Terpenes
Revised by Ellen Holland, with additional content provided by Royal Queen Seeds
Terpenes are major components of cannabis resin, just as they make up the largest percentage of aromatic essential oils contained in most plants. The scent of most flowers, herbs, and spices are composed of these oils. When therapists use plant oils in aromatherapy, or when they are used in natural incense, perfume, or other scents to set the mood, the aromas they create are from various combinations of terpenes. They have the power to energize, relax, focus, and ease anxiety.
The presence of terpenes is apparent when buds are pinched or rubbed between the fingers to volatilize (evaporate) them and release their scent. The signature odors of cultivars like Grapefruit, Silver Haze, Blueberry, and Real Skunk brings a recognition of the type of mental and physical effects that the sample is known to produce.
Plants produce terpenes for one of three reasons:
- to attract pollinators
- to repel or kill herbivores
- to attract predators of herbivores
These odor molecules, which are costly for the plant to produce, increase as the plant’s investment in reproduction increases. Before flowering, the odors are faint. As flowering progresses and the plant is more invested in protecting itself, the odor grows. As the bud ripens, whether seeded or sinsemilla (unpollinated), the odor increases substantially. Cannabis is wind-pollinated, so it doesn’t need to attract pollinators, and outdoors it is resistant to insect predation, as the odors are a signal to experienced mammals to stay away. This indicates that the odors deter animals that would eat the plant, including larger grazers. In other plants terpenes and essential oils are insect repellents, such as those derived from citronella, clove, and wintergreen, which makes them the base for many natural pest removal garden sprays.
By temporarily altering brain function, terpenes affect mood, sensitivity, and perceptions, including balance and pain.
Chemically speaking, terpenes are composed of repeating units of isoprene, which is a 5-carbon unit chain or ring with eight hydrogen atoms attached (C5H8). Terpenes use the simple isoprene units as blocks to build molecules with 10, 15, 20, 30, and 40 carbon units; they also twist and turn the molecular structure to form simple chains or three-dimensional (polycyclic) structures. Most significant for the cannabis plant, terpene pathways are key enzymatic steps in the plant’s production of THC. In addition, terpenes can form bonds with other molecules, which affects how animals and plants react to them. Depending on how terpenes stack against each other, they create different aromas.
