional double bond in the C-7 position, are prominent among their lipids.
Green sulfur bacteria Anaerobic photosynthetic bacteria that utilize sulfide or elemental sulfur to reduce CO2 and, in many cases, can also assimilate simple organic compounds. Their pigments include bacteriochlorophylls c, d, and e and isorenieratene and chlorobactene. They are capable of growing at relatively low light intensities and can live at greater depth in the water than most other photosynthetic organisms. (See anoxygenic phototrophs.)
Halophiles A group of archaea that inhabit highly saline environments such as natural salt lakes and artificial evaporation ponds from desalination plants, salt mines, and even the surfaces of preserved foods, such as salted meat or fish. Most halophiles require oxygen and obtain their energy and carbon from organic compounds.
Haptophyte Single-celled algae that are members of the Haptophyta phylum. (See coccolithophore.)
Heterotroph An organism that is dependent on organic compounds biosynthesized by other organisms for its carbon and, oft en, its energy. A heterotroph is a consumer in the food chain. (See autotroph.)
High-performance liquid chromatography (HPLC) Similar in principle to column chromatography, except the column is a narrow stainless steel tube (10–30 cm × 1–5 mm) that is packed with very fine grains or beads of silica, and the liquid mobile phase is pumped through the column at high pressure. Samples can be injected automatically, and for analytical HPLC are typically in the microliter range; solvents can be mixed and varied gradually during the analysis; and various detectors can be employed (ultraviolet-visible, fluorescence, mass spectrometer) for identification of the separated compounds. HPLC is particularly useful for analysis of high-molecular-weight and/or very polar organic compounds, but can also be used for the gross separation of bitumen into group-type fractions (nonaromatic hydrocarbons, aromatic hydrocarbons, heterocompounds) or separation of aromatic hydrocarbons into mono- di-, and tri-aromatic hydrocarbons.
Homologous series, homologue A series of chemically related compounds that diff er by the number of CH2 units in their carbon chains. True homologues have linear additions of CH2 groups; pseudohomologues may include alkyl group branches.
Hopane A saturated hydrocarbon in the hopanoid family.
Hopanoid A general term for a group of pentacylic triterpenoids with a particular fused ring structure composed of four six-membered and one five-membered ring. A wide spectrum of side groups may be attached. Bacteriohopanetetrol and derivatives are prominent bacterial cell constituents, and serve, among other things, to provide rigidity to the cell membrane. Their biosynthesis involves addition of a C5 sugar to the side chain on the fivemembered ring of a C30 hopanoid.
HPLC See high-performance liquid chromatography (HPLC).
Hydrocarbon A chemical compound consisting only of the elements carbon and hydrogen. The simplest hydrocarbon is methane (CH4).
Hydrolysis Cleavage of a chemical bond by the addition of a water molecule, usually in the presence of a base or acid catalyst. Important examples are the hydrolysis of an ester to yield a carboxylic acid and an alcohol, or hydrolysis of an amide to yield a carboxylic acid and an amine.
Hydropyrolysis Pyrolysis under pressure and in the presence of hydrogen gas and a catalyst (sometimes called hydrogenolysis). It eff ectively cleaves bonds at lower temperatures than traditional pyrolysis and is used to determine the structures and stereochemistry of compounds bound in kerogen. The kerogen may be flushed with hydrogen at even lower temperatures prior to actual bond cleavage in order to rid it of contaminants and bitumen.
Hydrothermal vent Fissures in the seafloor from which geothermally heated water gushes out into the ocean, found mostly in the tectonically active areas along mid-ocean ridges (see seafloor spreading) or in the vicinity of volcanoes. The dissolved metal ions and reduced chemicals in the hot water (up to 400°C) issuing from the vents provide abundant energy for chemosynthetic bacteria and archaea, which form the base of vibrant deep sea ecosystems. Sulfide-oxidizing bacteria are particularly abundant, often living in symbiosis with clams, mussels, and tubeworms.
