
CARBON DIOXIDE (CO2)
Carbon dioxide (CO2) and water are the two raw materials required for plant photosynthesis. CO2 constitutes roughly around 0.042% or 420 parts per million (ppm), and rising, of the Earth’s atmosphere at the time of writing.
Cannabis uses CO2 only in the presence of light. CO2 supplementation during the dark period has no benefit to the plant. Photosynthesis and CO2 absorption occur immediately after the plant receives light, and the plant’s stomatal pores open.
The plant mines CO2 from the air by opening its stomata, tiny organs found primarily on the underside of the leaf. The stomata are responsible for the gas exchange of oxygen (O2) and CO2 into and out of the plant, and for transpiration, or regulating how much water evaporates from the leaf through the pores. While human skin pores sweat to regulate temperature and moisture, plants use their stomata to regulate how much water evaporates from the leaf to cool off the plant.
Once CO2 is absorbed into the plant, it is directed to the chloroplasts—the plant organelles that contain light-absorbing pigments—where photosynthesis takes place.
Photosynthesis consists of a complex series of reactions in which light energy is used to convert CO2 and water to sugar, releasing O2 as a by-product.
The amount of CO2 in the air has a profound effect on the rate of photosynthesis and plant growth. As long as there is enough light to power the process, photosynthesis speeds up as the amount of CO2 in the air increases. Conversely, as the CO2 content of the air falls, photosynthesis slows to a crawl and virtually stops at a concentration of around 200 ppm, no matter the other conditions.
Plants that lack CO2 continue respiration and growth for a short time, until their sugars are used up, before slowing down their metabolism to conserve energy. This occurs on evenings following brightly lit days. The plant creates excess sugar, some of which is used to continue plant growth and maintain cellular function during the dark period. Only when light and more CO2 is available can the plant processes continue.
Outdoors, breezes and the exchange of gasses in the air constantly replace CO2 that plants consume. This provides enough atmospheric CO2 for vigorous growth. Until recently outdoor growers rarely thought of the gas as a limiting factor. Now, some farmers are supplementing outdoor gardens and fields.
In fact, the 420 ppm of CO2 found in the Earth’s atmosphere is on the low end of the continuum of C3 plants’ ability to use it as an ingredient for photosynthesis. Outdoor plants growing in the bright light of summer grow heavier and faster when supplemented with CO2.