Most of the aromas associated with plants are the result of terpenes and flavonoids, a group of natural substances found in many other plants including fruits, vegetables, tea, and wine that add to the color and flavor of the plant. Both flavonoids and terpenes are hydrocarbons, with either long carbon chains or carbon rings. Flavonoids are similar to terpenes and also play a role in the flavor and medicinal properties of cultivars by contributing anti-oxidative and anti-inflammatory properties. The health effects associated with cannabis are due to the synergistic effects of its components working together, a phenomenon known as the entourage effect.
Humans can smell and taste these compounds, but that is not the only way they are affected by them. Aromatherapy uses the inhalation of essential oils to regulate mood, sleep patterns, acuity, and healing processes. For example, lavender oil (rich in linalool) is a soothing agent and smooth muscle relaxant; rosemary is used to focus attention and provide a sense of satisfaction. These effects are a result of the combination of terpenes and other chemicals found in the oils of these plants.
While terpenes affect the brain in their own way, they also modify the effect of THC within the brain, adding subtleties to the high. Some terpenes may affect the high because they lock into receptor sites in the brain and modify its chemical output. A few, such as thujone, one of the main terpenes in wormwood (which is used to make absinthe), bind weakly to the CB1 receptor. Others alter the permeability of cell membranes or the blood brain barrier, allowing in either more or less THC. Others affect serotonin and dopamine chemistry by shutting off their production, affecting their movement, binding to their receptor sites, or slowing their natural destruction. Dopamine and serotonin, two of the main regulators of mood and attitude, are affected by some terpenes, as well as THC.
By temporarily altering brain function, terpenes can affect mood, sensitivity, and perceptions, including balance and pain. When terpenes are mixed, as they are in natural plant oils, they each play a role in affecting brain function. Some combinations may work synergistically and others antagonistically, but each “recipe” of terpenes affects moods and feelings in its own way.
Over 100 terpenes have been identified in cannabis, but there likely are more that have yet to be identified, especially considering the multiple variations of each terpene. For instance, the characteristic citrus odor found in orange and lemon rinds differs by type and even variety. Their terpenes, called limonenes, are mirror versions of each other. This is due to slight differences in the amounts of limonene, as well as other compounds that contribute to the variation in citrus scent profiles.
