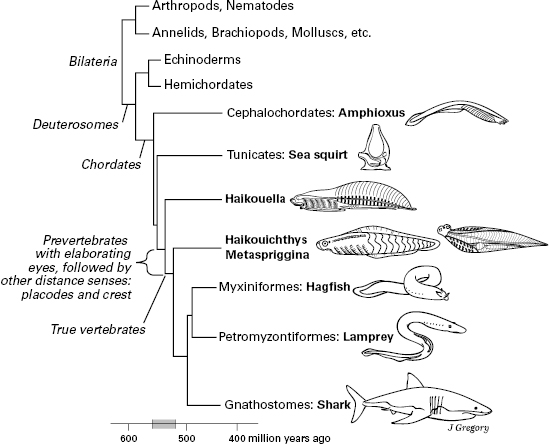
Figure 5.1 Relations and evolution of the chordate and vertebrate animals. Appearance of key sensory features is indicated at left. The animal sizes are not drawn to scale.
How ancient is consciousness? We will build the case that in vertebrates it evolved during the Cambrian explosion before 520 million years ago when critical advances in brain complexity made consciousness possible. If we are correct, this would make consciousness very old, much older than has typically been assumed. In this chapter and the next, we focus on the evolution of sensory consciousness of the external environment, that is, exteroceptive consciousness. In chapters 7 and 8, we discuss the evolution of sentience, the capacity for internal feelings and affects. But we will argue that the basic time frame for all forms of sensory consciousness is roughly the same.
Figure 5.1 is a phylogenetic tree that dates and shows the initial radiation of the vertebrates and other chordates. It marks the key advances in the evolution of the distance senses, the vertebrate head, and sensory consciousness, to be laid out in this chapter. Along with living animals, it shows the Cambrian fossil vertebrates and near-vertebrates that will help us to present our story (Haikouella, Haikouichthys, and Metaspriggina).

Figure 5.1 Relations and evolution of the chordate and vertebrate animals. Appearance of key sensory features is indicated at left. The animal sizes are not drawn to scale.
This chapter covers brain evolution because the Cambrian explosion that produced the vertebrates also produced the vertebrate brain.1 Amphioxus has shown us that the ancestral chordate already possessed the three-part brain (see figure 3.5), with a forebrain, midbrain, and hindbrain; and this brain pattern can even be reconstructed in the bizarre tunicates (see figure 3.4). In vertebrates, the three brain parts are much enlarged, more complex, and better differentiated (figure 5.2), and they contain high levels of well-developed neural hierarchies.
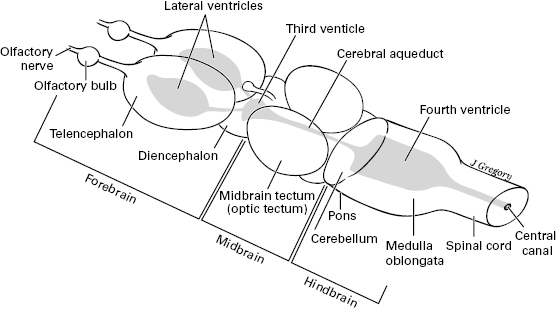
Figure 5.2 Main subdivisions and parts of the vertebrate brain (forebrain, midbrain, and hindbrain). Rostral is to the left. Modified from Butler and Hodos (2005). The hollow center is shaded.
Whereas figure 5.2 shows a generalized brain with the parts shared by all the vertebrates, figure 5.3 shows the brains of specific vertebrates, from fish to amphibian to reptile to bird to mammal. In addition, the human brain is shown in table 5.1. By labeling the brain structures that are held in common, figure 5.3 shows how these structures vary across the different vertebrates in relative and absolute size. These brain structures play a role through the rest of this book, so table 5.1 lays them out systematically and presents their functions. Then, table 5.2 shows which of the brain structures are also present in amphioxus and tunicates, and which ones are unique to the vertebrates. From this, the vertebrate-only structures will reveal the neural advances that occurred in the first vertebrates.
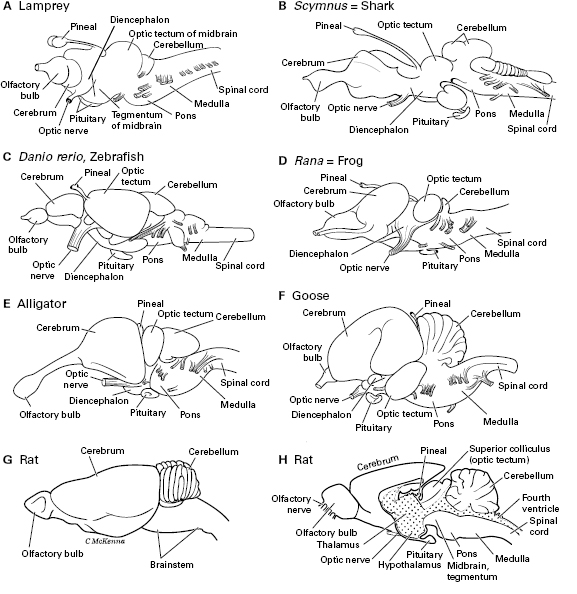
Figure 5.3 Brains of various groups of vertebrates in side view. Rostral is to the left. A. Lamprey. B. Shark, Scymnus. C. Zebrafish, Danio. D. Frog, Rana. E. Alligator. F. Goose, Branta. G. Rat, Rattus. H. Rat brain cut in half to show its interior; dotting shows the ventricles.