Raising the level of CO2 up to 0.15% (1500 ppm), or a little less than four times the amount usually found in the atmosphere, increases plant growth rate significantly.
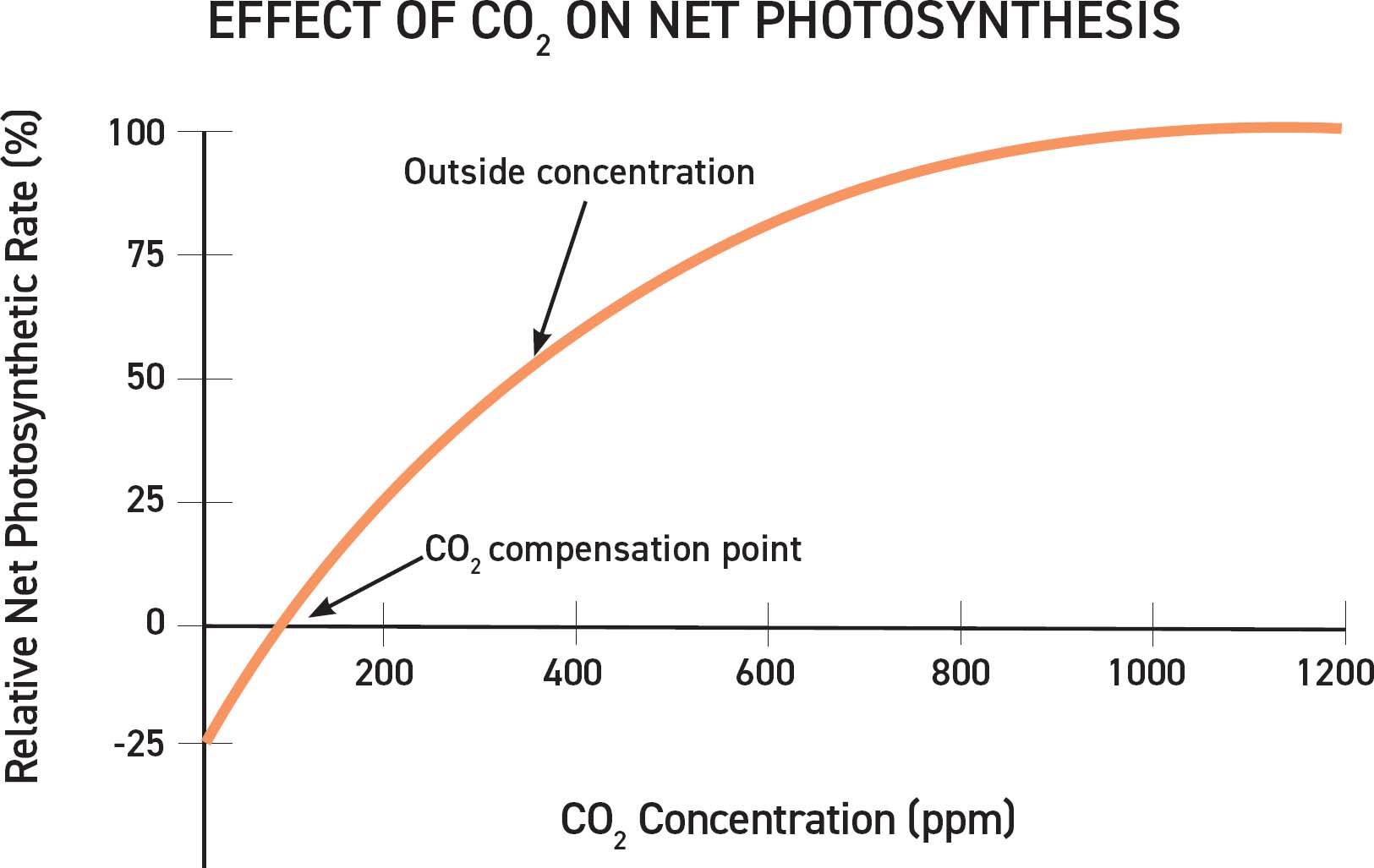
The orange line on this chart represents relative net photosynthetic rate at different CO2 concentrations. The rate of photosynthesis continues to increase quickly and then flattens out as the continued addition of CO2 no longer improves the photosynthetic rate.
Enhancing growth outdoors using increased CO2 is discussed at the end of the chapter. When plants are growing in an enclosed area, there is a limited amount of CO2 for them to use. Under bright lights, CO2 is used up quickly. Enclosed gardens with no ventilation are also rapidly depleted to the point where the photosynthetic rate slows to a virtual stop at 200 ppm. Only when more CO2 is added to the air does photosynthesis resume.
A closed closet or other small gardening space can be recharged with CO2 simply by opening the door or curtain to let in fresh air. This increases the CO2 content of the closet passively, as air naturally equalizes the concentrations of O2 and CO2 inside and outside the growing space, exchanging the higher O2 levels from the plants with CO2 from outside. Adding a small fan expedites the air exchange.
The rate of photosynthesis has the greatest increase as the CO2 level climbs from 0-200 ppm. Under low-light conditions the rate of photosynthesis continues to increase as CO2 rises to atmospheric levels. Increasing the CO2 concentration beyond that without increasing light intensity does not result in a higher rate of photosynthesis. The plant cannot take advantage of higher CO2 levels until the light intensity increases.
At high light intensity the photosynthetic rate increases as CO2 concentrations increase to atmospheric levels. The rate of increase declines a bit after that, but the photosynthetic rate continues to increase as CO2 levels reach 800 ppm. Increasing the light intensity encourages the plants to absorb even more CO2, which increases growth and yield. Above 800 ppm of CO2, photosynthesis continues to climb but at a slower rate, until it levels off at about 1,500 ppm.
CO2 Enrichment
Augmenting CO2 in the garden increases photosynthesis, resulting in faster growth and bigger yields. Optimal levels range between 800 and 1,500 ppm. These concentrations are much higher than atmospheric carbon dioxide levels and can be achieved only through supplementation. Plants do not absorb CO2 during the dark period, so CO2 enrichment is only implemented when the lights are on. Achieving optimized results also requires increased lighting and temperature.
