chapter three
materials
The dream bike is light, comfortable, durable, and costs nothing. Of course, it doesn’t exist. The people who identify the best materials for the parts of a bike, create the materials, and manipulate them into the optimum shapes and sizes like to be rewarded for their efforts and expertise. They have studied the behavior of materials and applied what has been discovered by scientists to perform engineering feats that have made bicycles among the most efficient of human inventions. Engineers create bicycles by understanding the structure, potential, and limitations of materials. They cheat nature by creating materials that have never existed without human intervention and form them into parts that we can rely on. Their’s is not a simple job, although it is made easier by the experiences of others. The engineers who have invented better ways to make an existing part or used new materials to introduce design innovations push the boundaries of bike making. This chapter provides the material evidence of their ingenuity.

What matter makes up a bicycle?
 Isn’t a bike just made out of stuff?
Isn’t a bike just made out of stuff?
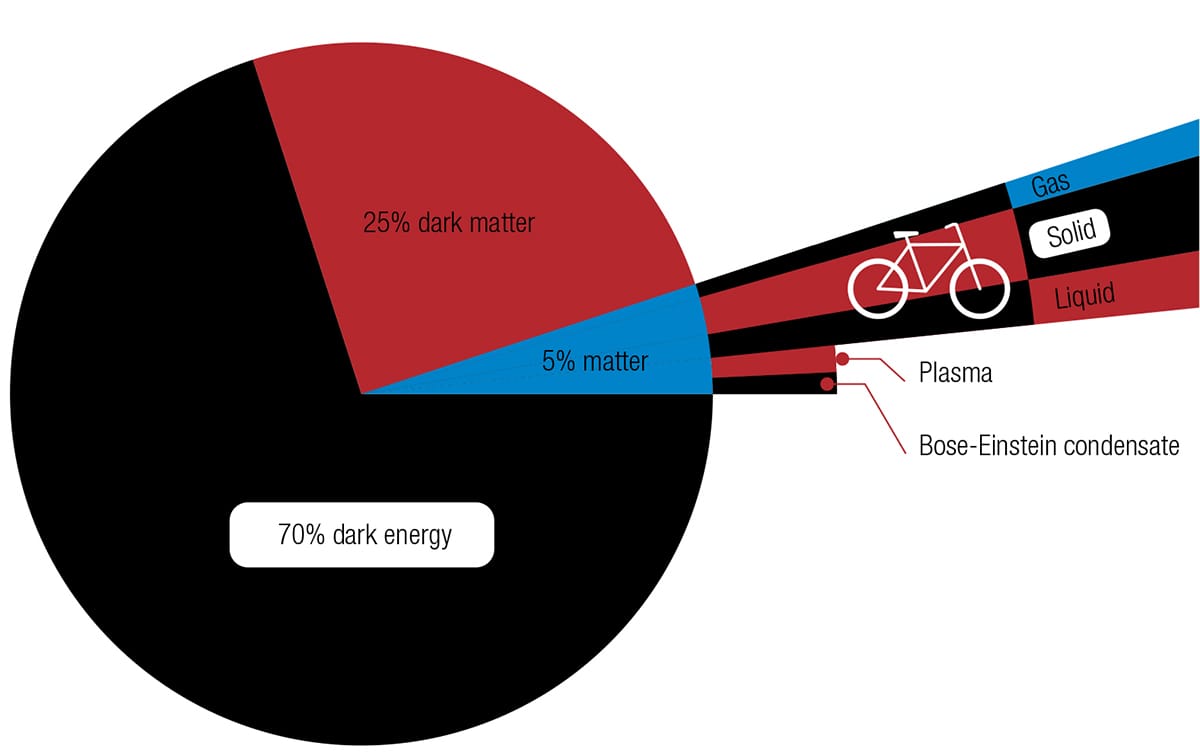
Whichever kind of bicycle you have, it is made of matter. Matter is what makes up the observable universe. Current theories suggest that nearly all of the universe consists of so-called dark energy and dark matter—neither has been directly observed, but their existence has been predicted from looking at how the remaining fraction behaves. It’s this remaining fraction that we know as matter, and it is categorized into five main states.
As far as cycling is concerned, the most obvious state is solid matter, such as metal, rubber, and plastic. Even though parts such as saddle covers and brake cables can be squashed, twisted, and bent, they are referred to as solid. The second state of matter, liquid, has become associated with bicycles only recently, with the workings of hydraulic brakes. The third state of matter, gas, has been an essential ingredient for comfortable and efficient riding ever since John Boyd Dunlop made the pneumatic tire popular in the nineteenth century. Suspension forks can also be pneumatic (which means that they use the properties of a compressed gas such as air). The fourth state of matter is plasma. While plasma makes up nearly all of the observable universe as part of stars and the space between them, it has not yet been harnessed for bicycles. Don’t rule it out, however, because it may soon have an influence on cycling aerodynamics. The fifth state of matter, the Bose-Einstein condensate, was not created on Earth until 1995 and hasn’t yet been applied to anything outside the laboratory, let alone to cycling.
All matter is made of atoms, or the chemical combinations of them called molecules, and has mass. The different states of matter are a result of how those atoms or molecules interact with one another and how they move about. Matter isn’t constant—it can change from one state (or phase) to another when conditions alter. This chapter explains how differences in the structure of matter determine the properties of everything your bicycle is made from.
Bike matter
Building blocks of everything All of the matter of the universe is, according to the standard model, made of building-block particles called quarks and leptons. Quantum field theory says these are held together by forces that are mediated by more particles, including photons, gluons, W and Z particles, and the Higgs boson. The latter is thought to give mass to all matter, and in July 2012 the discovery of a particle consistent with it was announced by scientists at CERN in Geneva, Switzerland.1
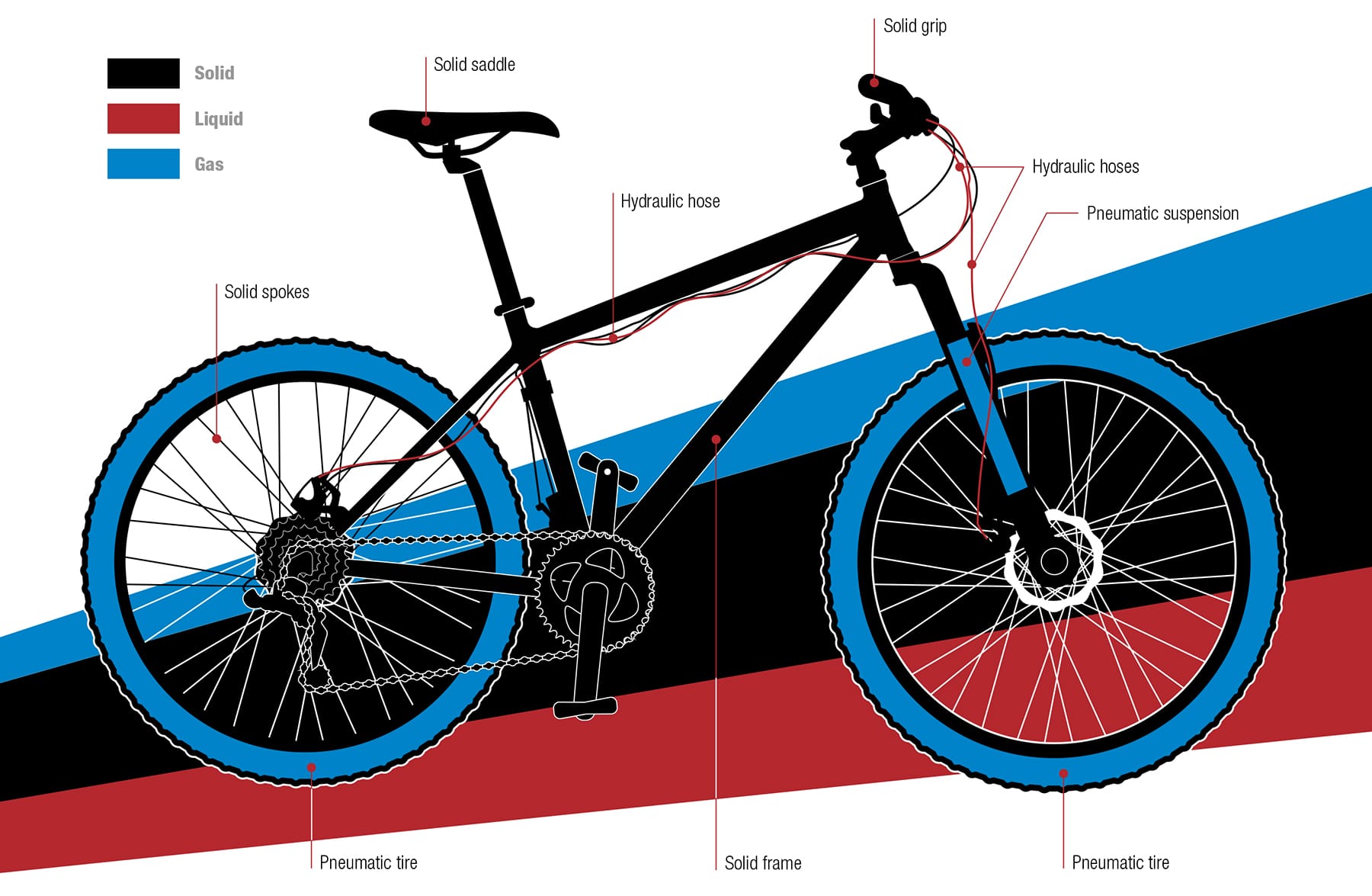
What’s my bike made of? The matter that is used to make bicycles is stable under normal conditions on the Earth’s surface, in that it does not change its state or phase. The first steerable two-wheelers harnessed matter only in its solid state, which is one reason why Draisines, Hobby Horses, and Ordinaries proved quite uncomfortable. It was only when two states of matter were put to work in combination that cycling became widespread. A flexible solid—rubber—was formed into a tube to contain a quantity of a gas—air—and wrapped around the rim. Liquids are of increasing interest to bicycle designers. They are used already to transmit braking forces from the levers to the pads, and there is research into how they could replace the conventional chain drive.
Why are metals strong?
 Why aren’t frames made of lightweight polystyrene?
Why aren’t frames made of lightweight polystyrene?
The solid matter used for many bicycle parts is metal. Metals aren’t the lightest of materials, but they are relatively strong, thanks to their atomic structure. The classical way to visualize an atom is as a swarm of negatively charged electrons orbiting around a nucleus consisting of an equal number of positively charged protons (plus, in all but a few elements, some uncharged neutrons). In metals, a number of electrons from each atom are so loosely bound to “their” nucleus that they are essentially free to drift among all the nuclei. This widespread sharing of electrons gives rise to electromagnetic forces that bind the metal atoms tightly together.
In general, the more of an atom’s electrons that are free to join this “sea” of electrons, the stronger the metal. A titanium atom has 22 electrons, while aluminum has only 13, but, more importantly, titanium’s atomic structure allows more of its electrons to join the “sea.” For this and other reasons, aluminum is weaker than titanium and many other metals and is not suitable for hard-working bike components such as ball bearings and chain links.
The most common metal element used for bicycles is iron (26 electrons, 26 protons, 30 neutrons), the major ingredient in steel alloy. It pops up everywhere, even on bikes with frames made of other metals or of carbon fiber-reinforced plastic. This is because, as a constituent of steel, iron is strong, hard, durable, and ductile. It can be rolled into tubes, machined with a thread, or drawn into wire. These characteristics are determined by its atomic structure. Trial and error have taught bicycle mechanics which metal is best for each part of a bike. Quantum mechanics explains why.
The bonds between metal atoms pull them into a repeating pattern (much like a collection of ball bearings settles together). Such a long-range pattern forms a crystalline structure. This tight packing makes the metal resilient, because it allows atoms to slide over each other, just as it’s easier to ride over closely packed cobblestones than ones with big gaps. The cobblestones on the Paris–Roubaix race are not just a test for riders; the infamous pavé also hammers the very atoms of their bikes. The right metals endure the punishment.
Atomic structures
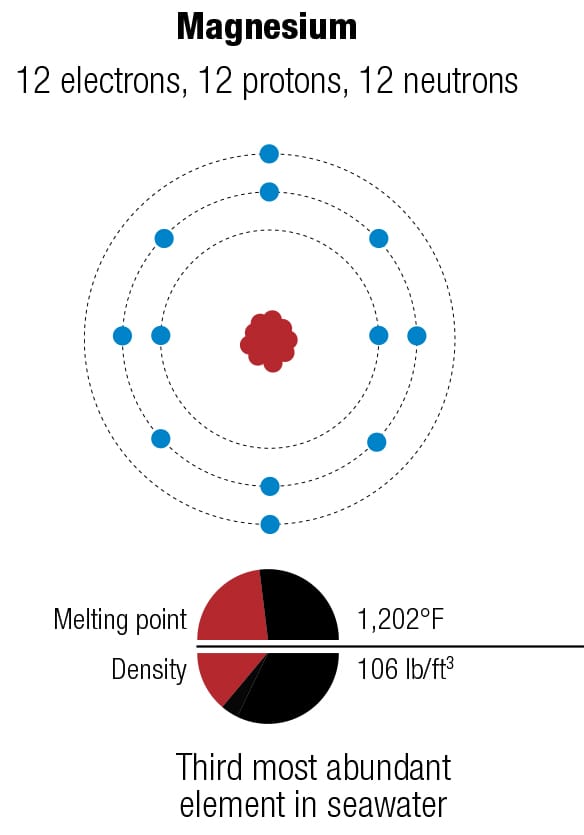
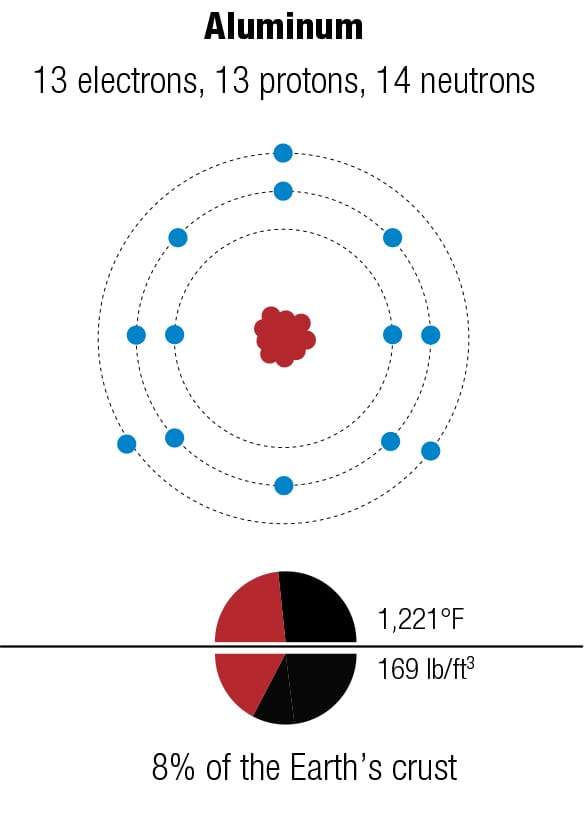
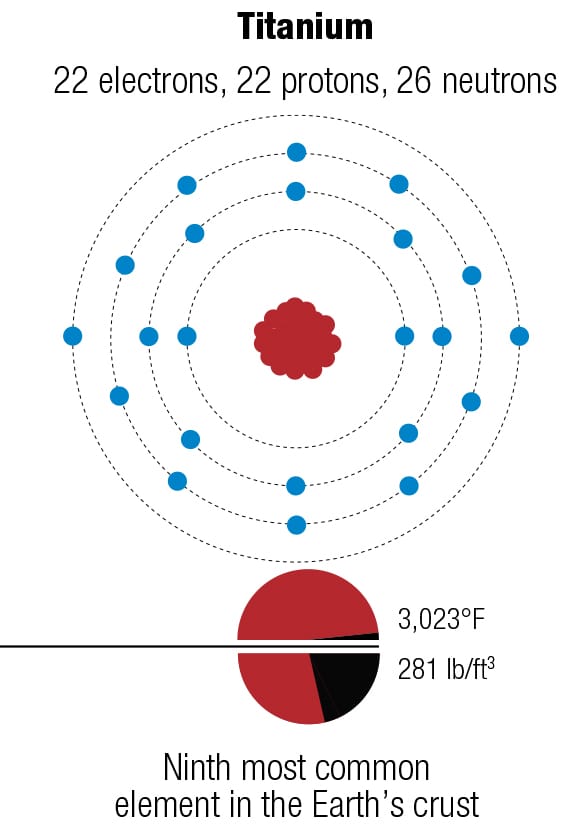
What is your bike made of? The four major metals used for bicycles—magnesium, aluminum, titanium, and iron—have different atomic structures, which lead to different properties, including crystal structures. They are rarely used in pure form because their properties can be improved by mixing them with other metals, as alloys. The diagrams represent the forms of each metal that are most abundant on Earth.
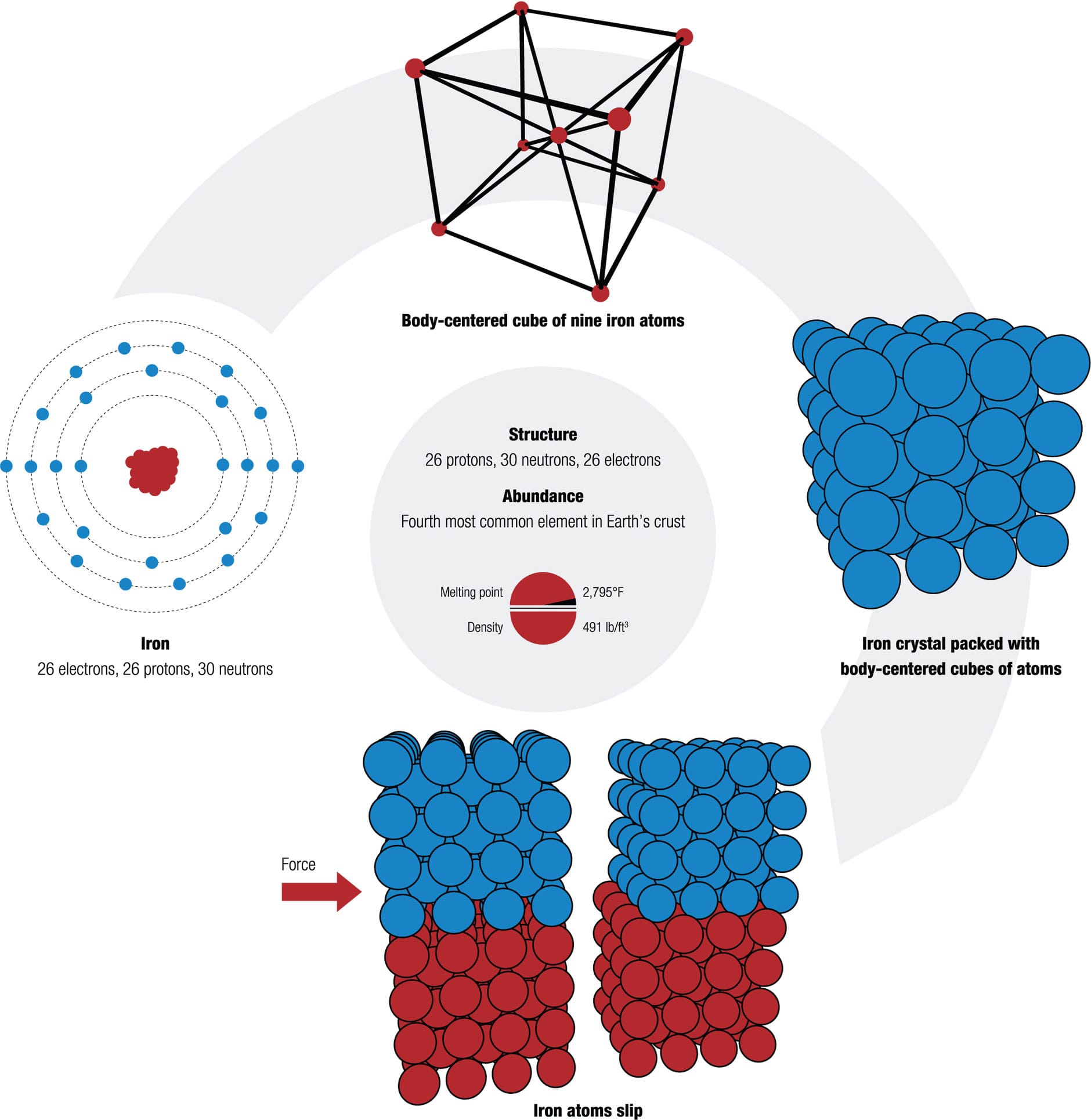
Hard but bendy Iron atoms bond strongly because they share some of their electrons. This attraction pulls the atoms close together in a so-called body-centered cubic pattern, with an atom at each corner of a cube and another in the center; this repeated pattern builds up a crystal of iron. When a force is applied to the crystal, the “sea” of delocalized electrons allows the atoms to slip past one another without experiencing the strong repulsive forces that can cause other materials to break. This gives iron good ductility—it can be bent and stretched without fracturing.