Hydrous pyrolysis Pyrolysis in the presence of water, usually done in an autoclave over extended periods. It is often applied to organic-matter–rich rocks to simulate petroleum formation in the laboratory, as it functions at lower temperatures than traditional pyrolysis.
Hydroxyl group The functional group (–OH) that characterizes alcohols.
Ice cores Obtained by drilling into the continental ice sheets of Greenland and Antarctica, ice cores extend thousands of meters into the ice sheets and comprise ice formed over 100,000 years or more. Tiny amounts of atmospheric gases were trapped in the pores of the ice when it crystallized and can be used to investigate how the earth’s atmosphere and climate have changed during the past several ice age cycles.
Infrared spectroscopy An analytical technique used to investigate the structure of organic molecules. Infrared light is passed through a sample of a pure organic substance (gas, liquid, or solid) inducing vibrations in the molecules, and examination of the transmitted light reveals how much energy was absorbed at each wavelength. The resulting absorption spectrum reveals details about the compound’s functional groups and overall structure.
Isolate (bacterial) The separation and enrichment of a single strain or selected mixture of bacteria in the laboratory; also refers to the strain isolated. (See culture.)
Isolation, isolate (chemical) The separation and purification of a single chemical compound from a mixture; also refers to the purified compound.
Isomerization The transformation of a molecule into its isomer. In this book, it refers to conversions between stereoisomers.
Isomers Chemical compounds with the same elemental composition but diff erent arrangement of the atoms. (See stereoisomers.)
Isoprenoid A general term for chemical compounds that are composed of repeating five-carbon-atom isoprene (2-methylbuta-1,3-diene) units. Also called terpenes or terpenoids. Monoterpenes have two isoprene units (C10), diterpenes have four (C20), triterpenes have six (C30), and so forth. Sesquiterpenes have three isoprene units (C15), sesterterpenes have five (C25), and polyterpenes consist of long chains of many isoprene units. The individual isoprene units are most commonly joined at opposite ends, or head-to-tail (regular isoprenoids), but may also be joined head-to-head or tail-to-tail. They can form open chains (acyclic isoprenoids) or ring compounds (cyclic terpenoids).
Isoprenoid glycerol ethers A group of lipid structures that occur in the membranes of archaea. Includes diphytanyl glycerol diethers (e.g., archaeol) and dibiphytanyl diglycerol tetraethers (oft en referred to in the geochemical literature as GDGTs for glycerol diphytanyl glycerol tetraethers).
Isopropyl An alkyl group wherein the three carbon atoms are arranged in a Y shape (−CH(CH3)2).
Isotopes The isotopes of an element have the same number of protons but a diff erent number of neutrons in their atomic nuclei. Thus they have diff erent atomic weights, but the same chemical affinities. For example, carbon-12 (12C) has 6 protons and 6 neutrons, whereas carbon-13 (13C) has six protons and seven neutrons, and carbon-14 (14C) has six protons and eight neutrons. (See δ13C, δ18O, fractionation.)
Jurassic A period in the Mesozoic era of the geologic timescale (206–142 million years ago) when dinosaurs dominated and the first birds appeared.
Kerogen The insoluble organic component in sediments. Kerogen is a macromolecular geochemical transformation product derived, over time, from decaying biomass in the sediments.
Ketone A class of chemical compounds characterized by the carbonyl group.
Lipid A chemical compound that is insoluble in water but soluble in organic solvents. Lipids are the main components of cell membranes and among the most plentiful compounds in biomass, together with carbohydrates and proteins. During diagenesis the decaying biomass becomes selectively enriched in lipids, which are likewise enriched in the kerogen, and become the most important starting material for the formation of petroleum during catagenesis.
Macromolecule A very large molecule that may be composed of diverse structural subunits, unlike a polymer, which consists entirely of repeating units.
Mass extinction The sudden extinction of a large number of types of organisms, as recorded by the fossil record in sedimentary rocks; in most cases, believed to be the result of rapid climate change associated with a catastrophic event such as a meteor impact or extreme volcanic activity.
Mass spectrometer (MS) The instrument used to perform mass spectrometry.