There are various ways to augment carbon dioxide in closed environment grow rooms. The most convenient way to do this is with a sensor, regulator, and tank kit. Other methods include using a sensor-regulated CO2 generator that burns propane or natural gas. Metabolic and chemical processes such as fermentation also produce CO2 and can be used to supplement a garden.
The use of CO2 involves serious life-safety issues; CO2 is regulated by OSHA as an asphyxiant. Any system used to apply it will need to include appropriate monitoring and alarm protocols, which are generally enforced by local authorities.
For both human and plant safety, food-grade bulk CO2 delivered in liquefied form is the safest source, is certified as an organic practice by the USDA, and will not lead to any product contamination during testing. The use of propane burners to generate CO2 gas is banned by some state and local fire codes. Note that incomplete combustion can produce deadly carbon monoxide, as well as ethylene, which is highly toxic to plants and can result in crop loss.
CO2 Tanks
The easiest way to supply the gas is to use a CO2 tank kit. The kit consists of a CO2 meter, pressure regulator, flow meter, and a solenoid valve. For most gardeners, 20 or 50 pound (9-22 kg) tanks (the weight of the gas) are the most convenient. Tanks can be bought or rented. Steel tanks weigh twice as much as aluminum tanks, so a steel tank that holds 20 pounds (9 kg) of CO2 has a gross weight of 50 pounds (22 kg), and an aluminum tank weighs about 40 pounds (18 kg) filled. The 50 pound (22 kg) tanks weigh, respectively, 170 and 110 pounds (77-50 kg) when filled.
Although CO2 tanks are a bit cumbersome to lug around and are more expensive than burning propane or natural gas, they are still the best solution for most growers. They don’t run the risk of degrading the garden environment, as compared with gas burners. Tanks release nothing but cool CO2, while CO2 generators release heat and water vapor, neither of which is helpful in most gardens.
Using burners to generate CO2 runs the risk of producing a small amount of ethylene gas. Ethylene (C2H4) is also a plant hormone that is bioactive at concentrations as low as one part per billion. Ethylene can have many effects on the plant depending on the stage of growth the plant is in. It causes faster fruit ripening.
CO2 tank systems use a regulator and emitter to control the amount of gas being released and, consequently, the concentration of CO2 in the garden. These are the parts for a complete CO2 supplementation system:
- A regulator that standardizes the pressure.
- An adjustable CO2 flow meter that controls the amount of gas released over a given time period.
- A solenoid valve that shuts the gas flow on or off.
These systems are usually sold as a single unit. Most systems include a CO2 sensor that constantly measures the ppm of CO2in the air and turns the flow on or off to maintain the ppm the gardner sets. These systems keep an accurate gauge of the CO2 in the air, eliminating guesswork and unwanted fluctuations.
CO2 System Setup
In most tank systems CO2 should be released just above the plants or circulation fans. The gas is heavier and cooler than the air, so it sinks. As it flows down, it reaches the top of the canopy first. This is where most of the light touches the leaves and where most of the plants’ CO2-consuming photosynthesis takes place. A good way to disperse the gas is by using inexpensive “soaker hoses” sold in plant nurseries and gardening stores. These soaker hoses have tiny holes along their length that disperse the gas.
Some systems circulate air through the plant canopy, drawing it up toward the ceiling. In this kind of system, the CO2 should flow from the tube just below the canopy, so it is pulled up to the top. Another method of enriching a space with CO2 is to add it to the air intake, so all new air is enriched. This is especially useful when a space has constant or frequent ventilation. Whichever setup is chosen, tanks should be placed in a space where they can easily be moved so that they can be refilled.