Table 5.1 Parts of the central nervous system of vertebrates, emphasizing the brain
| I. Spinal cord |
| Receives sensory inputs and sends motor outputs via the spinal nerves. Reflexes and simple movement commands involving the body below the head. |
| II. Brain, from caudal to rostral |
| A. Hindbrain, or rhombencephalon |
| 1. Medulla oblongata, or myelencephalon |
| • Attaches to last five of the twelve cranial nerves (8–12), so is involved with innervation of the head. |
| • Contains part of the reticular formation (see below), with “centers” for controlling autonomic (inner-body) functions such as swallowing, heart rate, blood pressure, and breathing rate. |
| 2. Metencephalon, or pons and cerebellum |
| a. Pons |
| • Attaches to three of the twelve cranial nerves (5–7), so is involved with innervation of head. |
| • Its reticular formation has centers for breathing, swallowing, and urinating. |
| • Its “pontine nuclei” work with the cerebellum. |
| b. Cerebellum |
| • Calculates how to make body movements smooth and coordinated. |
| • Helps with balance and posture. |
| B. Midbrain, or mesencephalon |
| 1. Tegmentum |
| • Attaches to one cranial nerve (3), which helps move the eyes to look at things. |
| • Its reticular formation starts some coarse body movements: midbrain locomotor center. |
| • Contains caudal parts of the striatum, which selects among basic motor programs (see below). |
| 2. Tectum (“roof”), or optic tectum (named superior colliculus in mammals) |
| • Visual reflexes and extensive visual processing. |
| • Also receives and processes other kinds of sensory information (touch, hearing, etc.). |
| • Isomorphic sensory maps. |
| • Attaches to one cranial nerve (4), which helps move the eyes to look at things. |
| 3. Periaqueductal gray, around the hollow center of the midbrain |
| • Signals the bodily responses of panic. |
| C. Forebrain, or prosencephalon |
| 1. Diencephalon |
| a. Pretectum |
| • Mediates responses of eye to changes in the intensity of light. |
| b. Thalamus (egg-shaped in figure 5.4) |
| • Relay center by which all other parts of the brain and spinal cord communicate with the pallium (see below), including with the cerebral cortex of mammals. |
| • Processes the information going through it. |
| • Role in alertness and wakefulness. |
| c. Epithalamus (most superior part of diencephalon) |
| i. Pineal organ |
| • Has light receptors that tell direction of the light and time of day or year, but does not form images. Secretes the hormone melatonin, which prepares body for night time of the day-night cycle. |
| ii. Habenula (habenular nucleus) |
| • Inhibits body movements, during sleep and to prevent behaviors that are inappropriate or dangerous. |
| d. Prethalamus, or subthalamus |
| • A part of the striatum, which selects among basic motor programs (see below). |
| e. Hypothalamus (most inferior part of diencephalon) |
| • Signals motor responses of affects or emotions. |
| • Second cranial nerve attaches here: optic nerve of vision. |
| • Retina of eye is an embryonic outgrowth of this part of brain. |
| • Pituitary gland attaches to its floor, contains the pituitary’s neurohypophysis. |
| • Main brain region for monitoring and controlling the autonomic or visceral functions; controls pituitary’s secretion of many hormones for such purposes. |
| • Has centers for thirst, hunger, temperature regulation, digestion. |
| • Controls some basic behaviors for survival, including sexual behaviors and biorhythms (generated by its suprachiasmatic nucleus). |
| 2. Telencephalon, with two cerebral hemispheres (see also figure 5.4) |
| a. Pallidum (ventrally located) |
| i. Striatum, or corpus striatum; essentially the same as “basal ganglia.” |
| • Selects which motor program should be carried out, among the many different motor programs that are coded in the brainstem and spinal cord: such programs are for breathing, chewing, swallowing, locomotion; fast but inflexible decisions about what movement patterns to perform.1 |
| ii. Septal nuclei, septum pellucidum |
| • Affects,2 moods, anxiety |
| iii. Amygdala, its pallidal or “subpallidal” part |
| • Affect2-directed responses and actions. Fear or fearlike behaviors. |
| b. Olfactory bulb and tract |
| • First cranial nerves (olfactory) attach to bulb. |
| • Initial smell processing occurs in the bulb. |
| c. Pallium (dorsally located; see esp. figure 5.4) |
| ii. Dorsal pallium |
| • In mammals, includes most of the cerebral cortex. |
| • Processes information from many senses, elaborate isomorphic maps in mammals. |
| • Complex decisions. |
| • Influences actions and behavior, some motor control (of fine, voluntary movements in humans). |
| • Cognition |
| • Some affective2 or emotional roles. |
| iii. Lateral pallium |
| • Olfactory processing of odors, important part of olfactory pathway. |
| • In mammals, is called the olfactory cerebral cortex. |
| iv. Medial pallium, hippocampus |
| • Constructs spatial memories of places and memories of objects and events. |
| v. Ventral pallium, or pallial part of amygdala |
| • Affective2 learning, especially of fears. |
| III. Other parts of nervous system, which involve multiple regions of the brain |
| A. Brainstem |
| • This is the medulla oblongata, pons, and midbrain, all continuous with one another. |
| B. Hollow center of spinal cord and brain (see figure 5.2) |
| 1. Central canal of spinal cord |
| 2. Ventricles of brain |
| a. Fourth ventricle, in hindbrain |
| b. Cerebral aqueduct, in midbrain |
| c. Third ventricle, in diencephalon |
| d. Lateral (first and second) ventricles, one in each cerebral hemisphere |
| C. Autonomic nervous system (ANS) |
| • Motor neurons that issue from the brain and spinal cord, reach the visceral organs and control inner-body functions: digestion, heart rate, urination, etc. |
| • Technically defined as the part of the peripheral nervous system that activates (or inhibits) the glands and smooth muscles in the organs of the body; also, the muscle of the heart wall. |
| • Parasympathetic and sympathetic divisions. Parasympathetic signals organs for “resting and digesting,” whereas sympathetic signals them for “fright, fight, or flight.” |
| • Much of this ANS output is controlled or influenced by the brain and spinal cord. |
| • Contrasts with the somatic motor system, which signals skeletal muscles to contract and move the limbs or body. |
| D. Reticular formation |
| • In the core of the brainstem: in medulla, pons, and midbrain. |
| • Contains the reticular activating system, for arousal, directing attention, and controlling the intensity of emotions (see figure 10.8). |
| • Signals and controls muscle contractions for basic or coarse body movements. |
| • Has centers that influence visceral functions via the ANS. |
| E. Greater limbic system (figure 5.4) |
| • For affects2 (and for emotions in humans). |
| • Motivates the basic behaviors for survival. |
| • Maintains visceral functions and a constant state of the inner body (homeostasis). |
| • In forebrain, includes amygdala, hippocampus, basal forebrain, septal nuclei, hypothalamus, and more. |
| • In brainstem, includes periaqueductal gray, reticular formation, and more. |
1. Striatum’s motor functions are described by Grillner et al. (2005).
2. Affects are positive and negative feelings (see chapters 1, 7, and 8).
Table 5.2 Vertebrate CNS versus that of tunicates and amphioxus: a comparison of parts: (+) present, (–) absent

The sources for amphioxus and tunicate structures are Burighel and Cloney (1997); Lacalli (2008); Mackie and Burighel (2005); Moret et al. (2005); Wicht and Lacalli (2005). See also chapter 3.
1. The visceral peripheral nervous systems of amphioxus and tunicates are different from the autonomic nervous system of vertebrates, with no contribution from the neural crest.
2. See the “neuropile” in amphioxus brain in figure 3.5B.
Here is a summary of tables 5.1 and 5.2, along with the brief survey of how brains vary across vertebrates. The hindbrain has a caudal part or medulla oblongata and a rostral part, the pons and cerebellum. The midbrain has a roof called the tectum and a tegmentum ventral to that. Together, the medulla, pons, and midbrain form the brainstem. The forebrain has a diencephalon (mostly thalamus and hypothalamus) and telencephalon (cerebrum, with two cerebral hemispheres). The brain is hollow with a connected system of fluid-filled ventricles in its center. Caudal to the brain is the spinal cord, whose hollow center is the central canal.
In vertebrate evolution, when the brain enlarged, this usually involved expansion of the cerebrum, especially of its dorsal part, the pallium or cerebral cortex, which is associated with higher mental functions. This expansion is best seen in the giant cerebrum of mammals and birds (figure 5.3F, G, and table 5.1), but also in some jawed fish and in reptiles (figure 5.3E). But not all enlargement involves the cerebrum. Any other part of the brain that performs a function at which a vertebrate species is expert can also be enlarged. For example, in fish with an extremely sharp sense of taste or of sensing electrical fields (electroreception),2 the parts of the brainstem that start to process these sensory stimuli are large.3
The cerebral pallium functions in memory and learning, fear, processing smells, complex processing of most kinds of sensory stimuli, making decisions, influencing body movements, and cognition. Attaching to the front of the cerebrum is the olfactory bulb for the initial processing of smells. This bulb is relatively largest in fish and amphibians and in some mammals like rats, who rely heavily on their sense of smell.