Mass spectrometry An analytical technique used to obtain information on molecular structures. There are many variations on the theme, but the most common application in organic geochemistry involves introduction of a pure substance or mixture, as a gas, into the high-vacuum system of a mass spectrometer (MS), either directly or as the effluent of a gas chromatograph. Here, the molecules are ionized by a high-energy beam of electrons that breaks the molecule into charged ions, including a molecular ion and charged fragments. These are separated according to their mass-to-charge ratios (m/z where z is 1 except for aromatic rings, which can carry more than one charge), and their abundance is registered by a detector. The results are displayed as a mass spectrum (ion intensity versus m/z) or mass chromatogram (total, summed selected, or selected single ion intensities versus time). Two mass spectrometers can be linked in series (MS-MS) to obtain more detailed information. Here, the ions formed in the first mass spectrometer are bombarded with atoms from an inert gas, such as argon, to induce further fragmentation, and this is recorded in the second mass spectrometer.
Maturity The degree to which the organic matter in a sediment or rock has been transformed under the influence of increasing burial depth and temperature. A certain level of maturity is required before petroleum generation by thermal cracking of kerogen can commence. Maturity is measured by various physical and chemical parameters, for example, vitrinite reflectance, carbon preference index of n-alkanes, and biomarker compound ratios.
Metabolite The generally small molecules produced as intermediates or products of biochemical reactions in a cell (metabolism). A primary metabolite is directly involved in normal growth, development, and reproduction, and common to many types of organisms, for example, sugars, amino acids, and nucleic acids. A secondary metabolite has some less universal function that nevertheless may be important to a particular organism’s long-term survival, for instance, pigments and antibiotics.
Methane hydrate A solid crystalline material composed of methane molecules trapped inside a cage-like lattice of water molecules that are held together by hydrogen bonds. Methane hydrates can form from bacterial or thermogenic methane, are stable at particular combinations of low temperature and high pressure, and are often found beneath the sediment surface along the continental margins, in some marine basins, and beneath the permafrost in polar regions.
Methane seep, methane vent A place where methane gas is seeping out of the sediments, often found along continental margins.
Methanogens A group of anaerobic archaea that obtain their energy from the reduction of carbon, rather than oxygen, and produce methane, rather than CO2, as a waste product. The most prominent known forms of methanogenesis employ CO2 or small organic molecules as their electron acceptors, and H2 or organic molecules, respectively, as electron donors. Both forms obtain their carbon from organic matter. Methanogens generally perform the last step in the decay of organic matter and are ubiquitous in sediments, soils, and natural waters where oxygen is absent.
Methanotrophic bacteria Aerobic bacteria with a wide range of diff erent morphologies (e.g., Methylomonas, Methylobacter, Methylococcus). They are widespread in soils, sediments, and natural waters. Common bacterial lipids such as iso-C17 and anteiso-C18 fatty acids and diploptene can serve as biomarkers when their δ13C values also point to a methanotrophic source. More specific biomarkers for methanotrophic bacteria include 3β-methyl bacteriohopanoids and 4α-methyl sterols. They are the only prokaryotes known to biosynthesize sterols in quantity.
Methanotrophs Bacteria or archaea (ANME) that obtain their energy and carbon from the oxidation of methane. The lipids of methanotrophs tend to be markedly depleted in carbon-13 due to their consumption of carbon-13–depleted methane.
Methyl group A one-carbon alkyl branch in a chemical structure (−CH3).
Methylene group A structural unit in a molecule consisting of a CH2 group either as part of a saturated chain (−CH2–) or as an unsaturated group at the end of a chain (=CH2).
Microbe, microorganism General terms for unicellular organisms, used to refer collectively to prokaryotes and/or unicellular eukaryotes.
Molecular ion The ion formed in mass spectrometry that is the starting molecule minus an electron (or, occasionally, plus an electron). Pseudomolecular ions are sometimes formed by the addition of a small charged particle (e.g., NH4+ or H–) in the mass spectrometer (secondary ionization), something that is particularly common in HPLC-MS.