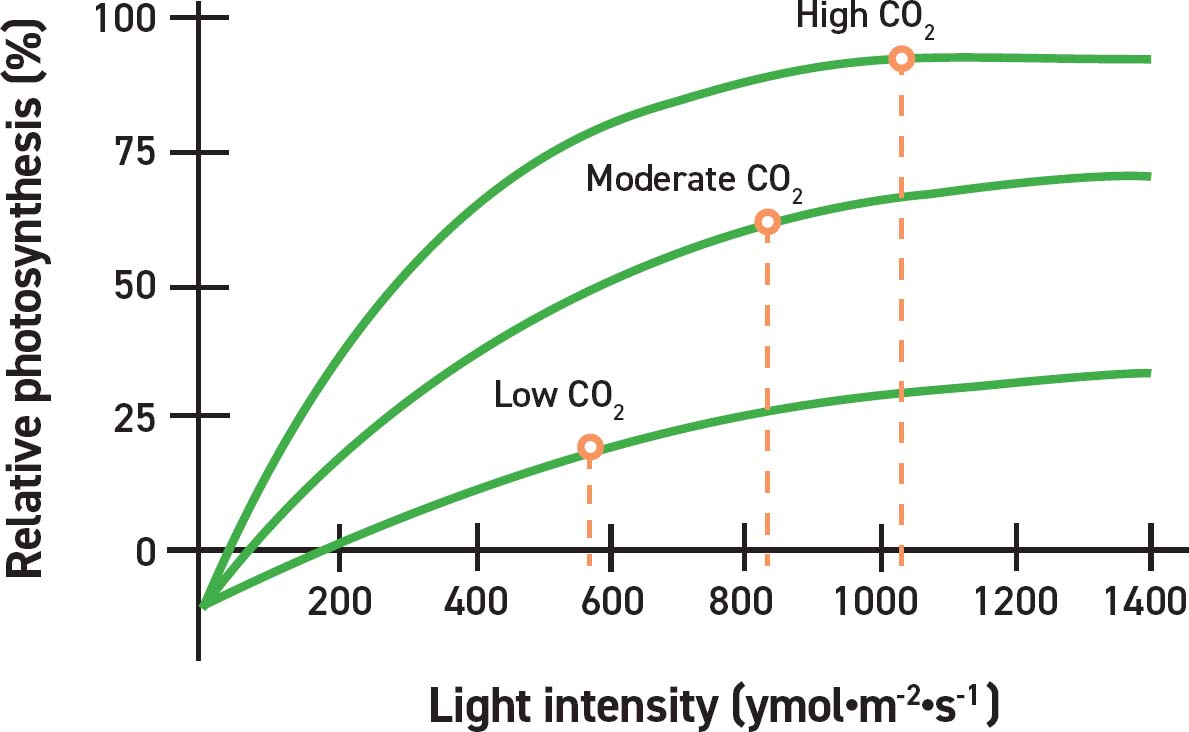
The three lines represent relative photosynthetic rates at various light intensities. The three curves represent the rates of photosynthesis when the ambient CO2 concentrations are low, medium, and high. The increase of relative photosynthetic rate is largest when the CO2 is highest and lowest when the CO2 concentration becomes a limiting factor. SOURCE: Erik Runkle, Michigan State University
Use of CO2 in Commercial Gardens
In many jurisdictions the application of CO2 to gardens is regulated for safety reasons. In some areas the use of portable 20 and 50 pound (9 and 23 kg) tanks are regulated, and safety precautions are in effect. They might include handling procedures as well as CO2 detectors, which alarm when the levels exceed 2,500 ppm, which is considered a precautionary health hazard. Sometimes small tanks must be held in a safety zone outside the structure. The gas is transported to the growing area via tubing.