What difference does alloying metals make?
 What are the virtues of being impure?
What are the virtues of being impure?
Pure metal elements are not usually used for bicycle parts. Most often they are enhanced to make metallic alloys that are stronger, lighter, and more durable. These properties are determined by the atomic structure of the metal crystals. A common way to change this structure is to heat the metal until it melts, then add small amounts of other elements. When the resulting alloy cools and solidifies, the extra ingredients affect the crystal structure. Sometimes the additive atoms replace the host metal atoms, and at other times they squeeze in between them. The speed and frequency of the heating and cooling process and the thickness of the material also influence the characteristics of the resulting alloy.
The most common alloy in a bike is steel; it’s the most popular material for the vast majority of bicycles and their components. Metallurgists have been alloying iron into steel for more than 160 years and there are well over 50 different recipes. Plain steel is made by adding carbon to iron. As the liquid mixture cools, a crystal compound called iron carbide aggregates in microscopic plates between crystals of pure iron. These make the steel stronger and lighter than iron, and other additions, such as chromium and molybdenum, can boost strength, reduce brittleness, and improve resistance to corrosion. Not all of the ingredients in a metal alloy may actually make any perceptible improvement to the performance of a bicycle, but they may help maintain the integrity of the material while it is being manufactured and then fabricated.
Iron isn’t the only metal commonly alloyed. Pure aluminum is very soft and is alloyed with magnesium, silicon, copper, and chromium to make it harder. A rival to the high-performance aluminum frame, one made from titanium alloy actually uses aluminum as a key ingredient, along with vanadium. The few magnesium frames on the market have zinc and manganese included in the alloys used.
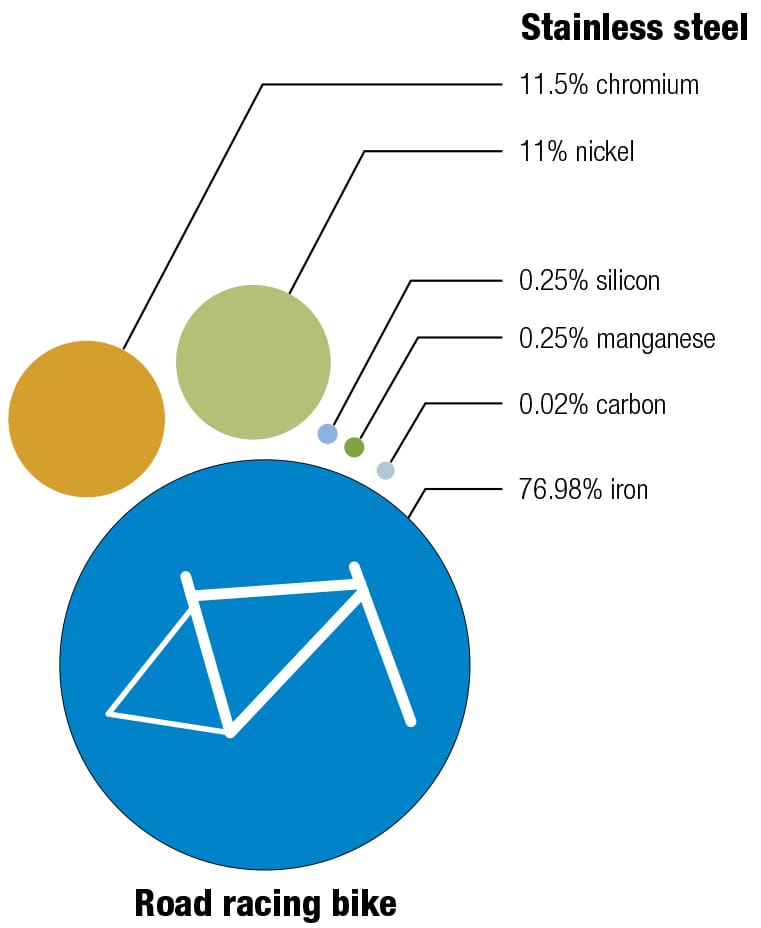
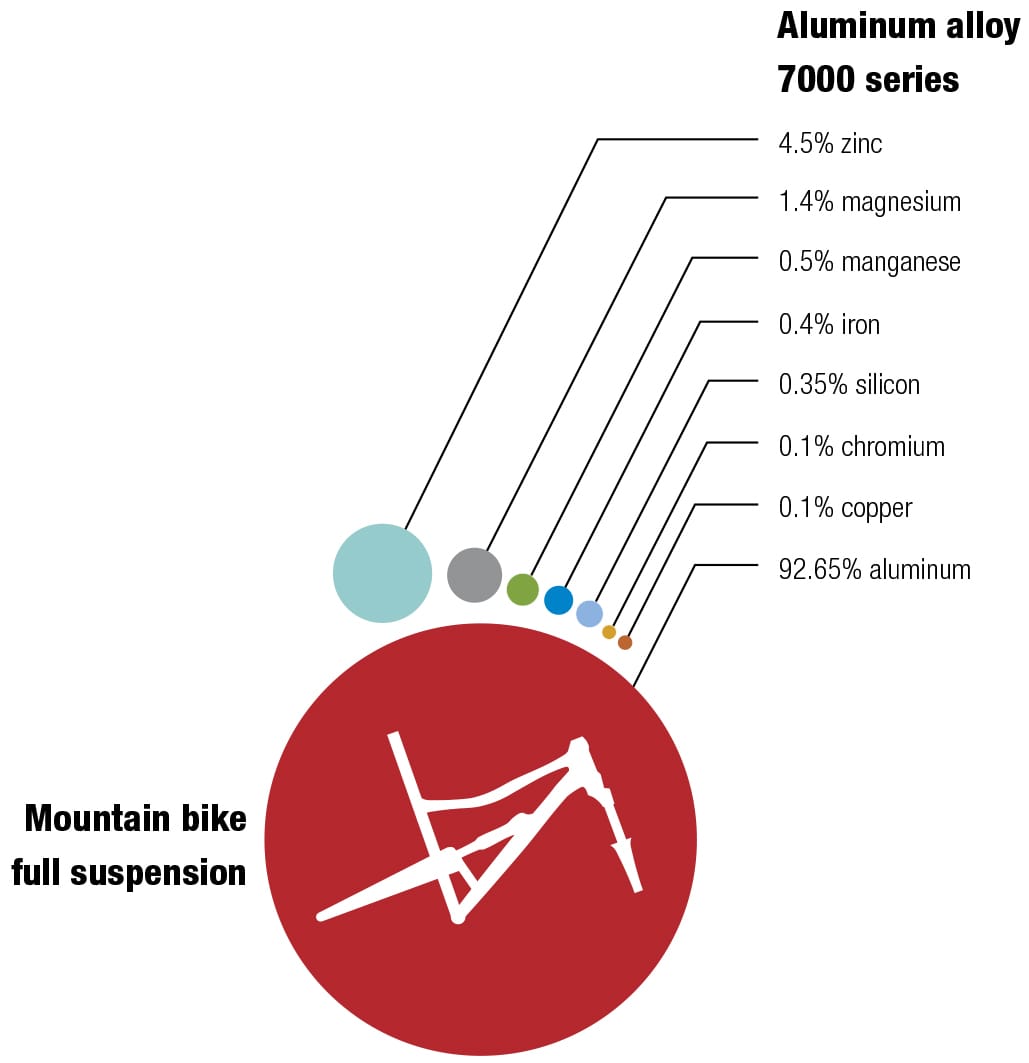
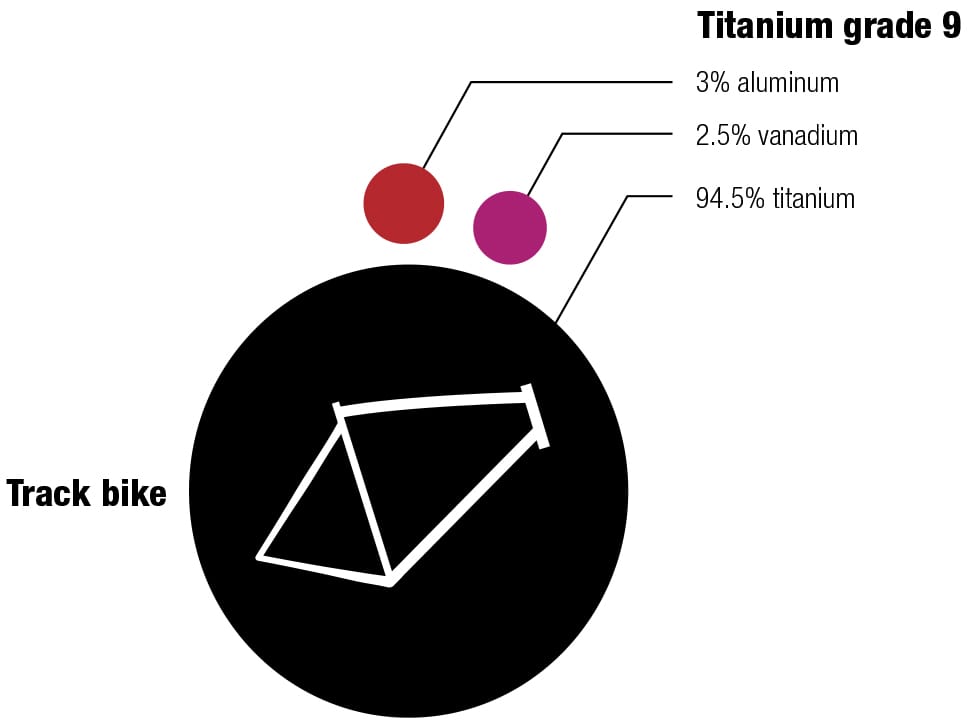
Frame alloys
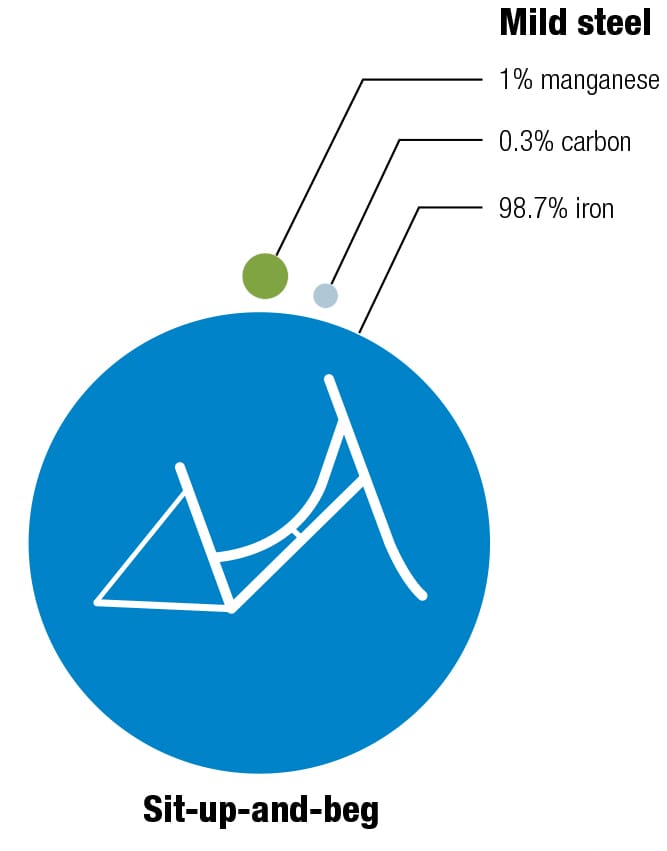
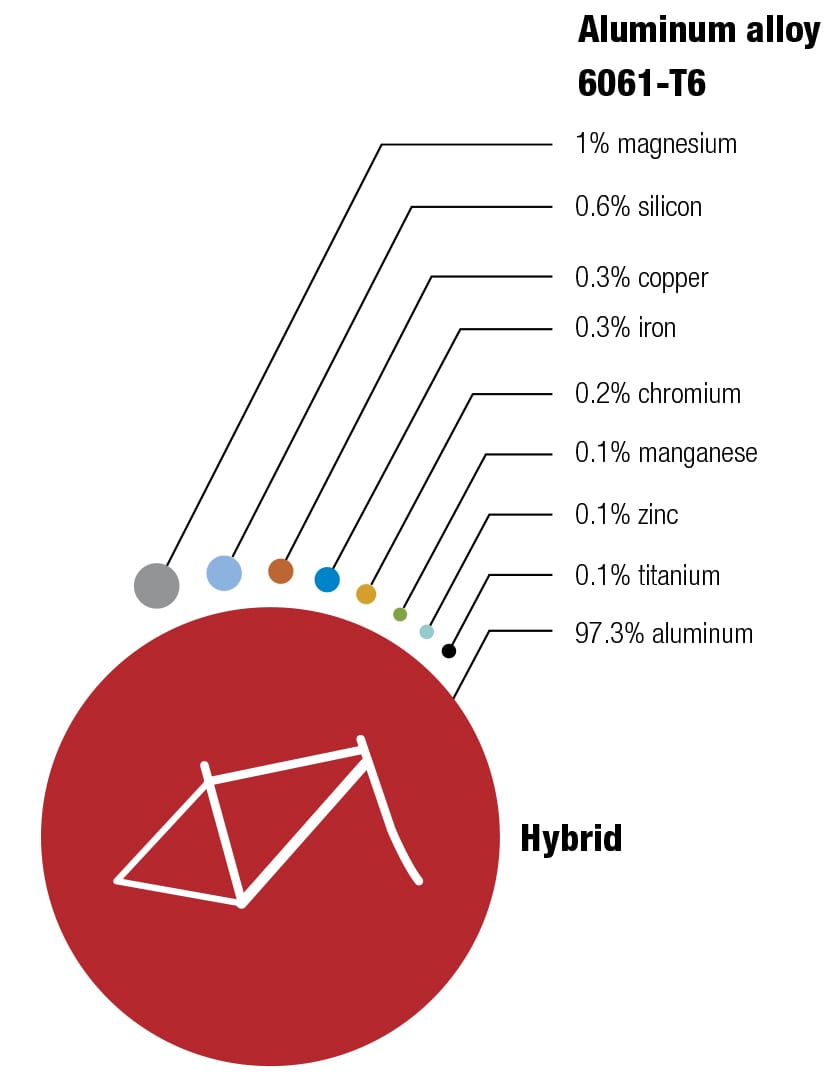
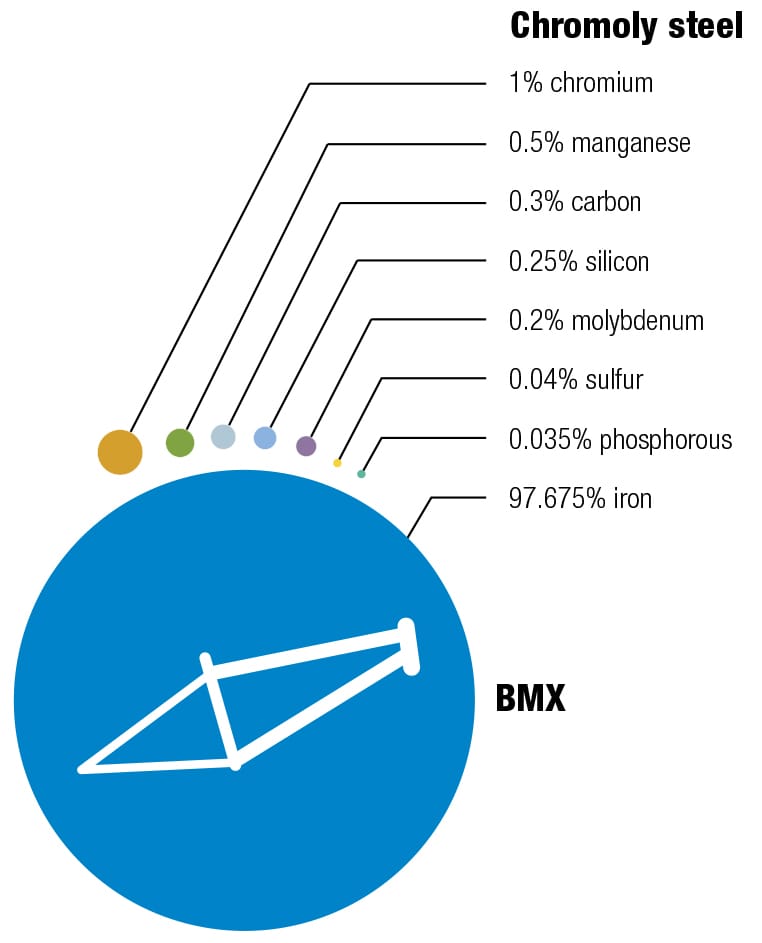
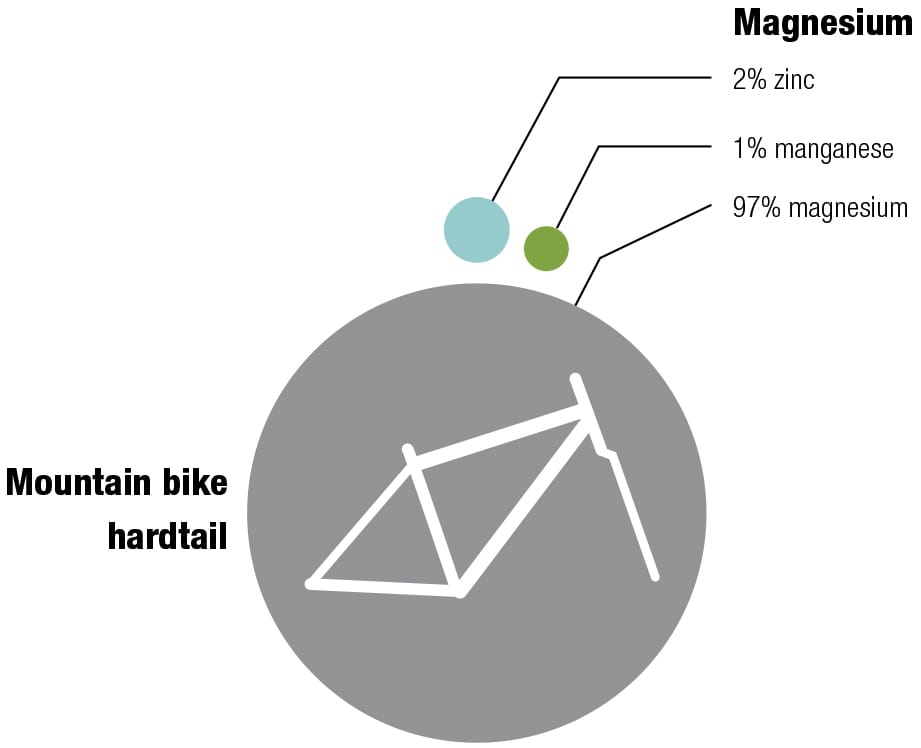
Here’s one of my favorite recipes Different alloys suit different bikes—it’s always a trade-off between price, weight, stiffness, and durability. The recipes for the various alloys have been perfected over decades, and metallurgists continually seek improvements by changing the mixes and the manufacturing and postproduction processes. These diagrams show the makeup of some typical alloys used for bike frames, with their ingredients listed by weight.
equipment: the frame High Wheeler (ca. 1870)
When Karl Drais invented the Hobby Horse in Mannheim, Germany, in 1817, he used wood from the European ash tree (Fraxinus), which was popular among coach builders because its grain is straight, easily shaped and joined, and has good overall strength relative to its weight. The material may have been familiar, but the bicycle’s design was a technological breakthrough—its two wheels were in line and, crucially, it had steering. Since then, frame design has been propelled by advances in materials and technology. With the right combination, the optimum bike frames are now sufficiently strong, light, durable, and stiff to race down alpine roads at 50 mph (80 km/h), cross fields of boulders, or land in one piece after a triple back flip.