Molecular sieve A substance that contains tiny pores of uniform size (e.g., 5 Å) used to separate the straight-chain compounds from the branched and cyclic ones in a solution. Straight-chain compounds such as n-alkanes and n-alkanols are trapped in the pore spaces, and the bulkier branched and cyclic compounds remain in solution. Various methods are used to purge the straight-chain compounds from the sieve. Minerals such as zeolite, porous glass, charcoals, and other substances are used. Crystals of urea can be used in a similar fashion (urea adduction).
Molecular weight The sum of the atomic weights of the elemental constituents of a molecule. In general chemistry the weighted average of the isotopes of each element is used, but when dealing with mass spectrometry the atomic weights of specific isotopes are used.
MS See mass spectrometer (MS).
Nomenclature A set of conventions used to unequivocally describe the structures of chemical compounds, as determined by the International Union of Pure and Applied Chemistry (IUPAC).
Oceanic anoxic event A period when the world’s oceans were depleted in oxygen and organic matter preservation in the sediments was enhanced, resulting in widespread deposition of black shales. Several such events occurred during the Cretaceous.
Oil shale A sedimentary rock that is rich in fossil organic matter but has not been buried deeply enough to generate petroleum. Oil can be produced from an oil shale by artificial heating (retorting).
Optical activity/rotation The tendency of certain substances to bend, or rotate, a ray of monochromatic plane-polarized light to the right or left . Optical activity is a property of chiral organic molecules such as amino acids, steroids, and triterpenoids.
Organic geochemistry See geochemistry.
Organic matter Remnants of decayed biomass.
Oxic Describes an environment where molecular oxygen is present. (See anoxic.)
Oxidation A chemical reaction involving the addition of an oxygen atom to a molecule, the removal of two hydrogen atoms from a molecule (as in the formation of a double bond) or, more generally, the loss of one or more electrons from a molecule. In a chemical reaction system, oxidation is always coupled with reduction; that is, of two reaction partners, one is oxidized whereas the other one is reduced (redox reaction).
PAH Polycyclic aromatic hydrocarbon. (See aromatic ring.)
pCO2 Partial pressure of carbon dioxide in the atmosphere. In paleoclimate research, past pCO2 levels are measured by analysis of the gases trapped in old layers of ice in the Greenland and Antarctica ice sheets. (See ice cores.)
Period See geologic timescale.
Per mil, ‰ Parts per thousand. Measurement scale for low numbers or concentrations like in isotope analysis.
Petroleum A general term for the products of the thermocatalytic transformation of kerogen during catagenesis. The most important products are natural gas and crude oil, which accumulate in large quantities in natural reservoirs in the subsurface. (See asphalt.)
Photic zone The upper layer of water in a lake or ocean where there is enough light for photosynthesis. The depth of the photic zone depends on the turbidity of the water and may range from a few meters to about 200 m in extremely clear water.
Photosynthesis The process by which carbon dioxide or bicarbonate are converted to biomass in plants, algae, and some bacteria, using light as an energy source.
Phylogenetic A way of classifying groups of organisms based on their evolutionary relationships, usually based on comparisons of their genetic information (phylogenetic relationship). A phylogenetic tree shows organisms that developed early in the history of life positioned near the roots and those that evolved later at higher positions of the tree. The main branches signify the three domains of life on Earth.
Phytane The diterpenoid (C20 acyclic isoprenoid) formed from phytol during diagenesis, probably the most abundant hydrocarbon on Earth. Phytol is readily cleaved from chlorophyll a, which is common to all plants, algae, and many bacteria, and produced in great quantities upon death of the organisms. Another common source may be the isoprenoid membrane lipids of archaea.
Phytoplankton Small or microscopic photosynthetic organisms (plants, algae, and bacteria) that live near the surface of lakes and oceans (e.g., coccolithophores, diatoms, dinoflagellates). They form the base of the marine food chain, its primary producers. (See primary productivity.)
Plankton, planktonic Refers to organisms that float or drift in the water of a lake or ocean, generally small organisms that serve as food for fish and other larger animals. Planktonic describes anything relating to or characteristic of such organisms. (See benthic.)