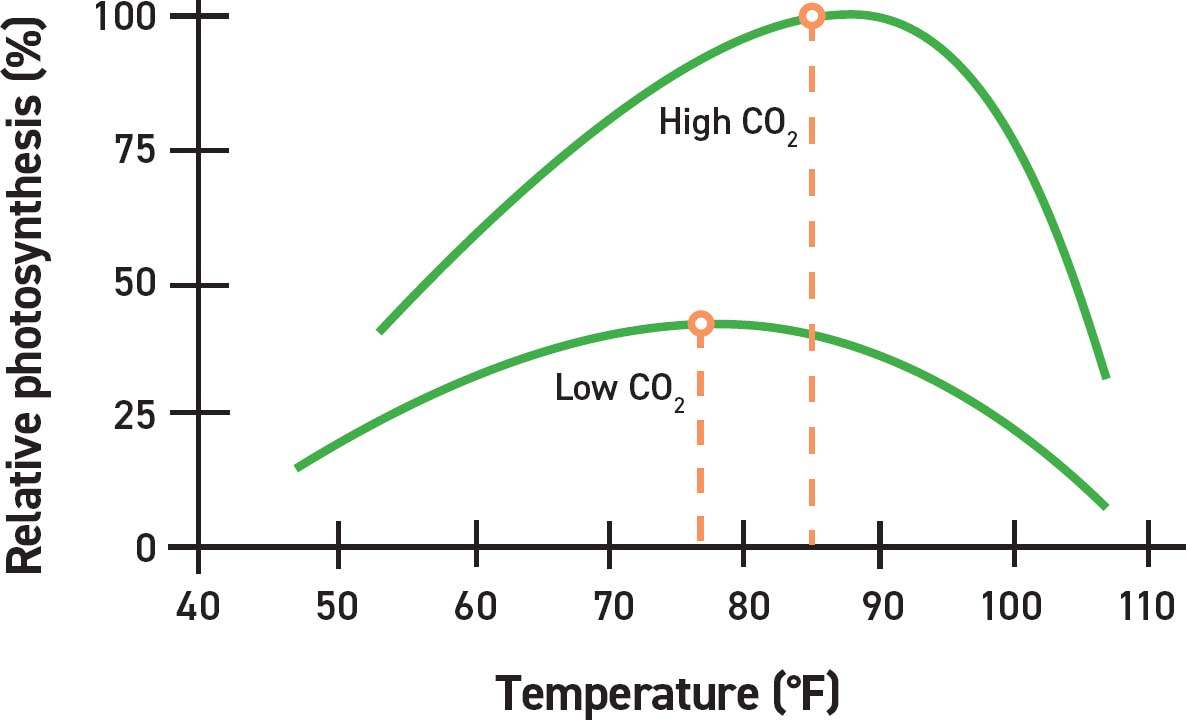
The lines represent the relative photosynthetic rate at high and low CO2 concentrations across various temperatures. The optimal (highest) rates are represented by the peaks of the curves. At low/ambient CO2 concentrations the optimal rate is closer to 78°F (26°C). At high CO2 concentrations the optimal rate is closer to 85°F (30°C). SOURCE: Erik Runkle, Michigan State University
Commercial cultivators often use micro-bulk CO2 tanks with capacities of thousands of liters of compressed, liquid CO2. These tanks are mounted on a pad on the exterior of the facility. The liquid carbon dioxide is converted to a gas and is either piped directly to sensor-controlled valves in each grow space or supplied via specialized HVAC systems that can simultaneously provide air conditioning, dehumidification, and supplemental carbon dioxide to each cultivation zone. These large-capacity tanks can last for many weeks, depending on the size and needs of the facility. Tanks can also be outfitted with a communications module that alerts the gas supplier when it is running low, so they can schedule on-demand, CO2 deliveries to the site.
CO2 Generators
Generators are much less expensive to operate than bottled CO2 injection systems. They create carbon dioxide inexpensively by burning either natural gas or propane. They are safe to be around and burn cleanly and completely without leaving toxic residues or creating carbon monoxide, a colorless, odorless, poisonous gas.
Generators emit CO2, water, and heat. Each pound of natural gas or propane burned produces about three pounds (1.36 kg) of CO2, one pound (0.45 kg) of water, and about 21,800 British thermal units (Btu’s) of heat. Other gasses and fuels produce different amounts of energy per unit burned.
If the growing area is cool or cold, the heat from a CO2 generator can be useful in keeping the space warm, as well as supplying CO2 and humidity to the garden. In a warm space, the generator’s heat must be dissipated to maintain the moderate temperature levels necessary for optimal growth. Some CO2 generators use water-cooling to absorb the heat. The heated water is cooled outside the garden area, eliminating the temperature problem. However, the CO2-enriched air still contains the moisture that was created.
Nursery supply houses sell large CO2 generators especially designed for greenhouses. Indoor garden centers typically sell smaller generators more appropriate for indoor gardens. Even a small generator unit can raise CO2 levels very quickly. Commercial use of CO2 generators in some areas has been banned, so check local and state regulations.