The first Hobby Horse weighed about 45 lb (20 kg). Today, the Union Cycliste Internationale (UCI), the sport’s governing body, prohibits racing bikes lighter than 15 lb (6.8 kg). In the intervening two centuries frame builders have fashioned metals, polymers, and man-made composites to allow riders to travel farther, faster, and for longer. Improved manufacturing methods have been adopted, such as extruding tubes from blocks of hot metal, varying the internal diameter of tubes to cut weight yet retain strength, and hydroforming aluminum into computer-designed profiles. Joining and assembling now uses TIG welding for titanium and totally automating fabrication with composites.
Computer-based engineering techniques such as finite element analysis, computational fluid dynamics, and genetic design algorithms have opened entirely new routes to frame designs that work better for the human rider. At the same time, the study of human kinetics has revealed how frame dimensions should be refined to maximize the cyclist’s potential.
Although the bicycle is a mature concept, the pace of change in frame design during the most recent three decades has driven the UCI to lay down strict, conservative rules to standardize the shape of a competitor’s bike. The World Hour Records set in the 1980s and 1990s by Francesco Moser, Graeme Obree, and Chris Boardman wouldn’t be recognized today because they were achieved on frames whose designs would not be legal. Inevitably, such rules will change. Not long ago, mountain bikes were unacceptable, and yet they’re now being ridden in the Olympics. Bicycles don’t have a reverse gear and neither does science.
Hobby Horse (ca. 1818) This was simply a beam with spoked wooden wheels attached, and the rider walked along with his legs on either side.
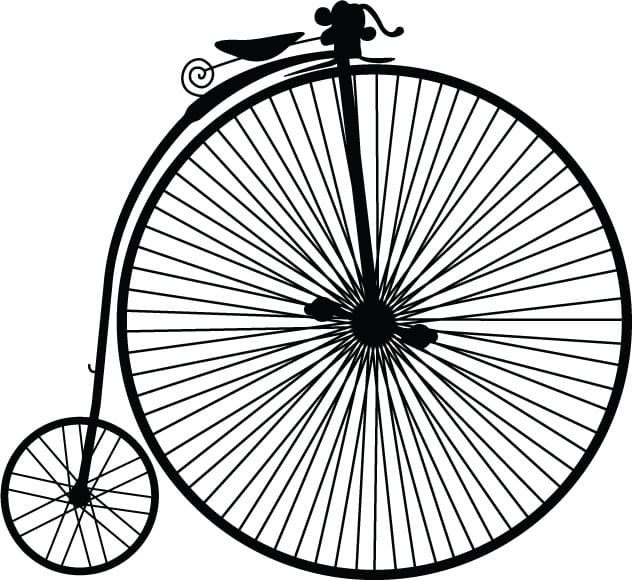
High Wheelers. (ca. 1870) The giant front wheel of this bicycle allowed the rider to cover a large distance with each rotation of the pedals.
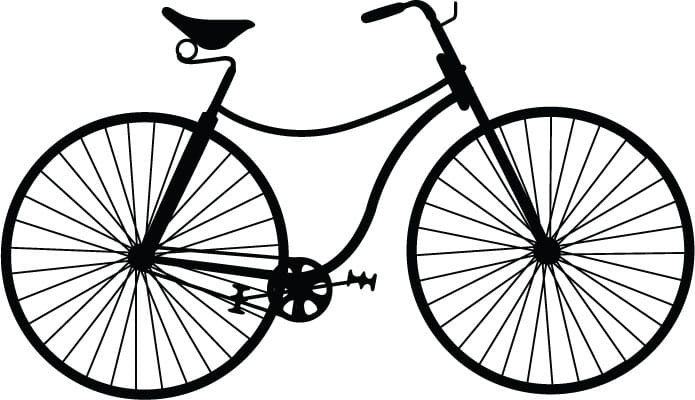
Safety machine (ca. 1885)
By the end of the nineteenth century, the modern bicycle had tangentially spoked, same-size wheels.

Road bike (ca. 1935)
Fierce rivalry between manufacturers led to lighter, stronger bikes with straight frame tubes, cross-laced spokes, and aerodynamic dropped handlebars.

BMX (ca. 1981)
Children often rode small- wheeled bikes off-road and specific models were first mass-produced in the 1970s.
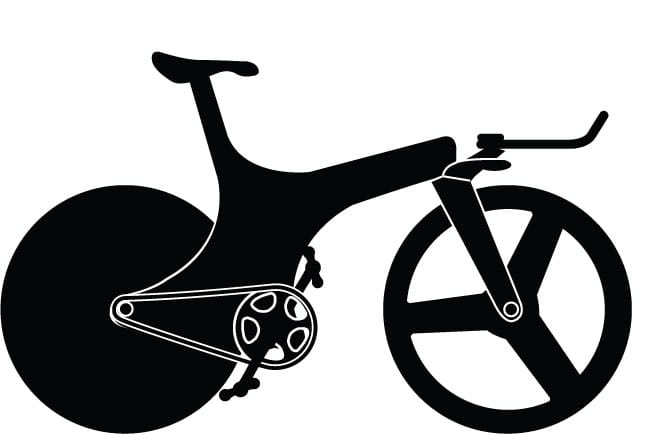
Lotus Type 108 (1992)
Engineer Mike Burrows perfected a carbon fiber monocoque so Chris Boardman could become the fastest cyclist ever.

Full suspension mountain bike (ca. 2000)
Full suspension absorbs the knocks of rough tracks, disc brakes aid control, and multiple gears ease pedaling.
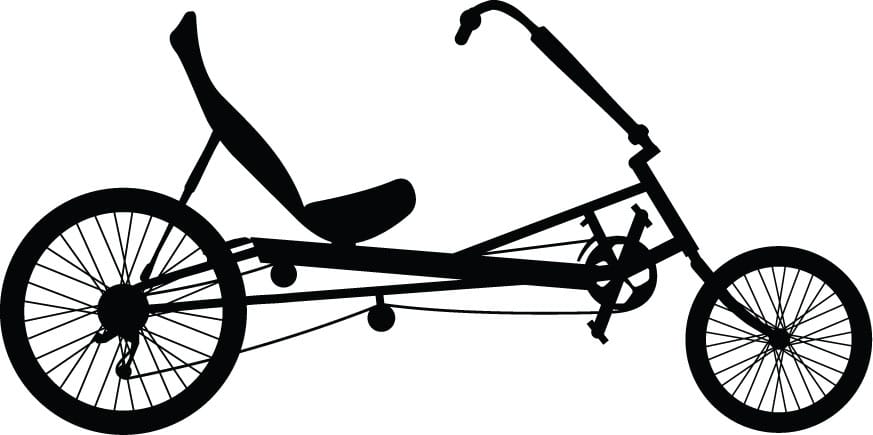
Long wheelbase recumbent (ca. 2003)
Low riding improves aerodynamics, so speeds far exceed those of diamond-frame bikes.
How is mechanical stiffness related to tube diameter?
 Why do some frames have porky tubes?
Why do some frames have porky tubes?
Bicycle manufacturers optimize the weight and rigidity of a bike by choosing frame tubes that use varying cross sections and dimensions at different places. Most frames are fabricated from metal alloys, but the tubes must be formed to the right shape to make the best of a material’s properties. Most makers still use hollow cylinders because this geometry minimizes the quantity of material required to produce the most resilient shape for a given weight—one that can withstand the many loads from different directions that are found in a hard-working bike. A square or rectangular cross-sectional tube shape cannot provide equal deformation or bending resistance in most directions unless more metal is used to reinforce it—and that imposes a weight penalty.
The diameter of a cylinder has a direct effect on its stiffness, and it is this dimension that has a significant influence on the rigidity of a frame. The rigidity of a material is defined by its Young’s modulus (or modulus of elasticity). Lab tests show that steel is about three times stiffer than aluminum, so it would seem logical that a piece of aluminum needs to be three times thicker than an identical steel piece to be equally rigid. However, cylinders are not flat pieces of metal, and their mechanical properties provide an important benefit. When the diameter of a hollow cylinder is increased, its stiffness increases in proportion to the third power of the diameter. In other words, when the diameter is doubled, stiffness increases eight-fold (23).
At the same time, weight only increases somewhere between two- and three-fold, depending on the thickness of the cylinder wall. So an aluminum frame tube needs to be roughly 1.44 times (1.44253 = 3) greater in diameter than a steel tube to fully compensate for its lower Young’s modulus, assuming there’s no difference in wall thickness. One crucial limit on tube stiffness is the functionality of the frame—there’s no point in having extremely stiff tubes if they are so fat that they get in the way of the cyclist’s limbs and the moving components.