The optic tectum of the midbrain is a center for processing visual information throughout vertebrates, though less so in mammals. The tectum also processes the other kinds of sensory information (except smell). It is a relatively large part of the brain in many fish (figure 5.3A, C). These facts make the tectum and cerebral pallium the main brain regions for processing information from the senses of vision, touch, hearing, and smell.
The cerebellum (not to be confused with the cerebrum) is a hindbrain structure that varies in size across vertebrates. It senses the movements of one’s own body and calculates how to make these movements smooth and coordinated, as well as helping with balance. It is largest in active and well-coordinated animals like birds and humans.
Mostly invisible from the outside is a deep or medial limbic system of the brain (the greater limbic system of Nieuwenhuys).4 All subdivisions of the brain contribute to the limbic system (figure 5.4). That is, it has parts in the brainstem, diencephalon, and telencephalon. It is involved in running core-body and basic survival functions, motivations, and affects. It includes the brain’s “primitive core region” described in chapter 3.
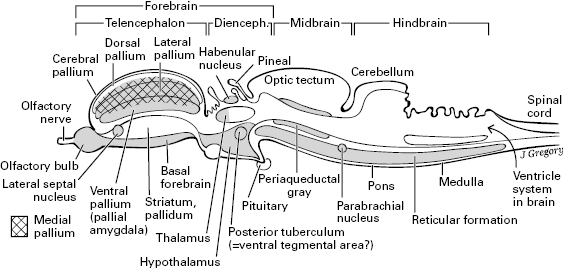
Figure 5.4 The limbic system, seen as the shaded structures in this mid-sagittal view of a generalized vertebrate brain. This figure also gives the best view of the parts of the telencephalon: the pallium, pallidum, and so on.
From the limbic core through the rest of the brain, we see much similarity of brain structure across all the vertebrates. The most obvious exception to this is the greater complexity of the cerebral pallium in mammals, birds, and reptiles.
Also note that vertebrates have critical brain structures that are lacking in amphioxus, our proxy for the earliest prevertebrates (table 5.2). This was a theme of chapter 3, but it becomes even clearer now that we have presented the vertebrate brain in more depth. Judging from the list in table 5.2, the most important brain structures that evolved anew in vertebrates are the cerebrum, a well-developed thalamus, the optic tectum, and the metencephalon (cerebellum and pons). All these new brain regions have major sensory-processing roles.
We will now reconstruct the senses of the prevertebrate ancestor, starting with light detection. Based on amphioxus from chapter 3 and our reconstruction of the original bilaterian worms from figure 4.5B, the early prevertebrates must have had light-sensitive cells or photoreceptors, some of which were partly shielded by a pigment screen, allowing them to tell the direction of light sources but not to form a visual image5 (see figure 5.5A, Stage II). For their other senses, early prevertebrates would have had mechanoreceptors in their skin, detecting various kinds of touch stimuli. We say this because amphioxus has a wide variety of such receptors.6 These prevertebrates also must have had nociceptors, for responding to noxious or harmful stimuli, as almost all animals do, and chemoreceptors for senses akin to smell and taste that detected odor and taste molecules dissolved in the water. The claim for chemoreceptors seems a risky deduction because scientists have not yet been able to find any definitive chemoreceptor organs in amphioxus and tunicates.7 But these chordates do have some of the genes associated with chemoreception,8 and chemoreceptors are thought to be a primitive character of all bilaterian animals. All the kinds of receptors—photoreceptors, mechanoreceptors, and chemoreceptors—would have been located in the body-surface layer of the prevertebrates, their skin, although some of the photoreceptors were in the brain and spinal cord.
Even though the vertebrates’ ancestor had these basic sensory systems that allowed some simple processing of environmental stimuli, we reconstruct these systems as entirely reflexive, as in amphioxus and tunicates. For conscious processing to arise, a more powerful and enlarged brain would have to evolve, with complex sensory hierarchies. The logical question, then, is how and why did this explosion in the sophistication of sensory processing occur?
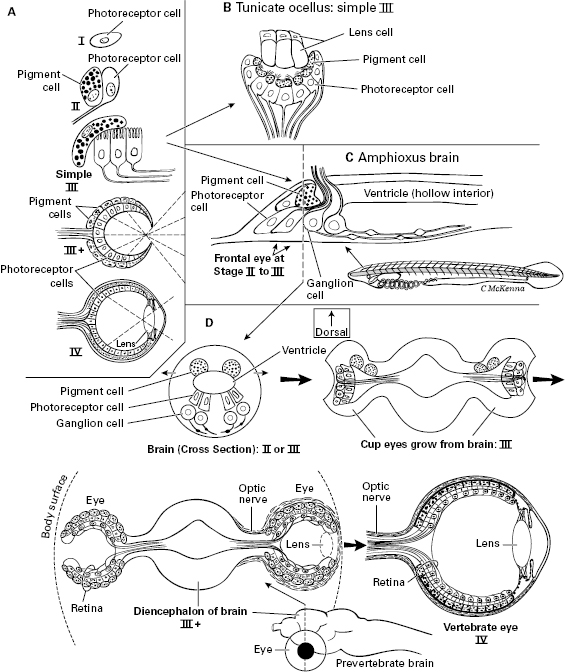
Figure 5.5 Stepwise evolution of the vertebrate eye. Reconstructed using amphioxus and tunicates, and from Dan-Eric Nilsson’s stages of eye evolution that were based on the range of eye types across the invertebrates. A. Nilsson’s four stages I–IV. B. Tiny ocellus “eye” of a tunicate larva (homologous to the pineal, “third-eye” of vertebrates). C. The small frontal eye of the amphioxus larva, in a side view of the brain. D. Proposed stages in vertebrate eye evolution starting from an amphioxus-like frontal eye. These cross sections were inspired by the art in articles by Trevor Lamb.
In the previous chapter, we deduced that the different distance senses of vertebrates evolved more or less together as Cambrian prevertebrates scrambled to detect and avoid the many new predators, most of which were arthropods. But in this nearly simultaneous appearance, did one of the senses actually evolve first, getting a head start, with the others soon following along?
One hypothesis is that light detection was the first of the distance senses to evolve sensory images. This hypothesis was proposed by Andrew Parker and built upon by Michael Trestman,9 both of whom focused on arthropods, although the claim applies to vertebrates as well. Parker called it the Light-Switch hypothesis and said that the appearance of true, image-forming eyes in arthropods allowed them to become the first predators, which then led to the Cambrian explosion itself. Trestman refined this to say that the new eyes did not merely form images, but formed spatial images that could identify discrete objects in the visual field and could reveal the positions, distances, and movements of these objects, as well as which objects lay in front of others. This spatial vision, he said, allowed prey to be spotted, tracked, struck, and manipulated with ease, and then placed in the mouth. Although the arthropods evolved such eyes first and threw the light switch, the vision of their prevertebrate prey quickly improved, via natural selection, to see the predators coming. The reasoning behind the vision-first hypothesis is that of all the senses, focused vision yields the most information about the environment, so it is most likely to have enabled the complex predatory activities and the evasive behavioral defenses of the Cambrian arms race. Once begun by vision, this race then led to the elaboration of other senses—smell, touch, and hearing—all of which improved survival in both predators (arthropods) and prey (prevertebrates).