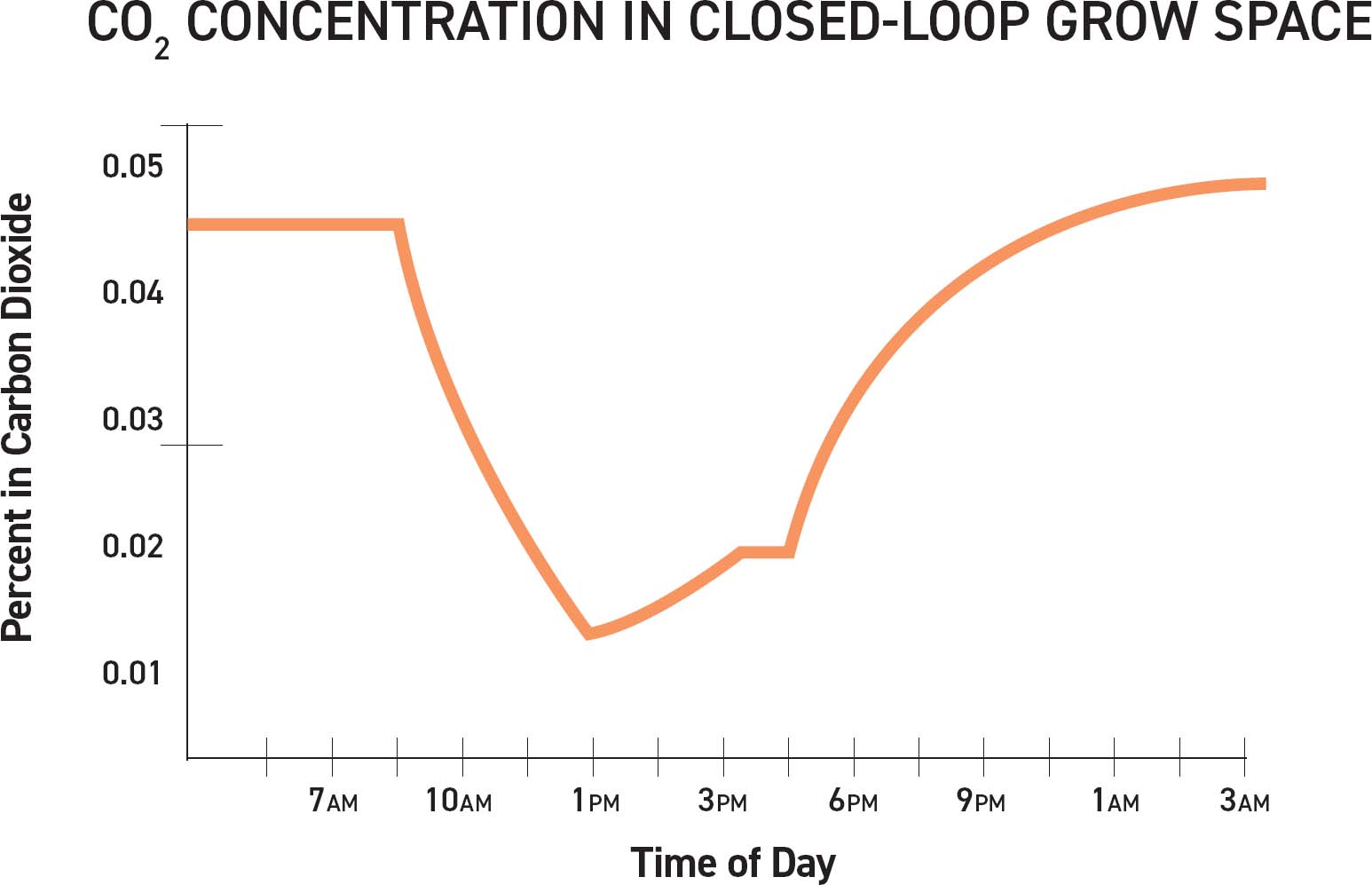
The orange line represents average CO2 concentrations throughout a 24-hour period in a closed-loop space with minimal ventilation. As lights turn on and photosynthesis starts, the CO2 concentration dips as the plants absorb CO2. Many plants close their stomata between 150 and 200 PPM CO2. The curve bottoms out when the plants stop photosynthesizing. As photosynthesis slows, the CO2 concentration increases. At night the plants are respiring and releasing CO2, which is illustrated by the higher than ambient concentrations of 420 PPM CO2 at night.