Mechanical properties of bike-frame materials
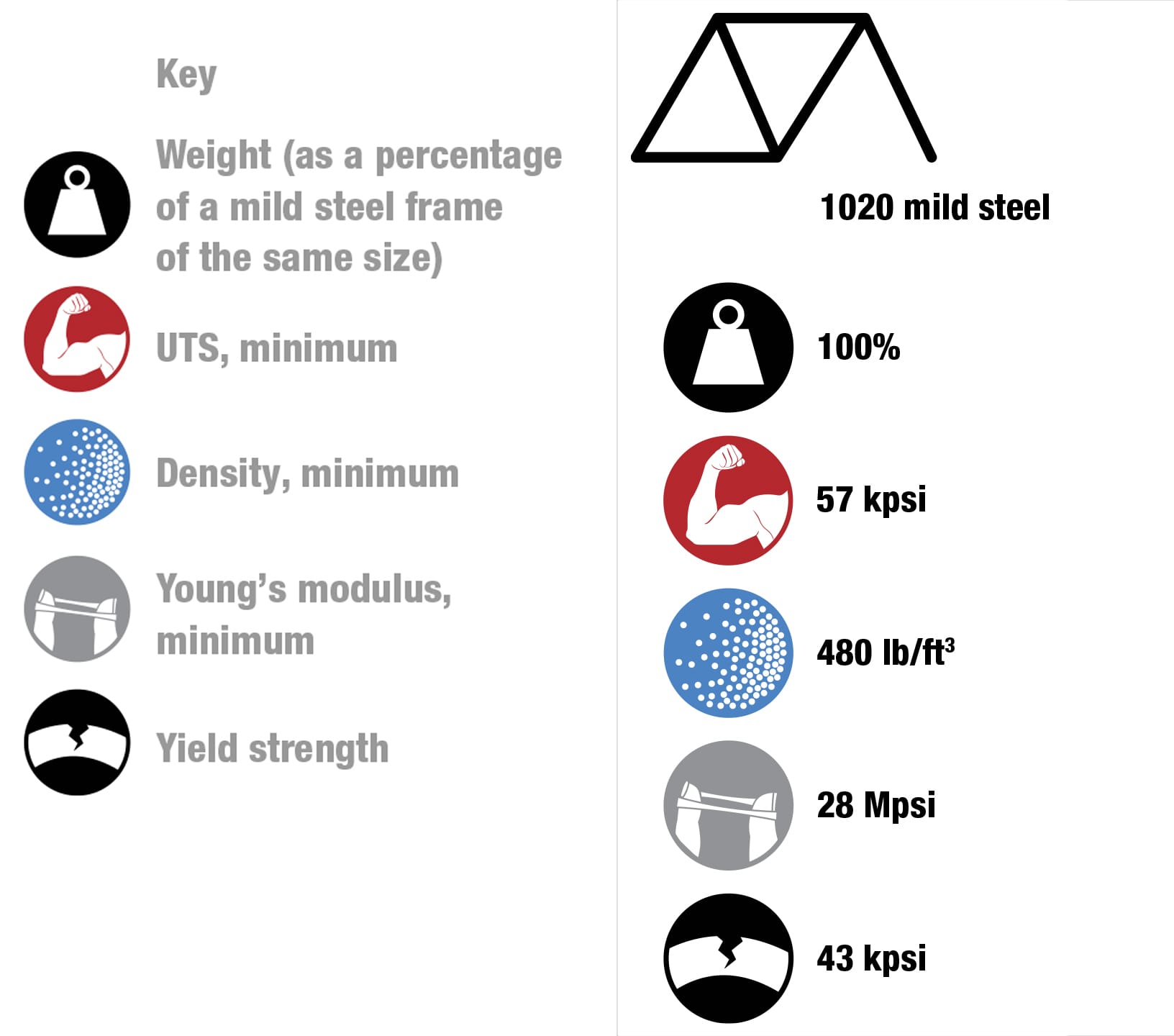
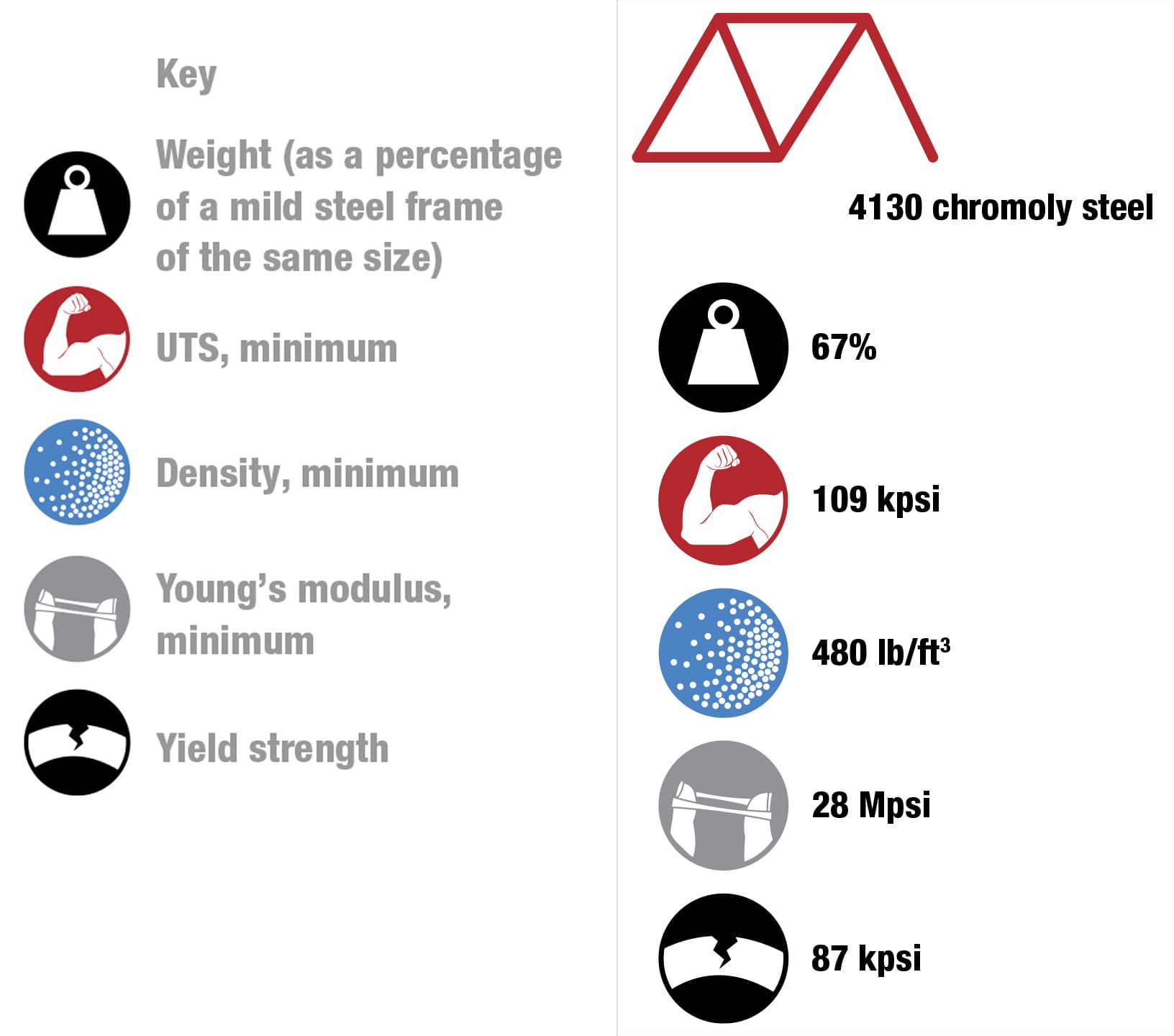
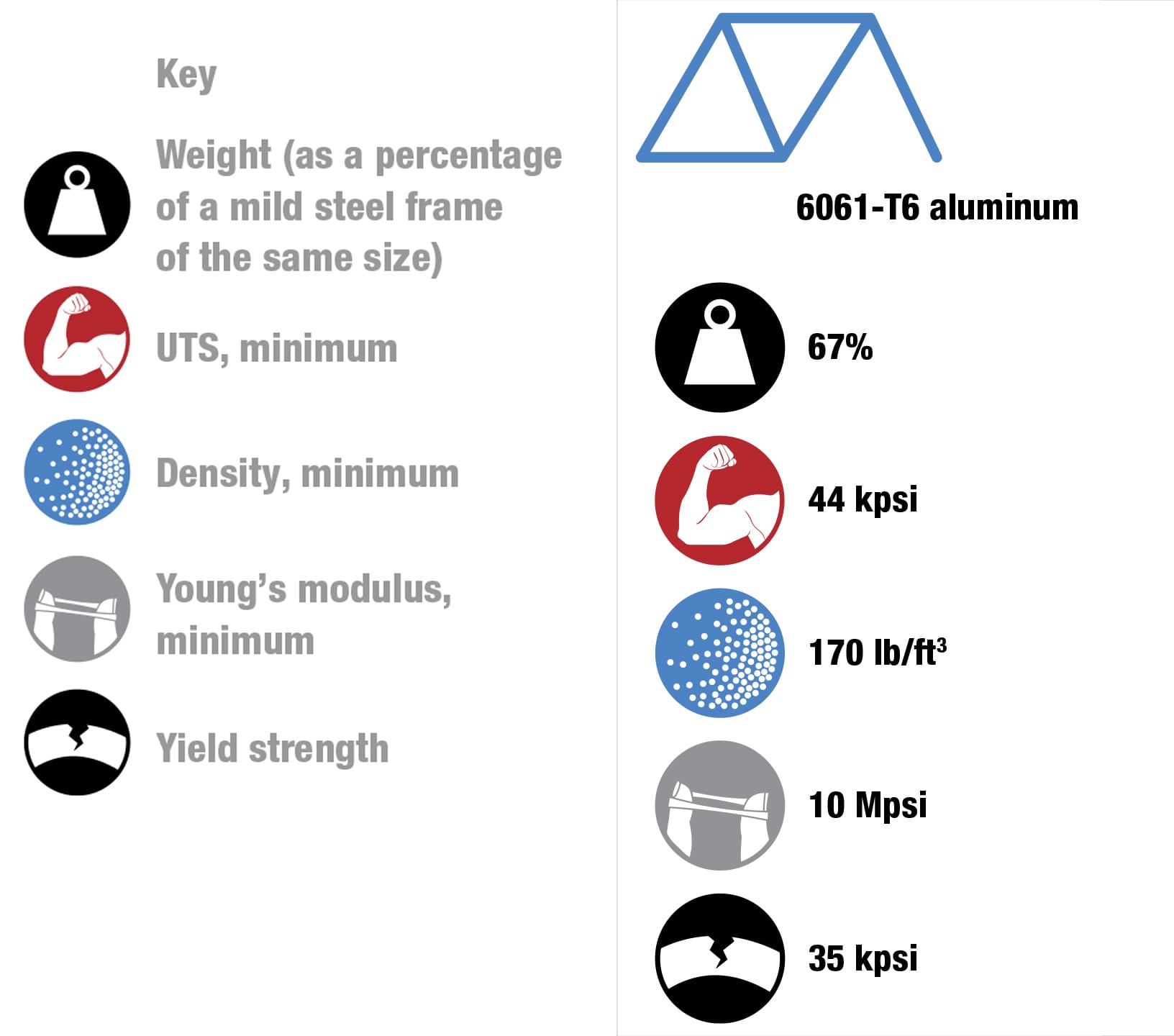
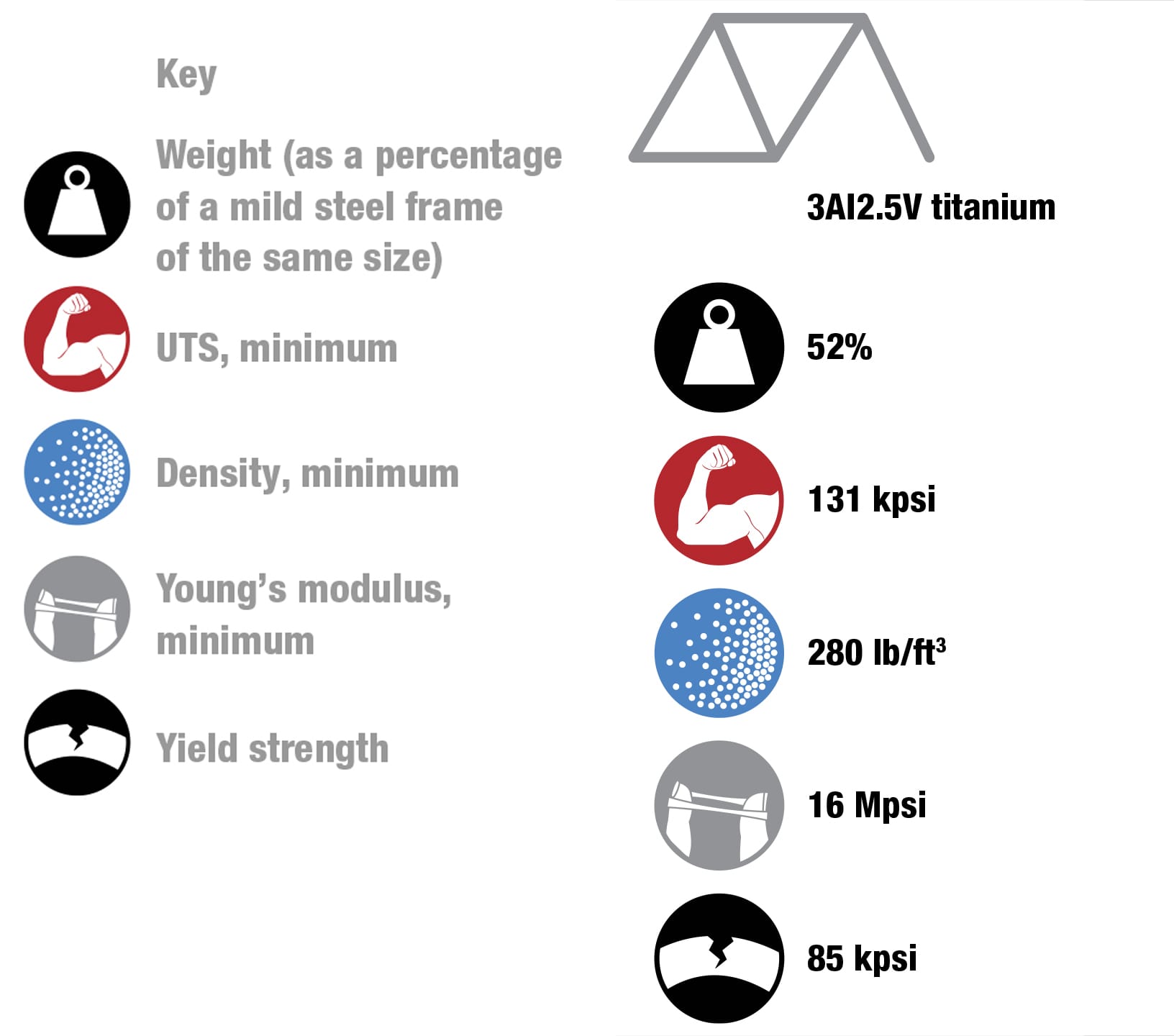
To each his own Every metal has its own properties and characteristics. The art of the frame builder is to balance these with the desired result—some cyclists want ultralight, stiff frames and others want rugged, more comfortable designs. These four alloys are the most commonly used for bike frames. To keep things simple, the data refers to straight gauge framesets with tube walls of constant thickness. The price of each material will vary according to market conditions. Nevertheless, based on historical data, it’s likely that relative prices will not change, increasing in the order from mild steel through to titanium. (1 kpsi = 1,000 psi, 1 Mpsi = 1,000,000 psi.)
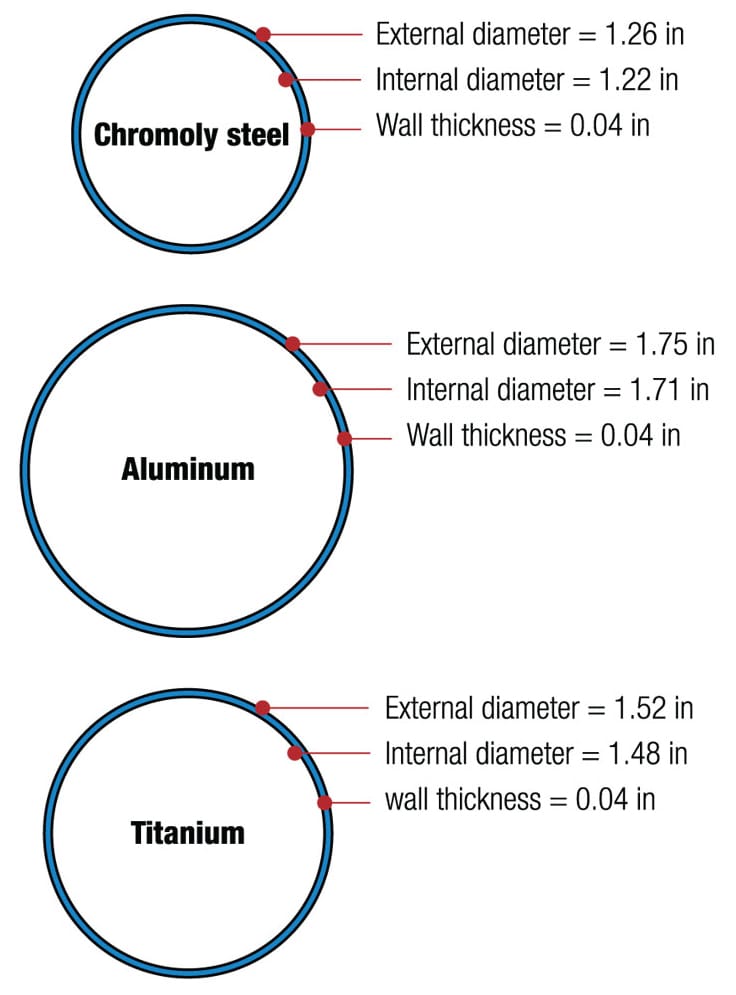
How fat to function? The stiffness of a frame tube changes according to the material and its dimensions—the diameter and wall thickness. If a steel head tube 1.26 in (31.7 mm) in diameter is to be replaced with another of aluminum or titanium but with the same wall thickness of 0.04 in (1 mm), the graphic reveals how much bigger the external diameters must be to compensate for the greater elasticity of the substitute metals.
How are solid metals joined?
 What sticks my frame together?
What sticks my frame together?
Nearly every metal frame is assembled by either welding or brazing the tubes together, and both methods involve turning solid metal into liquid and back again. The joints must be at least as strong as the tubes, and the frame builder achieves this by harnessing the complex chemistry of hot metal.
Welding involves heating two adjacent areas of metal until they melt and fuse, and then letting them cool and solidify. It’s complicated by the fact that molten metal may absorb gas molecules, which can weaken a joint considerably. Aluminum has a low melting point so it is easily welded, and an inert gas is used to shield it from potential contamination by gases in the air. For this process, gas tungsten arc welding (GTAW), also known as tungsten inert gas (TIG) welding, is used. Welding can reduce the strength of aluminum by 80 percent but this weakening can be reversed by heat treating the frame. Titanium especially needs a shield of inert gas to prevent oxygen, nitrogen, and hydrogen from diffusing into the hot alloy and making it brittle.
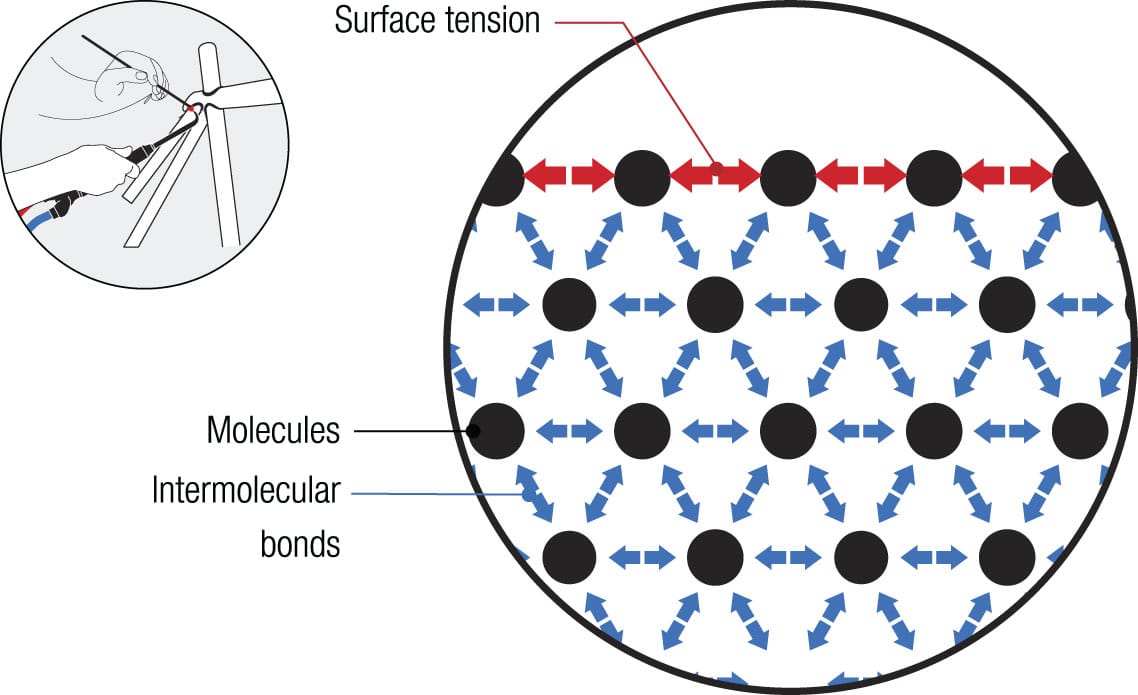
Brazing refers to a technique in which a small amount of a different metal, known as a filler, is used to hold the parts together. The filler is commonly brass or a silver/tin alloy of copper. The joint is heated, usually with an oxyacetylene gas torch, to a temperature hot enough to melt the filler but not the tube. When the filler melts, capillary action takes over and its molecules are drawn into the gap between the parts by a combination of adhesion forces (between filler and tube material) and surface tension (between filler molecules). This fills the gap and, as the brazing material cools, it solidifies into metal crystals that bind to the two parts of the joint. The advantage of brazing over welding is that it introduces a little more ductility (resilience) into a joint than welding, making it less prone to breakage.
Surface tension
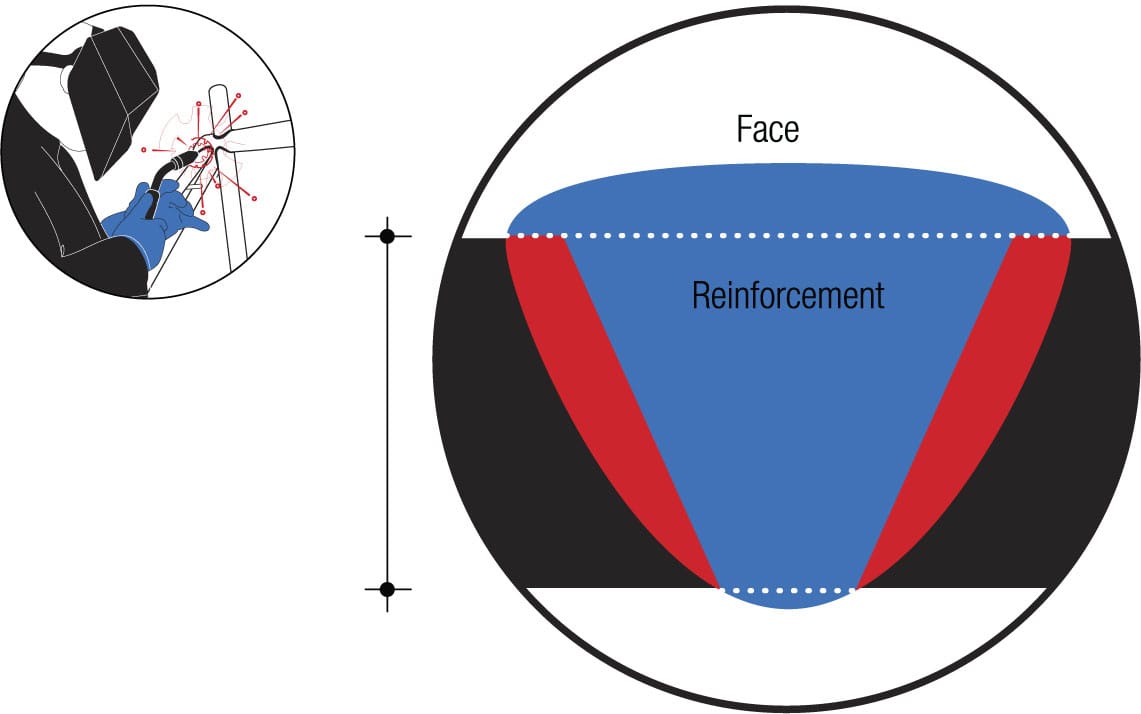
Exclusive partnership Welding melts metal with energy from a controlled electric arc and shields the working zone with an inert gas to exclude atmospheric gases that could contaminate and weaken the joint. A rod of material similar to the two tubes being joined is introduced to reinforce the junction. The fusion zone is created where material from the rod and the tube melt together and harden. The external surface of the weld is characterized by ripples, and the quality of the joint can be judged by their regularity.
Perfect marriage Brazing is commonly used by the custom frame builder to marry steel tubes. A rod of filler metal that liquefies at a temperature lower than the melting point of steel is heated with a torch, and capillary action draws it into the gap between the tubes. Traditionally, close-fitting steel sleeves or sockets, called lugs, are placed around the ends of the frame tubes to strengthen the junction, but fillet brazing doesn’t necessarily require them.
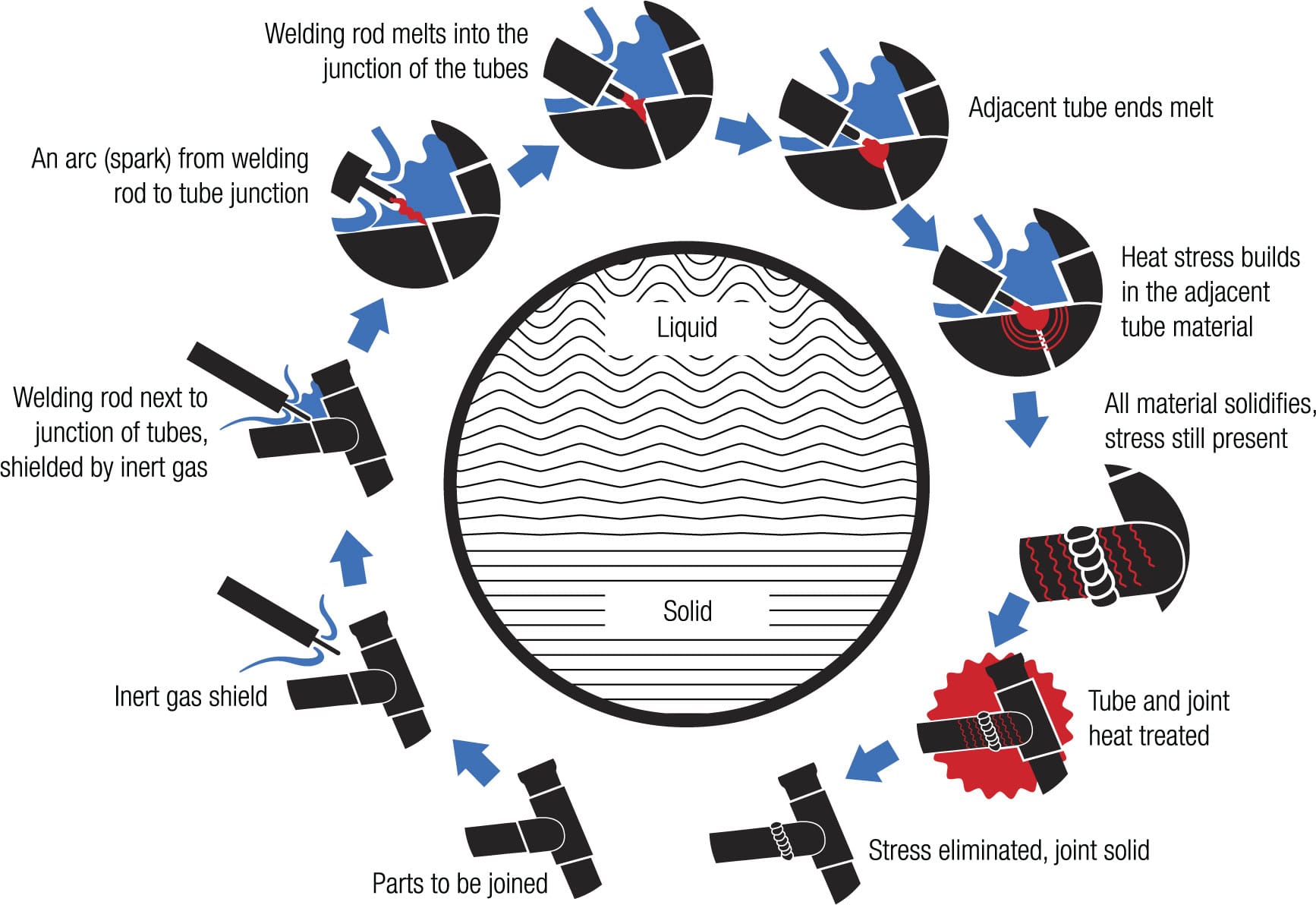
Stress reduction Structural components important to the strength of the bicycle melt during welding and some materials, such as aluminum, are susceptible to being weakened during the process. Heat treatment after the weld has cooled allows the molecules to restore their integrity.