By what anatomic steps did the image-forming eyes evolve in vertebrates (figure 5.5)? The fossil record is of no help until the last stages, so we must reconstruct the sequence using modern amphioxus and tunicates, plus some general trends in eye evolution that can be determined from other modern invertebrates. That is, dozens of groups of invertebrates have gone through the initial stages and evolved simple eyes independently, especially a variety of worms and snails, and the stages are mostly the same in all these animal groups.10 From such comparative-animal evidence, Dan-Eric Nilsson has divided eye evolution into four functional and structural stages, each of which could easily and logically lead to the next (I–IV in figure 5.5A).11 The four stages go from isolated photoreceptor cells (I), to an added pigment cell (II), to cupping (III and III+), to camera-style eye with high visual resolution (IV). Now for a more precise description of the stages.
Stage I is basic, nondirectional photoreception in which individual photoreceptor cells, unshaded by any pigment, just monitor the intensity of light. An animal in this stage can tell if it is night or day, or if it is in a dark place.
Stage II is directional photoreception. Here, the photoreceptor cells are partly shielded by pigment cells so they receive and sense light from some directions but not from others. This directionality lets the animal move away from or toward light, and lets it detect shadows of moving objects such as approaching predators. The ocelli (“little eyes”) of the blind, ancestral bilaterian shown in figure 4.5B would have been Stage II directional photoreceptors. The frontal “eye” of larval amphioxus, which is inside the brain and homologous to the eyes of vertebrates, is at this Stage II, or else barely into the next Stage III. We show this frontal eye of amphioxus in figure 5.5C (also in figure 3.5).
Stage III is low-resolution vision. A simple cupping of the photoreceptor and/or pigment cells is all that is needed to go from Stage II to Stage III (figure 5.5A, B, D). With the partial screen of light-blocking pigment, each photoreceptor cell only receives and responds to light that enters the cup from the unique direction that that particular photoreceptor faces. By assembling the inputs from all its photoreceptor cells, the eye forms an image—a crude one, but one that’s sufficient to track moving objects or to avoid bumping into things while swimming and navigating. Some animals with Stage III eyes have evolved a lens (figure 5.5B), not effective enough to focus an image but sufficient to bend and concentrate enough light onto the photoreceptor cells so that the eye can operate in dim light.
Figure 5.5D models how the prevertebrates went through this Stage III and beyond, with the eyecups enlarging as lateral outgrowths of the brain, and becoming the paired eyes. Note that in a proposed “III-plus” stage, the cupping became so deep that the eye acted like a pinhole camera and produced higher-resolution vision than did the shallower cup of the initial Stage III. Here our ancestors really began to see images—not perfectly, but well enough for earliest Cambrian times. Presumably their neo-arthropod predators, with which the prevertebrates coevolved, could not yet see any better. These two clades of visual unsophisticates fit an old saying, translated from its original Latin: “In the land of the blind, the one-eyed man is king.” And vision was rapidly improving.
Stage IV is high-resolution vision. Detailed vision required little more than changes in the lens so that this lens sharply focused images on the retina, and along with this a larger number of retinal photoreceptor cells. This is shown as the final step in figure 5.5D, labeled “Vertebrate eye.”
The new high-resolution images in the eye, if not to be useless, must have been accompanied by a great increase in visual processing in forebrain and midbrain. The detailed, isomorphic visual representations that resulted from such processing were the brain’s first conscious mental images, and they allowed the first vertebrates to perform a wide range of vision-guided behaviors. In this way, vision signaled the dawn of consciousness.
The retina of the vertebrate eye comes from the diencephalon (figure 5.5D), which means it is a part of the brain and belongs to the central nervous system. Therefore, even though the eye lies out near the body surface, it is not part of the peripheral nervous system (PNS), as one would expect. By contrast, all the other senses are received in the PNS.
We have taken the view that vision was the first of the distance senses to evolve in vertebrates and arthropods, but some of our colleagues, Roy Plotnick, Stephen Dornbos, and Junyuan Chen, disagree. They say that smell came first.12 They argue that at the start of the Cambrian, the ancestral worms lived under microbial mats on the ocean floor (as discussed in chapter 4), where odor is important but light cannot penetrate. They said that vision evolved later, when the animals started to swim above the ocean floor. But we counter that many ancestral worms must have grazed on top of the mats, where lots of light did reach. Plotnick and coworkers also argue that vision is not necessary for predators that live on the ocean bottom, pointing to modern starfish and snails. Our counterargument is that many visual predators do live in this environment, and they take advantage of the complex visual landscape of the ocean floor. Visual bottom dwellers among today’s arthropods and fish include lobsters, horseshoe crabs, flounders and stingrays. Finally, Plotnick and coworkers argue that the earliest fossil animals with image-forming eyes (such as trilobites) did not appear until 520 mya, which is 20 million years after the Cambrian began, so eyes must have evolved late. This argument seems unwarranted, however, because no body fossils of any animals at all are available from about 540 to 520 mya—an unfortunate gap in the rock record from which only small scales, shells, and fragments of the early animals are known13—meaning neither eyes nor smell organs could be known from this gap, whether they existed then or not.
We will reemphasize why we reason that vision was the key initial sense: because light provides so much more information. “Smell images” of space do exist14 and can be constructed by the vertebrate brain from odor plumes and concentration gradients of odor molecules emerging from smelly objects in the water, but these smell images cannot reveal the sharp borders of objects or the precise distances that light images can provide. And visual images are so much better for following objects that move fast, such as an attacking predator. Granted, vision does not work well in murky waters, but such murk is often caused by turbidity currents that also disperse and confuse odor signals, likewise interfering with the sense of smell.
However, smell is special in one important way. Unlike sights, sounds, or touch stimuli, smells linger after the source of the stimulus goes away. Odors remain, of body wastes, body secretions, and other spoor that is left behind by predators, mates, prey, and other animals. Smells tell of the past. Because smell has this unique time dimension, as soon as olfaction appeared natural selection linked it to memory: from this smell, I remember and recognize what odor-emitting object used to be here and still may be nearby, so I can know to avoid or approach it. The intimacy of this relationship explains why memory and smell structures evolved so near one another in the incipient telencephalon of the ancient vertebrate brain.
Now back to the argument that vision evolved before smell had elaborated beyond basic, primitive, chemoreception. In addition to the theoretical reasons, the physical evidence also favors this view. First, a method of identifying cell types by the genes they express has shown that the frontal eye of the amphioxus larva really is homologous to the vertebrate eye, in details ranging from its retinal cells to its pigment cells. Thus amphioxus has an “eye” while lacking any known organ of smell or hearing. Second, a study by Martin Šestak and his colleagues found that a surge of genes involved with vision evolved earlier on the tree of chordate animals than did the genes associated with the other senses such as smell, hearing and balance. Third, the candidate, fossil prevertebrates from Cambrian rocks show more evidence of eyes than of nasal (smell) organs.15
We will return to these fossils and to vision, but first we must explore the nonvisual distance senses of the ancient prevertebrates, the senses that evolved after vision. These other senses did not merely tag along as afterthoughts behind the blossoming of vision. Instead, their elaboration was part of a reorganization of the ancestral body and nervous system that contributed as much to the evolution of brain and consciousness in vertebrates as did the new visual images.