Now companies are creating cannabis extracts that use terpenes derived from cannabis as well as other botanical sources such as lavender and clove. These lab-created products can then be reverse engineered to recreate the chemical makeup of cultivars. While using terpenes extracted from something like mint instead of cannabis or hemp is more cost effective for the producer, the potential negative health effects of vaporizing botanical terpenes at high temperatures are still unknown.
Creating the flavoring for cannabis vape pens also includes the use of terpenes that originated in a lab, which are widely used in cologne and perfume. The safety of vaporizing lab-originated terpenes remains undetermined; however, they are chemically identical to terpenes produced in cannabis or other terpene-producing plants. These lab-created terpenes typically have more intense smells and flavors, which makes their effects additionally complex, since odor impressions may change based on terpene concentration.
About 10-30% of cannabis smoke resin is composed of assorted terpenes. Some terpenes appear only occasionally in cannabis, while others are found all the time. The percentage of particular terpenes and the ratios in which they are found vary by plant variety and are driven by the plant’s genetics.
Hops and both groups of cannabis contain similar complements of terpenes. The oil of common black pepper (Piper nigrum) also has a group of terpenes similar to cannabis (β-caryophyllene being the primary terpene). Terpenes are produced in the trichomes, the same glands where THC is produced.
Age, maturation, and the time of day can affect the amount, and perhaps ratios, of terpenes. One reason is their high volatilization rates at temperatures as low as 75°F (24°C). As plants mature, their odor gets more intense and sometimes changes as they ripen. Plants are constantly producing terpenes, but they volatize when exposed to sunlight and warm temperature. That means plants have more terpenes at the end of the dark period than after a full day of light. This can be easily tested by a home grower by checking a plant’s odor early in the morning and at the end of a sunny day. There will be more pungency earlier in the morning.
Climate and weather also affect terpene and flavonoid production. The same variety of cannabis can produce different quantities and perhaps even different types of oils, depending on the type of soil in which it is grown in or the fertilizers used. The terpenes described below are those generally most abundant in cannabis, though individual plants may differ widely both in total percentages of terpenes and in their ratios.
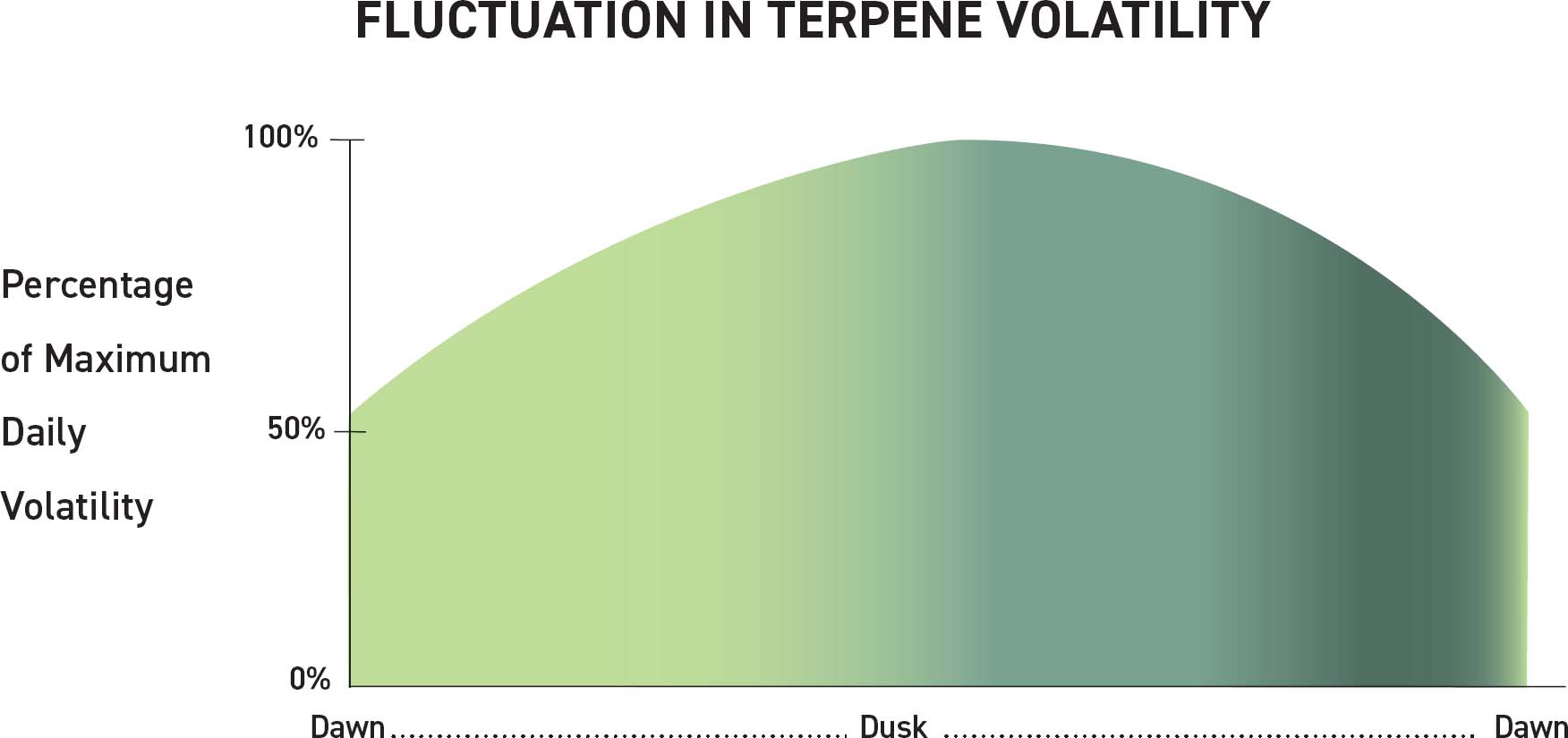
This chart represents fluctuation in terpene volatility over a 24-hour period. As the sun rises and/or temperatures increase, the volatility of terpenes will also increase. Terpene retention is prioritized during harvest, which is why harvests should occur at dawn, when the terpene volatility is at its lowest.