Solid/liquid cycles: brazing
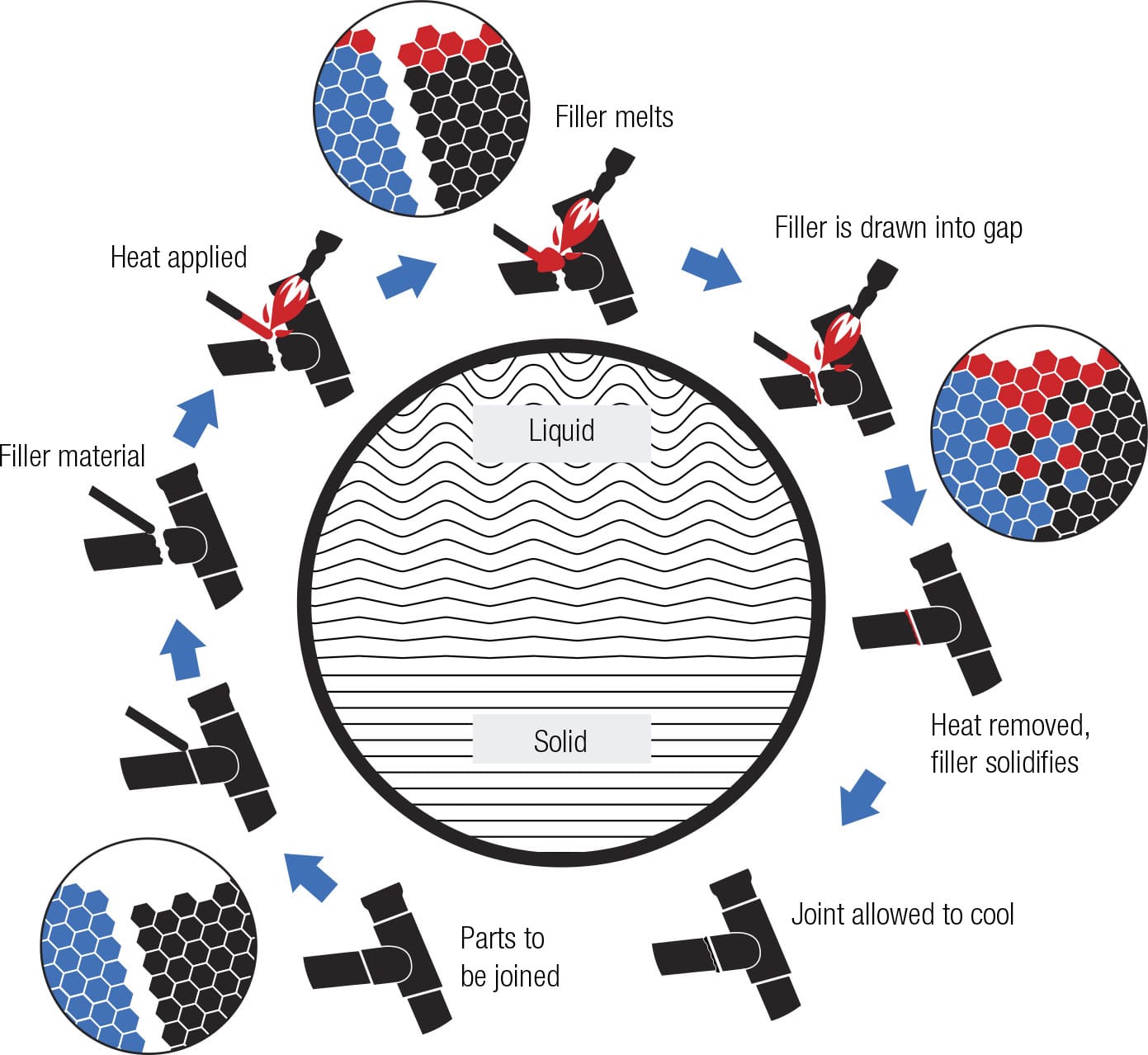
Joint venture The filler is the material that melts, flows into the crack between the tubes, and fuses with the solid metal on each side, frequently creating a bond that is actually more resilient than the frame tubes themselves.
equipment: frame tubes
The frame tubes of early bicycles were made of iron until steel alloys became available and affordable. Since that happened, in the second half of the nineteenth century, steel and its alloys have been used for the vast majority of bikes because they are relatively cheap and tolerate a wide range of fabrication processes. Aluminum became more popular in the late twentieth century, at the same time that titanium was first used by a few pioneers. Magnesium was tried in the 1980s but is still only offered by two manufacturers in the whole world. Away from the metal scene, composites have been growing for nearly 50 years and, in a more literal sense, so have bamboo frames.
Both the cheapest and the most expensive steel tubes are made from flat sheets rolled into cylinders and welded along their lengths. The weld is a weakness but is strengthened through heat treatment. Most other hollow metal frame tubing is seamless. For steel, a solid bar about 10 in (25 cm) in diameter and 35 in (90 cm) long is heated red hot, to more than 1,800°F (1,000°C), so it is compliant and can be rolled. To turn the hot, solid cylinder into a hollow tube, its end is pierced and drawn over a pointed steel rod called a mandrel. At the same time it passes through a conical hole, which reduces the external diameter. The process is repeated until a tube of the desired dimensions and a constant wall thickness—known as plain gauge—is obtained.
Frame tubing, however, needs to be strongest at its ends, so it is often made thinner along its central section to save weight. This is done by “butting.” In effect, the shaped mandrel, which defines the internal diameter and profile of the tubing, gets trapped inside because the walls at each end are too thick for it to be removed. The tube is then spun between offset reels. This is the clever part: While it has no significant effect on wall thickness or overall profile, it increases the external and internal diameters of the tube so the mandrel can slip out. The result is a thin-walled central section and thicker-walled ends.
Aluminum alloy tubing can be shaped more easily than steel. Profiles are hydroformed, with the sheet material placed inside a resilient mold and pressurized from inside by hydraulic fluid. This can also leave the aluminum alloy with a cosmetically better surface than other shaping methods.
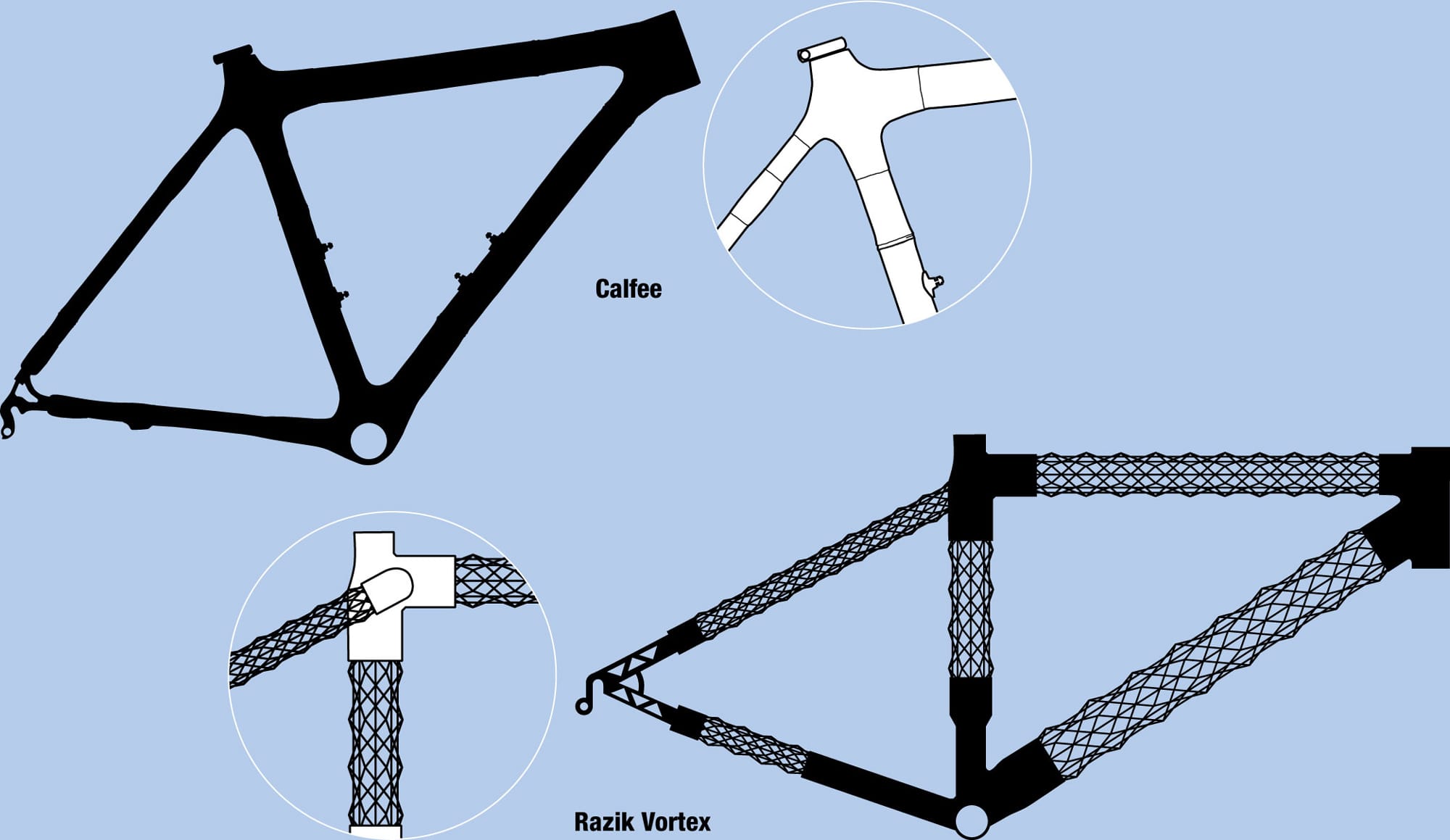
Tubes of the future New materials and manufacturing methods mean that frame tubes are changing. The versatility of carbon fiber has allowed Razik to produce hollow frame tubes that do not have solid walls, only a network of stiff, triangulated fibers. For its frame material, Calfee Design has adopted a natural composite—bamboo—and integrated modern components.
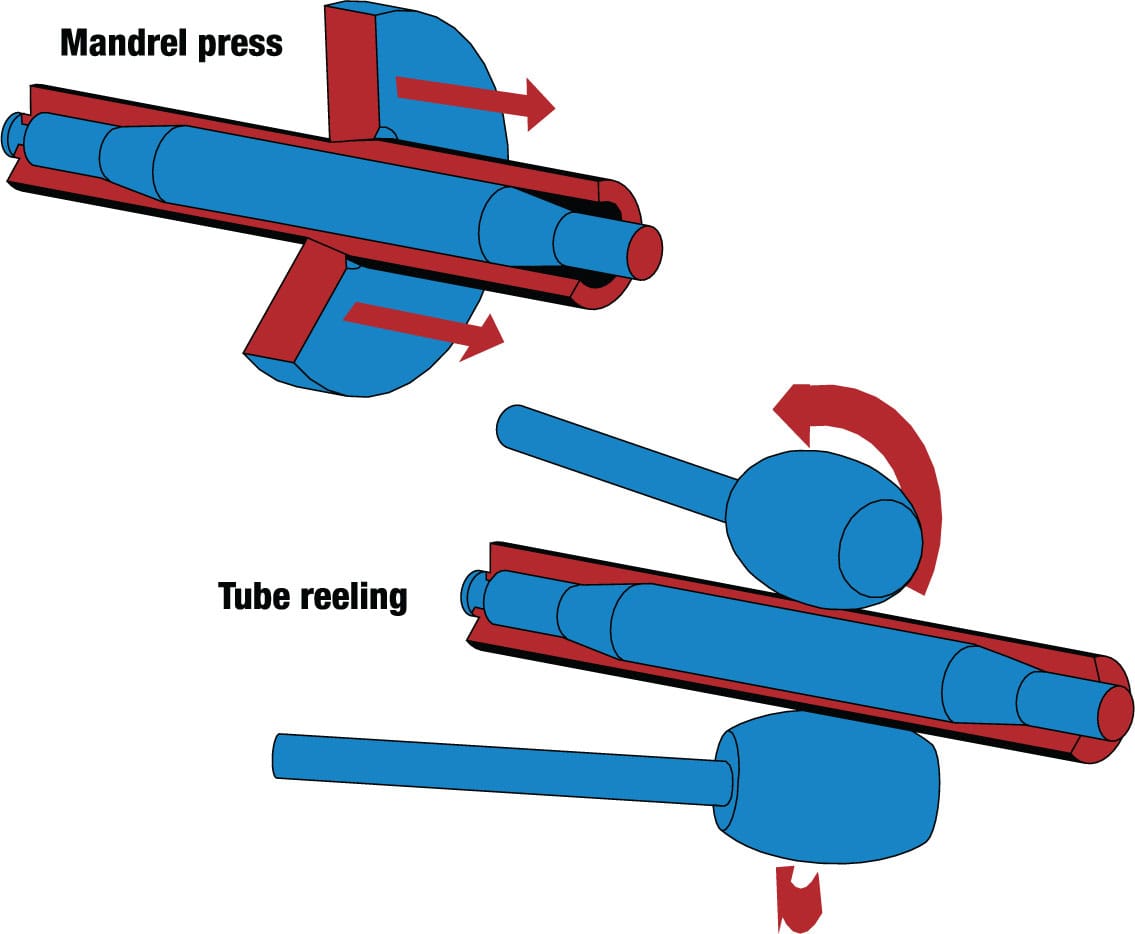
Butting To make the frame tube lighter, a mandrel press is used to make the walls of the central section thinner. Spinning the tube between offset reels increases the tube diameter temporarily, allowing the mandrel to be removed.
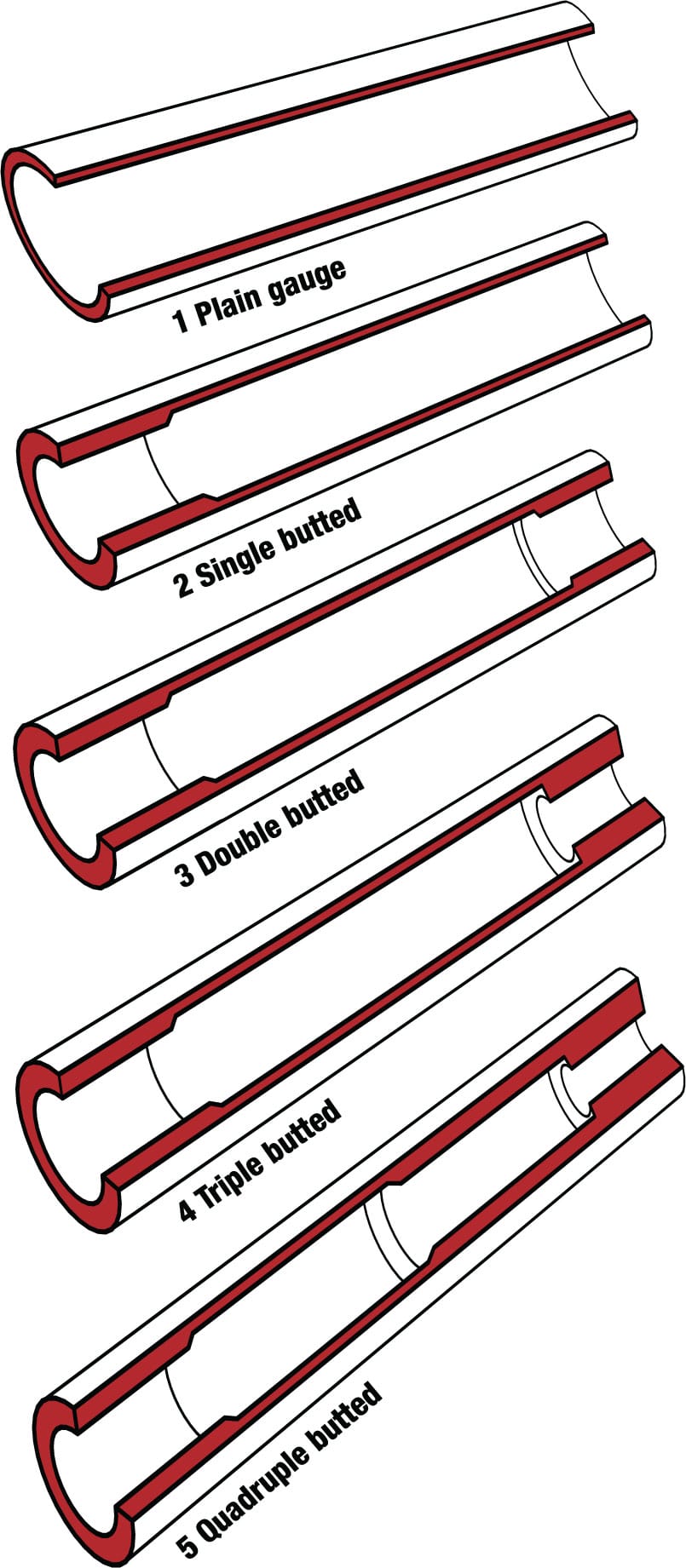
Tube profiles Conventional steel frame tubes have various profiles to save weight and retain strength and stiffness where they are most needed:
1 A tube without a butt has constant wall thickness, and is called a plain gauge tube.
2 A single-butted tube is thicker at one end.
3 Double-butted tubes are thin in the middle and thicker at both ends, with each end the same thickness.
4 Triple-butted tubes have ends of unequal thickness.
5 Quadruple-butted tubes have ends and midsections of varying thickness.
What is a polymer?
 What makes tires bendy?
What makes tires bendy?
Metals are not the only solid matter in bikes. Every single bicycle made also relies on polymers. If it’s on your bike and not made of metal, there’s a 99.5 percent chance it’s a polymer. Bikes were among the earliest inventions to utilize them; the natural rubber used for the first tires is a polymer. Other varieties such as plastics and foams are used for mudguards, saddles, cable sleeves, lever hoods, grips, bar tape, seals, valves, valve caps, jockey wheels, reflectors, and pedal platforms. Polymers are also crucial for some carbon fiber frames, disc wheels, and rims.