We have established that the focusing lens of the eye is essential for high-resolution vision and the evolution of the visual image. But where did this lens come from? Nor have we explained the body source of the neurons of the vastly improved nonvisual senses of vertebrates. Unexpectedly, these things (and more) came from brand new embryonic cells that evolved near the dawn of the vertebrates, specifically from neural crest and placodes (figure 5.6).16
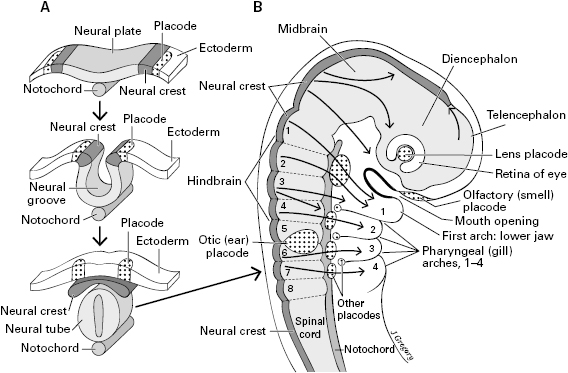
Figure 5.6 The embryonic tissues that are unique to vertebrates: neural crest and placodes. A. Time sequence of their formation and development in the back (dorsal side) of the embryo, arising from ectoderm near the neural tube. B. A vertebrate embryo, side view of its head and neck regions, showing the placodes and neural crest. The long arrows in the body show that the neural crest migrates widely.
Neural crest and placodes do not exist in any animals except the vertebrates,17 and they develop into so many important adult structures that they are widely recognized to be key contributors to the evolutionary success of the vertebrates. They especially contribute to the head and neck regions, and it is thought that their evolutionary origin led to the great complexity of the vertebrate head. They form in the early embryo, on the dorsal head and back, around the periphery of the neural plate, which is a long strip of surface tissue (ectoderm) extending from head to tail (figure 5.6A). The neural plate soon pushes down and folds into a neural tube, to become the spinal cord and brain. When this folding begins, ridges form at the margins, with the top of the ridge becoming the neural crest and the lateral walls giving rise to the placodes. As shown in figure 5.6A, the crest and placodes are paired on the right and left sides of the body.
We will discuss the neural crest first. Its cells develop into both nervous and nonnervous structures. They give rise to all the sensory neurons in the trunk and many in the head, especially neurons for the somatic senses of touch, pain, temperature, and so on. In addition, many crest cells migrate away from their original location in the back, to become widespread pigment cells called melanocytes, motor neurons of the gut, some of the skull, and more.
The placodes, whose official name is ectodermal or neurogenic placodes, develop in the head and neck region, where they mostly form sensory structures. The placodes are indicated by stippling in figure 5.6B. Most of them stay at the body surface, and overall they do not migrate as deeply or widely as does the neural crest. The olfactory placode gives rise to the receptor cells for smell in the nose, the lens placode gives rise to the lens of the eye, and the otic placodes give rise to the cells in the ear for the senses of hearing and balance. Other placodes contribute, along with neural crest, to the somatic sensory neurons of the head. They also form visceral sensory neurons to digestive organs including those to the taste buds, and the sensory cells of the lateral line (a line of mechanoreceptors in fish and water-dwelling amphibians, in the skin of their head and the lateral side of the body, for detecting vibrations in the water).18
Nearly all of the nonvisual sensory PNS of vertebrates comes from the placodes and neural crest. Amphioxus and tunicates, by contrast, have a sensory PNS but no crest or placodes; at best they only have rudimentary precursors of the crest and placodes that contribute little to their PNS.19 This difference implies that there was an almost complete replacement of the sensory PNS around the time of the dawn of the vertebrates. Our story of vertebrate sensory evolution has emphasized how giant eyes ballooned from the brain to gather vast quantities of visual information, but in some ways that was not as impressive as the near-total reboot of all the other senses, mechanoreceptive and chemoreceptive, that came with the neural crest and placodes.
When did the neural crest and placodes evolve, in relation to the stages of eye evolution mapped out in figure 5.5D? We deduce that they originated no later than between the low- and high-resolution stages (III and IV) in the eye sequence. Our reasoning is that a lens had to be present for that transition to sharp vision to occur, so the lens placode had to exist by then to form the lens.
The sensory revolution near the dawn of vertebrates affected the brain (figure 5.7). The new waves of sensory information arriving from the placodal and crest senses of olfaction, hearing, an increased sense of touch, and so on joined the flood of visual information from the image-forming eyes, demanding elaborate multisensory processing by the brain. As proposed by Lacalli, the resultant expansion of brain regions explains the differences between the vertebrate and amphioxus brains that we laid out in chapter 3 (figure 3.5).20 New, mainly dorsal regions of the brain appeared and enlarged. These are labeled “New” in figure 5.7B. From the dorsal and rostral part of the forebrain grew the new telencephalon, with the cerebrum. The vertebrate telencephalon receives the input of smell, so its origin was related to the appearance of this olfactory sense. The optic tectum of the midbrain enlarged to process visual information (along with other senses), and the dorsum of the hindbrain enlarged also, to process information from the senses of hearing and balance, and from the lateral line. Judging from all living vertebrates, each of the new and improved senses arrived in a neatly mapped, isomorphic arrangement. Thus, the retina, neural crest, and placodes supplied sensory input to the expanding dorsal parts of the brain, in which evolved the most elaborate sensory maps and images.
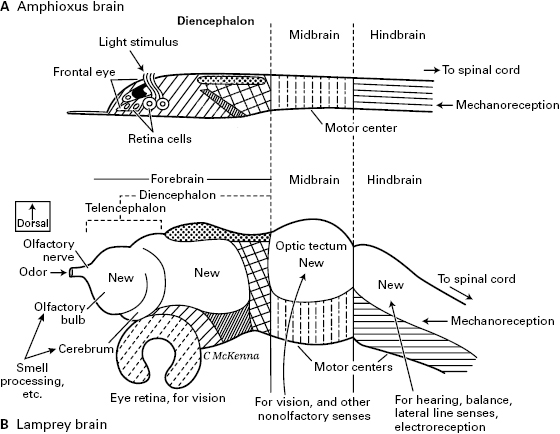
Figure 5.7 The brain of amphioxus (as a proxy for the ancestral, prevertebrate brain) compared to the brain of a lamprey (a proxy for the brain of the first true vertebrate). Identical hatching patterns mark the corresponding regions in the two brains. Notice the large “New” regions of the vertebrate brain. Largely after the studies of Thurston Lacalli.
We have proposed that the senses of vertebrates evolved in two overlapping waves: image-forming eyes grew out from the brain, and then the other senses elaborated from the placodes and neural crest. Our colleague Ann B. Butler actually proposed this first, in the year 2000, in her stepwise hypothesis of vertebrate origins.21 To illustrate her hypothesis, she reconstructed an intermediate stage between an amphioxus-like ancestor and the first true vertebrate, which she called the “cephalate” animal (figure 5.8A). Her hypothetical cephalate had evolved paired eyes and an enlarged brain with its visual system based on the diencephalon and midbrain. But it still lacked the other sensory systems of vertebrates, also lacked neural crest and placodes, and had no telencephalon. Its visual pathways, she proposed, served as a circuitry template for the new or improved sensory systems of smell, taste, touch, and hearing. That is, the sensory paths from the newly evolved crest and placodes adopted vision’s neuronal patterns in the brain. The sensory hierarchies of neurons that formed for the nonvisual senses mimicked the preexisting visual hierarchy.