Alternative Methods to Enrich Air with CO2
Other ways to bring CO2 into the garden space include chemical reactions and biological processes such as composting, fermentation, and animal respiration.
Vinegar & Baking Soda
When vinegar and baking soda are combined, they make a salt and release CO2.
In small gardens, a simple method for creating a controlled release of CO2 is to drip vinegar into a solution made of baking soda and water. As the vinegar combines with the solution, CO2 is released. Regulating the frequency of the drip controls the amount of CO2 generated. This is not cost effective because baking soda and vinegar are expensive to purchase in the quantities needed to create significant amounts of CO2.
To generate one cubic foot of CO2, combine three quarts of 5% vinegar with 3.7 ounces of baking soda. Generating one cubic meter of CO2 requires 106 liters of 5% vinegar and 3.7 kg of baking soda.
Fermentation
Fermentation is the process used to brew beer and make wine. All that is required to ferment alcohol and make CO2 is a jug or container, malt or another source of sugar, and yeast. Yeast are single-cell microorganisms that have been used in baking and fermenting alcoholic beverages for thousands of years. In fermentation, yeast digest sugar and release alcohol and CO2. A gross simplification of this process is:
(Sugar => Alcohol + Carbon Dioxide (CO2)
C6H12O6 => 2(CH3CH2OH) + 2(CO2)

A single TNB Naturals CO2 Enhancer can supplement 1,200 ppm of CO2 in a 144 square-foot (13 m2) space for up to two weeks. The Enhancer is made from 100% organic ingredients and is activated by adding four cups (1 liter) of lukewarm water and shaking. Shake daily for optimal performance. Canisters should be placed or hung above the canopy, as the CO2 will sink to the floor.
As yeast feast on the sugars, they release about half the weight of the sugars’ CO2, so one pound (~0.5 kg) of sugar yields about half a pound (0.25 kg), or 4.35 cubic feet (0.12 m3), of gas. The yeast complete their meal in about four days. Then the solution must be replaced.
Beer-Making Kit
Yeast have a hard time processing sugar as the alcohol content climbs. Most beer yeast slow down as the liquid approaches about 5-8% alcohol. The maximum amount of sugar that yeast can process well is about one-tenth of the weight of the water. A gallon of water weighs 8 pounds (3.6 kg), so about 13 ounces of sugar is used. A liter of water weighs 1,000 grams, so use 80 grams of sugar.
Human Respiration
Each breath taken by humans releases CO2. A 150-pound (68 kg) human produces an average of about 1 kilogram (2.2 lbs) CO2 per day. In an hour a human releases 0.326 cubic feet of the gas (0.0092 m3).
Outdoor CO2 Supplementation
Although carbon dioxide supplementation is a common practice when growing indoors, it is not as well known that outdoor supplementation is possible as well. Plants grown outdoors can benefit from CO2 supplementation. They will grow sturdier and produce higher yields. Plants grown in tunnels or in greenhouses also benefit from CO2 supplementation because they rapidly deplete the air of CO2 as they photosynthesize, so air enrichment is very effective at increasing their growth.
Because CO2 is expensive, it takes an automated prescriptive delivery system for it to be economical in ventilated environments. This requires “in-grow” sensors that measure light, temperature, humidity, and CO2 levels, so that the gas is only applied when it is needed. It’s also important to target the application of the CO2 directly into the foliar canopy, which is where plants take it in through stomatal pores in their leaves.