A polymer is a very large molecule (or “macromolecule”) built up by chemical combination of many small repeating units. The small molecules from which they are made are called monomers. Each monomer typically contains a “backbone” of up to around a dozen atoms in a line, with various side groups attached; the most common “backbone” atoms are carbon and oxygen. When thousands of monomers are linked together, the chain is referred to as a polymer. There are many kinds of polymers, because the chains can be built from different monomers, in different ways, and with different sequences, connections, and branches.
Polymer parts
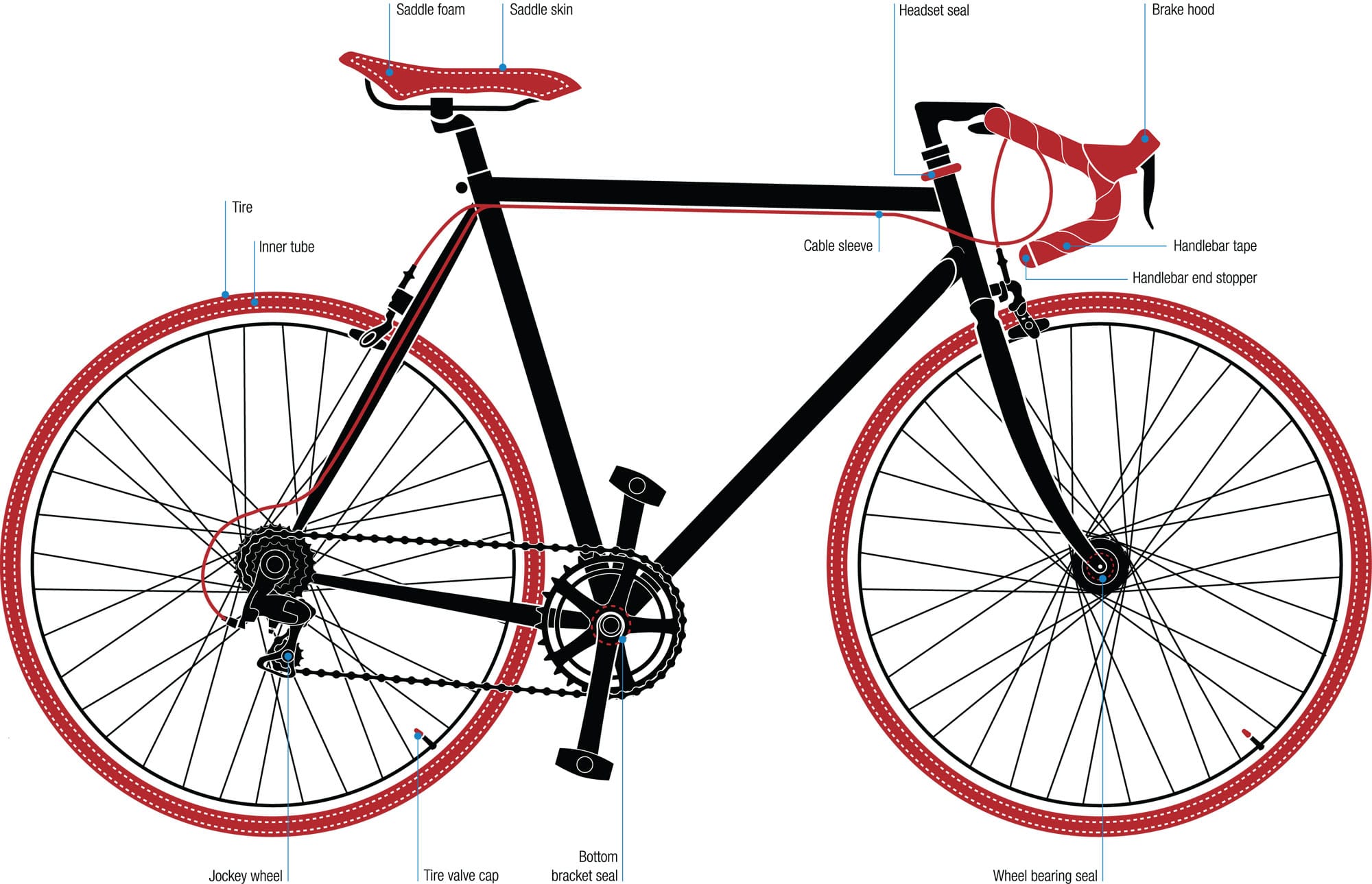
Polymer parts On conventional bikes, parts that aren’t metal are almost certainly made of polymers. Two of the rider’s contact points—the saddle and handlebars—have polymer covers for comfort. The tires and tubes are made of polymers because of their elasticity. Other parts are made of polymers because they are cheap, impermeable, or lightweight. High-end machines with carbon fiber frames are more polymer than metal, as are carbon fiber disc wheels.
Polymer properties such as strength and melting point can be dramatically different from the properties of their monomers, and they also depend on the length of the chains. Certain key properties such as elasticity and resistance to breakage are enhanced when these chains get entangled, like spaghetti in a bowl. Fillers, reinforcements, and additives are introduced to refine these properties, but the secret of polymer behavior—typically recovering undamaged from compressive, tensile, and torsional loads—is the length of their chains.
Natural polymers have shortcomings. For example, latex rubber from trees is too sticky and rots quickly, so all polymers on bikes are synthesized from petroleum. Synthetic rubber is heated in the presence of sulfur to cross-link the polymer chains, making it more resilient and durable—yet still elastic.
Polymer properties
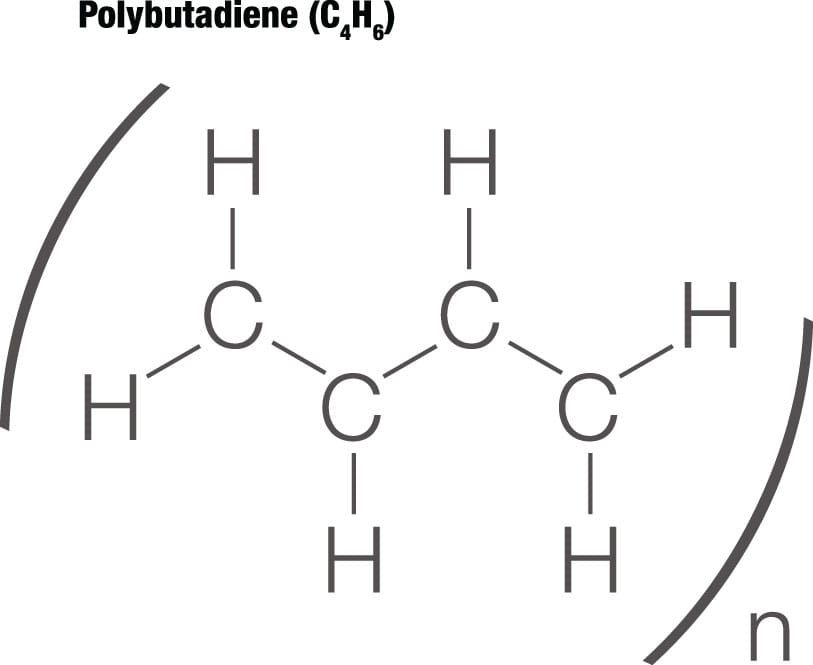
Polymer shapes Polymers can be linear or branched and their shapes affect their properties. Chemical diagrams such as this one communicate how they are arranged; the n indicates that this basic arrangement repeats.
How are composites constructed?
 What’s so special about carbon bikes?
What’s so special about carbon bikes?
Composites—two dissimilar solid materials that are bound together yet remain physically distinct—are increasingly used in bike frames, forks, stays, stems, bars, rims, disc wheels, and small components. One material is embedded in the other to combine the strength and low density of the former with the toughness of the latter and get something stronger, lighter, and stiffer than any single material. The most common pairing is a polymer matrix reinforced with carbon fibers. The fibers provide tensile strength (the fibers are typically more than a hundred times stronger in tension than the matrix alone) and the matrix is strong in compression.
The carbon fibers actually start out as a solid polymer, called polyacrylonitrile, which is melted so that strands can be drawn from it and heated to about 2,200°F (1,200°C). This drives off hydrogen and nitrogen atoms and leaves carbon filaments with diameters of around 200 to 300 millionths of an inch (5 to 8 micrometers, or millionths of a meter), a fraction of the diameter of a human hair. If heated higher, up to around 4,500°F (2,500°C), they become stiffer, “high modulus” filaments. The filaments are wound into threads that can be woven into fabric or spun around a mold. Fabric layers are then fashioned into the shape of the bike part. The matrix is usually a polymer known as an epoxy, a plastic that is melted to coat the fibers and then solidifies irreversibly. The quantity and location of the carbon fibers and epoxy matrix are adjusted to maximize strength and minimize weight. The length and alignment of the fibers can also be adjusted, in advance, to fine-tune the strength, weight, stiffness, and other properties of the finished product.
The number of possible combinations of fiber, matrix, alignment, processing, and shape means “carbon” frames can be phenomenally diverse in their appearance, properties, and performance.
Carbon frames
Carbon rules Once a black art, carbon fiber frames and components are now mass-produced, and their prices can rival those made of metal. Young’s modulus (stiffness) ranges from 20 to 90 Mpsi (135–600 GPa). Ultimate tensile strengths can be between 300 and 500 kpsi (2,200–3,500 MPa). Compare this with 4130 chromoly steel, which has a modulus of 28 Mpsi (190 GPa) and ultimate tensile strength of 109 kpsi (750 MPa). A basic plain-weave carbon fiber sheet has a stiffness-to-weight ratio 14 percent greater than chromoly steel and 18 percent greater than aluminum.
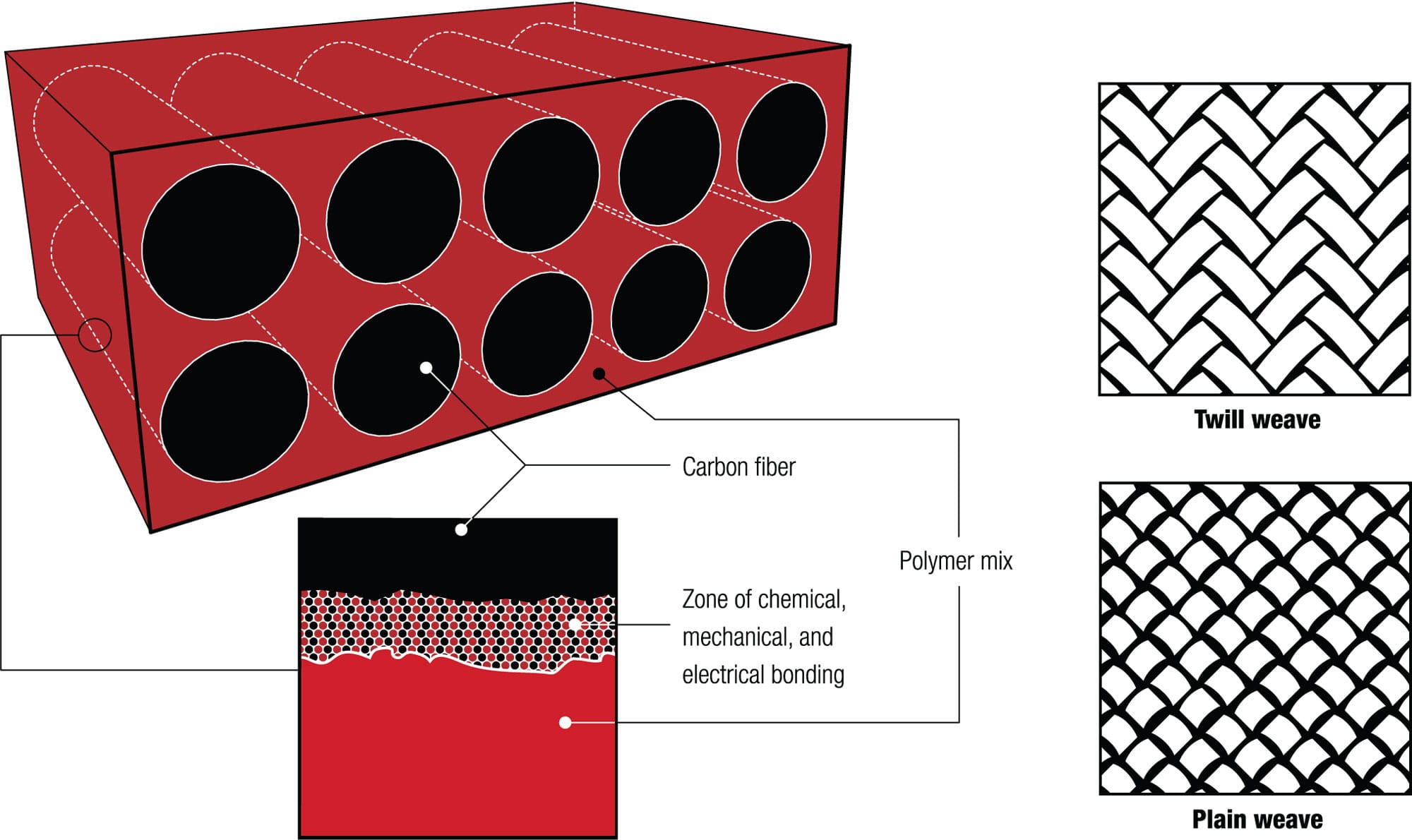
Carbon fiber magic The properties of carbon fiber-reinforced polymer varies according to weave pattern, fiber density, and matrix polymer. Carbon fibers can be aligned and woven to optimize the magnitude and direction of the tensile strength of a component. Compressive strength is provided by the plastic matrix, and it is important that this bonds well with the fibers. Many methods have been developed, including wet layup, vacuum bagging, resin transfer, matched tooling, insert molding, and pultrusion. Unlike metals, carbon fiber reinforced plastic doesn’t yield under excessive loads, so it bends and then snaps without the intermediate stage of permanent deformation. A broken piece of carbon fiber is not easily repaired, and a repaired component cannot be used with confidence if it is exposed to large dynamic loads, so it is usually discarded. Some bike manufacturers now recycle discarded fragments into smaller items.
What role do liquids play in cycling?
 Can I just go with the flow?
Can I just go with the flow?
Almost every material on a bicycle is liquid at some point during its manufacture. Metals and polymers go through liquid states as they are produced, and hydraulic fluids are pumped into some aluminum frames to expand them to fit their molds. Rubber and alloys are usually worked when they are molten. However, the only liquid found on most finished bikes is the lubricating fluid.
Under normal conditions, a liquid maintains its volume, but it can change its shape because its molecules are bound together more weakly than those in a solid, allowing it to move or flow. The rate of flow is affected by the forces between its molecules; these forces determine its viscosity, or “thickness.” Lubricating oil has a viscosity that is just low enough for it to flow easily into the gaps of a chain or bearing; it remains there because of forces of adhesion between the oil and the metal parts. The oil provides a thin film that reduces friction between metal surfaces which would otherwise rub and wear each other. It also provides a barrier between the metal and oxygen in the air, which would otherwise react in the presence of water to form a complex mixture of iron oxides (including FeO, Fe2O3, and Fe3O4) collectively referred to as rust. Another important function of oil was revealed in tests in a clean lab. Brand-new unlubricated chains were shown to be as mechanically efficient as lubricated ones, suggesting that, on the road, a lubricant’s main function is to fill microscopic gaps in the metal surfaces and stop contaminating particles getting in.2
A growing number of bikes also use hydraulic fluid in sealed hoses to transfer pressure from the brake levers to the pads or from the gear levers to the derailleurs. The fluid is usually a mineral oil of a viscosity chosen to transfer pressure consistently and repeatedly. The fluid must have a high boiling point for riding long descents—the constant pressure when braking continuously can raise the fluid temperature significantly.