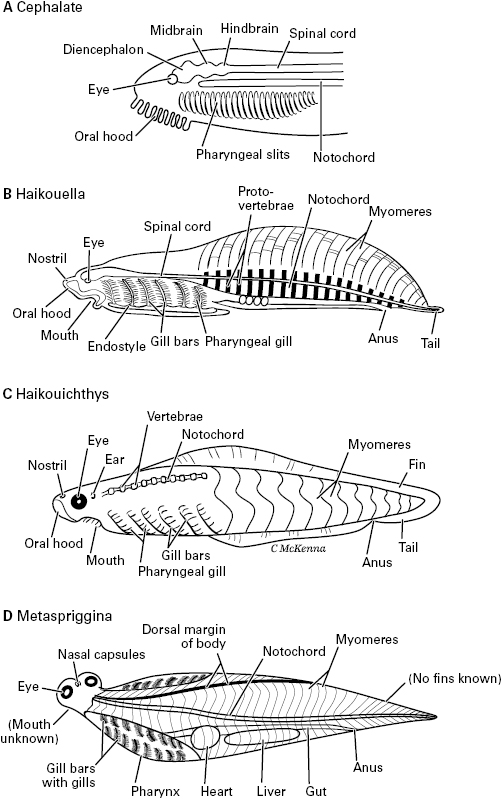
Figure 5.8 Prevertebrates and early vertebrates. A. The hypothetical “cephalate” animal, conceived by Ann Butler as a sort of intermediate between amphioxus and true vertebrates. B. Haikouella, a fishlike fossil animal. C. Haikouichthys, an Early Cambrian fossil fish. D. Metaspriggina, a Middle Cambrian relative of Haikouichthys.
About the time Butler presented her cephalate model, some fossil animals by which to test it were being discovered. As pictured in figure 5.8B, these Cambrian fossils are the soft-bodied, blade-shaped, inch-long yunnanozoans from the Chengjiang Shale of the Yunnan province in southern China.22 This shale is 520 million years old and contains the oldest known whole-body fossils of bilaterian animals (discussed in chapter 4). The best-preserved and most informative yunnanozoan fossils are of a species named Haikouella lanceolatum (figure 5.8B). The specimens of Haikouella were interpreted by Junyuan Chen, coauthor Mallatt and their colleagues as fish-like and the closest relative of vertebrates (figure 5.1).23 By this assessment, it had a notochord, paired eyes, and a large brain with diencephalon and hindbrain parts, with all these structures in the same positions as in living fish such as lampreys. But Haikouella differed from vertebrates in having no skull or ear capsules, and only hints of a telencephalon and nostrils (for smell) were evident.
With its paired eyes and well-developed diencephalon but no ear and an uncertain “nose,” Haikouella seems to fit well with the vision-first idea and with the proposed cephalate stage in which the crest and placodal senses had not yet evolved. Yet, it had fishlike gill bars, which develop from neural crest, and a dotlike lens in the center of each eye, which develops from the lens placode. This suggests that Haikouella was closer to the vertebrates than was the cephalate, as the latter was originally conceived. Haikouella’s eyes were small, just a fifth of a millimeter in diameter. This is much larger than the tiny frontal “eye” of amphioxus, whose diameter is a hundredth of a millimeter, but still was probably too small to have formed a well-focused image.24 Other, clearly visual Cambrian animals, mostly arthropods, had eyes closer to a millimeter or larger.25 Still, the fact that Haikouella had paired eyes at all, lateral to the diencephalon rather than within it, gives some support to the vision-first hypothesis.
Haikouella and the other yunnanozoans are controversial, and some paleontologists think their “eyes” are just artifacts of fossilization that never existed in life, and that the yunnanozoans are not even chordates.26 If that should turn out to be true, it leaves another group of inch-long fossil animals from the Chengjiang Shale, Haikouichthys and its close relatives,27 as most likely to reveal the early evolution of the vertebrate nervous system (figure 5.8C). Unfortunately, Haikouichthys has a name that sounds like Haikouella, which naturally causes many to confuse these two different animals with each other. The best way to avoid confusion is to remember that “-ichthys” means “fish” (a vertebrate), and “-ella” is the dimunitive ending meaning “little-bit,” or “not fully a vertebrate.” Since it was discovered and described as a vertebrate by Degan Shu and his coworkers in the late 1990s, Haikouichthys has generated surprisingly little controversy among scientists. It is almost universally agreed to be a true vertebrate, a jawless fish, because its fossils show vertebrae, obvious eyes with a diameter of 0.6 millimeter, and ear and nasal (olfactory) capsules. Its fossils also show the characteristic gill bars, gill pouches, body fins, and curved myomeres of vertebrate fish. Without hesitation, we used Haikouichthys as a representative Cambrian vertebrate in chapter 4 (figures 4.4D and 4.3B). With eyes that probably formed visual images, which presumably went to its brain for processing, Haikouichthys had visual consciousness in our view. Recall that the 520 million-year-old Chengjiang Shale yields the oldest good record of bilaterian animals, meaning that consciousness has existed since that time.
More recently, paleontologists Jean-Bernard Caron and Simon Conway Morris identified another Haikouichthys-like fish, Metaspriggina, from the slightly younger Burgess Shale of British Columbia in Canada (505 mya).28 Metaspriggina is shown in figure 5.8D. We drew it as if twisted, with its head up but its trunk shown in typical, lateral view. The head of Haikouichthys looks almost exactly like this in top view,29 supporting a close relation between these two Cambrian fish.
Although Haikouichthys and Metaspriggina tell much about the first true vertebrates and even suggest when consciousness arose, they reveal less about how the senses and brain evolved along the road to vertebrates. First, their brains are not preserved—although their eyes and noses imply that they had a vertebrate brain. Second, though Haikouichthys and Metaspriggina lived among the most ancient bilaterians for which we have body fossils, each was already too good of a vertebrate, a full vertebrate in every way that can be learned from their fossils, so they cannot reveal the prevertebrate stages of sensory evolution. To uncover those stages, we must rely on the theory-driven logic from earlier in this chapter, namely the logic that favors the vision-first view, and on the yunnanozoans, which show the brain expansion.
To summarize the chapter so far, somewhere between 560 and 520 mya, the unpaired frontal “eye” of a creature like amphioxus, which recorded the direction of light and moving shadows, evolved into the vertebrate “camera eyes” that recorded images in high resolution (figure 5.5D), a process that was completed by 520 mya. The isomorphic visual images were processed by the expanding brain into mental images, which we propose marks the arrival of consciousness. Other distance senses followed and entered conscious perception. As an attempt at more precise dating (figure 5.1), we deduce that the larger-eyed Haikouichthys and Metaspriggina were conscious but the smaller-eyed yunnanozoans may not have been. The first vertebrates had the first consciousness.
Butler proposed that as good vision evolved in the prevertebrates and the brain took on visual processing, the original reflex paths gained more levels of neurons in a well-organized, isomorphic, and hierarchical pattern. Soon thereafter, the nonvisual senses formed chains of processing neurons that copied the visual pattern. Indeed, all the different sensory systems of vertebrates are similar to each other in this way. The similarities are shown in figures 5.9 through 5.12, which portray the visual, touch (somatosensory), auditory, and olfactory pathways, respectively.30 Then, table 5.3 shows how each pathway can fit into the same, five-part scheme, with the circled numbers in the pathways in the figures corresponding to the column numbers in the table (1–5). The sensory paths are illustrated for mammals, as is standard procedure, although in mammals the topmost parts are more complex than in fish, amphibians, or reptiles. The paths of fish and amphibians are included in table 5.3 and shown in figure 6.4.