Hops

Mango
β-Myrcene is the most prevalent terpene found in most varieties of cannabis, but little is found in hemp. It is also present in high amounts in hops, lemongrass, West Indian bay tree (used to make bay rum), verbena, and the plant from which it derives its name, Myrcia. Myrcene is often referred to as one of the most “earthy” smelling terpenes, with musky notes somewhat similar to cloves. Additionally, it has a slight odor of red grape and balsamic and a hint of mixed spice. Myrcene also appears in small amounts in the essential oils of many other plants and can be found abundantly in mangoes. Its odor is variously described as clove-like, earthy, nutty, green-vegetative, and citrus. The various odors are the result of slight differences in the essential oil’s chemical makeup. All of these odors are commonly used to describe cannabis.
Myrcene is a potent analgesic, anti-inflammatory, and antibiotic. It blocks the actions of cytochrome, aflatoxin B, and other pro-mutagens that are implicated in carcinogenesis. It is also present in small amounts in many essential oils associated with antidepressant and uplifting effects.
Myrcene is a monoterpene that is an essential precursor to forming other secondary terpenes. The compound has been known to compose up to 50% of the total terpene content found in individual cultivars. Cultivars containing more than 0.5% myrcene are most likely to induce sedative effects classically attributed to indicas.

Lemon

Orange

Grapefruit
Limonene is found in citrus peels, mint, juniper, rosemary, pine, and cannabis. It is used in food, drugs, cosmetics, and biotechnology. Limonene is often the second, third, or fourth most prevalent terpene in cannabis resins. Citrus peel resins are a familiar source of limonene: the oil explodes into the air when a fruit is peeled. The exact odor is determined by the structure of the terpenes. Plants use limonene to repulse predators. For instance, flies have a group of receptors wired directly to the fly brain that are similar in function to the taste buds on human tongues. One of them detects noxious chemicals and responds to limonene as a toxin.
Limonene is one of the most commonly used terpenes. It appears as an ingredient in food, drugs, cosmetics, detergents, and cleansers, and it also finds applications in the biotechnology industry. After myrcene, limonene is the most abundant terpene in most cannabis cultivars.
Limonene has very low toxicity, and humans rarely encounter adverse effects from it. However, just like many terpenes and solvents, limonene may irritate skin and the respiratory system, causing symptoms such as watery eyes and vasodilation.
Limonene has antibacterial, antifungal, and anticancer effects. It inhibits the RAS cancer gene cascade, which promotes tumor growth. Since limonene is such a potent antifungal and anticancer agent, it is thought to protect against the Aspergillus fungi and carcinogens sometimes found in cannabis smoke streams.
Limonene synergistically promotes the absorption of other terpenes by penetrating cell membranes. In humans, limonene’s design facilitates a direct response by quickly permeating the blood-brain barrier. The result is increased systolic blood pressure. In tests, participants reported an increase in alertness and restlessness. Various limonene analogs can cue the brain to sexuality, buoyancy, or focused attention. Limonene sprays are used to treat depression. This terpene interacts positively with cannabinoids THCa, CBDa, CBCa, CBC, and CBG, and with other terpenes such as caryophyllene and linalool. Further, the increased cell permeability caused by limonene facilitates the assimilation of other substances by the human body.
Limonene assists in modulating the body’s immune system, resulting in an anticancer effect. It plays a role in healing damaged skin and regenerating cellular tissues. Limonene has potent anti-inflammatory properties, making this terpene a promising option in the treatment of certain forms of cancer.

Black pepper

Cloves

Cotton
β-Caryophyllene is a major terpene found abundantly in black pepper (15-25%), clove (10-20%), and cotton (15-25%). It is found in smaller percentages in several other herbs and spices. It has a sweet, woody, dry-clove odor and tastes pepper-spicy with camphor and astringent citrus backgrounds. It contributes to black pepper’s spiciness and is used industrially to enhance tobacco flavor.
β-Caryophyllene, ingested in large amounts, blocks calcium and potassium ion channels. As a result, it impedes the pressure exerted by heart muscles. Applied topically, it is an analgesic and one of the active constituents of clove oil, a preferred treatment for toothache. It docks on the CB2 receptor site, the same site for which CBD has an affinity. Thus, it may help reduce inflammation. It may also contribute to giddy, goofy, and good feelings induced by cannabis use.
Caryophyllene has also been found to play the role of a cannabinoid by targeting the CB2 receptor. Cannabinoids that target the CB2 receptor can potentially help treat disorders such as arthritis and multiple sclerosis, without the inconvenience of psychotropic effects. The CB2 receptor is involved in the regulation of emotional behavior and could be a potential therapeutic target when it comes to anxiety and depression. Caryophyllene may reduce inflammatory pain responses and also reduce spinal neuroinflammation.