Hydraulic fluids
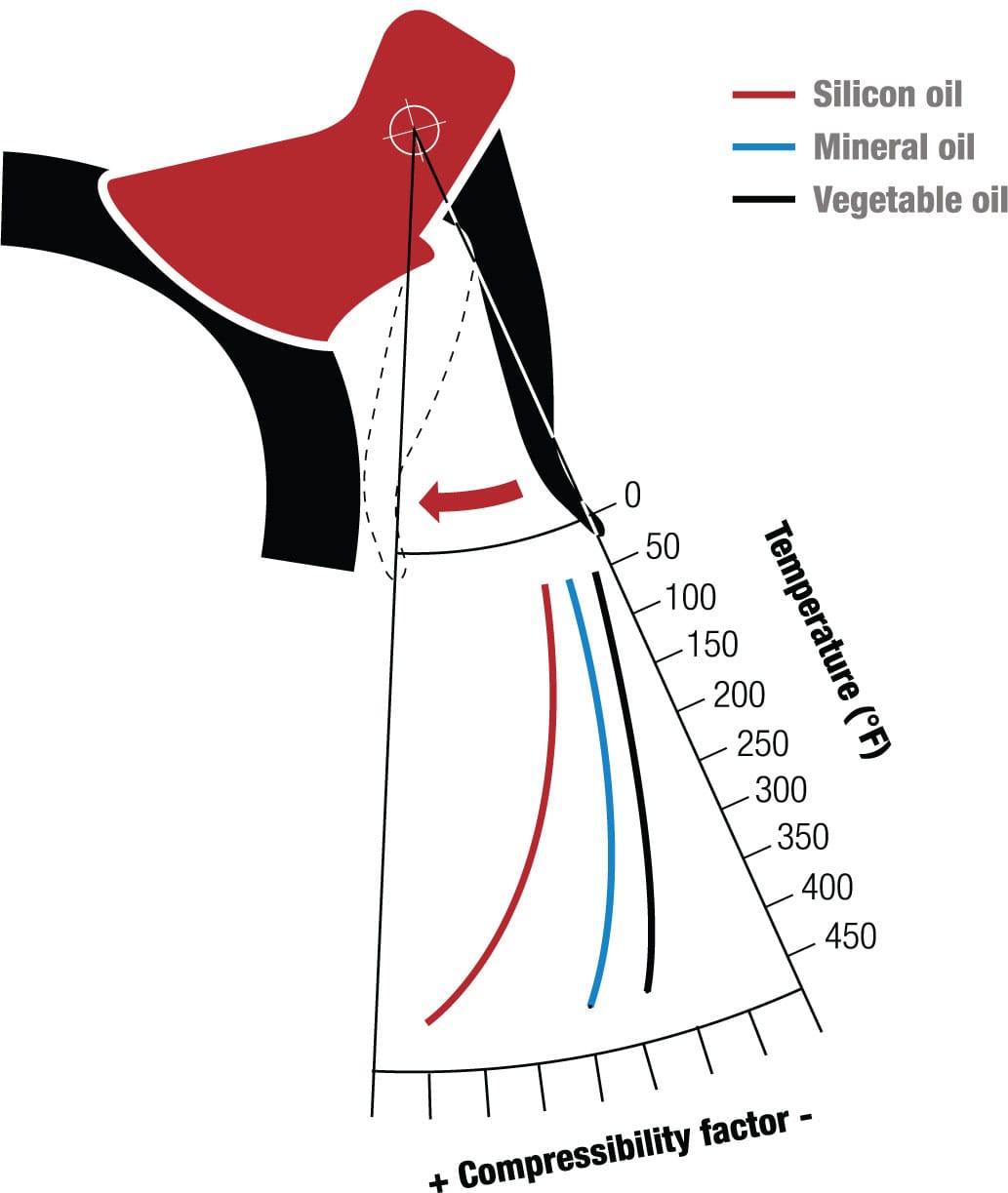
Hot and bothered Fluid compression varies with temperature and the type of fluid used. The warmer it gets, the more the hydraulic fluid can be compressed. This reduces its response under load so brakes can “fade” at high temperatures.3
Power remaining after loss through friction
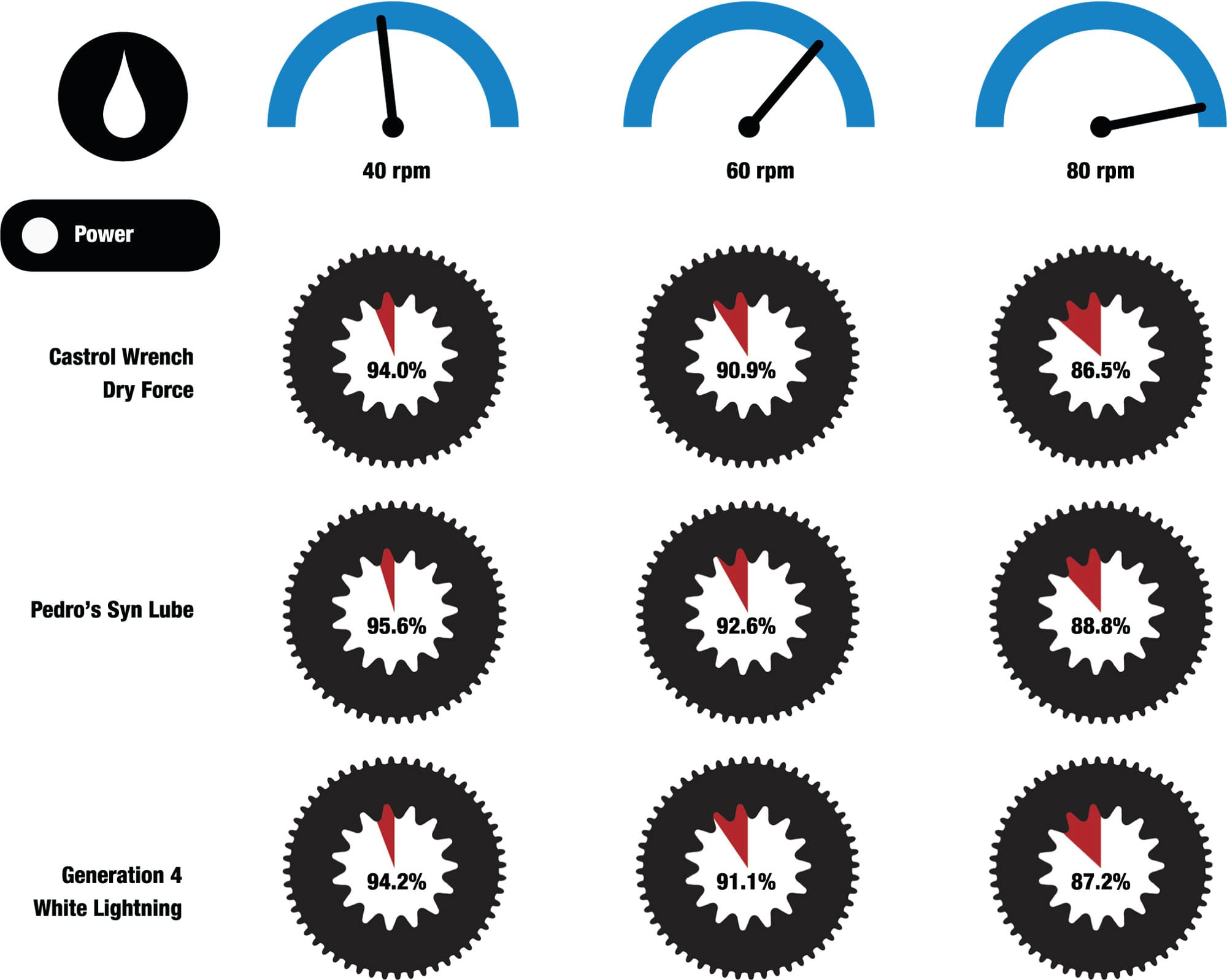
One lube as good as another? In the lab and with a brand-new chain, it’s been shown that lubricants are not significantly different from one another when it comes to reducing the friction of a chain. When 74 ft·lb/s (100 W) of power was applied at the front chainring, the loss through friction at the rear cluster was very similar whatever the lubricant (the percentages indicate the power remaining after the loss through friction). However, real life is dirtier and harsher than in a laboratory and, as a lubricant ages and becomes contaminated, it can affect the longevity of the moving components.4
Metals in close-up
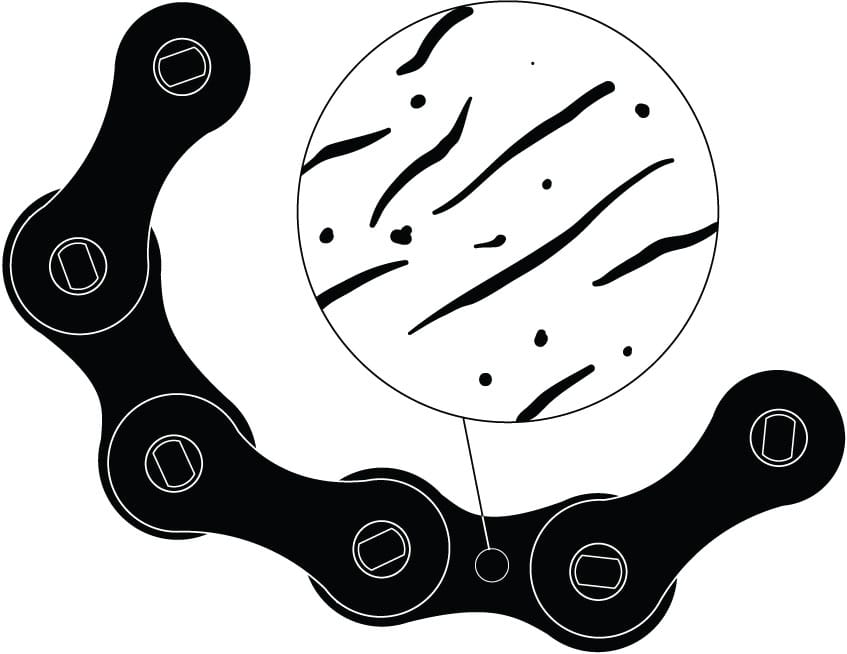
Roughed up A smooth-looking metal surface has microscopic ridges and pits. Lubricant serves to reduce the friction of these elements.
How do gases affect riding?
 Why does my pump get hot?
Why does my pump get hot?
Gas both impedes and facilitates cycling. Felt as a headwind, air is a gas that gets in the way, but without it cyclists would suffocate. Apart from all of the bike’s solids, and their own body, the average rider is also transporting about 0.1 ft3 (3 liters) of air. Roughly half of this is trapped inside the hollow frame tubes, forks, stays, and handlebars, with the other half inside the tires.
A gas has mass, although, luckily, very little compared to most solids and liquids, but it does count. A material becomes a gas when the kinetic energy of its molecules (which depends on temperature) is sufficient to overcome the attractive forces between them.
There is a specific relationship between pressure, volume, and temperature for any gas, and this is demonstrated every time you inflate a tire. Molecules are in constant motion; in a solid, they may just jiggle, but in a gas they hurtle about, colliding and transferring kinetic energy (energy of motion) to one another and to the walls of their container. Pushing the piston of the pump reduces the space in the barrel and, therefore, raises the density of molecules, so they suffer more frequent collisions. The classical theory of gases associates this frequency with pressure, so pushing gently on the piston makes the pressure in the pump (and the tire) go up.
What’s more, when you push the piston in the pump, you give some of your own kinetic energy—through the moving piston—to the gas molecules in the pump. Using a simple model of a gas as tiny elastic spheres in random motion, one can show that its temperature is proportional to the average kinetic energy of those molecules: the faster they’re moving, the warmer the gas. Some of this energy is imparted to the walls of the pump, so the pump warms up, too. The harder and faster you pump, the more energy you provide and the more noticeable the rise in temperature.
Pumping molecules
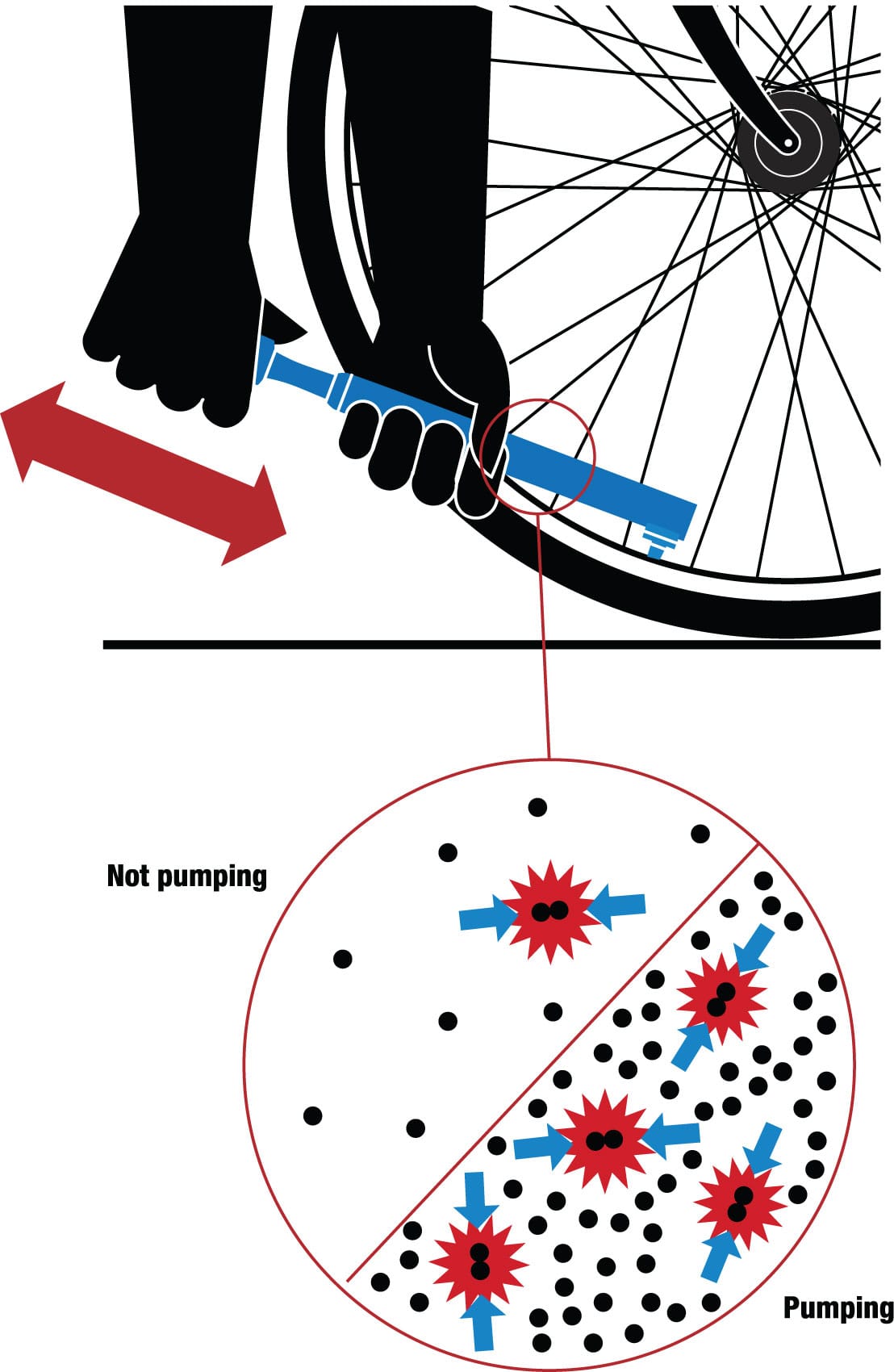
Pressure up, temperature up Put gas suddenly under pressure with a hand pump and it heats up as molecules acquire more kinetic energy. That’s what happens when you pump up your tires.
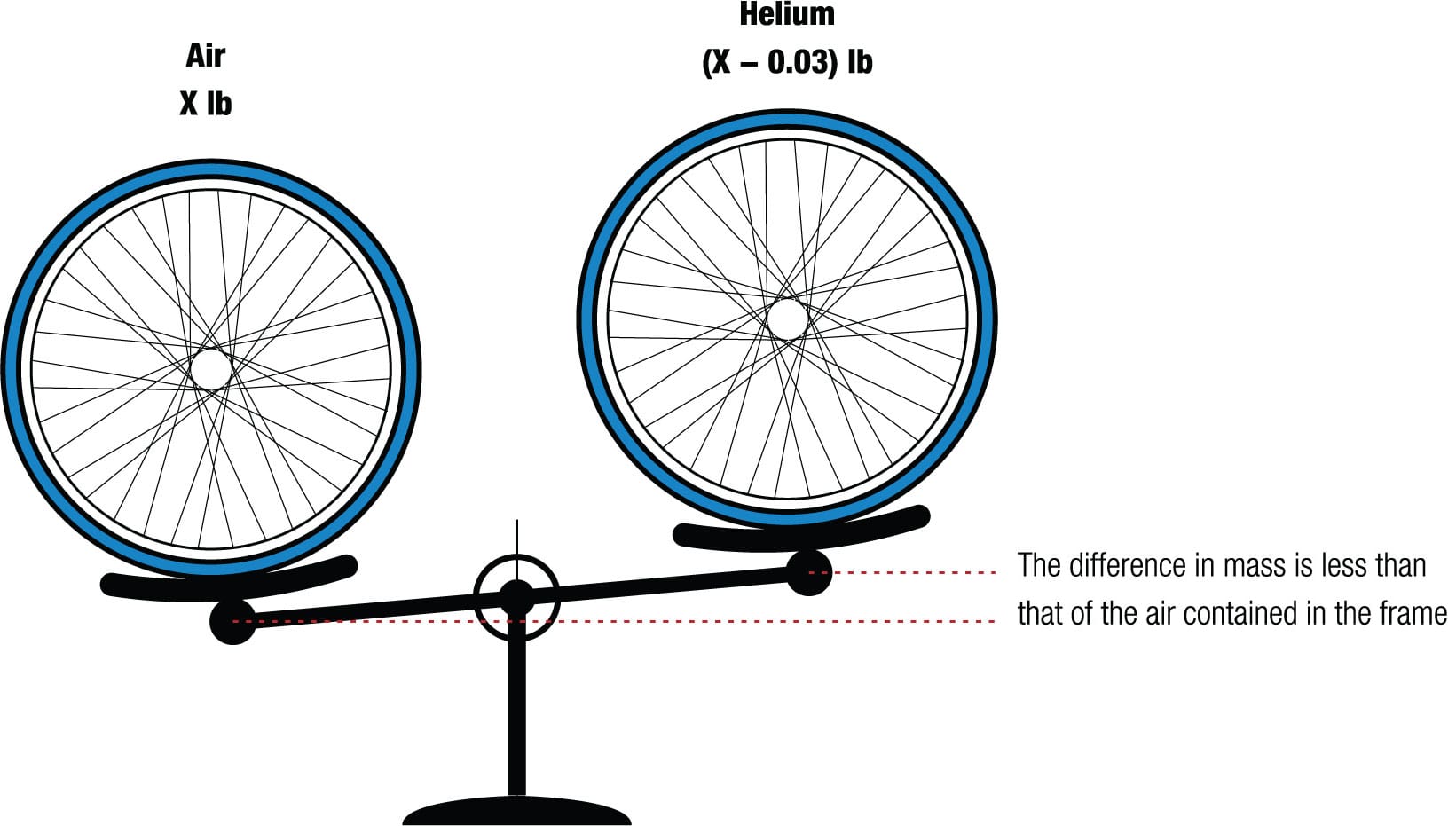
Up, up, and away As he was preparing for a world hour record bid in Mexico in 1972, Eddy Merckx, the great racing cyclist, decided he wanted helium in his tires because it would cut weight by about 0.03 lb (14 g). But according to his bike builder, Ernesto Colnago, he couldn’t get any.6 Nevertheless, Merckx set a new record and it stood for 12 years.
Men’s hour records—best human effort
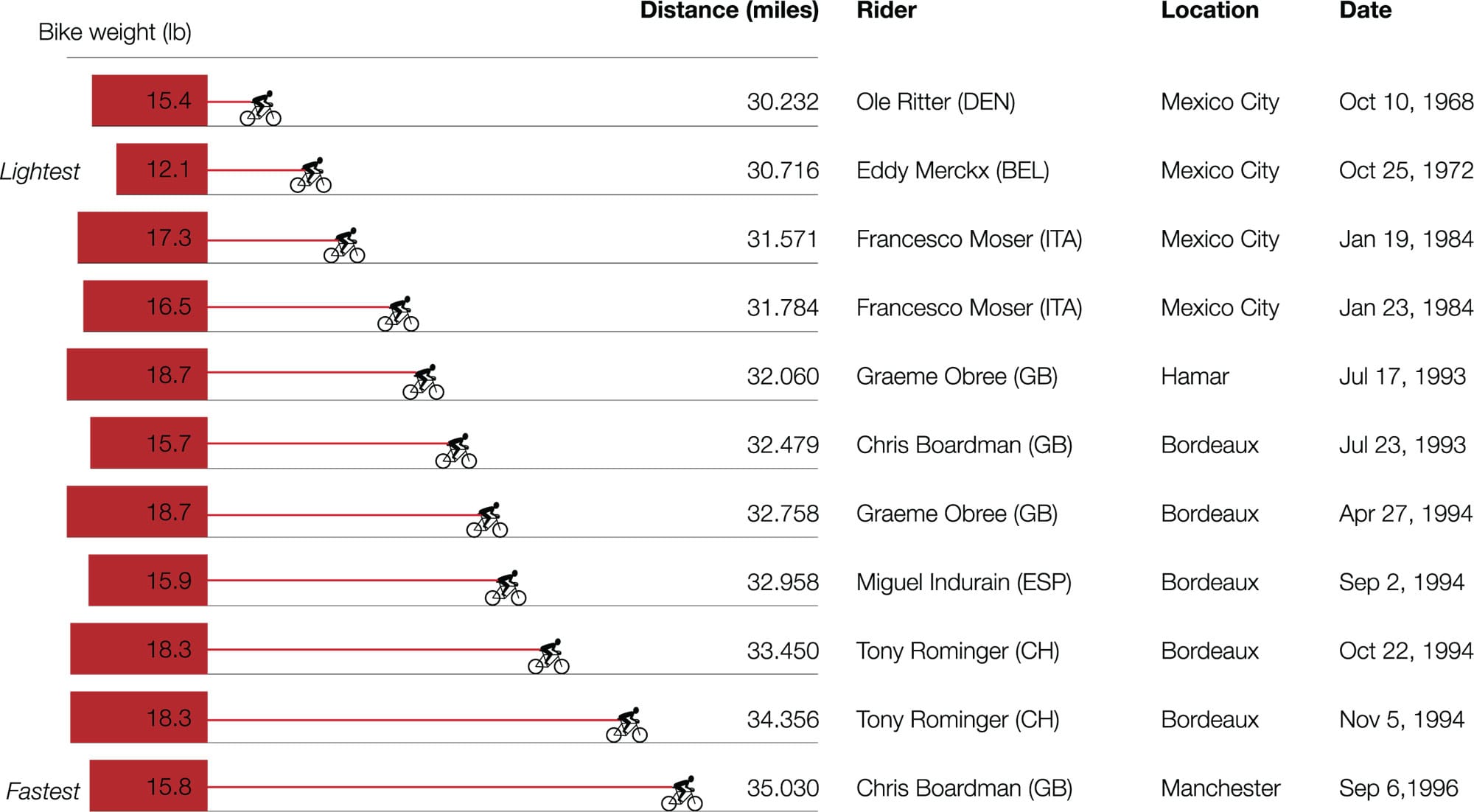
Unbearable lightness Frame weights vary over time, due to advances in technologies and regulatory restrictions. They do not correlate directly with record times.7
Why might plasma be the future of bike materials?