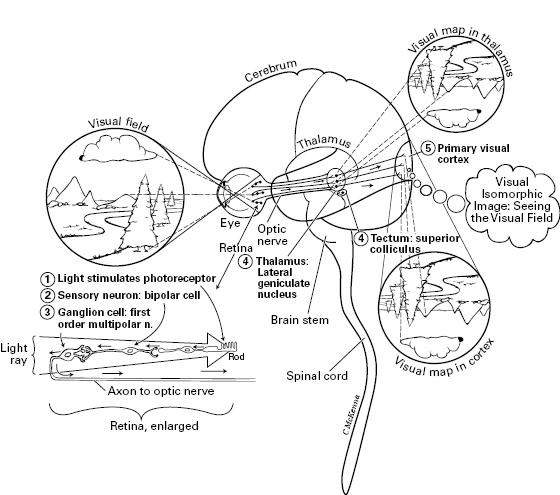
Figure 5.9 Vision: visual pathway in the human. Path goes from where light stimulates a photoreceptor (left), to bipolar sensory neuron, to ganglion cell, to thalamus (or to superior colliculus = optic tectum), to primary visual cortex of the cerebrum. Throughout the pathway, the nervous structures are arranged according to a map of the visual field, as projected onto the retina, an isomorphic feature called retinotopy. Numbers correspond to the column numbers in table 5.3. The visual image might emerge higher in the cerebral cortex than is shown here, and the same may be true for the other senses shown in figure 5.10 to 5.12 (Panagiotaropoulos et al., 2012; Pollen, 2011).
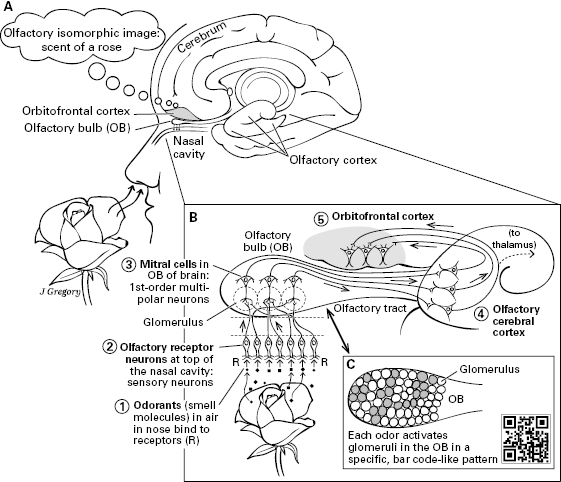
Figure 5.12 Smell: olfactory pathway in the human. A. Overview showing the medial part of the cerebrum in relation to a nose inhaling the scent of a rose. B. Path goes from smell molecules stimulating smell receptors (R), through sensory neuron, to mitral cells in the olfactory bulb (OB), to the olfactory cerebral cortex, to the orbitofrontal cortex. C. Inset showing how an odor activates a specific set of glomeruli in the olfactory bulb, as a scent’s “bar code.” In this pathway, the neural structures are arranged according to maps that indicate specific odors.
Table 5.3 Summary of the major sensory pathways of vertebrates
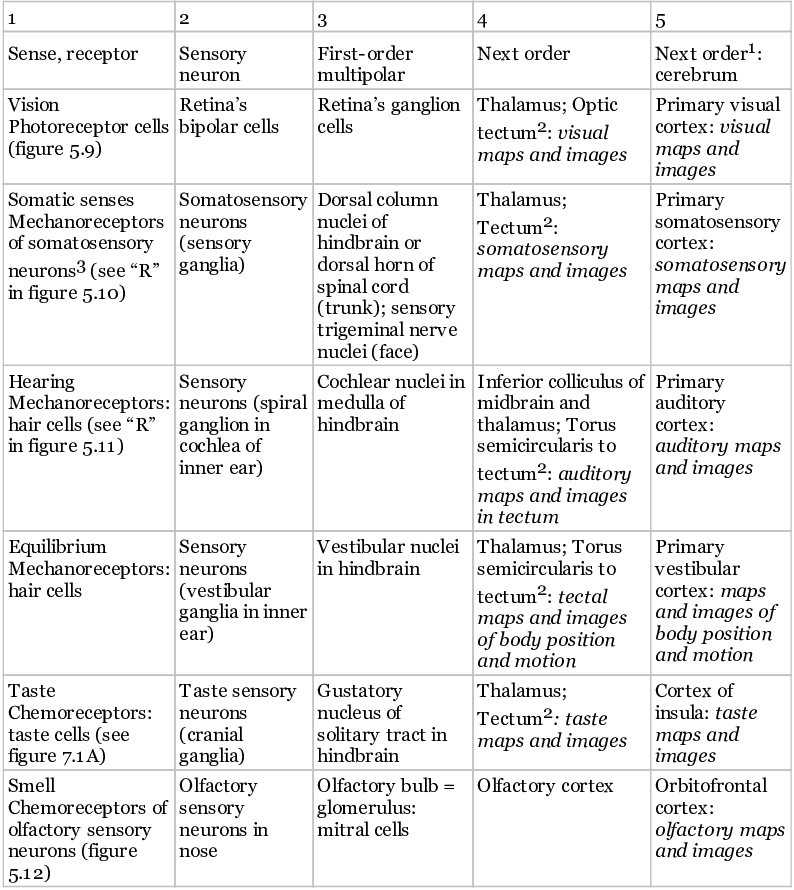
1. “Next order: cerebrum” applies only to the mammals (for all senses but smell), and, in mammals, to still higher-order processing of all senses, leading up to the frontal lobes of the cerebral cortex. It is likely that further processing in these higher areas and a merging of all classes of sensory information there are required for complete, multisensory conscious images in mammals: Boly et al. (2013); Kandel et al. (2012); Maier et al. (2008); Pollen (2011).
2. “Tectum” here means in fish and amphibians. In chapter 6 we argue that in these vertebrates, the images for these senses are in the optic tectum with help from the thalamus, but no higher.
3. For the general inner-body senses, pain, and affective senses, see chapters 7 and 8, especially figure 7.1.
The pathways and the figures are intricate and can seem overwhelming to those learning them, reminding us of a frequent complaint of our young neurobiology students who ask, “Why is the vertebrate brain, which is the most complicated thing in the whole universe, so detailed and challenging to learn?”—a question that answers itself. But we will keep things as simple as possible. We will consider just the general, shared features of the different sensory pathways and how they fit together in the basic parts of the brain.
The shared features are as follows. All the sensory pathways start with sensory information that stimulates receptors (1), which signal sensory neurons (2). The sensory neurons then send the signal to first-order multipolar neurons (3) in the central nervous system. Then in most cases these neurons project to further neurons (4) in the midbrain’s “tectum” (= superior colliculus) or in the thalamus; and the thalamus projects to the higher processing center (5), the cerebrum, which is the “cortex” in each figure. For the exceptions, the auditory path has an extra neuron (thus, “4A” and “4B” in figure 5.11), and the main olfactory path does not go to the thalamus or tectum (figure 5.12).31 Without exception, however, all the sensory paths show isomorphic organization through their successive levels, which is important in mapped sensory consciousness. This isomorphism is emphasized in all four figures, 5.9 through 5.12.