If done right, CO2 enrichment can generate yield improvements of 50% or more. This is because it does the following:
- Helps create larger root structures, healthier stems, and more leaves during the vegetative state
- Improves a plant’s heat-tolerance by up to 10°F (~5°C), allowing photosynthesis to continue at high summer temperatures when plants would normally “shut down”
- Helps reduce plant transpiration and therefore foliar canopy humidity (think mold)
- Supplies added carbon during the flowering stage, leading to bigger and denser buds
Large Outdoor Spaces
CO2 release is most efficient in large fields, where it is less likely to leave the cultivation site, even if it is moved by a breeze. Because CO2 is heavier than air, it tends to move more horizontally rather than vertically. If it’s released in a field, it will spread to other plants rather than be lost to the air.
Releasing CO2 to rows of plants in a field during calm, sunny weather can increase yields by 50% or more. Coordinating CO2 release to fields from CO2-generating facilities will remove it from the atmosphere and may result in carbon credits.

ExHale by Garden City Fungi is a natural source of CO2 that creates a steady sustainable supply that can meet the enrichment needs of enclosed gardens of all sizes, from small personal to large-scale commercial gardens. Inside each ExHale cultivator bag is a non-fruiting mycelial mass that is growing on organic matter. This mycelial mass cultivates CO2, and the micro-porous breather patch releases it continually into the garden. No manure is used, so it is odorless.
Outdoor CO2 Tanks
CO2 from tanks can be applied to plants growing outdoors, in small and large gardens as well as in fields. To enrich the air around a plant outdoors, run a CO2 line from a tank up the stem of the plant so that the gas disperses around the canopy. CO2 is heavier than air and is cool coming out of the tank, so it will sink after it is released. It is best to set up the CO2 line at the level of the canopy to be as close to where the leaves absorb CO2.
There are several conditions that should be taken into consideration, particularly light, temperature, and wind velocity. CO2 is most effective in warm conditions. It will help when the canopy leaf temperature is above 70°F (21°C) and becomes more effective as it climbs into the 80s. Ideal conditions for this method are sunny or very bright with low wind speed, especially in small gardens.
There are several commercial systems that regulate release based on three factors: light, temperature, and the amount of CO2 in the space’s atmosphere. Sensors and switches can be installed that are sensitive to weather conditions, so CO2 is turned on only when there is bright light, little wind, and warm temperatures. Very often there is a period daily during summer and fall when the sun is shining and there is little wind. This most often occurs between 11 a.m. and 4 p.m., when the sun’s rays are most intense.
These systems utilize appropriately sized CO2 tanks that are refilled by gas companies in much the same way as propane is delivered.
In small gardens it works best when the emitters are placed around the top of the plant in a Christmas tree-like spiral draped down around the plant, or by placing the emitters along the central stem. Emitters can also be placed on the top of the canopy of rows of smaller plants.
Compost Piles
Compost piles produce considerable CO2 and can be placed in the center of a densely planted outdoor garden. As the CO2 is emitted by the pile, breezes will carry it through the plants. A small compost container can be placed under a large plant. Because compost piles generate heat, the emerging gas will be warmer than the surrounding air and will drift up through the plant’s canopy. Moist organic mulches or thin layers of compost can also be placed over the soil. When they are provided with a bit of nitrogen, the microorganisms they hold become very active and release extra CO2. Their activity is regulated by temperature, so they produce more CO2 during the warmer daylight hours, rather than at night.
Compost can be used to increase the CO2 content of both outdoor and greenhouse gardens. It is not necessarily smelly and yields a large amount of CO2. Using compost or vermicompost (compost with worms) to generate CO2 is environmentally friendly. When it’s ripe, the compost can be used to enrich the soil or to make compost tea.
About a sixth to a quarter of a compost pile starting wet weight is converted to CO2, so a 100 pound (45 kg) pile could contribute 33-50 pounds (15-22 kg) of carbon to the gas. Carbon makes up about 27% of the weight and volume of the gas, and oxygen makes up 73%, so that the total amount of CO2 created is 61 to 93 pounds (27.6-42 kg), produced over a 60-day period, in warm conditions. That comes to two to three pounds (0.9-1.3 kg) or 16-25 cubic feet (0.45-0.7 m3) a day. The gas is supplied both day and night, so much of it is produced when the plants won’t utilize it.
Adding a heavy layer of organic compost or mulch on top of the soil provides warmth and protection from the elements while increasing the CO2 content around the plants as microorganisms feed on it, releasing the gas.