 Will the stuff of stars boost bike speed?
Will the stuff of stars boost bike speed?
Plasma is commonly known as the fourth state of matter. It’s a little like a gas in that the particles move around relatively freely, but it differs because thermal or other energy is intense enough to strip electrons off some of its atoms, leaving a swarm of ionized (charged) and nonionized particles. In fact, this mix makes up some 99 percent of the matter in the observable universe—it’s what stars are made of—and could, one day, help you ride faster.
Scientists have successfully used plasma to influence the airflow over aircraft wings and control the flight path without using wing flaps. The plasma is created using electrodes that pass electrical energy through the air, similar to the way a charge passes through the gas of a neon tube. By precisely positioning the electrodes and controlling the energy they discharge, plasmas of the desired volume, location, and duration are formed—and modify the airflow over the plane. The technology has been trialed with small unmanned drones and the power requirements are not large, so it is plausible that the technique could be applied to cyclists and their bikes.8
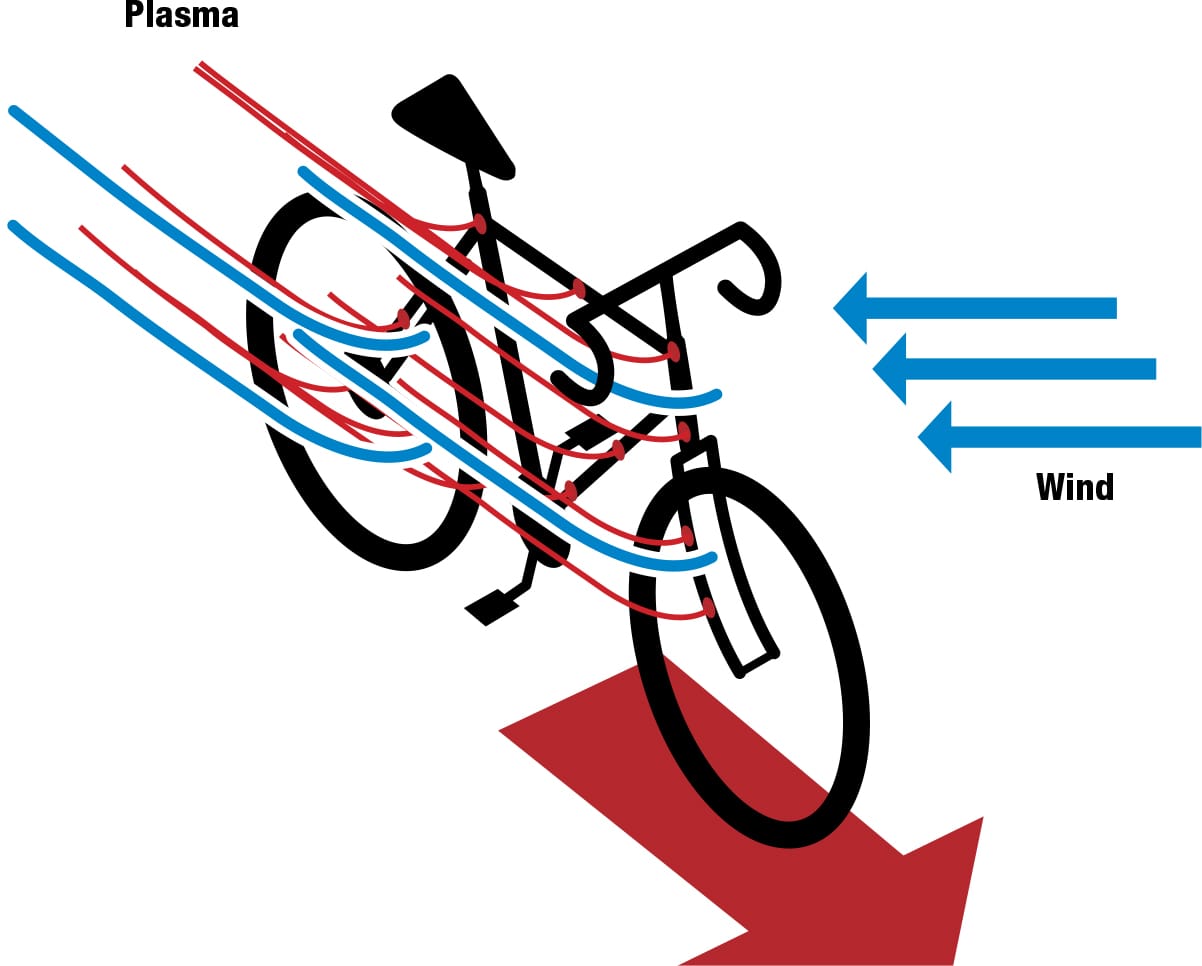
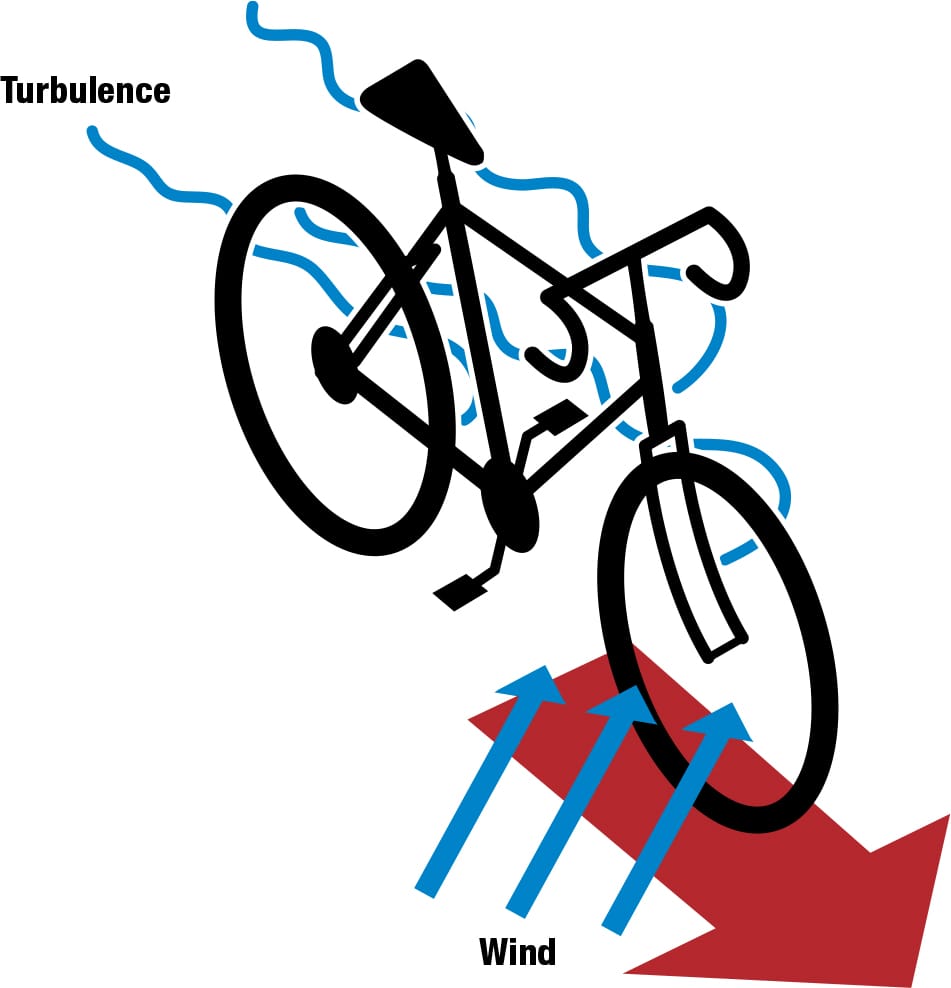
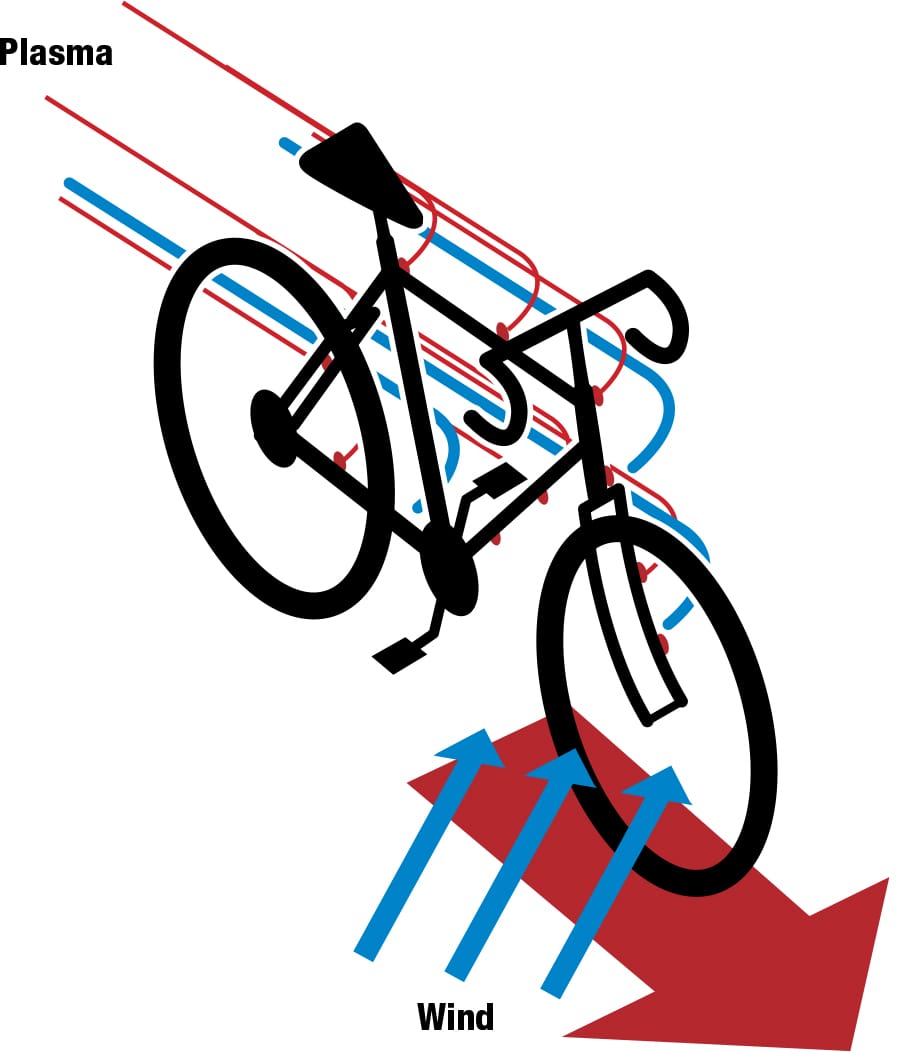
A mesh of plasma generators could be embedded in a composite frame, fork, handlebars, and disc wheels, even in the cyclist’s clothing and helmet. These could help create dynamic virtual streamlining, which could change shape and location as the bike changes orientation in relation to the air flow, potentially reducing turbulence and optimizing the cyclist’s aerodynamics in any gusts and breezes. Although still hypothetical, the suggestion is that it could reduce the power needed to overcome aerodynamic drag by some 40 ft∙lb/s (50 W). Aerospace brought advances in alloy and composite technology to cycling; plasma could follow in their slipstream.
Plasma effects
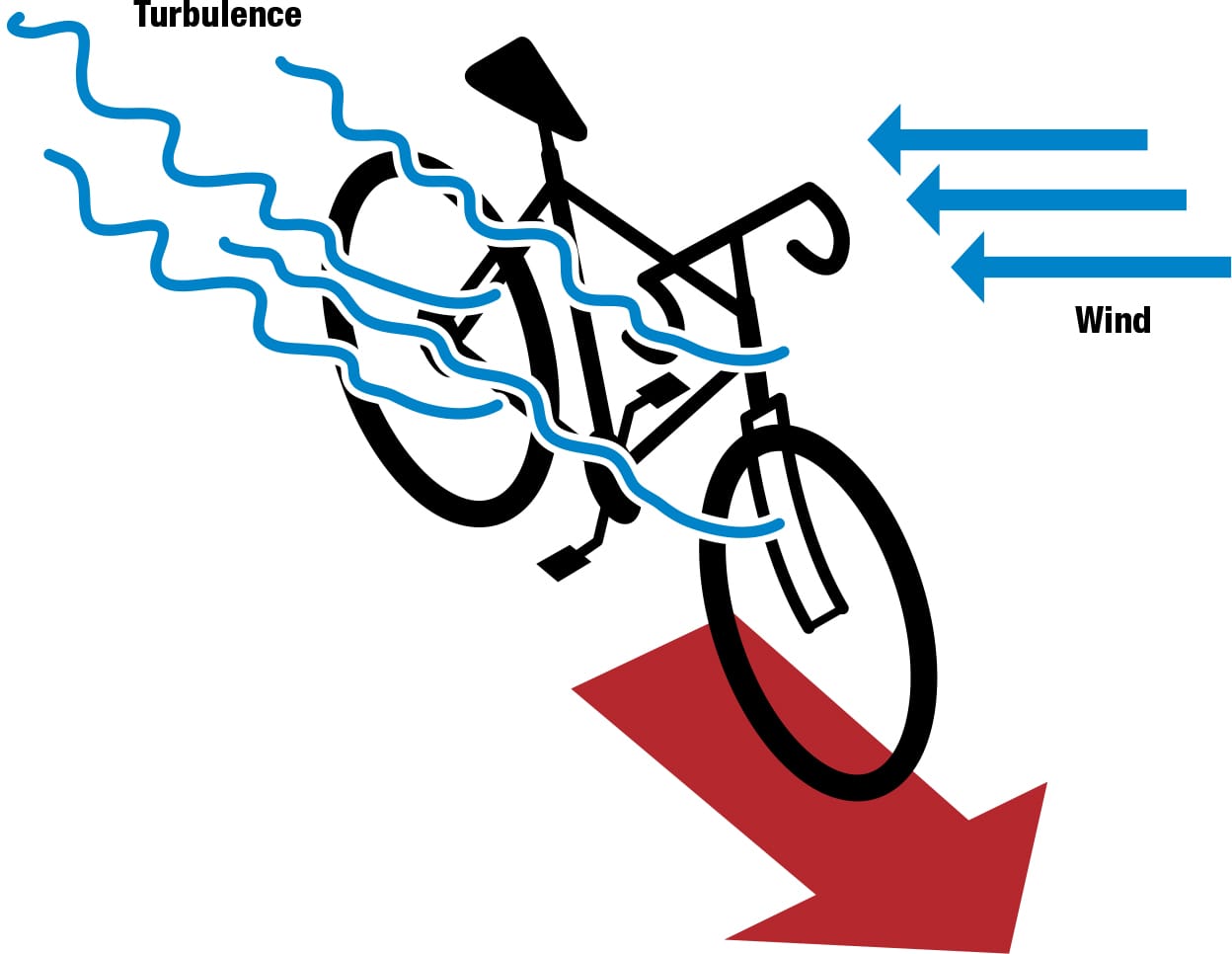
Riding with plasma A mesh incorporated in the cyclist’s clothing and helmet and in the bike’s frame and disc wheels could react dynamically to wind, creating plasma fields to reduce turbulence and improve aerodynamics.
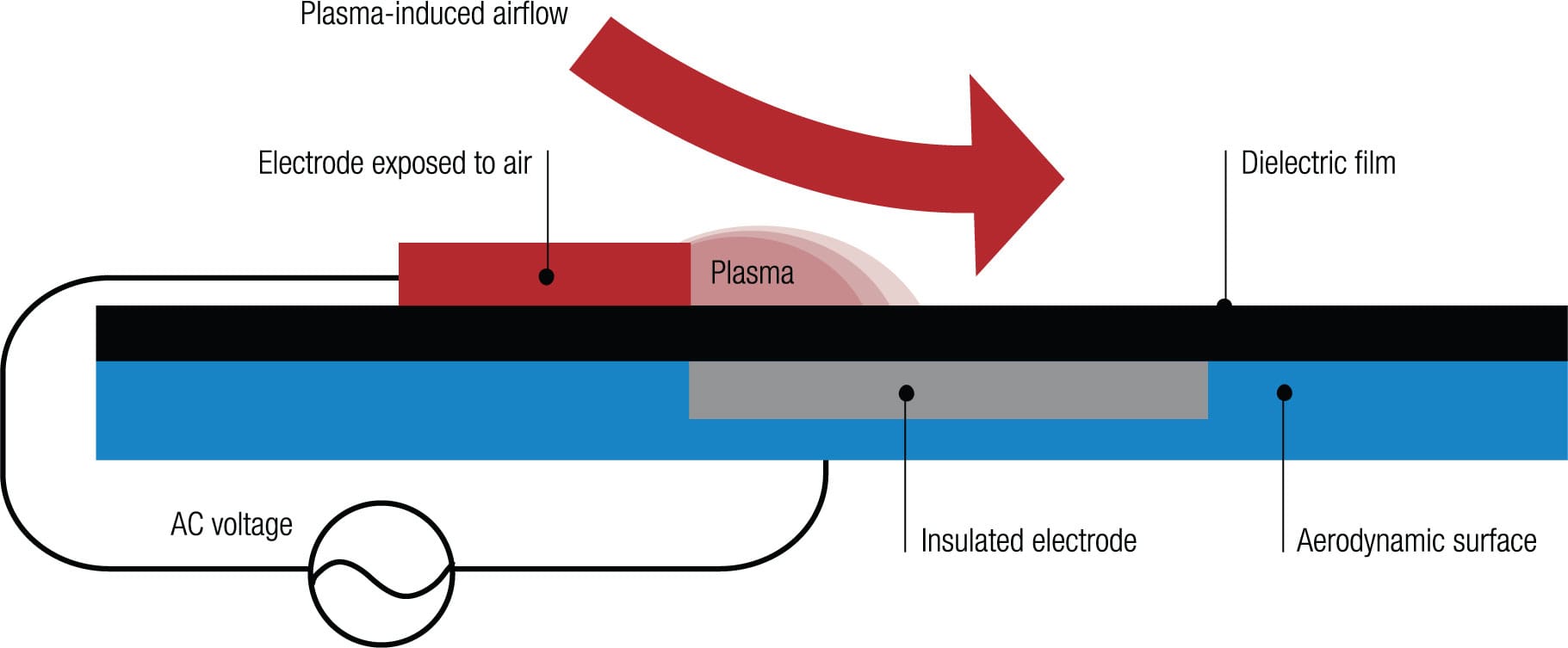
Making plasma Plasma can be made using a DBD (dielectric barrier discharge) plasma actuator. When an AC voltage of sufficiently large amplitude is applied across two electrodes, one exposed to the surrounding air and the other encapsulated in a dielectric material, the air ionizes in the region of the strongest electric field. This typically occurs at the edge of the electrode that is exposed to the air. In the presence of the electric field gradient, the ionized air (plasma) exerts a force on the ambient air, which has the effect of altering the virtual aerodynamic shape of the surface around the actuator.