Plate tectonics A theory describing movements in the earth’s crust that accounts for the changing positions of the continents and oceans over geologic time. The earth’s lithosphere (crust plus rigid part of the mantle) is broken up into jigsaw-like pieces called tectonic plates, which move slowly about the planet. These plates move away from each other at mid-ocean ridges. Where oceanic plates converge, one plate slides beneath the other; where two continental plates collide, mountains form. Earthquakes, volcanic activity, mountain building, and oceanic trenches occur along plate boundaries.
PMI Pentamethylicosane; a saturated acyclic C25 isoprenoid hydrocarbon found in sediments, believed to derive from the corresponding unsaturated hydrocarbons (pentamethylicosenes, with up to five double bonds) made by methanogenic and methanotrophic archaea.
pO2 Partial pressure of oxygen in the atmosphere.
Polar Describes molecules with a high affinity for water or solvents like methanol (which are likewise composed of polar molecules). Polarity stems from the presence of atoms with free electrons (e.g., oxygen, nitrogen, sulfur) in compounds like alcohols and carboxylic acids or, to a lesser extent, the delocalized electrons in aromatic hydrocarbons.
Polycyclic aromatic hydrocarbons (PAHs) See aromatic ring.
Pore water The water that fills the spaces between grains of sediments. Special techniques must be used to “squeeze” it out of the sediments for analysis.
Porphyrin An organic compound, derived from the diagenetic transformation of chlorophyll, with four nitrogen-bearing pyrrol rings and a fully aromatic ring system that makes it very stable. Porphyrins can survive thermocatalytic cracking of kerogen and are found in deeply buried sedimentary rocks and crude oil.
ppm Parts per million. Measurement scale for very low numbers or concentrations.
Primary productivity The total amount of photosynthetic biomass production by autotrophic organisms. often refers to the production of biomass by phytoplankton in the photic zone of the ocean.
Prokaryote An organism with an uncompartmentalized cell structure, lacking a cell nucleus or any other membrane-enclosed structure. A general term used to refer to all noneukaryotic life, that is, a collective term for bacteria and archaea. (See eukaryote.)
Protokerogen A chemically ill-defined precursor of kerogen formed during early diagenesis. The term “humic matter” is often used in the same sense, to refer to structurally ill-characterized high-molecular-weight organic material in soils or in the ocean.
Proton A subatomic particle with an electric charge of one positive fundamental unit. The nucleus of an atom is made up of protons and neutrons together. A proton is formed when an atom of hydrogen, whose nucleus contains one proton and no neutrons, loses an electron. A molecule that releases protons is an acid. Protons released from acids can catalyze many chemical reactions. (See carboxylic acid.)
Proxy A parameter that makes use of variables that can be empirically determined to estimate some other variable that cannot be measured directly. Proxies are often used to assess and quantify conditions in the geological past. Examples include the 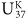 or TEX86 proxies for sea surface temperatures, and the δ18O proxy for global ice mass and ocean temperatures.
or TEX86 proxies for sea surface temperatures, and the δ18O proxy for global ice mass and ocean temperatures.
Purple sulfur bacteria Anaerobic photosynthetic bacteria that utilize sulfide or elemental sulfur to reduce CO2 and, in many cases, can also assimilate simple organic compounds. They are commonly found in sulfur springs or in the anoxic zones of lakes, where their large blooms give the water a conspicuous purple color. Their pigments include okenone and bacteriochlorophylls a and b. (See anoxygenic phototrophs.)
Pyrolysis A method for analyzing kerogen or other recalcitrant organic materials that involves breaking them up into their chemical constituents by heating in the absence of any chemical reactants, in particular molecular oxygen. Flash pyrolysis usually involves rapid heating in a stream of helium. (See hydrous pyrolysis, hydropyrolysis.)
Racemic Denotes a 1:1 mixture of the two mirror-image forms of a chiral molecule.
Reduction A chemical reaction involving the addition of hydrogen (e.g., to a double bond to form a single bond) or removal of oxygen, or, more generally, the acceptance of one or more electron(s). (See oxidation.)