Table 5.3 is good for showing the shared features of the pathways for the different senses and how they all have the same plan, but it is not ideal for showing how many neuronal levels are in each pathway. The latter is of interest because we seek the minimum number of levels a sensory hierarchy can have to produce consciousness. We realize that number of levels can only be a crude indicator of consciousness because the amount of processing within and between the levels in a neural hierarchy is as important as the total number of levels. But level number is far easier to measure and count than is within-hierarchy communication, so we will adopt it as a “marker,” to seek the requirements for consciousness.
Upon first consideration, the minimum number of neuronal levels in a conscious sensory hierarchy seems to be four. In humans, the only animals known with certainty to be conscious, this value of four is obtained by counting neurons in the somatosensory pathway of figure 5.10 (where we counted the somatosensory cortex as one of the neuron levels), and in the smell pathway of figure 5.12 (where we counted the orbitofrontal cortex as a level). Five neuronal levels, by contrast, characterize the human visual pathway in figure 5.9 (if the photoreceptors are counted as neurons and the primary visual cortex is also counted as a level) and also the hearing pathway of figure 5.11 (again, where the primary auditory cortex is included as a level). We must stress that the actual number of neuronal levels for consciousness may be higher than this; there is much debate over whether consciousness really emerges in the primary sensory areas, as traditionally thought, or else higher in the cortex.32 More, higher-order processing areas may be required, leading all the way up to the prefrontal cortex.
If conscious sensory images can emerge in the optic tectum of fish and amphibians, as we claim in table 5.3 and we investigate in chapter 6, then the minimum number of neuronal levels in a sensory hierarchy is only three. Such a three-neuron chain makes up the somatosensory pathway in these particular vertebrates, from (1) the sensory neurons to (2) relay neurons in the spinal cord or brainstem to (3) the optic tectum (see figure 6.4, the pathway named “s”). However, four levels characterize the all-important visual and hearing pathways to the tectum of the fish and amphibians, and the smell pathway to the dorsal pallium (see pathways “v” and “oL” in figure 6.4). Again, the actual number of levels may be higher because extensive sensory processing occurs within the tectum.33
We conclude that sensory hierarchies can be conscious if they have four or more levels of neurons projecting to (and including) the highest processing area, or in some cases, just three levels.
To summarize this chapter, the evolution of image-forming eyes in vertebrates led to the first complex sensory hierarchy, to brain expansion, “mental images,” and the “dawn of consciousness.” The known, fishlike Cambrian fossils are consistent with this view. Eye evolution and the visual hierarchy were closely followed by the appearance of additional game-changing structures, the placodes and neural crest, so that similarly constructed hierarchies of smell, touch, hearing, and so on arose and enabled these early brains to create integrated “multisensory images,” thereby enriching sensory consciousness. Isomorphic organization characterized the successive levels of these hierarchies, contributing a mapped order to the resultant sensory images. Upon counting from these hierarchies in various living vertebrates, we deduced that the minimum number of neuronal levels required for consciousness is four in mammals (figures 5.9 through 5.12), and three or four in fish (figure 6.4).
Our concept of neurobiological naturalism postulated a set of special neurobiological features required for sensory consciousness (table 2.1). In modeling how the simple reflex pathways of an amphioxus-like ancestor expanded into complex sensory hierarchies with isomorphic organization as the vertebrates evolved, we have shown how the first vertebrates could have met these requirements and been conscious. But we have not yet considered any specific living vertebrate that might give clues to the origin of consciousness. For that purpose, the next chapter will explore the nervous system of the lamprey, which is in the lineage of living vertebrates that arose first. We will show how the optic tectum of its brain could hold the key to sensory consciousness.
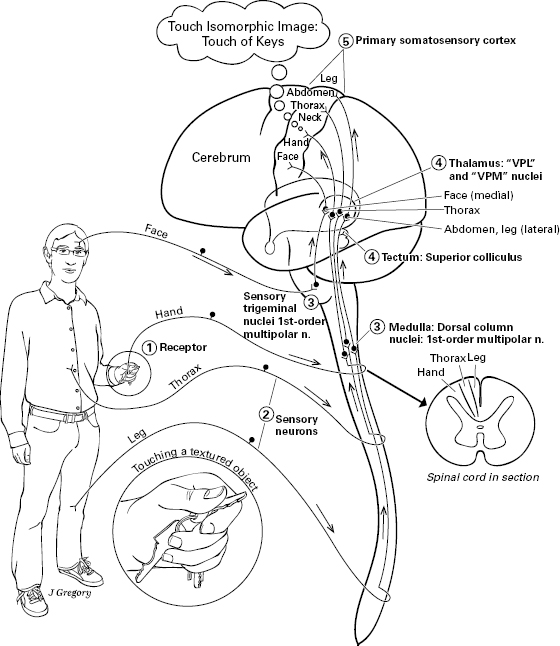
Figure 5.10 Touch: somatosensory-touch pathway in the human. Path goes from a textured object stimulating mechanoreceptors, through sensory neuron, to dorsal column nuclei in medulla oblongata of hindbrain, to thalamus (or to superior colliculus = optic tectum), to primary somatosensory cortex of the cerebrum. Throughout the pathway, the neural structures are isomorphically arranged according to a map of the body, a feature called somatotopy.
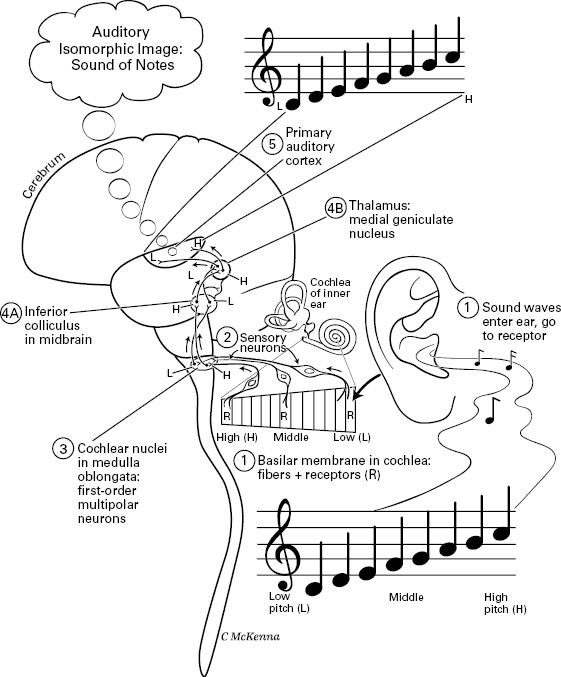
Figure 5.11 Hearing: auditory pathway in the human. Path goes from sound waves stimulating receptor cells (R) in the cochlea of the inner ear, to sensory neurons, to cochlear nuclei, to inferior colliculus, to thalamus, to primary auditory cortex of the cerebrum. Throughout the pathway, the neural structures are arranged according to a map of the basilar receptor-membrane in the cochlea, which in turn is mapped by tone (high, middle, and low pitch), an isomorphic feature called tonotopy.