Pine

Rosemary

Basil

Dill
α- and β-Pinene provide the familiar odor associated with pine trees and their resins. α-Pinene is responsible for the distinct scents in pine needles and the herb rosemary, whereas β-pinene is the molecule responsible for the unmistakable smells of basil and dill. Pinene is likely to give the true skunk varieties, ones that stink like the animal, much of their odor.
Pinenes are the primary component in turpentine and are found in noticeable amounts in many other plant essential oils including rosemary, sage, and eucalyptus.
Pinene is one of the most researched terpenes. It is effective at protecting the lungs against certain types of viral infection. Pinene is used medically as an expectorant and topical antiseptic. It crosses the blood-brain barrier easily, where it acts as an acetylcholinesterase inhibitor, that is, it inhibits activity of a chemical that destroys an information-transfer molecule, resulting in better memory. Largely due to the presence of pinene, rosemary and sage are both considered “memory plants.” Concoctions made from their leaves have been used for thousands of years in traditional medicine to retain and restore memory.
It is an anti-inflammatory as well as a bronchodilator, so each breath results in increased oxygen absorption. The invigorating experience of taking a deep breath while hiking through a pine forest may be a function of pinene’s bronchodilator function.
It increases focus, self-satisfaction, and energy, and is known to increase alertness and to ease the potential short-term memory loss associated with consuming high amounts of THC. It can also help to combat negative sensations, such as paranoia.

Sage

Wormwood

Thyme
Borneol smells much like the menthol aroma of camphor and is easily converted into it. It is found in small quantities in many essential oils. Commercially, it is derived from Artemisia plants such as wormwood and some species of cinnamon.
Borneol is one of several terpenes that insects and parasites hate. Humans, on the other hand, are usually quite attracted to these unique scents. The camphor-like overtones of many of the Haze cultivars are unmistakable. The high of these varieties has a calming effect, in addition to its psychedelic aspects, which is indicative of the relatively high concentrations of borneol present in these varieties. It is considered a “calming sedative” in Chinese medicine. Borneol is used to relieve certain kinds of pain, facilitate digestion, improve blood circulation, and even to help treat respiratory diseases. Its refreshing action is used for fever reduction and other cooling purposes. In Asia, this substance is also used as a preventative treatment for cardiovascular diseases.
Borneol naturally occurs in ginger, rosemary, sage, camphor, marjoram, thyme, mugwort, and other plants. Its oxidation generates the substance commonly known as camphor, which can itself be extracted from plants like camphor laurel and used as a source of borneol.
Borneol has anti-inflammatory, antioxidant, anticoagulant, and anesthetic properties. Research is beginning to reveal neuroprotective and antioxidant properties as well (Hur, Pak, Koo, and Jeon 2012).
Small amounts of borneol naturally contained in cannabis and other herbs are completely safe, while pure borneol can irritate the eyes, skin, and respiratory tract. Pure borneol is also harmful if swallowed.
Borneol increases the bioavailability of other active compounds and improves the transportation of substances toward brain cells.
Δ3-Carene has a sweet and hearty smell with notes of lemon, musk, and pine. It can be found in basil, pepper, cedar, pine, rosemary, and turpentine. This bicyclic monoterpene can be effectively extracted from several plants, and it’s a component of many essential oils used in aromatherapy. It’s also used in cosmetics, as a food flavoring, and as an insect repellent.
This terpene has some valuable properties still under investigation. Among its potential benefits for the body and brain are anti-inflammatory and antifungal effects. One of the most interesting therapeutic characteristics of carene is its role in supporting bone healing. Together with its anti-inflammatory action, carene speeds up bone repair and growth, especially in cases of malnutrition or injury.
Like other terpenes, carene is nontoxic, but it may cause irritation when inhaled. The cannabis flower often develops carene among its protective chemical compounds, though it is not one of the most abundant terpenes. Carene may contribute to the dry eye and dry mouth experienced by cannabis users. In aromatherapy, cypress oil, which is high in Δ3-carene, is used to dry excess fluids, tears, runny noses, excess menstrual flow, and perspiration.
Juniper essential oil is mainly composed of pinene and carene. Both are known to have powerful antifungal abilities and have been tested as treatments for candida, aspergillus, and dermatophytes fungi causing infections of the skin, hair, and nails.