Resin 1. A hydrocarbon excretion of many plants, particularly conifers. Usually a viscous liquid containing a variety of terpenes, often used in varnishes and adhesives. 2. The polar heterocompounds in bitumen.
Rubisco Ribulose-1,5-bisphosphate carboxylase/oxygenase. An enzyme that catalyzes the first step of the Calvin cycle of photosynthesis, incorporating CO2. It is the first step in carbon fixation in C3 plants.
Sapropel An organic-matter–rich sedimentary rock, often used synonymously with black shale, but generally used for younger, less consolidated sediments. Sapropels are found in the sedimentary records of the Mediterranean Sea and the Black Sea, as well as in many lake deposits.
Saturated Refers to chemical compounds with no double bonds or aromatic rings. (See unsaturated.)
Scanning electron microscope (SEM) An instrument that produces high-resolution images of very small items (e.g., fossils of unicellular organisms). Its focus has a great depth of field so that the images appear three-dimensional.
Seafloor spreading The movement of two tectonic plates away from each other as new basaltic ocean floor (oceanic crust) forms at mid-oceanic ridges such as the mid-Atlantic Ridge. (See plate tectonics.)
Sea surface temperature (SST) An important parameter in oceanography and climate research. Proxies used to estimate past sea surface temperatures include the 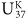 index, TEX86, and, to some extent, δ18O.
index, TEX86, and, to some extent, δ18O.
Sediment The solid material that collects at the bottom of lakes, rivers, and oceans. It is generally composed of the carbonate or siliceous shells of aquatic organisms, clay, mineral rock fragments, and organic matter.
Sedimentary rock The lithified form of sediment produced by the pressure and heat experienced during the deep burial that results from continuing sedimentation and subsidence.
Shale A fine-grained sedimentary rock that easily fractures along bedding planes and consists of clay minerals or, more generally, clay-sized particles that may also include carbonate.
Sponge An animal of the phylum Porifera. Sponges are sessile, mostly marine filter feeders that pump water through their bodies to filter particles out of the water for feeding. Their lipids include C30 sterols with a propyl or isopropyl group at C-24 in the side chain.
Standard In analytical chemistry, a chemical substance that has an authentic composition and structure obtained by chemical synthesis or by isolation from a natural source (e.g., a plant or a geological sample). Standards are used to unequivocally identify an unknown chemical compound.
Sterane The alkane form of a steroid.
Stereochemistry The study of the spatial orientation of atoms in molecules and the reactions that change that orientation; often used synonymously with configuration, to refer to the particular orientation of atoms in a molecule.
Stereoisomers Two or more forms of a molecule that diff er only in the relative spatial orientation of the atoms. The specific orientations at a given atom are designated R or S according to a convention that defines the relative hierarchy of its attached atoms and groups of atoms. If the atom in question is part of a ring structure, then the two configurations are designated α or β, depending on whether the attached hydrogen atom or group of atoms points down from or up from the plane of the ring structure, which is again defined by a chemical convention related to the hierarchy of the attached groups. The configurations of amino acids are traditionally designated L or D based on an older chemical convention.
Steroid A class of polycyclic chemical compounds with a particular fused ring structure composed of three six-membered rings and a five-membered ring. They may have a wide range of side groups and functional groups attached. Steroids are biosynthesized by plants (phytosterols) and animals and may enter the geological record after the death of these organisms. Sterols from organisms are converted to sterenes during early diagenesis, and organic matter in deeply buried sediments and crude oils contains steranes and aromatic steroid hydrocarbons.
Stratigraphy The characterization of sedimentary rock layers, or strata. Many diff erent parameters are used, including rock type, fossils, age, magnetic properties, isotope signatures, and biomarkers. (See dating techniques.)
Subsidence The downward movement of the earth’s surface due to an increasing load of sediments or tectonic movements such as earthquakes and faulting.
Sulfate-reducing bacteria A morphologically and phylogenetically diverse group of anaerobic bacteria that obtain their energy from the reduction of sulfate and oxidation of organic acids, alcohols, or molecular hydrogen. They are widespread in aquatic and terrestrial environments.