Lily of the Valley

Lavender
Linalool has a floral scent reminiscent of spring flowers such as lily of the valley, but with spicy overtones. It is refined from lavender, neroli, and other plants. Humans can detect its odor in the air at rates as low as one part per billion.
Linalool acts on the human body in a number of beneficial ways. It has been used as a natural soporific, anti-inflammatory, anxiolytic, and topical skin application for millennia.
Insomniacs of the Renaissance and continuing to the present keep parcels of lavender under their pillows. It is still used to treat scars today.
Linalool is being tested for treating several types of cancers. It is a powerful anti-inflammatory and analgesic. It is also a component of several sedating essential oils, which makes sense, since it is shown to be a smooth muscle relaxer. In tests on humans who inhaled it, it caused severe sedation. In tests on rats, it reduced their activity by almost 75%.

Catnip

Peppermint
Pulegone has a minty-camphor odor and flavor that is used in the candy industry. It is found in catnip, peppermint, and pennyroyal, among other plants. It is implicated in liver damage when used in very high dosages. It is found in tiny quantities in cannabis. Pulegone is an acetylcholinesterase inhibitor, that is, pulegone interferes with the action of the protein that destroys acetylcholine, the chemical the brain uses to store memory. Pulegone may counteract THC’s effect of lowering acetylcholine levels, which may result in forgetting less if THC is accompanied with pulegone.

Ginger

Ginseng
Humulene is also found in hops, basil, coriander, cloves, ginseng, and ginger. It has a woodsy, earthy flavor with spicy undertones. Humulene gets its name from the genus name for hops, which is Humulus. Hops is also found in a recognizable plant family: Cannabaceae. A very hoppy beer such as a Double IPA sometimes smells of cannabis because of humulene and other such terpenes.
Humulene is always part of the cannabis terpene profile. Therefore, this prolific oil plays a part in all the modified genotypes of different cultivars.
While this terpene may be known best for the aroma and flavors it provides to beer through hops, it’s used for its anti-inflammatory properties and analgesic effects that block pain and relieve fear and stress.
Humulene is an isomer of β-caryophyllene, meaning that they have the same chemical formulation, but their bonds are arranged differently. In this way, both terpenes are known for the potential to suppress cancer tumors.
Humulene is an antibacterial agent and has anticancer and anti-inflammatory properties. It is frequently invoked as an appetite suppressant. This may lead to its more widespread use in the future.
Humulene is released when hops are steeped and can be used as an effective sedative. In the same vein, pepper and ginseng both contain humulene and are used as natural antibiotics.
Tea tree

Marjoram

Cardamom
Terpinolene is characterized as having herbaceous piney scents and tastes combined with hints of citrus and floral notes. It’s found in other plants and herbs such as cardamom, conifers, cumin, lilac, marjoram, nutmeg, rosemary, sage, and tea tree. Also known as Δ-terpinene, terpinolene’s woody aroma is often accompanied by citrusy and floral tones, which vary by isomer. Terpinolene’s strong scent is used in fragrances for household products to provide pine and lemon fragrances in cleaners.
Termites excrete terpinolene as an alarm pheromone to signal a threat to the colony. Terpinolene is antibacterial and antifungal and has a sedative effect, so it can be used to reduce insomnia. Terpinolene is often found in sativa-dominant cultivars and is one of the primary components of the terpene profiles found in Jack Herer cultivars.
Terpineol has a pleasant odor that varies between apple blossom, citrus, lilac, and lime and is used in perfumes and soaps for fragrance.
Terpineol is a minor constituent of many plant essential oils and is derived commercially by processing other terpenes. It may account for the couch-lock effects of some cannabis, although terpineol’s odor is not usually associated with body highs. That may be explained by the fact that terpineol is often found in cannabis with high pinene levels, as mentioned above. Its odor is masked by the pungent woodsy aromas of pinene. It is one of the terpenes that induces sleepiness but goes undetected, hidden by stronger odors.

Jasmine

Lemongrass
Nerolidol is a sesquiterpene, which means it is less volatile and more aromatic than monoterpenes and other evaporative substances. Several plants contain nerolidol in their essential oils, including citrus peel, ginger, jasmine, lavender, lemongrass, tea tree, and many other plants. It has a floral woodsy odor with a hint of citrus. Plants produce it in response to insect attacks.
Nerolidol is used in many industrial products including candies, cleansers, cosmetics, and perfumes. It has antifungal, antibacterial, anxiolytic, and antioxidant properties. It is effective against parasites, spider mites, head lice, and other pests. It is also known for potent antimalarial properties.
Humans can safely ingest and inhale nerolidol. It has been traditionally used for its relaxing, sedative, and sleep-assisting effect. It’s also commonly used as a food-flavoring agent. Cultivars containing substantial amounts of it are antidepressant, ease social interactions, and seem to promote positive attitudes.
Nerolidol is nontoxic and enhances skin penetration of cannabis topicals. Formulators of balms, creams, and lotions might consider it for improving absorption of topicals when choosing ingredients.

Chamomile
α-Bisabolol is produced in moderate amounts only in chamomile flowers and a few other plants, such as the Candeia tree (Vanillosmopsis erythropappd), Myoporum crassifolium, and some cannabis cultivars.
It is a sedative and an antidepressant that works well for stress reduction.
Bisabolol is a monocyclic sesquiterpene alcohol. It has a warm floral aroma similar to honey, apples, and chamomile. Bisabolol has anti-inflammatory, anti-irritant, antioxidant, antimicrobial, antifungal, and analgesic properties. As with some other terpenes, it penetrates the skin easily and enhances the absorption of other molecules.
Bisabolol stimulates gastrointestinal tract receptors, so it has a calming effect on the stomach. It bonds with specific chemical messengers, including inflammatory proteins, reducing the production of inflammatory cytokines. Bisabolol has also shown to be effective against leishmaniasis, an infection caused by parasites (Corpas-López, Morillas-Márquez, et. al. 2015).

Parsley
Ocimene is a tropical terpene that has a sweet, fruity, woody odor that evokes flavors of citrus, guavas, mangos, papayas, and pine. It’s found in fragrant herbs such as mint, parsley, and basil. It plays a key role in the social regulation of honeybee colonies, leading researchers to look into the idea of drug-sniffing bees.
Ocimene is believed to have a variety of medicinal benefits. It has anti-inflammatory and antifungal properties.

Eucalyptus

Mugwort
Eucalyptol/Cineole is the main ingredient in eucalyptus oil. It has a camphor-minty odor similar to pulegone. It is also found in other fragrant plants and in minor amounts in cannabis. It’s responsible for the pleasurable and cooling scents found in eucalyptus, mint, rosemary, tea tree, mugwort, bay leaves, sweet basil, sage, and some varieties of cannabis.
Eucalyptus oil is considered centering, balancing, and stimulating. It’s probably the stimulating and thought-provoking part of the cannabis smoke stream. It’s used to increase circulation and pain relief, among other topical uses. Eucalyptol/cineole easily crosses the blood-brain barrier and triggers a fast olfactory reaction.
Eucalyptol/cineole is found in many mouthwashes and cough syrups. It helps to control the secretion of mucus, making it a good terpene to encourage optimal breathing. Like the name implies, it’s also found in eucalyptus and contains minty, cool tones. This terpene is also known as cineol and is showing promise in treating memory loss associated with Alzheimer’s disease.
Eucalyptol/Cineole has been studied intensely and has been shown to have numerous potential applications within the domain of medicine. Eucalyptol/cineole may contribute to improved cognition and have a positive effect on Alzheimer’s due to its potential to enhance memory and learning. Cineole may also alleviate another element of Alzheimer’s. It has been reported to decrease the inflammation caused by amyloid beta plaques. Additionally, it is antifungal, antibacterial, antioxidant, and has anticancer properties (Murata, Shiragami, Kosugi et. al. 2013).
Terpene Interaction
The way terpenes interact with one another and their resulting effect on brain activity provide fascinating territory for another level of exploration and creativity for seed breeders. By learning the odors of the terpenes, breeders can predict the mind-altering properties that each lends to a bud.