Symbiosis A mutually beneficial association of diff erent organisms.
Syngenetic Formed contemporaneously with sediment deposition. Used to diff erentiate organic matter that derives from the environment where the sediments formed and is approximately the same age, from that which may have become incorporated in a rock much later.
Tectonic Denotes a process related to movements of the earth’s crust, for example, earthquakes and volcanic activity. (See plate tectonics.)
Terpene, terpenoid See isoprenoid.
Terrestrial Originating on the planet Earth (used in some texts to distinguish continental from marine organisms and material, but not here).
Tetraether See isoprenoid glycerol ethers.
TEX86 A measure of the relative abundances of ring-containing tetraethers that correlates with the temperature of the water where the crenarchaea that produced them lived. It is used as a proxy parameter for the determination of past sea surface temperatures.
Thin-layer chromatography (TLC) An analytical technique used to separate mixtures of organic compounds, based on the same principles as column chromatography, except that the stationary phase (silica gel or aluminum oxide) is a coating on an aluminum or glass plate. A drop or two of the mixture to be analyzed is applied near the bottom of the plate, which is then placed standing in a shallow pool of solvent (mobile phase) inside a glass chamber. The solvent then moves slowly up the plate by capillary action, separating the mixture according to polarity into single compounds or groups of compounds. These can be viewed by spraying the plate with some substance that reacts with the organic compounds to dye them, or by pretreating the stationary phase such that they can be viewed with a UV lamp. The separated compounds can be scraped off the plate and extracted from the silica for further analysis.
Total organic carbon (TOC) Typically used as a measure of the total amount of organic matter in a rock or sediment, determined by combustion at high temperature and measurement of the amount of CO2 produced.
Triterpenoid See isoprenoid. Pentacyclic triterpenoids of the oleanane, lupane, and ursane families are biomarkers of flowering plants; those of the hopane family are biomarkers of bacteria.
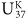 A measure of the degree of unsaturation of C37 alkenones that is directly correlated with the temperature of the water where the coccolithophores that produced them lived. The slightly modified
A measure of the degree of unsaturation of C37 alkenones that is directly correlated with the temperature of the water where the coccolithophores that produced them lived. The slightly modified 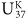 ’ index most widely used disregards the tetraunsaturated alkenone and is simply the ratio of diunsaturated to di- and triunsaturated alkenones. Both indices serve as a proxy for past sea surface temperatures.
’ index most widely used disregards the tetraunsaturated alkenone and is simply the ratio of diunsaturated to di- and triunsaturated alkenones. Both indices serve as a proxy for past sea surface temperatures.
Unsaturated Refers to a chemical compound containing one or more double bond(s) or aromatic rings. (See saturated.)
Upwelling The upward movement of cold, deep, usually nutrient-rich water. It is produced in areas where the winds create off shore or divergent surface currents, such as along the western boundaries of continents and along the equator. Coastal upwelling occurs along the coasts of Southwest Africa, Western Australia, California, and West Africa. Seasonal upwelling driven by the monsoon occurs off the coast of Oman and in the Arabian Sea. The influx of nutrients leads to high primary productivity in these regions.
Water column A term used by oceanographers and geochemists to refer to the conceptual column of water, from the surface of the water to the surface of the sediments, often divided into layers or zones of diff erent densities depending on temperature and salinity. When such layers are pronounced and don’t mix, the water column is said to be stratified.
Wax ester An informal name used for any compound that is composed of a long-chain alcohol linked by an ester bond to a long-chain fatty acid, often of similar length. Wax esters typically range from 32 to 56 carbon atoms in length, and are found in animal, plant, and microbial tissues, where they may serve as energy stores, waterproofing, or lubrication.
Younger Dryas A relatively brief period (about 1,300 years) of exceptionally cold climate that suddenly interrupted the warming phase from the last glacial to the present interglacial about 11,000 years ago.
Zooplankton Planktonic animals that graze on phytoplankton. The most abundant zooplankton are microscopic in size, but the term includes everything from copepods to shrimp and jellyfish.